Abstract
Visual imaging modalities, videofluoroscopic swallow study (VFSS) and fiberoptic endoscopic evaluation of swallow, for assessment of oropharyngeal dysphagia have been part of the speech language pathologist’s (SLPs) armamentarium for the diagnosis and treatment of dysphagia for decades. Recently, the addition of high-resolution manometry (HRM) has enabled the SLP to evaluate pharyngeal pressures and upper esophageal sphincter relaxation. Taken together, the use of visual imaging modalities with HRM can improve interpretation of swallowing physiology and facilitate more effective treatment planning. The goal of this article is to describe a clinical paradigm using HRM as an adjunct to VFSS, by the SLP, in the assessment of complex dysphagia. Moreover, in three cases described, the value of manometric measurements in elucidating swallowing imaging studies and documenting physiologic change in response to treatment is highlighted. As technology in this area is evolving, so will the clinical use of HRM by the SLP. Limitations of current HRM systems and applications are discussed.
Keywords: Deglutition, Deglutition disorders, High-resolution manometry, Pharynx, Upper esophageal sphincter
Introduction
Swallowing is a dynamic, biomechanical process that cannot be comprehensively represented through a single diagnostic approach. Visual imaging modalities, including videofluoroscopic swallowing study (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES), are considered primary evaluative tools for diagnosing and characterizing oropharyngeal dysphagia. Both provide visualization of anatomic and temporal relationships within the pharyngeal mechanism relative to the bolus. Penetration and aspiration of contrast have been identified and rated reliably by both VFSS and FEES [1–4]. Beyond characterization of contrast entering the airways, clinical interpretation of swallowing physiology via video images remains subjective and fraught with challenges in both intra- and inter-rater reliability [5–9]. Bolus residue remaining after the swallow heralds deglutitive inefficiency, though detailed image analysis of biomechanical causes has been impractical in the clinical setting and thus confined to the research laboratory [10]. Individual clinicians often bear the onus of image interpretation in complex dysphagia, where multifactorial physiologic impairments are not clearly delineated by the functional consequences of aspiration or bolus residue. VFSS and/or FEES often fall short in defining physiologic characteristics of oropharyngeal dysphagia for selected patients, compromising precise treatment planning.
Muscular function during swallowing produces exertion of initial force to the bolus, space creation, nasopharyngeal and glottis closure, upper esophageal sphincter (UES) opening, bolus movement, and clearance pressure maintenance. In normal swallowing, these roles are carried out with exquisite temporal accuracy [11–16]. Technologic advances have availed high-resolution manometry (HRM) for measurement of the spatiotemporal features of pharyngeal swallowing [17, 18] and it has been utilized in research to define swallowing pressure events in fine temporal resolution [19–25]. HRM is an established and standardized clinical tool in the categorization of esophageal motility disorders [26]. The use of HRM concurrent with oropharyngeal swallowing imaging as a standard tool in clinical care has been recognized as an emerging model in its early development [18]. Speech language pathology practices within the diagnosis and treatment of swallowing disorders are poised for the adoption of HRM. The American Speech-Language-Hearing Association, ASHA [27] has identified pharyngeal manometry as an area of emerging practice for the speech pathologist. Further, there are clinical precedents for the use of transnasal procedures by speech pathologists, including FEES, videostroboscopy, and esophageal insufflation testing. The ASHA Scope of Practice document [28] defines a broad spectrum of speech language pathologist (SLP) clinical practices “using instrumentation (e.g., videofluoroscopy, electromyography, nasendoscopy, stroboscopy, endoscopy, nasometry, computer technology) to observe, collect data, and measure parameters of communication and swallowing or other upper aerodigestive functions.” Dysphagia treatment planning and outcomes documentation demand objective measurement of targeted pharyngeal function now clinically feasible with HRM. A clinical model for the application of HRM by the speech pathologist is presented in three case studies to embody the roles of this developing tool in the diagnosis and treatment of complex dysphagia.
Methods
HRM Program Development
SLPs participated in a structured HRM mentorship and competency training. Criteria for staff eligibility to initiate training included having obtained a Certificate of Clinical Competence and achieved competency in both VFSS and FEES. The latter criterion ensured experience with patient selection for and performance of transnasal endoscope passage, a skill set directly applicable to performing HRM. A competency checklist was developed to reflect the knowledge and skill set required for safe catheter placement and removal, equipment use, and interpretation of spatiotemporal HRM plots (“Appendix: Competency in HRM” section). Resources for gaining knowledge and skills included recommended readings [11–21, 23–25], equipment training session, and mentor-supervised procedures on at least three normal volunteers and five patients. An otolaryngology head and neck surgeon experienced in performing and interpreting HRM supervised the first phase of competency training.
An existing collaborative multidisciplinary clinic model for dysphagia evaluation was expanded to incorporate HRM in assessment by the speech pathologist. Comprehensive evaluation is performed by a speech pathologist specializing in swallowing disorders, including imaging with VFSS or FEES, and is immediately followed by an otolaryngologist’s examination. In addition to determining diagnosis, this patient-centered model culminates in collaborative treatment planning, including behavioral and medical/surgical considerations. HRM has also been applied by the speech pathologist for baseline and post-therapy outcome measurements in conjunction with imaging studies. HRM has served as a biofeedback tool for swallowing maneuver training, including effortful swallowing and Mendelsohn maneuver instruction.
Selective application of HRM has been performed to ensure the procedure is warranted and feasible (Fig. 1). Eligibility criteria are listed in Table 1. Most HRM evaluations are recommended and performed following VFSS or FEES to determine whether evidence of pharyngeal weakness or UES dysfunction is present. Current HRM systems do not feature synchronization of imaging with spatiotemporal plots to allow concomitant viewing and analysis. For this reason, separate procedures have been performed for most patients to preserve operational efficiency in the videofluoroscopy suite when no benefit is otherwise gained by performing concomitant procedures. An exception is when referrals have been received for evaluation of known Zenker’s diverticulum, as these are scheduled for concurrent videofluoroscopic swallowing evaluation (VFSS) with HRM. Videofluoroscopic guidance of manometer catheter placement for patients with a diverticulum has proved necessary to navigate catheter passage through the UES into the esophagus.
Fig. 1.
HRM decision matrix
Table 1.
Patient eligibility criteria for HRM
| Capable of following complex instructions |
|---|
| No recent facial trauma |
| No recent nasal, pharyngeal, laryngeal, or esophageal surgery |
| No known nasal, pharyngeal, or esophageal obstruction |
Equipment and Catheter Placement
A solid-state high-resolution manometer with impedance has been utilized (ManoScanZ HRM System, Sierra Scientific Instruments, Los Angeles, CA). Manometry catheters feature a 4.0-mm diameter with 36 circumferential pressure channels and 18 impedance channels. The system supports archiving of examinations with spatiotemporal plot visualization in both real-time and playback modes. While automated analysis of esophageal pressures is featured, adaptation for pharyngeal swallowing evaluation currently requires methodical manual analysis of pressure plots [21].
The patient is prepped for catheter insertion with topical application of 2 % viscous lidocaine hydrochloride solution in the naris judged by the patient to be most patent for ease of catheter passage. Lubrication of the catheter with a water-soluble lubricant further assists passage. During blind passage of the catheter, where no visualization of passage is necessary, the patient is instructed to chin tuck while performing sequential water swallows until the length of the catheter has been inserted. For patients with dysphagia who cannot swallow safely in this manner, endoscopic viewing of catheter passage is performed to safeguard against laryngeal entry and achieve effective passage through the UES. Alternatively, for patients with a Zenker’s diverticulum who undergo catheter passage under videofluoroscopic guidance, a smaller-bore catheter without impedance channels provides the advantage of greater rigidity for maneuvering the catheter tip past the diverticulum. Once the catheter has been inserted to the point where the most proximal sensor has entered the nares, it is secured with tape to the patient’s nose for the duration of the manometric examination (Fig. 2). Use of this proximal sensor as a landmark assures adequate sensor placement to capture velar pressures proximally, with the distal tip of the catheter remaining within the esophagus. Dry swallows are gauged online for appropriate pressure quiescence at the UES during dry swallows and propagation within the esophagus to verify catheter placement.
Fig. 2.

Manometric catheter placement
Protocol for Evaluation
Nonswallow tasks are recorded to provide benchmarks for channels measuring nasopharyngeal region pressures against the catheter, including/ka/repeated three times and cough. Saline is utilized as bolus medium to allow for display of impedance, which can indicate the presence of residual saline remaining in the pharynx or esophagus following the swallow. However, the role of impedance measures within the pharynx has remained uncertain as measures have not correlated temporally to represent bolus clearance in a standard manner as utilized and verified at the UES and esophagus [29]. Measured volumes are presented via syringe, with the patient instructed to hold bolus volumes prior to swallowing. Upon the command to swallow, the administering clinician labels the spatial–temporal plot to allow identification of trials during review. Volumes typically assessed include three presentations of 1, 5, and 10 mL each, and one 20-mL volume and consecutive swallows via straw as appropriate. Swallowing trials may be expanded to include assessment of postural strategies, swallowing maneuvers, or other textures. Upon completion of data collection, the manometry catheter is removed.
Analysis
The ManoScanZ HRM System allows for review of spatiotemporal plots by scrolling along the temporal axis. Display may be modified to allow higher temporal resolution review, with an 8-s screen typically used for pharyngeal analysis. Impedance overlay display may be toggled on or off; for the purpose of clear graphic representation of pharyngoesophageal pressure propagation, spatiotemporal plots in this report do not include impedance overlay. The SLP identifies labeled bolus trials, and manual examination is performed utilizing a mouse analysis tool to select areas or points along the swallow plot for measurement.
Specific channels are identified to guide measures, allowing the examiner to define nasopharynx, tongue base, and UES regions in a standard manner across the recorded study (Fig. 3). The nonswallow tasks performed, including/ ka/repetitions and cough, allow for identification of velar elevation against the catheter. The velopharyngeal region is typically defined by at least two channels at the superior aspect of the pharyngeal spatiotemporal plot. Just inferior to these two channels, the base-of-tongue region can be identified. Resting pressures of the UES against the catheter are easily identified as the band of pressure spanning between swallowing efforts. Cricoid rise together with pharyngeal shortening will result in UES movement along the catheter during swallowing trials. UES opening away from the catheter is immediately followed by the post-swallow pressure region, providing a landmark for identifying UES superior excursion and the channel for measurement of minimum pressures during UES opening.
Fig. 3.
Normal swallowing spatiotemporal plot
Manual measurements calculated to characterize swallowing events include duration (s) of swallow and UES opening; mean maximum closure and bolus clearance pressures (mmHg) at the velopharynx, tongue base region, and UES; and mean minimum pressure (mmHg) during UES opening.
Clinical Roles of HRM
Three patients chosen to illustrate the role of HRM in oropharyngeal dysphagia diagnosis are described in Table 2. All had undergone multiple prior imaging studies at other hospitals. Each presented to our clinic seeking a second opinion regarding dysphagia treatment, with the most recent imaging record provided for review. During the interval between dysphagia diagnosis and presentation to our clinic, two had encountered aspiration pneumonia. Two of the three remained strictly NPO, taking all nutrition and hydration via gastrostomy tube, with the goal of discontinuing tube feeding. The third patient had remained dependent upon nectar liquids, with a goal of advancing his diet to allow thin liquids. All had participated in behavioral therapy with a SLP in the months immediately preceding consultation with our clinic without success in achieving their stated goals.
Table 2.
Patient demographics
| Patients | Gender | Ages | Diagnosis | FT/ duration | Baseline diet | Prior imaging | Therapy |
|---|---|---|---|---|---|---|---|
| 1 | Male | 62 | Squamous cell carcinoma right TVF s/p excisional biopsy 10 years post-radiotherapy Xerostomia |
n/a | Moistened general diet, nectar liquids | VFSS | TSE |
| 2 | Female | 58 | Squamous cell carcinoma BOT 12 years post-chemoradiation Lingual atrophy Tongue base, laryngeal scarring UES stricture s/p dilation |
6 months | NPO | VFSS | TSE, NMES |
| 3 | Male | 65 | Left lateral medullary stroke (Wallenberg’s syndrome) Left vocal fold paralysis 6 months post-stroke |
6 months | NPO | VFSS | TSE |
NPO nil per os, TSE traditional swallowing exercise, NMES neuromuscular electrical stimulation (VitalStim)
In each clinical example, the decision to use HRM was based upon the presence of bolus residue after swallows in reviewed VFSS studies, as depicted in the HRM decision matrix (Fig. 1). Endoscopic visualization of catheter passage was utilized as none of the featured participants was capable of consecutive swallows of thin liquids via straw without aspiration. All tolerated catheter placement and HRM protocol well without side effects monitored during and after the procedure.
Differentiating Impairment
Six months prior to his referral to our clinic, Patient 1 suffered a leg fracture requiring hospitalization. During his hospitalization, complications of aspiration pneumonia were reported. Swallowing studies during and following his hospitalization demonstrated silent aspiration of thin liquids likely related to a history of head/neck cancer treatment more than 10 years prior to his injury (Table 2). Review of outside videofluoroscopic imaging revealed penetration during and aspiration after swallows due to reduced laryngeal closure and pyriform sinus stasis. There was no radiographic evidence of UES stricture or opening impairment. FEES examination was pursued in our clinic to provide additional information on laryngeal structure and function, evaluate aspiration and bolus stasis patterns, and explore positioning strategies. A head turn to the right with chin tuck was applied during visualization, with apparent success in eliminating the volume of bolus residue in the pyriform sinuses, and addition of a supraglottic swallow strategy eliminated penetration to the vocal folds. The bolus residue that remained in the hypopharynx without the postural strategy could have resulted from either reduced pharyngeal constriction or impaired UES opening related to prior radiotherapy. Neither FEES nor VFSS could objectively differentiate which of these impairments might be the root cause of stasis and resultant aspiration. HRM was recommended to specify the mechanism by which the head turn was effective in improving bolus clearance from the pyriform sinuses so that treatment planning may specifically target the identified dysfunction.
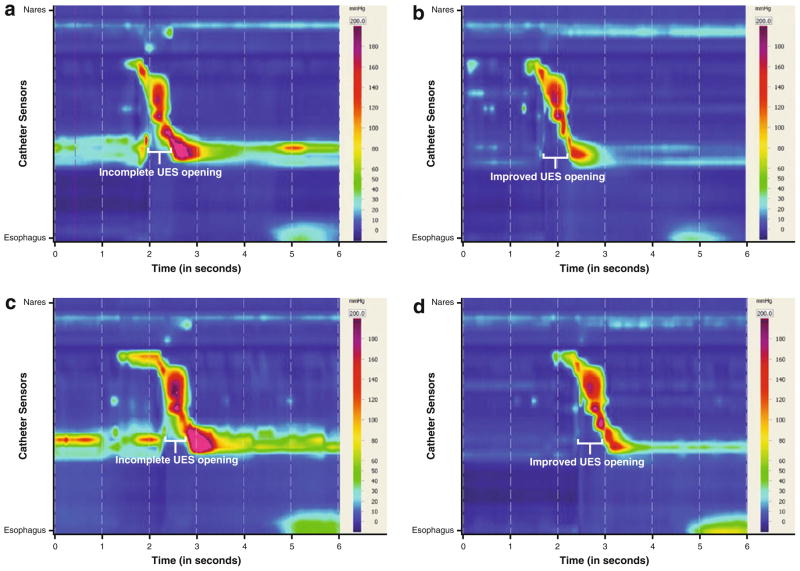
Three 1-mL volumes of saline were tested in neutral and head turn positions, and three 5-mL volumes were tested with supraglottic swallow with and without head turn right with chin tuck. The supraglottic swallow was used with the 5-mL boluses to manage penetration to the vocal folds secondary to reduced supraglottic closure. Spatiotemporal plots for the two bolus volumes and head turn right with chin tuck condition are presented in Fig. 4. Corresponding pressure measurements are listed in Table 3. In the neutral position condition for both 1- and 5-mL boluses, a lack of adequate UES opening was visually represented by continuous pressure along the catheter plotted at the UES during bolus transit (Fig. 4a, c). Conversely, in the head turn right with chin tuck condition, the UES opening was visible between the same points in Fig. 4b, d. Other collected pressure maximums in the nasopharyngeal and tongue base regions were within normal limits compared to normative values [19]. These HRM measures, when considered with the FEES results, suggest that the head turn right achieves a consistent biomechanical effect on the UES opening to increase bolus clearance from the hypo-pharynx during swallows and to prevent aspiration after swallow. Use of the head turn right with chin tuck combined with a supraglottic swallow strategy was presented as the compensatory treatment plan for returning to thin liquids at meals. Swallowing therapy was prescribed to provide adequate training in the use of the head turn combined with the supraglottic swallow maneuver prior to diet advancement, which was achieved within 3 weeks following the initial evaluation. Post-therapy assessment with VFSS 4 weeks after the initial FEES verified safe swallowing of thin liquids taken in small volumes with the applied strategies. Therapy interventions and outcomes are listed in Table 4. The patient’s penetration–aspiration score for 5 mL of thin liquid improved from 8 to 4 over the 4-week period. The patient pursued continued therapy with his local SLP, with repeat VFSS recommended following an 8-week exercise regimen to determine the need for swallowing strategies over the long term.
Fig. 4.
Spatiotemporal plots, Patient 1: 1 mL saline (a), 1 mL saline using head turn right with chin tuck (b), 5 mL with supraglottic swallow strategy (c), 5 mL with SGS and head turn right with chin tuck (d)
Table 3.
Manometric measures: Patient 1
| Bolus volumes (mL) | Mean (±SD) nasopharyngeal region maximum (mmHg)
|
Mean (±SD) tongue base region maximum (mmHg)
|
Mean (±SD) post-swallow pressure maximum (mmHg)
|
Mean (±SD) UES opening pressure minimum (mmHg)
|
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Head turn right | Baseline | Head turn right | Baseline | Head turn right | Baseline | Head turn right | |
| 1 | 158.2 ± 10.9 | 150.1 ± 28.6 | 150.9 ± 30.9 | 170.4 ± 29.0 | 279.3 ± 8.7 | 172.9 ± 10.2 | 14.6 ± 2.0 | 0.8 ± 1.7 |
| 5 (SGS) | 118.6 | 124.7 ± 1.4 | 204.2 | 185.0 ± 8.6 | 408.2 | 201.5 ± 45.7 | 16.8 | 3.2 ± 2.8 |
SGS supraglottic swallow maneuver, SD standard deviation
Table 4.
Treatment and outcomes following HRM
| Patients | Dysphagia characteristics | Interventions | Treatment intervals (weeks) | FT | Post- treatment diet |
|---|---|---|---|---|---|
| 1 | Delayed pharyngeal response, hyolaryngeal excursion, UES opening | Head turn right with chin tuck—thin liquids only Supraglottic swallow, Shaker exercise | 4 | – | Moistened general diet, thin liquids |
| 2 | Oral transit, tongue base propulsion, pharyngeal sequencing, hyolaryngeal excursion, timing and duration of UES opening | Exercise regimen: lingual resistance, Shaker exercise, effortful swallow, Mendelsohn maneuver, supraglottic swallow; lean to left—all textures | 26 | N | Diced 1/4 in. solids, thin liquids |
| 3 | Tongue base propulsion, pharyngeal constriction, hyolaryngeal excursion, glottal closure, cricopharyngeal relaxation | Exercise regimen: lingual resistance, Shaker exercise, effortful swallow, Mendelsohn maneuver; cricopharyngeal myotomy; head turn left with chin tuck—all textures | 16 | N | Mechanical soft diet, nectar liquids |
Measuring Treatment Outcomes
Patient 2 described swallowing a general diet with thin liquids until about 1 year prior to consultation. She suffered decline in her swallowing ability and significant health impact over the first 6 months of that year until she agreed to gastrostomy tube placement during hospitalization for malnutrition and dehydration. Her medical history included chemoradiation treatment for tongue base squamous cell carcinoma 12 years prior (Table 2). In the midst of her functional decline, ENT examinations and biopsies were performed with no evidence of tumor recurrence. Outside VFSS imaging reported mild oral and severe pharyngeal phase dysphagia characterized by impaired hyolaryngeal excursion, impaired pharyngeal transit, reduced UES opening, and resultant aspiration of thick and thin liquids. The patient underwent UES dilation to 36 Fr 4 months prior to this evaluation to address stricture, and repeat dilation to 39 Fr a month later. The patient participated in swallowing treatment for 6 weeks at an outside hospital immediately following the second dilation (Table 2).
FEES examination was performed in our clinic to evaluate current swallowing function and better characterize laryngeal function. Velopharyngeal closure appeared to be effective during speech tasks as judged by range of velar and lateral nasopharyngeal wall movement. The absence of epiglottic structure was identified with significant scar banding extending from the tongue base to the arytenoids bilaterally. A greater amount of pyriform sinus space was appreciated on the left side of the pharynx to accommodate stasis. Head turning to either side was ineffective in improving bolus transit and swallowing safety. Trials of 5 mL of nectar liquid were presented with a lean to left with reduced penetration and eliminated aspiration. Application of a supraglottic swallow maneuver was added to facilitate clearance of bolus material from the exposed and scarred larynx. Thin liquids were aspirated despite the strategy used, and the use of pudding resulted in significant stasis extended from the tongue base to pyriform sinuses. FEES imaging was unable to clearly indicate why the bolus material remained throughout the pharynx after swallows. HRM was recommended to provide objective measures of pharyngeal and UES functioning.
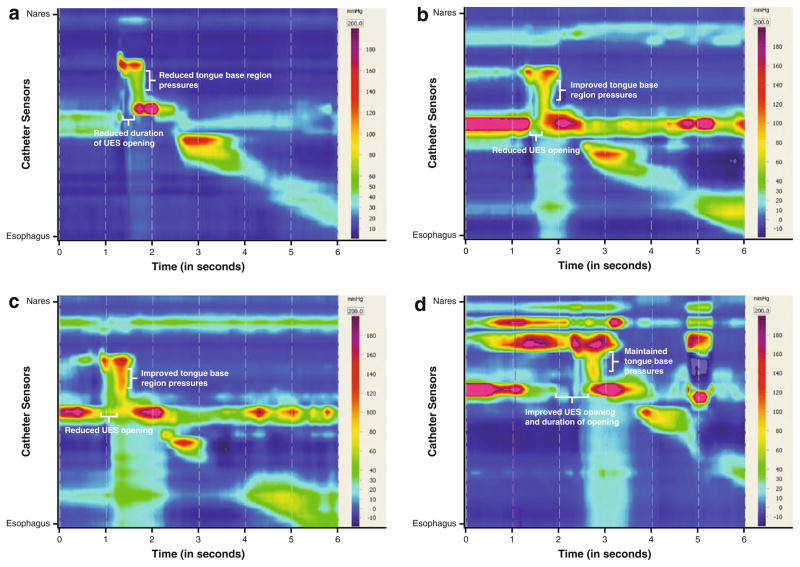
HRM was performed at the initial visit following FEES, with volumes of saline limited to 1 mL because of the risk of aspiration identified on imaging studies (Table 5). Spatiotemporal plots exhibited a relatively vertical appearance to pressure propagation, with velopharyngeal region, tongue base region, and hypopharyngeal region pressures occurring concurrently, suggesting a loss of temporal motor sequencing (Fig. 5). Nasopharyngeal region pressures were within normal limits [19]. Pressures in the tongue base region indicated reduced contact with the catheter, with a mean maximum of 78.2 (±11.5 SD) mmHg while swallowing 1-mL boluses without strategy use. Mean UES post-swallow clearance pressure maximum was within normal limits [19], but the timing of this pressure presented an obstructive pattern during bolus transit, with the peak concurrent with the tongue base region maximum pressures. Mean UES opening minimum pressures indicated incomplete opening with a residual pressure minimum of 14.5 (±3.8) mmHg, with shortened duration of opening at 0.3 s [19]. Given the reduced hyolaryngeal excursion assessed on prior VFSS, reduced hyolaryngeal mechanical pull on the UES was diagnosed as contributing to a poor UES opening. Her reduced tongue base region pressures may not generate an adequate bolus tail transit through the fibrotic pharynx and override elevated UES pressures and a poorly coordinated opening.
Table 5.
Manometric measures: Patient 2
| Bolus volumes (mL) | Mean (±SD) nasopharyngeal region maximum (mmHg)
|
Mean (±SD) tongue base region maximum (mmHg)
|
Mean (±SD) post-swallow pressure maximum (mmHg)
|
Mean (±SD) UES opening minimum pressure (mmHg)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Tx | Detrain | Baseline | Post-Tx | Detrain | Baseline | Post-Tx | Detrain | Baseline | Post-Tx | Detrain | |
| 1 | 158.8 ± 27.7 | 159.5 ± 22.1 | 229.5 ± 6.5 | 78.2 ± 11.5 | 106.3 ± 12.9 | 104.9 ± 28.6 | 483.7 ± 4.7 | 185.9 ± 92.3 | 676.0a ± 0 | 14.5 ± 3.8 | 31.7 ± 3.6 | 16.7 ± 15.4 |
| 5 (LTL) | – | 171.6 ± 6.3 | 263.4 ± 14.2 | – | 91.4 ± 16.1 | 81.4 ± 6.7 | – | 336.4 ± 22.9 | 633.5 ± 73.6 | – | 34.4 ± 6.9 | 7.4 ± 7.8 |
| 10 (LTL) | – | 243.0 ± 1.6 | – | 76.0 ± 4.6 | – | – | 670.8 ± 9.1 | – | – | 14.5 ± 1.8 | ||
LTL lean to left, Post-Tx post-therapy and surgical intervention 16 weeks after initial evaluation, Detrain detraining 26 weeks following initial evaluation, no therapy for 14 weeks, SD standard deviation
Sensor ceiling reached on each of three trials
Fig. 5.
Spatiotemporal plots, Patient 2: 1 mL, baseline (a), 1 mL, 12 week post-therapy (b), 5 mL, 12-week post-therapy, with lean to left (c), 5 mL, 26 weeks detraining, with lean to left (d)
These detailed HRM findings guided establishment of measurable behavioral therapy goals to increase bolus driving pressures at the tongue base and target lower UES minimum pressures during bolus transit to achieve improved swallowing efficiency. FEES results verified the lean to the left strategy to compensate for laryngeal structural abnormality and avoid aspiration of nectar thick liquids. Intensive swallowing therapy was prescribed in a 12-week home regimen of repetitive strengthening exercise (lingual resistance, Shaker exercise), swallow-specific exercise (effortful swallow, Mendelsohn maneuver), and daily trials of nectar liquids in restricted volumes with lean to left.
VFSS and HRM were repeated following a 12-week exercise regimen and again at 26 weeks following an equivalent period of detraining. Improved pressures at the tongue base region were documented at 12 weeks and appeared to be maintained above 100 mmHg for 1-mL volumes of saline at 26 weeks (Table 5). Use of the leaning position with the supraglottic swallow allowed 5-mL boluses of saline to be presented with HRM at the post-therapy point, and at 26 weeks the strategy allowed 10-mL boluses to be tested. An anticipated inverse bolus volume effect at the tongue base region was evident for larger volumes tested [19]. Functional improvement was evident at each of the 12-week post-therapy and 26-week detraining measurement points on both VFSS and HRM. Patient 2’s penetration–aspiration score for 5 mL of thin liquid improved from 7 to 2 over the 26-week period. The patient’s therapy interventions and outcomes are listed in Table 4. It is important to note the variability in the duration of the opening of the UES among the HRM testing points. Repeat dilations were not performed in the detraining interim. The significant expansion of diet textures and oral feeding volumes following the patient’s 13-week evaluation may have further trained the UES for a longer opening, though the timing of post-swallow pressures appeared unchanged. The spatiotemporal plot representing 5 mL of saline after detraining (Fig. 5d) demonstrates a marked increase in duration of velopharyngeal elevation and UES opening compared to her initial and post-therapy evaluation points. These differences represent behavioral changes in her swallowing function that had evolved during the detraining period and likely contributed to her improved functional outcomes.
Guiding Behavioral and Surgical Interventions
Patient 3 was referred for collaborative swallow, voice, and ENT evaluation 6 months after a left lateral medullary stroke (Table 2). He reported participation in both inpatient acute rehabilitation and outpatient rehabilitation at outside facilities. He suffered aspiration pneumonia during his acute hospitalization but had maintained stable respiratory status since hospital discharge 5 months before seeing us. A vocal fold injection had been performed within the week following the stroke without improvement to vocal function as per patient report.
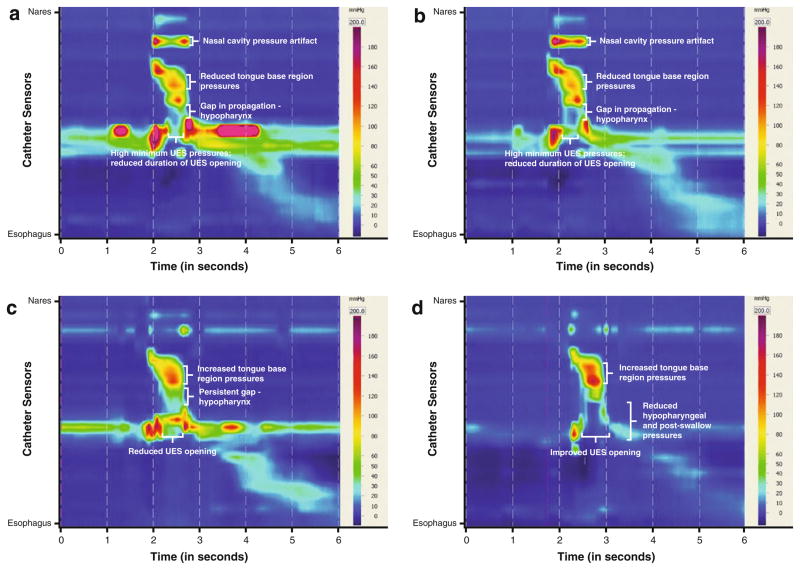
In consultation with our clinic, vocal function and ENT evaluations revealed severe dysphonia due to left vocal fold paralysis. His last VFSS, performed at an outside facility 2 weeks prior to consultation, was reviewed; it showed poor hyolaryngeal excursion, weakened pharyngeal constriction, and UES dysfunction resulting in aspiration of thickened liquids and severe stasis with pudding despite the use of a head-turning strategy. HRM was recommended to provide objective measures of pharyngeal pressures and UES opening in the conditions of head neutral and head turn to left with chin tuck to guide behavioral and medical/surgical interventions. Nasopharyngeal baseline pressures appeared to have been within normal limits [19] in both neutral and head-turning positions at 180.0 (±2.3) and 153.6 mmHg, respectively (Table 6). Tongue-base-region pressures were below normal values [19], i.e., 110.5 (±3.5) and 106.2 mmHg for 1-mL volumes in neutral and head-turning positions, respectively. The UES opening did not appear to respond to the application of the head turn left and chin tuck. Minimum UES pressures were above normal values [19] at 14.2 (±7.2) mmHg without the positioning strategy and 16.8 mmHg with the positioning strategy, indicating UES pressure along the catheter during bolus transit in both conditions. Based upon these data, the surgeon proposed the addition of endoscopic cricopharyngeal myotomy with CO2 laser to the already planned surgical intervention of left Gore-Tex medialization thyroplasty with left arytenoid adduction, to address presumed failure of cricopharyngeus relaxation related to medullary stroke. Intensive behavioral intervention was recommended to train the patient to perform the head turn strategy and initiate a swallowing exercise regimen.
Table 6.
Manometric measures: Patient 3
| Bolus volumes (mL) | Mean (±SD) nasopharyngeal region maximum (mmHg)
|
Mean (±SD) tongue base region maximum (mmHg)
|
Mean (±SD) post-swallow pressure maximum (mmHg)
|
Mean (±SD) UES opening minimum pressure (mmHg)
|
||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Post-Tx | Baseline | Post-Tx | Baseline | Post-Tx | Baseline | Post-Tx | |
| 1 | 180.0 ± 2.3 | 97.2 ± 3.8 | 110.5 ± 3.5 | 113.4 ± 6.2 | 342.5 ± 112.6 | 131.8 ± 62.0 | 14.2 ± 7.2 | 36.9 ± 9.2 |
| 1 HTL-CT | 153.6 | 91.8 | 106.2 | 142.9 | 173.2 | 30.2 | 16.8 | 1.0 |
| 5 HTL-CT | – | 91.0 ± 7.3 | – | 113.2 ± 4.3 | – | 37.4 ± 5.3 | – | 6.5 ± 0.5 |
Post-Tx post-therapy and surgical intervention 16 weeks after initial evaluation, HTL-CT head turn left with chin tuck, SD standard deviation
HRM findings guided behavioral therapy planning by establishing measurable goals for increasing tongue base region bolus-driving pressures, and increasing duration and degree of UES opening during bolus transit. During his initial HRM, the patient performed the effortful swallow and Mendelsohn maneuver to determine exercise potential for an intensive home program. He was capable of volitionally increasing duration of pressures against the catheter at the velum, tongue base region, and hypopharynx. Intensive swallowing therapy was prescribed prior to and following surgery in a home regimen of repetitive strengthening exercise (Table 4). VFSS performed 1 week after cricopharyngeal myotomy (1 month after initial evaluation) demonstrated Patient 3’s ability to safely advance oral feeding to 4-oz. boluses of minced solids and nectar liquids three times daily using the head turn left with chin tuck. Intensive swallowing exercise was continued in therapy. His recent surgery was a contraindication for performing HRM at this point in his treatment (Table 1). VFSS with HRM was repeated 16 weeks after initial evaluation, which was 12 weeks after surgical intervention and initiation of oral feeding. Velopharyngeal pressures against the catheter were reduced across all trials; this may represent reduced compensatory effort to preserve pharyngeal pressures. Mean tongue-base-region measures remained relatively unchanged in the neutral position following surgery and behavioral therapy, but when using a head turn left and chin tuck, his tongue-base-region measures increased to 142.9 mmHg for 1 mL of saline. Of greatest significance was the difference in the UES opening between the neutral position and head turn left with chin tuck (Fig. 6c, d). At the 16-week evaluation point, minimum UES pressures during bolus transit increased to above his baseline UES pressures in the neutral position. However, with a head turn left and chin tuck applied, his mean minimum UES pressures dropped to near-normal values [19]. These patterns suggest that the patient’s UES opening following cricopharyngeal myotomy had been specifically trained to the head-turn positioning condition. The patient was taught the critical need for the use of the head turn with chin tuck application and shown the measurable differences in UES opening under each condition. Functional outcomes for this patient included discontinued tube feedings with tube removal and return to a modified oral diet (Table 4). The patient’s penetration–aspiration score for 5 mL of thin liquid improved from 8 to 1 over the 16-week period, though trace aspiration of 10-mL volumes or greater has persisted. Vocal fold medialization was successful, though left vocal fold atrophy together with vocal fold plane asymmetry has persistently compromised glottic closure. The patient has since undergone further vocal fold injection and has continued to undergo swallowing therapy with the goal of swallowing larger volumes of thin liquids safely.
Fig. 6.
Spatiotemporal plots, Patient 3: 1 mL baseline (a), 1 mL using head turn left with chin tuck baseline (b), 1 mL post-therapy and surgical intervention (c), 1 mL post-therapy and surgical intervention using head turn left with chin tuck (d)
Discussion
The use of HRM in clinical diagnosis and treatment remains in its early development. HRM offers possibilities in identifying patterns of impairment, where spatiotemporal profiles may herald underlying etiologies for oropharyngeal dysphagia. In the clinical examples presented, HRM provided insight into the biomechanical causes for bolus retention within the valleculae and pyriform sinuses to guide behavioral or surgical interventions. Timing and degree of UES opening away from the manometry catheter can be objectively represented when radiographic or endoscopic signs are vague, such as in complex fibrosis of the swallowing mechanism or with brainstem stroke as represented in this clinical application. HRM offers the opportunity to plan and demonstrate measurable progress with behavioral and surgical interventions, which, when combined with imaging study ratings of aspiration, can objectively represent physiologic change resulting from treatment rendered.
Placement of the HRM catheter requires patient motivation and full cooperation, and thus a selective application model was presented. Greater severity or complexity of oropharyngeal dysphagia, with hallmarks on imaging that warrant HRM, is most appropriately targeted with this relatively invasive procedure. Up to now, there have been no studies that characterize potential side effects of HRM or quantify the patient experience. As this tool is adapted for oropharyngeal dysphagia assessment, it will be important to establish tolerance and side effects to better weigh the potential risks of the procedure in individual patients against benefits gained in treatment planning.
It has become imperative to systematically determine whether combining HRM data collection with swallowing imaging studies improves intra- and inter-rater reliability in interpretation of pharyngeal and UES function on VFSS and FEES. Given the unique visual perspectives of each imaging technique, exploring the combination of either with HRM may render a different value. Visualization of the spatial relationships within the pharynx and UES during VFSS has offered an advantage in interpretation of HRM data in this early model. Each of the cases presented had recent VFSS images available for review to assist in HRM interpretation. HRM in turn determined the cause of bilateral pyriform sinus stasis in the first patient as related to reduced UES opening, and measured a physiologic benefit of the strategy when applied. The distorted anatomy of the second patient precluded ease of interpreting radiographic or endoscopic signs of bolus material remaining after swallows. The ability to measure pressure against the catheter through the scarred, fibrotic pharynx and UES provided objective data regarding tongue base, pharyngeal, and UES function. Insight into the swallow-specific training that results from intensive behavioral swallowing therapy was evident in the third patient’s HRM measurements, bringing objective dimension to pre- and post-treatment outcome measures.
Technologic advancements are necessary for optimal clinical application of HRM. At this point in time, options for synchronizing imaging studies with manometric data are too technically complex to be efficiently applied in standard clinical swallowing care. Manual synchronization of imaging with HRM is possible following completion of a study but can be labor intensive. Accurate and simple synchronization of manofluoroscopic evaluations will promote reliability of defined regions along the HRM catheter. Automated analysis holds the promise of standardizing measures, with algorithms that can maximize use of spatiotemporal data in representing swallowing physiology [21, 30, 31]. The value of impedance data in the pharynx may be greater when combined with manometric measures in composite analysis [29, 32]. Ongoing research is needed to provide the evidence base that can define the role of HRM in swallowing care delivery. Widespread clinical application will require expanded age-specific normative data for pharyngeal HRM that can be based upon larger sample sizes. Ultimately, defining spatiotemporal plot regions, measurement standards, and disordered profiles may drive a diagnostic classification system for pharyngeal dysphagia that is analogous to the Chicago classification of esophageal motility [26].
Application of HRM in comprehensive swallowing evaluation presents the opportunity for improved objective measurement of interventions targeting functional swallowing improvement. The third patient described here embodied the challenge faced by surgeons and swallowing clinicians when combined treatment modalities are necessary to achieve functional improvement. HRM provides objective data to assist communication of patient status and eligibility for interventions among dysphagia team members. Combining HRM measures with the described imaging signs may substantiate interpretation and better define the role of behavioral treatment to optimize surgical outcomes. As third-party payers demand more detailed baseline and therapy outcome documentation, the objective data provided by HRM has the promise of substantiating clinical outcomes achieved through behavioral and surgical interventions.
HRM complements instrumental swallowing imaging studies by providing objective measures of pharyngeal and UES pressures, clarifying muscular function and physiology for interpretation of imaging. Improving the accuracy of swallowing evaluation is essential to increasing treatment efficacy. The cases presented demonstrate the promise of HRM to empower clinicians in interpretation of swallowing imaging and documentation of physiologic outcomes of treatment.
Acknowledgments
This work was funded in part from the Diane M Bless Endowed Chair, Division of Otolaryngology-Head and Neck Surgery, University of Wisconsin Madison and from the NIDCD grant NIH 4 R33 DC011130-03.
Appendix: Competency in HRM
| Competency: High Resolution Manometry (HRM) | |||
|---|---|---|---|
| Eligibility: □ CCC-SLP | □ VFSS competency achieved | □ FEES competency achieved | |
| Does Not Meet Competency Requirements | Meets Competency Requirements | Comments | |
| Knowledge Required: The candidate will describe-- | |||
| Scope of SLP manometry practice as defined by state and national professional guidelines and regulations | |||
| Indications and contraindications for a manometric examination in oropharyngeal dysphagia | |||
| Appropriate dosage, risks associated with and contraindications of use of topical anesthetic during manometric examination | |||
| Signs of appropriate and inappropriate functioning of manometric and recording equipment | |||
| Troubleshooting strategies for catheter placement and manometric sensor functioning | |||
| Normal and abnormal manometric findings in terms of swallowing anatomy and physiology | |||
| Evidence-based practice related to use of HRM in diagnosis and treatment of oropharyngeal dysphagia | |||
| Role of HRM in biofeedback and education of patients, family and caregivers | |||
| Appropriate timing for re-evaluation of swallowing pressures with manometric examination | |||
| Skills Required: The candidate will demonstrate-- | |||
| Operation, maintenance and disinfection of HRM equipment | |||
| Rationale for HRM in a specific patient as based on obtaining complete medical, surgical and swallowing history | |||
| Preparation of bolus media in accordance with facility-specific protocol, with appropriate consideration of individual aspiration risk | |||
| Patient instruction regarding the goals for HRM examination | |||
| Safe and effective application of topical anesthetic | |||
| Catheter insertion technique that prevents complications and causes minimal patient discomfort | |||
| Patient instruction of protocol tasks for efficient and complete examination | |||
| Administration of bolus volumes/types in a measured and logical presentation | |||
| Evaluation of swallowing physiology as represented by spatiotemporal plots and manually derived pressure and impedance measures | |||
| Assessment of postures and maneuvers based upon imaging and manometric findings | |||
| Monitoring of possible risks related to the examination | |||
| Manometer catheter removal technique that prevents complications and causes minimal patient discomfort | |||
| Interpretation and documentation of HRM findings in a written report | |||
| Ability to integrate HRM findings into dysphagia diagnosis and treatment plan | |||
| Training: Normals (3 minimum) | Signature |
|---|---|
| Supervised Procedures (5 minimum) | Signature |
|---|---|
| I have witnessed this clinician's knowledge and skills for performing high resolution manometry (HRM) and attest to his/her achievement of critical competency in HRM: | |
| signature _________________ date:__________________ | |
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose.
References
- 1.Rosenbek JC, Robbins JA, Roecker EB, et al. A penetration–aspiration scale. Dysphagia. 1996;11:93–8. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 2.Aviv JE. Prospective, randomized outcome study of endoscopy versus modified barium swallow in patients with dysphagia. Laryngoscope. 2000;110:563–74. doi: 10.1097/00005537-200004000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Kelly AM, Drinnan MJ, Leslie P. Assessing penetration and aspiration: how do videofluoroscopy and fiberoptic endoscopic evaluation of swallowing compare? Laryngoscope. 2007;117:1723–7. doi: 10.1097/MLG.0b013e318123ee6a. [DOI] [PubMed] [Google Scholar]
- 4.Colodny N. Interjudge and intrajudge reliabilities in fiberoptic endoscopic evaluation of swallowing (FEES) using the penetration–aspiration scale: a replication study. Dysphagia. 2002;17:308–15. doi: 10.1007/s00455-002-0073-4. [DOI] [PubMed] [Google Scholar]
- 5.Kuhlemeier KV, Yates P, Palmer JB. Intra- and interrater variation in the evaluation of videofluorographic swallowing studies. Dysphagia. 1998;13:142–7. doi: 10.1007/PL00009564. [DOI] [PubMed] [Google Scholar]
- 6.McCullough GH, Wertz RT, Rosenbek JC, et al. Inter- and in-trajudge reliability for videofluoroscopic swallowing evaluation measures. Dysphagia. 2001;16:110–8. doi: 10.1007/PL00021291. [DOI] [PubMed] [Google Scholar]
- 7.Scott A, Perry A, Bench J. A study of interrater reliability when using videofluoroscopy as an assessment of swallowing. Dysphagia. 1998;13:223–7. doi: 10.1007/PL00009576. [DOI] [PubMed] [Google Scholar]
- 8.Stoeckli SJ, Huisman TA, Seifert B, et al. Interrater reliability of videofluoroscopic swallow evaluation. Dysphagia. 2003;18:53–7. doi: 10.1007/s00455-002-0085-0. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox F, Liss JM, Siegel GM. Interjudge reliability in video-fluoroscopic studies of swallowing. J Speech Hear Res. 1996;39:144–52. doi: 10.1044/jshr.3901.144. [DOI] [PubMed] [Google Scholar]
- 10.Pauloski BR, Rademaker AW, Kern M, Shaker R, Logemann JA. The feasibility of establishing agreement between laboratories for measures of oropharyngeal structural movements. J Med Speech Lang Pathol. 2009;17:9–19. [PMC free article] [PubMed] [Google Scholar]
- 11.Hiss SG, Huckabee ML. Timing of pharyngeal and upper esophageal sphincter pressures as a function of normal and effortful swallowing in young healthy adults. Dysphagia. 2005;20:149–56. doi: 10.1007/s00455-005-0008-y. [DOI] [PubMed] [Google Scholar]
- 12.Kendall KA, McKenzie S, Leonard RJ, et al. Timing of events in normal swallowing: a videofluoroscopic study. Dysphagia. 2000;15:74–83. doi: 10.1007/s004550010004. [DOI] [PubMed] [Google Scholar]
- 13.Mendell DA, Logemann JA. Temporal sequence of swallow events during the oropharyngeal swallow. J Speech Lang Hear Res. 2007;50:1256–71. doi: 10.1044/1092-4388(2007/088). [DOI] [PubMed] [Google Scholar]
- 14.Perlman AL, Palmer PM, McCulloch TM, et al. Electromyographic activity from human laryngeal, pharyngeal, and submental muscles during swallowing. J Appl Physiol. 1999;86:1663–9. doi: 10.1152/jappl.1999.86.5.1663. [DOI] [PubMed] [Google Scholar]
- 15.Steele CM, Huckabee ML. The influence of orolingual pressure on the timing of pharyngeal pressure events. Dysphagia. 2007;22:30–6. doi: 10.1007/s00455-006-9037-4. [DOI] [PubMed] [Google Scholar]
- 16.Van Daele DJ, McCulloch TM, Palmer PM, et al. Timing of glottic closure during swallowing: a combined electromyographic and endoscopic analysis. Ann Otol Rhinol Laryngol. 2005;114:478–87. doi: 10.1177/000348940511400610. [DOI] [PubMed] [Google Scholar]
- 17.Takasaki K, Umeki H, Enatsu K, et al. Investigation of pharyngeal swallowing function using high-resolution manometry. Laryngoscope. 2008;118:1729–32. doi: 10.1097/MLG.0b013e31817dfd02. [DOI] [PubMed] [Google Scholar]
- 18.Nativ-Zeltzer N, Kahrilas PJ, Logemann JA. Manofluorography in the evaluation of oropharyngeal dysphagia. Dysphagia. 2012;27:151–61. doi: 10.1007/s00455-012-9405-1. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman MR, Ciucci MR, Mielens JD, et al. Pharyngeal swallow adaptations to bolus volume measured with high-resolution manometry. Laryngoscope. 2010;120:2367–73. doi: 10.1002/lary.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffman MR, Mielens JD, Ciucci MR, et al. High-resolution manometry of pharyngeal swallow pressure events associated with effortful swallow and the Mendelsohn maneuver. Dysphagia. 2012;27:418–26. doi: 10.1007/s00455-011-9385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mielens JD, Hoffman MR, Ciucci MR, et al. Automated analysis of pharyngeal pressure data obtained with high-resolution manometry. Dysphagia. 2011;26:3–12. doi: 10.1007/s00455-010-9320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omari TI, Dejaeger E, van Beckevoort D, et al. A method to objectively assess swallow function in adults with suspected aspiration. Gastroenterology. 2011;140:1454–63. doi: 10.1053/j.gastro.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Takasaki K, Umeki H, Kumagami H, et al. Influence of head rotation on upper esophageal sphincter pressure evaluated by high-resolution manometry system. Otolaryngol Head Neck Surg. 2010;142:214–7. doi: 10.1016/j.otohns.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Takasaki K, Umeki H, Hara M, et al. Influence of effortful swallow on pharyngeal pressure: evaluation using a high-resolution manometry. Otolaryngol Head Neck Surg. 2011;144:16–20. doi: 10.1177/0194599810390885. [DOI] [PubMed] [Google Scholar]
- 25.McCulloch TM, Hoffman MR, Ciucci MR. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann Otol Rhinol Laryngol. 2010;119:369–76. doi: 10.1177/000348941011900602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJPM, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.American Speech-Language-Hearing Association. [Accessed 25 Nov 2012];Report on emerging areas of clinical practice. 2008 http://www.asha.org/academic/reports/EmergingAreasClinicalPractice.htm.
- 28.American Speech-Language-Hearing Association. [Accessed 1 Jan 2013];Scope of practice in speech–language pathology [Scope of Practice] 2007 http://www.asha.org/policy.
- 29.Omari TI, Rommel N, Szczesniak MM, Fuentaealba S, Dinning PG, Davidson GP, Cook IJ. Assessment of intraluminal impedance for the detection of pharyngeal bolus flow during swallowing in healthy adults. Am J Physiol Gastrointest Liver Physiol. 2006;290:G183–8. doi: 10.1152/ajpgi.00011.2005. [DOI] [PubMed] [Google Scholar]
- 30.Mielens JD, Hoffman MR, Ciucci MR, et al. Application of classification models to pharyngeal high-resolution manometry. J Speech Lang Hear Res. 2012;55:892–902. doi: 10.1044/1092-4388(2011/11-0088). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omari TI, Papathanasopoulos A, Dejaeger E, et al. Reproducibility and agreement of pharyngeal automated impedance manometry with videofluoroscopy. Clin Gastroenterol Hepatol. 2011;9:862–7. doi: 10.1016/j.cgh.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Hoffman MR, Mielens JD, Omari TI, et al. Artificial neural network classification of pharyngeal high-resolution manometry with impedance data. Laryngoscope. 2013;123(3):713–20. doi: 10.1002/lary.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]