Abstract
µ-SIIIA, a novel µ-conotoxin from Conus striatus, appeared to be a selective blocker of tetrodotoxin-sensitive sodium channels in frog preparations. It also exhibited potent analgesic activity in mice, although its selectivity profile against mammalian sodium channels remained unknown. We have determined the structure of µ-SIIIA in aqueous solution and characterized its backbone dynamics by NMR and its functional properties electrophysiologically. Consistent with the absence of hydroxyprolines, µ-SIIIA adopts a single conformation with all peptide bonds in the trans conformation. The C-terminal region contains a well-defined helix encompassing residues 11–16, while residues 3–5 in the N-terminal region form a helix-like turn resembling 310 helix. The Trp12 and His16 side chains are in close proximity, as in the related conotoxin µ-SmIIIA, but Asn2 is further away. Dynamics measurements show that the N-terminus and Ser9 have larger magnitude motions on the sub-ns timescale, while the C-terminus is more rigid. Cys4, Trp12 and Cys13 undergo significant conformational exchange on µs - ms timescales. µ-SIIIA is a potent, nearly irreversible blocker of NaV1.2, but also blocks NaV1.4 and NaV1.6 with submicromolar potency. The selectivity profile of µ-SIIIA, including poor activity against the cardiac sodium channel, NaV1.5, is similar to that of the closely related µ-KIIIA, suggesting that the C-terminal regions of both are critical for blocking neuronal NaV1.2. The structural and functional characterization described in this paper of an analgesic µ-conotoxin that targets neuronal subtypes of mammalian sodium channels provides a basis for the design of novel analogues with an improved selectivity profile.
INTRODUCTION
Toxins from the genus Conus are valuable tools in elucidating the physiological functions of their targets and in probing the size and shape of their cognate binding sites {Jones, 2000 #3; Norton, 2006 #2}. Conotoxins that target voltage-gated sodium channels (VGSCs)1, which are responsible for the influx of sodium ions during action potentials in excitable tissues, represent a particularly good example. Four families of conotoxins target these channels, causing activation (ı-conotoxins), inhibition (µ- and µO-conotoxins) or inhibition of fast-inactivation (δ-conotoxins). Among the most extensively studied of these in terms of their interactions with the channel are the µ-conotoxins. These toxins bind to Site 1 on VGSCs {Cestele, 2000 #4}, which is located on the extracellular surface of the pore-forming α-subunit and also binds the guanidinium alkaloids tetrodotoxin (TTX) and saxitoxin. Of the nine α-subunits cloned from mammals {Catterall, 2000 #71; Goldin, 2001 #5}, six bind TTX with high affinity (IC50 in the nM range), and three, NaV1.5, NaV1.8, and NaV1.9, are classified as TTX-resistant (IC50 > 1 µM).
The µ-conotoxins are a validated source of selective sodium channel blockers. The first µ-conotoxin characterized, µ-GIIIA, targets mainly the skeletal muscle subtype NaV1.4 {Cruz, 1985 #6; Moczydlowski, 1986 #7; Safo, 2000 #8; Li, 2003 #9}. µ-PIIIA also has a strong preference for NaV1.4, but can block other TTX-sensitive subtypes as well, albeit with lower affinities {Safo, 2000 #8; Shon, 1998 #12; Nielsen, 2002 #13}. Recently, a new group of µ-conotoxins was discovered that targets neuronal sodium channels with greater selectivity and potency than µ-GIIIA or µ-PIIIA {West, 2002 #14; Keizer, 2003 #66; Bulaj, 2005 #87; Zhang, 2007 #88; Lewis, 2007 #100}. This group includes µ-SmIIIA, which irreversibly inhibited TTX-resistant sodium currents in amphibian sympathetic and dorsal root ganglion (DRG) neurons {West, 2002 #14}, as well as µ-KIIIA and µ-SIIIA {Bulaj, 2005 #87}. The selectivity of these new µ-conotoxins towards mammalian sodium channel subtypes was defined only recently, when it was shown that µ-KIIIA was a potent blocker of the neuronal subtype NaV1.2, and that several amino acid residues, including Lys7 and His12, were critical for activity {Zhang, 2007 #88}. Thus, the new group of µ-conotoxins consisting of µ-KIIIA, µ-SIIIA and µ-SmIIIA is a promising source of subtype selective blockers of neuronal sodium channels.
Relatively little is known about biological activity or structural properties of µ-SIIIA. µ-SIIIA was shown to potently block TTX-insensitive sodium currents in amphibian DRG and sympathetic neurons, but it was a relatively poor inhibitor of TTX-sensitive currents recorded from frog skeletal muscle preparations {West, 2002 #14}. Micromolar concentrations of µ-SIIIA (5–25 µM) partially blocked TTX-sensitive currents in mice DRG neurons, whereas 1 µM of the peptide blocked almost completely A-compound action potentials in mouse sciatic nerve preparations {Green, 2007 #86}. µ-SIIIA appeared to significantly reduce the acute and the inflammatory pain responses in the formalin assay in mice at doses < 1 mg/kg following intraperitoneal administration {Green, 2007 #86}. These results strongly suggested that µ-SIIIA has therapeutic potential as a potent blocker of mammalian sodium channels, but the subtype selectivity or structural properties of this µ-conotoxin remained unknown.
Previous NMR studies of µ-conotoxins have found evidence of conformational flexibility, manifested in line broadening of one or more resonances {Lancelin, 1991 #15; Wakamatsu, 1992 #17; Hill, 1996 #18}, although this was not the case for µ-SmIIIA {Keizer, 2003 #66}. However, when µ-SmIIIA was subjected to molecular dynamics simulations, the resulting structure showed several differences from the one calculated on the basis of NMR restraints {Bulaj, 2005 #87}. In particular, the side chain of Arg2 moved away from that of Trp14, in conflict with the observed NOEs between these side chains {Keizer, 2003 #66}, and the first loop (between Asn5 and Gly9) sampled a larger region of conformational space than in the NMR structure. Apart from these differences, the rest of the backbone was largely constant throughout the simulation, and consistent with the NMR structure. Nonetheless, these observations raise questions about the flexibility of µ-conotoxin structures, which are germane to their use as a basis for modelling new structures in this superfamily and for the design of non-peptidic mimetics {Green, 2007 #86}.
NMR relaxation measurements provide a means of characterising protein solution dynamics over a broad range of timescales. Experimental procedures for measuring 15N relaxation parameters (R1, R2 and steady-state 15N-{1H} NOE) as well as protocols for data analysis using Modelfree formalism are well established for the study of protein backbone dynamics at ps to ns timescales {Palmer, 1997 #56; Fischer, 1998 #61}. There is also compelling evidence that protein dynamics in solution, particularly on the ms timescale, correlate closely with protein function {Feher, 1999 #58; Volkman, 2001 #59; Eisenmesser, 2002 #89}. This is significant because one of the mechanisms underlying protein function and protein-protein interaction is conformational exchange, which typically occurs at the sub-ms to ms timescales {Frauenfelder, 1988 #60}.
Most of these studies have been conducted using isotopically enriched proteins produced in bacteria grown on minimal media. Toxins with multiple disulfide linkages such as the µ-conotoxins can be expressed and isotopically labelled in heterologous expression systems, eg. {Goldenberg, 2001 #83}, but most toxins that are the subject of structural studies are produced by solid-phase synthesis without labelling. With the widespread availability of NMR cryoprobes, it has become straightforward to obtain 13C and 15N chemical shifts for unlabelled samples {Buczek, 2007 #72; Ellison, 2008 #73}, and these parameters are useful in defining backbone and potentially also side chain dihedral angles {Cornilescu, 1999 #65}. Given that 13C has a higher natural abundance and sensitivity than 15N (1.11 and 0.37 %, and sensitivities for the same number of nuclei relative to 1H of 1.59×10−2and 1.04×10−3, respectively), it has also become feasible to monitor 13C relaxation on unlabelled samples. In the past this has required large sample tubes and/or concentrated samples (e.g. {Oldfield, 1975 #82; Norton, 1977 #77}), but such studies can now be undertaken in a reasonable time with ca 1 mM samples. In this paper we describe the results of a 13C NMR relaxation study of the conotoxin µ-SIIIA, which was undertaken to determine whether such measurements could detect internal motions in this class of toxin on timescales relevant to conformational averaging and biological activity. In order to interpret the relaxation data we have also determined a high-resolution structure for µ-SIIIA and compared it with the structures of other µ-conotoxins.
EXPERIMENTAL
Peptide Synthesis & Sample Preparation
µ-SIIIA was synthesized, folded and purified using the protocols described previously {Bulaj, 2005 #87}. The peptide was purified using C18 reversed-phase HPLC and its identity confirmed by mass spectrometry. Samples used for structure determination and 13C relaxation measurements were prepared by dissolving µ-SIIIA in 350 µl of 95% H2O/5% 2H2O or 100% 2H2O, respectively, to a final concentration of approximately 0.9 mM. The pH was adjusted to 4.2 without allowances for isotope effects.
NMR Spectroscopy
For structure determination, 2D TOCSY (spin-lock time 60 ms), NOESY (mixing time 250 ms) and DQF-COSY spectra were recorded on a Bruker DRX600 spectrometer at 25 °C. Complex data matrixes of 2048 퀇 225 for TOCSY, 2048 퀇 300 for NOESY and 2048 퀇 300 for DQF-COSY and 40,112 and 40 scans per t1 increment in the indirect detected dimension, respectively, were used. Assignments of aliphatic carbon resonances were established from 13C HSQC, 13C HMQC and 13C HMQC-TOCSY {Palmer, 1991 #90} spectra whereas backbone 15N resonances of µ-SIIIA were assigned from a 15N HSQC spectrum based on the corresponding amide proton chemical shifts, which were well resolved in the F2 (1H) dimension. All of these heteronuclear correlation spectra were recorded at natural abundance on a Bruker Avance500 using a TXI cryoprobe. 13C R1, R2 and 13C-{1H} NOE were also measured at natural abundance on the Bruker Avance500 at 283, 298 and 310 K using standard pulse sequences in the Bruker pulse sequence library. Relaxation-weighted 13C-HSQC spectra were acquired in an interleaved manner, i.e. a 3D data set was acquired with the pulse sequence stepping through the relaxation durations prior to signal averaging and the time increments of the indirectly detected dimension of the spectra. Twelve relaxation delays, including two duplicated delays, ranging from 10 to 800 ms for R1 and 22.4 to 268.8 ms for R2, respectively, were measured. A complex data matrix of 2048 퀇 32 together with 48 scans per t1 increment for R1 and R2 and 192 for the NOE were used. The recycle times were 2.8 s for R1 and R2 and 4.0 s for the steady-state 15N-{1H} NOE. All NMR spectra were processed using TOPSPIN (Version 1.3, Bruker BIOSPIN) and analyzed using XEASY (Version 1.3; {Bartels, 1995 #55}).
Structure Calculations
Volumes of cross peaks in a 250 ms mixing time NOESY spectrum recorded at 25 °C were converted to distance restraints using the script CALIBA supplied with CYANA {Herrmann, 2002 #23}. The resultant values were then scaled by a factor of 1.2 to allow for potential effects of spin diffusion. Restraints were added for all three disulfide bonds (Cys3-Cys13, Cys4-Cys19 and Cys8-Cys20). Backbone ϕ angle restraints were derived from 3JHNNA coupling constraints measured from the DQF-COSY spectrum (3JHNHα > 8 Hz, ϕ = −120±30° 3JHNHα < 6 Hz, ϕ = −60±30°) and combined with those derived from the program TALOS {Cornilescu, 1999 #65} based on chemical shifts of (N, Hα, Cα and Cβ) of µ-SIIIA. All other residues were restricted to a negative ϕ value except for Gly6 and Gly7, which were not restrained. Initial structures were generated using the torsion angle dynamics program CYANA {Herrmann, 2002 #23} for refinement of structural restraints. The final distant angular restraints were then used to calculate a family of 400 structures in XPLOR-NIH {Schwieters, 2003 #24} using standard distance geometry and simulation annealing scripts. Residue pyroGlu was added into the XPLOR library as previously {Keizer, 2003 #66}. A group of 50 structures out of those 400 calculated structures with lowest NOE energies were then further refined using simulated annealing. The resultant structures were further refined in a water box built around the peptide and then energy minimized on the basis of experimental distance and angular restraints as well as the geometry of bonds, angles and impropers. A family of 20 structures, based on their stereochemical energies and NOE energies, was then chosen for structural analysis using programs PROCHECK {Laskowski, 1996 #85} and MOLMOL {Koradi, 1996 #84}. This family of final structures and associated structural restraints have been deposited in the BioMagResBank database {Ulrich, 2008 #103} with accession no. 20023.
Analysis of 13C relaxation data
13C relaxation rates R1 and R2 were obtained by fitting peak intensities at a series of relaxation durations to a two-parameter single exponential decay curve using the program SigmaPlot. Errors of the relaxation rates were standard deviations from fittings in SigmaPlot. The steady-state 13C-{1H} NOE values were calculated from peak intensity ratios obtained from spectra acquired in the presence and absence of proton saturation with uncertainties of 5 % for peak intensities determined from background noise of the spectra {Farrow, 1994 #67}.
13C relaxation parameters were first analyzed using Modelfree formalism. Given that the rotational correlation time of µ-SIIIA is in the low ns range, fittings to the extended spectral density function which includes a slower timescale of internal motion were not attempted. 13C R1, R2 and NOE were fitted to one of three models of (1) S2, (2) S2 and τe and (3) S2 and Rex using criteria similar to model selection approach commonly used in 15N relaxation analysis {Mandel, 1995 #68}.
In contrast to Modelfree analysis, reduced spectral density mapping extracts information on molecular dynamics without approximations made regarding molecular shape and global rotational behavior. While reduced spectral density mapping has been commonly used in the analysis of backbone 15N relaxation data, it is more limited for 13C relaxation data than for 15N. It has been noted previously that, while reduced spectral density mapping introduced few errors for 15N relaxation analysis, it was less valid for 13C as the derivation of reduced spectral density functions (Eqs 1b–1c) assuming spectral density function J(Ω) is flat around ΩH, i.e J(ΩH-ΩN) = J(ΩH) = J(ΩH+ΩN) for 15N and J(ΩH-ΩC) = J(ΩH) = J(ΩH+ΩC) for 13C, respectively {Atkinson, 1999 #91}. Nevertheless, it provides a complementary picture of the local dynamics of the molecule to that obtained from Modelfree analysis, provided the value for J(1.563ΩH) is not interpreted {Slupsky, 2007 #64}.
The values of spectral density functions J(0), J(ΩC) and J(1.56ΩH) were calculated from measured 13C R1, R2 and steady-state NOE values similar to those described previously for reduced spectral density mapping of 15N relaxation parameters.
| (1a) |
| (1b) |
| (1c) |
| (1d) |
where d=(µ0hγCγH/(8π2))/<r3CαH<, c=ΩC(σ║-σ⊥)/31/2, µ0 is the permeability of free space, h is Planck’s constant, γH and γC are the gyromagnetic ratios of 1H and 13C, respectively, rCαH = 1.09 Ǻ is the average amide bond length, and (σ║-σ⊥) = 25 ppm is the chemical shift anisotropy for 13Cα nuclei {Wei, 2001 #104}.
Electrophysiology of mammalian NaV clones expressed in Xenopus oocytes
Oocytes expressing VGSCs were prepared and two-electrode voltage clamped essentially as described previously {Zhang, 2007 #88; Fiedler, 2008 #102}. Briefly, oocytes were placed in a 30 µl chamber containing ND96 and two-electrode voltage clamped with a holding potential of −80 mV. To activate Na channels, the membrane potential was stepped to a value between −20 and 0 mV (depending on NaV subtype) for a 50 ms period every 20 sec. To apply toxin, the perfusion was halted, 3 µL of toxin solution (at ten times of the final concentration) was applied to the 30 µL bath, and the bath manually stirred for about 5 s by gently aspirating and expelling a few µL of the bath fluid several times with a micropipette. Toxin exposures were in static baths to conserve material. On-rate constants were obtained from the slopes of kobs versus [peptide], where kobs was determined from the single-exponential fit of the time course of block by a given toxin concentration. Off-rate constants were determined from single-exponential fits of the time course of recovery from block following toxin washout. All recordings were made at room temperature (~21 °C).
RESULTS
Chemical Shift Assignments
A one-dimensional 1H NMR spectrum of µ-SIIIA in aqueous solution (Figure S1) showed a single major species with good spectral dispersion. Chemical shifts assignments were obtained for resonances from all amide and aliphatic 1H, aliphatic 13C and backbone 15N of µ-SIIIA. These assignments have been deposited in the BioMagResBank database (accession number 20023) and are tabulated in Supplementary Material (Table S1). Plots of secondary chemical shifts of backbone HN, N, Cα and Hα versus the sequence of µ-SIIIA are shown in Figure S2; the Hα and Cα shifts for residues 11–16 are consistent with the presence of α-helical structure in this region of the molecule.
Solution Structure
Parameters characterizing the final 20 structures of µ-SIIIA and structural statistics are summarized in Table 1. The final 20 structures fit well with experimentally derived distance and angle constraints and are well defined over the entire length of the polypeptide, with the N-terminal pGlu1 and Asn2 being slightly less ordered than the rest of the polypeptide chain. A summary of backbone RMS deviations and backbone angular order parameters of µ-SIIIA is shown in Figure S4 and stereo views of the structures are shown in Figure 1.
Table 1.
Structural statistics for µ-SIIIA in aqueous solution at 298 K.
| Distance restraints | 164 |
| Intra (i = j) | 53 |
| Sequential (|i – j| = 1) | 68 |
| Short (1 < |i – j| < 6) | 31 |
| Long | 12 |
| Dihedral restraints | 22 |
| Energies (kcal mol/1) a | |
| ENOE | 7.5 ± 0.8 |
| Deviations from Ideal Geometry b | |
| Bonds (Å) | 0.0065 ± 0.0002 |
| Angles (°) | 0.743 ± 0.014 |
| Impropers (°) | 0.551 ± 0.021 |
| RMS Deviations (Å) c | |
| All heavy atoms | 1.22 |
| Backbone heavy atoms (N, Cα, C’) | 0.74 |
| Average pairwise RMS deviations | |
| All heavy atoms | 1.83 ± 0.43 |
| Backbone heavy atoms (N, Cα, C’) | 1.06 ± 0.36 |
| Ramachandran plot d | |
| Most favored (%) | 91.6 |
| Allowed (%) | 8.4 |
| Additionally allowed (%) | 0 |
| Disallowed (%) | 0 |
The values for ENOE are calculated from a square well potential with force constants of 50 kcal mol−1 Å2.
The values for the bonds, angles, and impropers show the deviations from ideal values based on perfect stereochemistry.
RMSD over the backbone heavy atoms (N, Cα, C) of all residues.
As determined by the program PROCHECK-NMR {Laskowski, 1996 #25} for all residues except Gly and Pro.
Figure 1.
µ-SIIIA structure. (A) Stereo view of backbone µ-SIIIA (a family of 20 final solution structures of µ-SIIIA superimposed over backbone heavy atoms (N, Cα and C’) of all 20 residues). The letters N and C refer to the N and C termini, respectively. (B) Stereo view of closest-to-mean structure in the family of 20 structures shown in (A) with backbone of µ-SIIIA in light grey and side chains of several residues highlighted in colour. Those residues with side chains highlighted are also labeled. The three disulfide bonds (residues 3–13, 4–19 and 8–20) are shown in yellow.
The main secondary structure element is a well-defined C-terminal helix (residues 11–16), but the N-terminal residues 3–5 also appear to form a helix-like turn resembling 310 helix. The side chain of Lys11, which may be important for binding to Site 1 on VGSC in µ-SIIIA, is only partially exposed owing to is proximity to the side chains of Trp12 and Asp15.
13C Relaxation Parameters and Rotational Correlation Times
After excluding Gly6 and Gly7, as well as residues whose measurements were compromised by the residual water resonance in the directly detected dimension (F2), 13C R1, R2 and 13C-{1H} NOE values were measured for a total of 15, 16, and 16Cα of µ-SIIIA at 283, 298 and 310 K, respectively, as shown in Figure 2. A 13C-1H HSQC spectrum of µ-SIIIA at 298 K, representative decay curves of R1 and R2, and all measured 13Cα relaxation parameters are given in Supplementary Material (Figure S5, Figure S6 and Table S2). The average values of R1, R2, and NOE as well as effective rotational correlation times of µ-SIIIA estimated from the average 13C R2/R1 ratio (residues with angular parameters Sϕ and Sψ > 0.9 and their R2/R1 values within one standard deviation of the averaged R2/R1 ratio) are listed in Table 2. The rotational correlation time of µ-SIIIA from hydrodynamics calculations based on its closest-to-mean structure is also shown.
Figure 2.
Cα 13C relaxation parameters, R1, R2 and NOE, of µ-SIIIA measured at 283 K (●), 298K (▲) and 310K (♦). Data are not shown for Gly6 and Gly7, and residues whose resonances lay too close to the residual water resonance in the F2 dimension (Figure S4 and Table S2).
Table 2.
Summary of average 13C relaxation parameters, rotational correlation times and order parameters of µ-SIIIA
| T (K) |
<R1> (s−1) |
<R2> (s−1) |
<NOE> | <R2/R1> a | τc (ns) |
τc’ b (ns) |
<S2> c (overall) |
<S2> d (helix 11–16) |
|---|---|---|---|---|---|---|---|---|
| 283 | 3.91 ± 0.43 | 14.38 ± 6.59 | 1.30 ± 0.13 | 3.04 ± 0.41 (11/15) | 2.29 ± 0.25 | 2.72 | 0.91 ± 0.17 | 0.97 ± 0.01 |
| 298 | 4.28 ± 0.54 | 8.07 ± 2.2 | 1.38 ± 0.11 | 1.87 ± 0.43 (16/16) | 1.45 ± 0.19 | 1.80 | 0.80 ± 0.21 | 0.85 ± 0.02 |
| 310 | 4.12 ± 0.63 | 6.06 ± 1.59 | 1.54 ± 0.12 | 1.45 ± 0.22 (16/16) | 0.98 ± 0.15 | 1.32 | 0.76 ± 0.20 | 0.84 ± 0.07 |
Average value over resides whose R2/R1 within 1.5 SD of averaged value of R2/R1 of all measured residues, number of residues satisfy the criteria versus total number of residues whose 13C relaxation parameters are measured is given in the parentheses.
Effective rotational correlation times of µ-SIIIA from hydrodynamic calculation, using program HYDRONMR with a radius of atomic elements 3.1 Å and viscosities of 100% 2H2O
Rh = 9.0 Å. Previously published values of viscosities for 100% 2H2O of 1.679×10−3, 1.110×10−3, and 0.850×10−3 N s m−2 at 283, 298, and 310 K, respectively, were used {Cho, 1999 #69}.
Averaged over a group of 12 residues with order parameters determined at all three temperatures.
Average value over the helical region (residues 11–16)
Dynamics Parameters
Dynamics parameters of µ-SIIIA resulting from Modelfree analysis and reduced spectral density mapping from its 13Cα relaxation parameters measured at 283, 298, and 310K are summarized in Figures 3 and 4, respectively, and tabulated in Supplementary Material (Tables S3 and S4). Average S2 values over the entire molecule (from residues with S2 values available at all three temperatures) and over the C-terminal helical region (residues 11 –16) are also summarized in Table 2.
Figure 3.
Dynamics parameters of µ-SIIIA. (A) S2, (B) τe, and (C) Rex at 283 K (●), 298 K (▲) and 310 K (♦) derived from 13C Cα relaxation parameters shown in Figure 2 using the Modelfree formalism.
Figure 4.
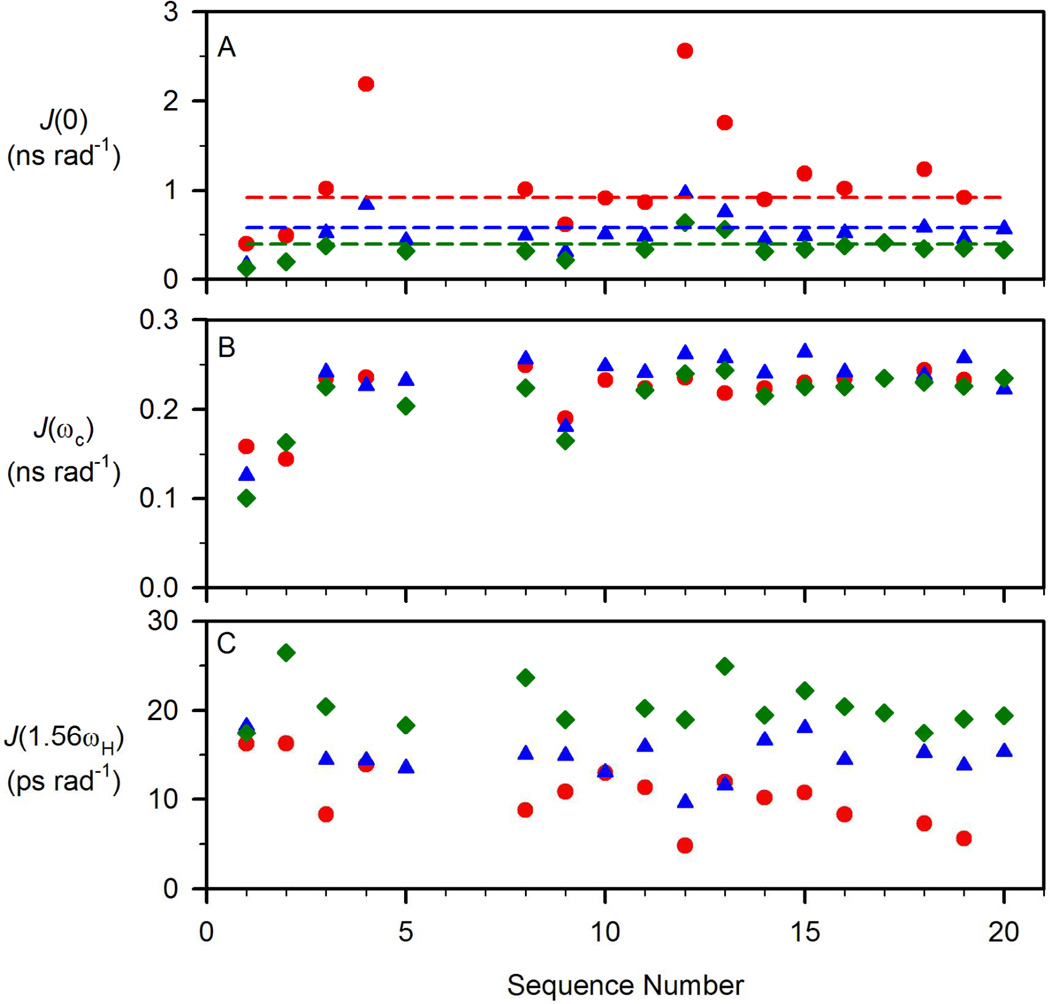
Reduced spectral density functions of µ-SIIIA. (A) J(0), (B) J(ΩC), and (C) J(1.563ΩH) at 283 K (●), 298 K (▲) and 310 K (♦) calculated from 13C relaxation parameters depicted in Figure 2 using Eqs 1a–1d. Dashed lines in (A) corresponding to (2/5)τm, indicating J(0) values for completely rigid molecule at the given rotational correlation times (283 K in red, 298 K in blue, and 310 K in green, respectively, for µ-SIIIA).
Temperature Dependence of Dynamics Parameters
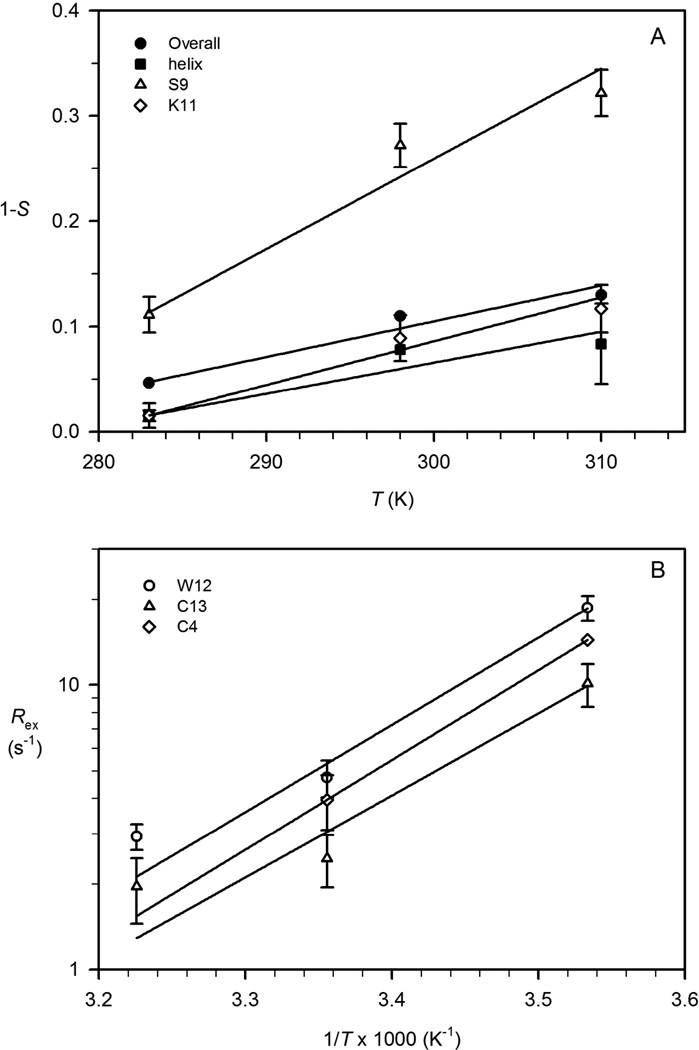
The observed decrease in order parameters at higher temperature reflects increased amplitudes of motion of the Cα-H bond vectors, with several residues (Cys8 and Ser9, and to a lesser extent Lys11, Arg14 and Asp15) exhibiting more pronounced temperature dependence than the average over the entire polypeptide chain or the C-terminal helical region (residues 11–16) (Figure 3). Figure 5 summarizes the temperature dependence of the order parameters (shown as 1- S) of µ-SIIIA for the average over the entire molecule, the C-terminal helical region, Ser9 and Lys11 (Figure 5A), as well as the temperature dependence of apparent chemical exchange, Rex, for Cys4, Trp12 and Cys13 (Figure 5B). Ribbon presentations of µ-SIIIA with its structural characteristics and dynamics parameters highlighted are shown in Figure 6.
Figure 5.
Temperature dependence of chemical exchange and order parameters for µ-SIIIA. (A) Temperature dependence of chemical exchange are shown as Arrhenius plot, (lnRex versus 1/T), for resides Cys4 (◊), Trp12 (○), and Cys13 (∆). (B) Order parameters are shown as (1-S) versus T for (●) the average value of overall sequence (12 residues with S2 values at all three temperatures were determined), (■) the C-terminal helix (residues 11–16), (∆) Ser-9, and (◊) Lys11.
Figure 6.
(A) Ribbon diagram of µ-SIIIA (closest-to-mean) with three disulfide bridges (Cys3-Cys13, Cys4-Cys19 and Cys8-Cys20) highlighted in yellow. (B) Mean structure of the final family of 20 structures of µ-SIIIA (BioMagResBank access code 20023) with radii of backbone atoms proportional to their averaged displacement to the mean structure. Note that this structure is not the same as that shown in parts A, C and D of this figure. (C) Ribbon diagram of SIIIA (closest-to-mean) with radii of backbone atoms proportional to 1-S2, where S2 is the order parameter resulted from Modelfree analysis of 13Cα relaxation parameters measured at 298 K (Figure 3). Residues 2, 6, 7 and 17, whose S2 values were not determined, are shown in grey with their bond radii shown as the averaged values from their neighboring residues. (D) Backbone Cα atoms of µ-SIIIA, shown in spheres, are color coded based on their Rex values at 283 K. Color scheme: Rex > 10 s−1 in red, 0 s−1 < Rex < 10 s−1 in pink, and residues whose dynamics data were not determined in grey. This figure was prepared using MOLMOL {Koradi, 1996 #26}.
Functional Studies
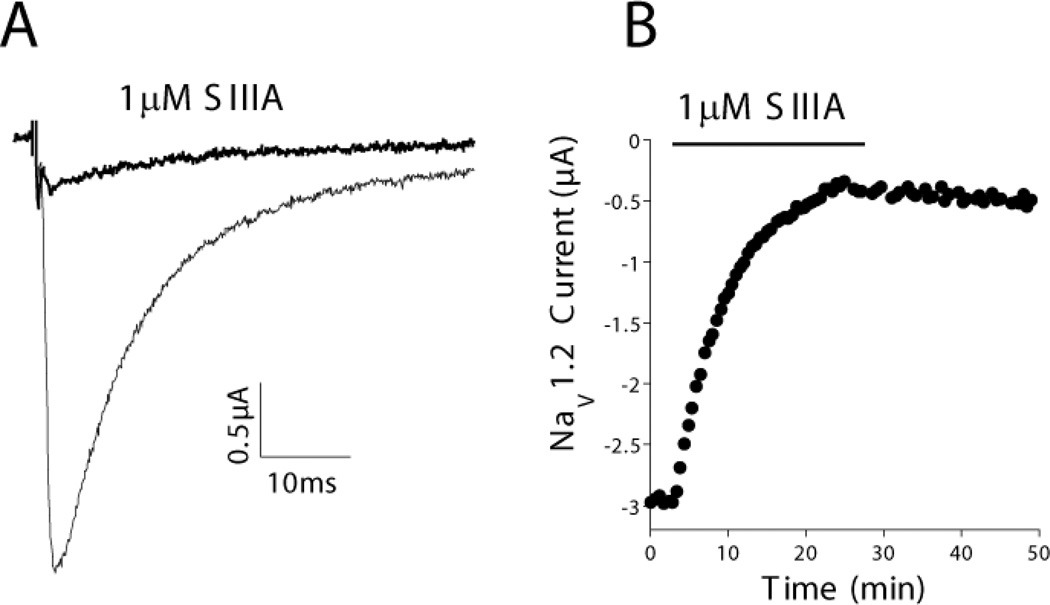
The activity of µ-SIIIA on cloned α-subunits of rat (r) or mouse (m) sodium channels expressed in oocytes was assessed by voltage-clamp protocols. Table 3 summarizes dose-dependent inhibition by µ-SIIIA of seven sodium channels subtypes, including skeletal muscle rNaV1.4 and heart muscle rNaV1.5. Four neuronal subtypes, rNaV1.1, rNaV1.2, rNaV1.3, mNaV1.6, were blocked by µ-SIIIA with IC50 values ranging from < 0.76 to 11 µM. The block of rNaV1.2 was only very slowly reversible (Figure 7); in contrast, the block of rNaV1.1, rNaV1.3 and mNaV1.6 was readily reversible. Of the channels tested, rNaV1.4 was blocked with the fastest on-rate and second slowest off-rate. Inhibition of rNaV1.5 and rNaV1.7 was characterized by very low affinity, with IC50 values of 251 and 65 µM, respectively. Of the five IC50 values determined in Table 3, the corresponding Kd values (calculated from koff/kon) for four were within a factor of 2. For NaV1.7, however, the Kd (15 µM) was four-fold lower than the IC50; we attribute this discrepancy to the fact that the block of NaV1.7 had the slowest on-rate and a relatively slow off-rate, which created a long period to reach steady-state and therefore a less reliable determination of IC50.
Table 3.
Inhibition by µ-SIIIA of cloned sodium channels expressed in Xenopus oocytes. Rate constants were determined as described under Experimental. Standard deviation and 95% confidence intervals (95% C.I) were calculated from at least three independent experiments using Prism software.
| Sodium channela |
kon (µM−1.min−1) |
koff (min−1) |
IC50(95% C.I.) (µM) |
|---|---|---|---|
| rNaV1.1 | 0.011 ± 0.004 | 0.2 ± 0.06 | 11 (9.3–12.6) |
| rNaV1.2 | 0.10 ± 0.015 | Irreversibleb | N.A.c |
| rNaV1.3 | 0.017 ± 0.002 | 0.12 ± 0.018 | 11 (9.0–12.9) |
| rNaV1.4 | 0.29 ± 0.036 | 0.04 ± 0.008 | 0.13 (0.11–0.16) |
| rNaV1.5 | --d | --d | 251 (204–308)e |
| mNaV1.6 | 0.06 ± 0.012 | 0.082 ± 0.006 | 0.76 (0.69–0.85) |
| rNaV1.7 | 0.005 ± 0.0005 | 0.075 ± 0.012 | 65 (57.8–73.1) |
α-Subunit cloned from rat (r) or mouse (m).
Although the block was essentially irreversible within the experimental time frame, the koff was estimated as 0.0047 ± 0.0015 min−1 based on residual recovery after 20 min of wash and assuming exponential decay.
Not available because slow kinetics precluded steady state from being achieved within the experimental time frame.
Unable to be determined because block was too small and apparent kinetics too fast to be measured accurately.
Extrapolated value assuming 100% block at saturating peptide concentration.
Figure 7.
µ-SIIIA blocks rNaV1.2 expressed in Xenopus oocytes. Oocytes were two-electrode voltage clamped as described under Experimental. (A) Representative recordings, each trace represents the average of 5 responses before (control, gray trace) and during (black trace) exposure to 1 µM µ-SIIIA. (B) Time course of block and recovery; black barabove plot indicates when µ-SIIIA was present.
DISCUSSION
Conotoxins µ-SmIIIA, µ-KIIIA and µ-SIIIA are potent sodium channel blockers, indeed the latter two were shown recently to possess potent analgesic activity in the inflammatory pain assay in mice {Zhang, 2007 #88; Green, 2007 #86}. µ-KIIIA and µ-SIIIA share high sequence homology in the C-terminal part of their structure. Consistent with this, the selectivity profile of µ-SIIIA for the various NaV subtypes was similar to that described previously for µ-KIIIA {Zhang, 2007 #88}. Both were nearly irreversible blockers of NaV1.2, and reversible blockers of skeletal muscle subtype NaV1.4. However, the neuronal subtype NaV1.7 was more readily blocked by µ-KIIIA (Kd 0.29 µM {Zhang, 2007 #88}), than by µ-SIIIA (IC50 65 µM or Kd 15.0 µM; see Table 3 and last paragraph of Results), suggesting that either Ser13 in µ-KIIIA is an important determinant of the interactions with Nav1.7 (the homologous residue in µ-SIIIA is Ala17), or that some minor conformational differences between the two peptides may be responsible for their different potencies. In any event, our current functional data suggest that the N-terminal region of µ-conotoxins SIIIA, KIIIA and SmIIIA is important for determining subtype selectivity in these peptides, whereas the C-terminal region contains a common pharmacophore for the interactions with the neuronal subtypes of sodium channels.
Comparison with Structures of µ-SmIIIA and Other µ-Conotoxins
The structures of several µ-conotoxins have been solved previously, including µ-SmIIIA (PDB access code: 1Q2J){Keizer, 2003 #66}, µ-PIIIA (1R9I){Nielsen, 2002 #13}, µ-GIIIA (1TCJ){Wakamatsu, 1992 #17} and µ-GIIIB (1GIB){Hill, 1996 #18}. A sequence alignment of these µ-conotoxins is shown in Figure 8 along with a comparison of their structures. Superposition of these five structures over their C-terminal halves is significantly better than that over their N-terminal halves (Figure 8B). As noted by Keizer et al. {Keizer, 2003 #66}, this is at least partly a consequence of the trans conformation for the peptide bond preceding residue 8 in µ-SmIIIA and the major form of µ-PIIIA, as opposed to the cis conformation in µ-GIIIA, µ-GIIIB and the minor form of µ-PIIIA. All peptide bonds in µ-SIIIA are also in the trans conformation. The C-terminal region of µ-SIIIA contains a well-defined helix (residues 11–16), and the N-terminal region contains a helix-like turn resembling 310 helix at residues 3–5.
Figure 8.
Sequence alignment and structure comparison of µ-conotoxins. (A) Amino acid sequences of µ-SIIIA, µ-SmIIIA, µ-PIIIA, µ-GIIIA, and µ-GIIIB. Numbers shown at the top are those for µ-SIIIA. Z represents pyro-glutamate and O hydroxyproline. Conserved cysteines are highlighted in yellow and partially conserved residues in light grey. The Arg or Lys residues in the C-terminal half of the polypeptide (boxed in dark blue) have been suggested to play a key role for the potency of these peptides. (B) Structures of µ-SIIIA ((BioMagResBank access code 20023), µ-SmIIIA (PDB access code: 1Q2J), µ-PIIIA (PDB access code: 1R9I), µ-GIIIA (PDB access code: 1TCJ), and µ-GIIIB (PDB access code: 1GIB) with side chains of Arg or Lys shown in dark blue and disulfide bonds in yellow. All these structures are shown in a similar orientation after superimposition over backbone heavy atoms (C, Cα, N) of aligned residues (Figure 8A); superposition over the Cα and Cβ atoms of the six Cys residues gave a similar result. Side chains of Asn2, Trp12 and His 16 of µ-SIIIA and Arg2, Trp14 and His18 of µ-SmIIIA are shown in magenta. Figure 8B was generated using the program MOLMOL {Koradi, 1996 #26}.
In µ-SmIIIA the indole ring of Trp14 is flanked by the imidazolium ring of His18 on one side and the guanidinium moiety of Arg2 on the other {Keizer, 2003 #66}, these side chain interactions presumably being favoured not only by interactions between the π orbitals of the indole and imidazolium rings, but also by aromatic-cation interactions. The equivalent Trp in µ-SIIIA, Trp12, is also close to His16 (Figure 1B) but these residues are some distance away from residue 2, which is Asn in µ-SIIIA. The Hδ2 resonance of His16 of µ-SIIIA has a very similar chemical shift to that of His18 in µ-SmIIIA (Table S1), implying that it is influenced by ring-current shifts from the Trp indole ring as a consequence of their close proximity in both structures. Analysis of ring current shifts on the final family structures of µ-SIIIA performed using the program MOLMOL {Koradi, 1996 #26} resulted in very similar upfield shifts for resonances from CαH, CβH and the ring protons of His16, as previously reported for His18 in µ-SmIIIA {Keizer, 2003 #66}.
Comparison with Model Structure
Models of both µ-SIIIA and µ-KIIIA were generated in the course of MD simulations performed to gain insight into the potential structural consequences of deleting a number of residues in the first loop (the region flanked by the second and third Cys residues) {Bulaj, 2005 #87}. Figure S7 compares stereo views of the µ-SIIIA model with our solution structure. The C-terminal halves of the two are very similar (backbone RMSD 1.07 Å for residues 11–20, compared with 2.58 Å for residues 1–10), and the helical region (residues 11 –16) is conserved. The difference seen in the N-terminal region may be attributed to apparent structural differences between µ-SIIIA and µ-SmIIIA, as the model structure of µ-SIIIA was generated based on the reported structure of µ-SmIIIA. As described in the Introduction, the simulated structure of µ-SmIIIA showed several differences from the structure determined from NMR data {Keizer, 2003 #66}.
Backbone Dynamics of µ-SIIIA
Reduced flexibility is observed for µ-SIIIA at lower temperatures, as reflected in its overall S2 (Figure 3A) and J(0) values (Figure 4A). Relatively lower S2 values compared with the average over the entire backbone were observed for pyro-Glu1, Asn2 and Ser9. The angular order parameters Sϕ and Sψ (Figure S3) also show relatively lower values for pyro-Glu1 and Asn2 but not for Ser9. It appears that the N-terminus shows larger magnitude motions on the sub-ns timescale, while the C-terminus is more rigid, although the difference is small if the first two residues are ignored. As disulfide bridges connect Cys3 and Cys4 to the C-terminal half of the molecule, there is no simple correlation between the locations of the disulfides and relative backbone flexibility. Greater flexibility is anticipated near the chain termini, which is in accord with our observations for residues 1 and 2, but the C-terminal residue, Cys20, participates in a disulfide bond and has S2 values essentially identical with other residues in the C-terminal half. The only other residue to show greater motion on the sub-ns timescale is Ser9 (Figure 3A), which is located in the middle of the polypeptide chain and adjacent to Cys8.
Conformational Exchange of µ-SIIIA- Motion on the Microsecond to Millisecond Timescales
Larger J(0) values were observed for the backbone at 283 K compared with 298 and 310 K, indicating reduced sub-ns motions of the Cα-H bond vectors at lower temperature (Figure 4A). Slower µs to ms motions due to conformational exchange experienced by backbone Cα may also be reflected in the spectral density function at angular frequency, Ω= 0, which give rise to elevated values for J(0). For µ-SIIIA, significantly higher J(0) values were observed for Cys4, Trp12 and Cys13 at 283 K, which agrees very well with the result from Modelfree analysis that significant apparent Rex terms are needed in order to fit the relaxation data of these residues (Figure 3C).
The interpretation of Rex in terms of chemical/conformational exchange contributions to the transverse relaxation can be significantly affected by the anisotropic global reorientation of the molecule. However, in µ-SmIIIA, both Modelfree analysis and reduced spectral density mapping (which does not assume a particular model from rotational reorientation of the molecule) indicate that Cys4, Trp12 and Cys13 can undergo significant exchange on µs to ms timescales. It has been shown previously that the sign of dRex/dT indicates whether microscopic exchange is faster or slower than 3.2/τcp, where τcp is the interval between two consecutive 180° pulses in the CPMG segment of the pulse sequence used for 13Cα R2 measurements {Mandel, 1996 #92}. As can be seen in Figure 5B, the magnitudes of Rex for Cys4, Trp12 and Cys13 decrease with increasing temperature. Thus, for a τcp of 900 µs for µ-SIIIA, an estimate of kex > 3.2/τcp = 3.6×103 s−1 can be obtained {Mandel, 1996 #92}.
Given that Trp12 and Cys13 are adjacent to the biologically important Lys11, their observed propensity for conformational exchange implies that structural changes in this region may be possible upon binding to the sodium channel. As Lys11, Trp12 and Cys13 are part of the C-terminal helix, the conformation of this helix may also be subject to change upon sodium channel binding. Two of the residues showing significant exchange, Cys4 and Cys13, are involved in disulfide bridges (although not to each other), raising the possibility that conformational transitions involving disulfide bonds may contribute to the observed exchange processes {Otting, 1993 #94}. The presence of conformational exchange and reduced angular order parameters in the loop between Cys8 and Cys13 is also not a simple function of loop size, as the least flexible region in the molecule is actually the longest loop, encompassed by Cys13 and Cys19.
The backbone dynamics of an 15N-labelled Ω-conotoxin MVIIA precursor have been studied previously using 15N relaxation measurements {Goldenberg, 2001 #83}. The backbone proved to be well ordered on the nanosecond time scale but residues 9–15 experienced conformational exchange processes with a time constant of about 35 µs. These observations were consistent with the results of 13C relaxation measurements on unlabelled Ω- MVIIA by Atkinson et al. {Atkinson, 2000 #93}, who detected conformational exchange in the loop between Cys8 and Cys15 and suggested that this was associated with conformational exchange of the Cys8-Cys20 disulfide bridge. As this region of the Ω-conotoxins contains residues essential for Ca2+ channel binding {Norton, 1999 #95; Nielsen, 2000 #96}, it is possible that these exchange processes may influence activity.
Conclusions
The structure determined in this study for µ-SIIIA extends our understanding of the conformational space available to this class of conotoxins. Its well-defined C-terminal region closely matches the corresponding regions of all µ-conotoxins investigated to date, but its N-terminal half is distinct, sharing some features with the related µ-conotoxin µ-SmIIIA but showing a more extended structure for the N-terminal residues. Internal motion of the backbone has been detected over various timescales, with pyroGlu1, Asn2 and Ser9 showing larger magnitude motions on the ps-ns timescale and Cys4, Trp12 and Cys13 undergoing conformational exchange on the µs to ms timescale. Even the presence of three disulfide bonds in a short polypeptide of just 20 residues is not sufficient to confer rigidity on the polypeptide backbone. Indeed, it may even be that conformational averaging associated with the disulfide bridges is the cause of the observed conformational exchange, as also suggested for Ω-MVIIA {Atkinson, 2000 #93}. An awareness of the presence of conformational exchange is valuable in several ways. Interpreting measured NOEs as structural restraints requires some caution if either or both of the interacting nuclei are from regions undergoing such exchange. Knowing which regions of a structure are potentially flexible is also important if modelling studies are to be undertaken based on that structure. And, most important of all, it must be taken into account when using structures determined in solution as a basis for understanding receptor binding or constructing mimetics {Baell, 2001 #98}. Whether conformational exchange serves any important biological function, for example in allowing the toxin to undergo required conformational rearrangements as it engages its cognate binding site, or whether it is simply an adventitious consequence of the presence of multiple disulfide bridges in certain environments, remains to be established. One way of addressing this question is to replace some of the disulfide bridges with less flexible homologues, and means to achieve this are now available {Robinson, 2007 #99}. In parallel with such efforts, greater access to backbone relaxation data afforded by high-field spectrometers equipped with more sensitive probes should contribute to a better understanding of the relationships among structure, flexibility and activity. Our results on the structure and dynamics of µ-SIIIA also provide a foundation for further structure-function studies on this therapeutically important group of conotoxins.
Supplementary Material
ACKNOWLEDGMENT
We thank Alex Morrison for his help with conopeptide synthesis, Alan Goldin for the clones for NaV1.1 - 1.6, Gail Mandel for the NaV1.7 clone, and Brian Smith, Chris MacRaild and Jeff Babon for helpful discussions.
Footnotes
This work was supported in part by National Institute of General Medical Sciences grant GM 48677 (to B.M.O.). R.S.N. acknowledges fellowship support from the Australian National Health and Medical Research Council.
Chemical shift assignments and the family of structures for α-SIIIA have been deposited in the BioMagResBank with accession number 20023.
Abbreviations: µ-GIIIA, µ-GIIIB, µ-conotoxins GIIIA and GIIIB, respectively, from Conus geographus; µ-KIIIA, µ-conotoxin KIIIA from C. kinoshitai; µ-PIIIA, µ-conotoxin PIIIA from C. purpurascens; µ-SIIIA, µ-conotoxin SIIIA from C. striatus; µ-SmIIIA, µ-conotoxin SmIIIA from C. stercusmuscarum; MD, molecular dynamics; RMS, root mean square; TTX, tetrodotoxin; VGSC, voltage-gated sodium channel.
SUPPORTING INFORMATION AVAILABLE
NMR data and structure comparisons referred to in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Jones RM, Bulaj G. Conotoxins: New vistas for peptide therapeutics. Curr. Pharm. Des. 2000;6:1249–1285. doi: 10.2174/1381612003399653. [DOI] [PubMed] [Google Scholar]
- 2.Norton RS, Olivera BM. Conotoxins down under. Toxicon. 2006;48:780–798. doi: 10.1016/j.toxicon.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Cestele S, Catterall WA. Molecular mechanisms of neurotoxin action on voltage-gated sodium channels. Biochimie. 2000;82:883–892. doi: 10.1016/s0300-9084(00)01174-3. [DOI] [PubMed] [Google Scholar]
- 4.Catterall WA. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 5.Goldin AL. Resurgence of sodium channel research. Annu.ReV. Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 6.Cruz LJ, Gray WR, Olivera BM, Zeikus RD, Kerr L, Yoshikami D, Moczydlowski E. Conus geographus toxins that discriminate between neuronal and muscle sodium channels. J. Biol. Chem. 1985;260:9280–9288. [PubMed] [Google Scholar]
- 7.Moczydlowski E, Olivera BM, Gray WR, Strichartz GR. Discrimination of muscle and neuronal Na-channel subtypes by binding competition between [3H]saxitoxin and μ-conotoxins. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5321–5325. doi: 10.1073/pnas.83.14.5321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safo P, Rosenbaum T, Shcherbatko A, Choi DY, Han E, Toledo-Aral JJ, Olivera BM, Brehm P, Mandel G. Distinction among neuronal subtypes of voltage-activated sodium channels by μ-conotoxin PIIIA. J. Neurosci. 2000;20:76–80. doi: 10.1523/JNEUROSCI.20-01-00076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li RA, Ennis IL, Xue T, Nguyen HM, Tomaselli GF, Goldin AL, Marban E. Molecular basis of isoformspecific μ-conotoxin block of cardiac, skeletal muscle, and brain Na+ channels. J. Biol. Chem. 2003;278:8717–8724. doi: 10.1074/jbc.M210882200. [DOI] [PubMed] [Google Scholar]
- 10.Shon KJ, Olivera BM, Watkins M, Jacobsen RB, Gray WR, Floresca CZ, Cruz LJ, Hillyard DR, Brink A, Terlau H, Yoshikami D. μ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J. Neurosci. 1998;18:4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nielsen KJ, Watson M, Adams DJ, Hammarstrom AK, Gage PW, Hill JM, Craik DJ, Thomas L, Adams D, Alewood PF, Lewis RJ. Solution structure of μ-conotoxin PIIIA, a preferential inhibitor of persistent tetrodotoxin-sensitive sodium channels. J. Biol. Chem. 2002;277:27247–27255. doi: 10.1074/jbc.M201611200. [DOI] [PubMed] [Google Scholar]
- 12.West PJ, Bulaj G, Garrett JE, Olivera BM, Yoshikami D. μ-conotoxin SmIIIA, a potent inhibitor of tetrodotoxinresistant sodium channels in amphibian sympathetic and sensory neurons. Biochemistry. 2002;41:15388–15393. doi: 10.1021/bi0265628. [DOI] [PubMed] [Google Scholar]
- 13.Keizer DW, West PJ, Lee EF, Yoshikami D, Olivera BM, Bulaj G, Norton RS. Structural basis for tetrodotoxin-resistant sodium channel binding by μ-conotoxin SmIIIA. J. Biol. Chem. 2003;278:46805–46813. doi: 10.1074/jbc.M309222200. [DOI] [PubMed] [Google Scholar]
- 14.Bulaj G, West PJ, Garrett JE, Watkins M, Zhang MM, Norton RS, Smith BJ, Yoshikami D, Olivera BM. Novel conotoxins from Conus striatus and Conus kinoshitaiselectively block TTX-resistant sodium channels. Biochemistry. 2005;44:7259–7265. doi: 10.1021/bi0473408. [DOI] [PubMed] [Google Scholar]
- 15.Zhang MM, Green BR, Catlin P, Fiedler B, Azam L, Chadwick A, Terlau H, McArthur JR, French RJ, Gulyas J, Rivier JE, Smith BJ, Norton RS, Olivera BM, Yoshikami D, Bulaj G. Structure/function characterization of μ-conotoxin KIIIA, an analgesic, nearly irreversible blocker of mammalian neuronal sodium channels. J. Biol. Chem. 2007;282:30699–30706. doi: 10.1074/jbc.M704616200. [DOI] [PubMed] [Google Scholar]
- 16.Lewis RJ, Schroeder CI, Ekberg J, Nielsen KJ, Loughnan M, Thomas L, Adams DA, Drinkwater R, Adams DJ, Alewood PF. Isolation and structure-activity of μ-conotoxin TIIIA, a potent inhibitor of tetrodotoxin-sensitive voltagegated sodium channels. Mol. Pharmacol. 2007;71:676–685. doi: 10.1124/mol.106.028225. [DOI] [PubMed] [Google Scholar]
- 17.Green BR, Catlin P, Zhang MM, Fiedler B, Bayudan W, Morrison A, Norton RS, Smith BJ, Yoshikami D, Olivera BM, Bulaj G. Conotoxins containing nonnatural backbone spacers: Cladistic-based design, chemical synthesis, and improved analgesic activity. Chem. Biol. 2007;14:399–407. doi: 10.1016/j.chembiol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 18.Lancelin JM, Kohda D, Tate S, Yanagawa Y, Abe T, Satake M, Inagaki F. Tertiary structure of conotoxin GIIIA in aqueous solution. Biochemistry. 1991;30:6908–6916. doi: 10.1021/bi00242a014. [DOI] [PubMed] [Google Scholar]
- 19.Wakamatsu K, Kohda D, Hatanaka H, Lancelin JM, Ishida Y, Oya M, Nakamura H, Inagaki F, Sato K. Structure-activity relationships of μ-conotoxin GIIIA: Structure determination of active and inactive sodium channel blocker peptides by NMR and simulated annealing calculations. Biochemistry. 1992;31:12577–12584. doi: 10.1021/bi00165a006. [DOI] [PubMed] [Google Scholar]
- 20.Hill JM, Alewood PF, Craik DJ. Three dimensional solution structure of μ-conotoxin GIIIB, a specific blocker of skeletal muscle sodium channels. Biochemistry. 1996;35:8824–8835. doi: 10.1021/bi960073o. [DOI] [PubMed] [Google Scholar]
- 21.Palmer AG. Probing molecular motion by NMR. Curr. Opin. Struct. Biol. 1997;7:732–737. doi: 10.1016/s0959-440x(97)80085-1. [DOI] [PubMed] [Google Scholar]
- 22.Fischer MWF, Majumdar A, Zuiderweg ERP. Protein NMR relaxation: Theory, applications and outlook. Prog. Nucl. Magn. Reson. Spectrosc. 1998;33:207–272. [Google Scholar]
- 23.Feher VA, Cavanagh J. Millisecond-timescale motions contribute to the function of the bacterial response regulator protein Spo0F. Nature. 1999;400:289–293. doi: 10.1038/22357. [DOI] [PubMed] [Google Scholar]
- 24.Volkman BF, Lipson D, Wemmer DE, Kern D. Two-state allosteric behavior in a single-domain signaling protein. Science. 2001;291:2429–2433. doi: 10.1126/science.291.5512.2429. [DOI] [PubMed] [Google Scholar]
- 25.Eisenmesser EZ, Bosco DA, Akke M, Kern D. Enzyme dynamics during catalysis. Science. 2002;295:1520–1523. doi: 10.1126/science.1066176. [DOI] [PubMed] [Google Scholar]
- 26.Frauenfelder H, Parak F, Young RD. Conformational substates in proteins. Annu. ReV. Biophys. Biophys. Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- 27.Goldenberg DP, Koehn RE, Gilbert DE, Wagner G. Solution structure and backbone dynamics of an ω-conotoxin precursor. Protein Sci. 2001;10:538–550. doi: 10.1110/ps.30701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buczek O, Wei D, Babon JJ, Yang X, Fiedler B, Chen P, Yoshikami D, Olivera BM, Bulaj G, Norton RS. Structure and sodium channel activity of an excitatory I1-superfamily conotoxin. Biochemistry. 2007;46:9929–9940. doi: 10.1021/bi700797f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellison M, Feng ZP, Park AJ, Zhang X, Olivera BM, McIntosh JM, Norton RS. R-RgIA, a novel conotoxin that blocks the R9R10 nAChR: Structure and identification of key receptor-binding residues. J. Mol. Biol. 2008;377:1216–1227. doi: 10.1016/j.jmb.2008.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cornilescu G, Delaglio F, Bax A. Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J. Biomol. NMR. 1999;13:289–302. doi: 10.1023/a:1008392405740. [DOI] [PubMed] [Google Scholar]
- 31.Oldfield E, Norton RS, Allerhand A. Studies of individual carbon sites of proteins in solution by natural abundance 10948 Biochemistry, Vol. 47, No. 41, 2008 Yao et al. carbon 13 nuclear magnetic resonance spectroscopy. Relaxation behavior. J. Biol. Chem. 1975;250:6368–6380. [PubMed] [Google Scholar]
- 32.Norton RS, Clouse AO, Addleman R, Allerhand A. Studies of proteins in solution by natural-abundance carbon-13 nuclear magnetic resonance. Spectral resolution and relaxation behavior at high magnetic field strengths. J. Am. Chem. Soc. 1977;99:79–83. doi: 10.1021/ja00443a016. [DOI] [PubMed] [Google Scholar]
- 33.Palmer AG, Cavanagh J, Wright PE, Rance MJ. Sensitivity improvement in proton-detected 2-dimensional heteronuclear correlation NMR-spectroscopy. J. Magn. Reson. 1991;93:151–170. [Google Scholar]
- 34.Bartels C, Xia T, Billeter M, Güntert P, Wüthrich K. The program XEASY for computer-supported NMR spectral-analysis of biological macromolecules. J. Biomol. NMR. 1995;6:1–10. doi: 10.1007/BF00417486. [DOI] [PubMed] [Google Scholar]
- 35.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE assignment using the new software CANDID and the torsion angle dynamics algorithm DYANA. J. Mol. Biol. 2002;319:209–227. doi: 10.1016/s0022-2836(02)00241-3. [DOI] [PubMed] [Google Scholar]
- 36.Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 2003;160:65–73. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- 37.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 38.Koradi R, Billeter M, Wüthrich K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graphics. 1996;14:51–55. 29–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- 39.Ulrich EL, Akutsu H, Doreleijers JF, Harano Y, Ioannidis YE, Lin J, Livny M, Mading S, Maziuk D, Miller Z, Nakatani E, Schulte CF, Tolmie DE, Kent Wenger R, Yao H, Markley JL. BioMagResBank. Nucleic Acids Res. 2008;36:D402–D408. doi: 10.1093/nar/gkm957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farrow NA, Muhandiram R, Singer AU, Pascal SM, Kay CM, Gish G, Shoelson SE, Pawson T, Forman-Kay JD, Kay LE. Backbone dynamics of a free and a phosphopeptide-complexed Src homology-2 domain studied by 15N NMR relaxation. Biochemistry. 1994;33:5984–6003. doi: 10.1021/bi00185a040. [DOI] [PubMed] [Google Scholar]
- 41.Mandel AM, Akke M, Palmer AG. Backbone dynamics of Escherichia coli ribonuclease Hi: Correlations with structure and function in an active enzyme. J. Mol. Biol. 1995;246:144–163. doi: 10.1006/jmbi.1994.0073. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson RA, Lefevre JF. Reduced spectral density mapping for proteins: Validity for studies of 13C relaxation. J. Biomol. NMR. 1999;13:83–88. doi: 10.1023/A:1008323420405. [DOI] [PubMed] [Google Scholar]
- 43.Slupsky CM, Spyracopoulos L, Booth VK, Sykes BD, Crump MP. Probing nascent structures in peptides using natural abundance 13C NMR relaxation and reduced spectral density mapping. Proteins. 2007;67:18–30. doi: 10.1002/prot.21294. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Lee DK, Ramamoorthy A. Solid-state 13C NMR chemical shift anisotropy tensors of polypeptides. J. Am Chem. Soc. 2001;123:6118–6126. doi: 10.1021/ja010145l. [DOI] [PubMed] [Google Scholar]
- 45.Fiedler B, Zhang MM, Buczek O, Azam L, Bulaj G, Norton RS, Olivera BM, Yoshikami D. Specificity, affinity and efficacy of ı-conotoxin RXIA, an agonist of voltage-gated sodium channels NaV1.2, 1.6 and 1.7. Biochem. Pharmacol. 2008;75:2334–2344. doi: 10.1016/j.bcp.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyberts SG, Goldberg MS, Havel TF, Wagner G. The solution structure of eglin c based on measurements of many NOEs and coupling constants and its comparison with X-ray structures. Protein Sci. 1992;1:736–751. doi: 10.1002/pro.5560010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pallaghy PK, Duggan BM, Pennington MW, Norton RS. Three-dimensional structure in solution of the calcium channel blocker ω-conotoxin. J. Mol. Biol. 1993;234:405–420. doi: 10.1006/jmbi.1993.1595. [DOI] [PubMed] [Google Scholar]
- 48.Mandel AM, Akke M, Palmer AG., III Dynamics of ribonuclease H: Temperature dependence of motions on multiple time scales. Biochemistry. 1996;35:16009–16023. doi: 10.1021/bi962089k. [DOI] [PubMed] [Google Scholar]
- 49.Otting G, Liepinsh E, Wüthrich K. Disulfide bond isomerization in BPTI and BPTI(G36S): An NMR study of correlated mobility in proteins. Biochemistry. 1993;32:3571–3582. doi: 10.1021/bi00065a008. [DOI] [PubMed] [Google Scholar]
- 50.Atkinson RA, Kieffer B, Dejaegere A, Sirockin F, Lefevre JF. Structural and dynamic characterization of ω-conotoxin MVIIA: The binding loop exhibits slow conformational exchange. Biochemistry. 2000;39:3908–3919. doi: 10.1021/bi992651h. [DOI] [PubMed] [Google Scholar]
- 51.Norton RS, Pallaghy PK, Baell JB, Wright CE, Lew MJ, Angus JA. The polypeptide ω-conotoxin GVIA as a basis for new analgesic and neuroprotective agents. Drug DeV. Res. 1999;46:206–218. [Google Scholar]
- 52.Nielsen KJ, Schroeder T, Lewis R. Structure-activity relationships of ω-conotoxins at N-type voltage-sensitive calcium channels. J. Mol. Recognit. 2000;13:55–70. doi: 10.1002/(SICI)1099-1352(200003/04)13:2<55::AID-JMR488>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 53.Baell JB, Forsyth SA, Gable RW, Norton RS, Mulder RJ. Design and synthesis of type-III mimetics of ω-conotoxin GVIA. J. Comput.-Aided Mol. Des. 2001;15:1119–1136. doi: 10.1023/a:1015930031890. [DOI] [PubMed] [Google Scholar]
- 54.Robinson AJ, Elaridi J, Van Lierop BJ, Mujcinovic S, Jackson WR. Microwave-assisted RCM for the synthesis of carbocyclic peptides. J. Pept. Sci. 2007;13:280–285. doi: 10.1002/psc.840. [DOI] [PubMed] [Google Scholar]
- 55.Cho CH, Urquidi J, Singh S, Robinson GW. Thermal offset viscosities of liquid H2O, D2O and T2O. J. Phys.Chem. B. 1999;103:1991–1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.