Abstract
MicroRNAs are implicated in many biological and pathological processes and are emerging as key actors in lung health and disease. Specific patterns of dysregulated microRNAs have been found in idiopathic pulmonary fibrosis (IPF), an untreatable interstitial lung disease of unknown etiology. IPF is characterized by dramatic and extensive phenotypic changes in the lung that include alveolar cell hyperplasia, fibroblast proliferation and formation of myofibroblast foci, deposition of extracellular matrix, and changes in lung transcriptional programming. Here, we discuss the latest insights about the role of microRNAs in lung fibrosis with a focus on the contribution of animal models of disease to the derivation of these insights.
Introduction
IPF is a progressive and devastating disorder characterized by the unremitting accumulation of activated fibroblasts in the lung leading to scarring and ultimately respiratory failure. The prevalence of IPF is estimated at 20/100 000 for men and 13/100 000 for women with a survival time from 2 to 5 years (after diagnosis) [1,2]. Our group has a long-standing interest in understanding the mechanisms governing how gene expression is regulated in IPF [3,4]. A relatively, new and exciting field of study is the role played by microRNAs, small non-coding RNAs that function as crucial checkpoints in the regulation of gene expression [5]. We and others have identified multiple microRNAs that are differentially expressed in IPF patients compared to normal individuals, thus suggesting a distinct and reproducible IPF ‘microRNA signature’ which might be crucial for the pathogenesis of the disease. Understanding how microRNAs regulate their targeted genes is critical to devising rational and highly targeted therapies to treat IPF. Despite advances in microRNA-related research, the role of microRNAs in IPF is only just emerging [3]. Our efforts have focused on understanding on how these molecules may contribute to the IPF phenotype employing both animal and in vitro model systems of pulmonary fibrosis. The fact that many microRNAs and genes found in mice have molecular and functional homologues in vertebrates, including humans, means that genetic discovery in animal models may be generalizable to our understanding of IPF and to identifying potential therapies for this challenging disease. Our approach is to identify genes and microRNAs that are differentially expressed in human lung fibrosis. We then move to animal and in vitro systems to understand how these or microRNA contribute to the pathogenesis of the human disease. We design experiments that perturb these gene or microRNAs employing genetic or pharmacologic strategies. The ultimate goal of our work is to translate our data back to our patients who suffer from IPF. In this review, we will follow a similar approach. First we will briefly review the biology of microRNAs. We will then discuss specific microRNAs that are differentially expressed in IPF and how these microRNAs have been studied in vitro and in vivo. We will then conclude by suggesting strategies by which microRNAs may be tested in animal systems as new clinical tools in IPF.
Biosynthesis and mechanism of microRNAs expression
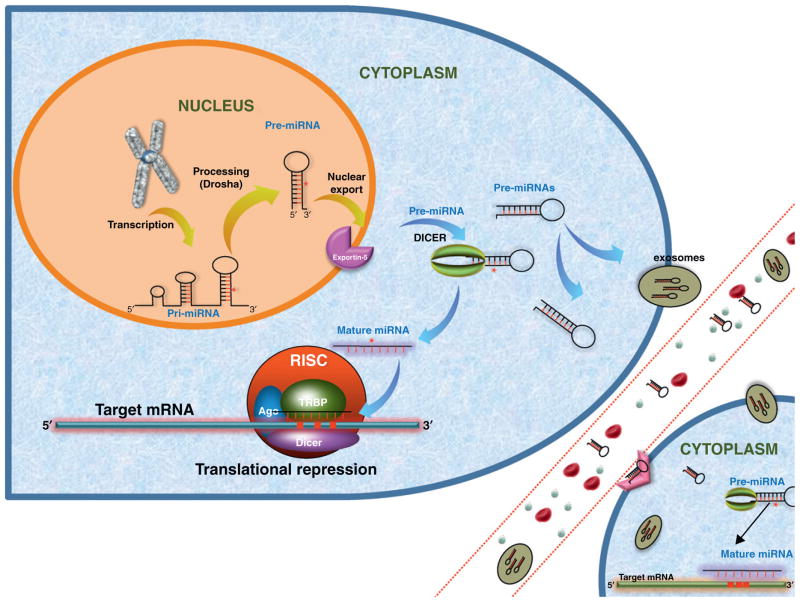
The discovery of let-7, a small RNA, added a new layer of complexity to traditional concepts of gene expression [6]. MicroRNAs are highly conserved among species and comprise a class of small non-coding RNAs of approximately 19–22 nucleotides in length. They have been shown to regulate gene expression at the level of transcription and are predicted to regulate at least 60% of human genes [7]. MicroRNAs are first transcribed in the nucleus by RNA polymerase III as pri-miRNA molecules [8]. These pri-miRNAs, approximately 2000 base pairs (bp) in length, are next processed by the enzyme drosha, a ribonuclease (RNase III), to produce a 200 bp pre-miRNA precursor [9]. These nascent microRNAs are transported from the nucleus to the cytoplasm by the nuclear export receptor exportin-5 [10]. Once in the cytoplasm, they are processed by a second RNase III endonuclease, dicer, generating a double stranded RNA (dsRNA) structure of 19–22 nucleotides [11]. This microRNA duplex is unwound into the mature single-strand form and is recognized by the RNA-induced silencing complex (RISC), which carries the mature microRNA to its complementary 3′ or 5′-untranslated region (UTR) or open reading frame (ORF) or promoter regions of the targeted mRNA (Fig. 1), usually resulting in translational repression or target degradation and gene silencing [12].
Figure 1.
Biogenesis and function of microRNAs. After transcription in the nucleus, pre-miRNA molecules are released into the cytoplasm and can be processed further by Dicer to form the mature miRNA which is selectively recognized by RISC complex to regulate its target mRNA. In addition, recent findings have demonstrated that pre-miRNAs can be packaged and transported using exosomes and multivehicle bodies or other (not fully explored) pathways together with RNA-binding proteins.
MicroRNAs in idiopathic pulmonary fibrosis: From human to mouse
miR-155
The earliest observation of microRNAs in pulmonary fibrosis did not follow our paradigm but rather began with observations in mice. This first report suggesting a role of microRNAs in lung fibrogenesis, published by Pottier and collaborators [13], demonstrated that miR-155 down-regulated expression of keratinocyte growth factor (KGF/FGF7) in normal human lung fibroblasts. The expression of KGF is crucial for maintaining the alveolar epithelial cell phenotype and may be protective in bleomycin injury [14]. Bleomycin-sensitive mice (C57bl/6) injured with bleomycin showed significant increases in miR-155 expression compared to the bleomycin-resistant Balb/c strain. The authors also showed that increased expression of miR-155 was correlated with the severity of the lung fibrosis [13]. So far, differential expression of miR-155 has not been demonstrated in IPF [15].
Let-7 family
Pandit et al. [16] have shown that let-7 is down-regulated in a large cohort of patients with IPF and in mice following bleomycin injury. The authors demonstrated that let-7d was directly inhibited by TGFβ signaling in epithelial cells. Inhibition of let-7 microRNA caused expression of mesenchymal markers, consistent with epithelial mesenchymal transition (EMT). In vivo inhibition of let-7 family in the lung was associated with epithelial expression of mesenchymal markers and deposition of collagen without additional injury. This suggests that the dysregulation of let-7 independent of injury to the lung may lead to increased expression of crucial pro-fibrotic factors in lung [16]. A proven target of let-7 is high mobility group AT-hook 2 (HMGA2), a transcription factor that has been implicated in the EMT program [17]. While EMT does not appear to be a source of matrix-producing cells in the lung [18], EMT may be crucial for promoting fibrosis [19]. HMGA2 is up-regulated in the lung with IPF [16]. This suggests that let-7 inhibition leads to increased expression of HMGA2 which may lead to activation of pro-fibrotic programs in the lungs [17]. If down-regulation of let-7 indeed initiates pro-fibrotic programs in the lung, reconstitution of let-7 in the lung may be a promising strategy of therapy for IPF. Studies that use let-7 mimics or inhibitor of let-7 targets in animal models of fibrosis will be required to address this question.
miR-29 family
There are three members of the miR-29 family including miR-29a, miR-29b and miR-29c [20]. They are highly expressed in normal lungs and down-regulated in several lung diseases such as cancer and pulmonary fibrosis [21,22]. In vitro and in vivo studies have shown that miR-29 is negatively regulated by TGF-β/SMAD3 signaling. Xiao et al. [23] have shown that administration of miR-29 before installation of bleomycin prevents pulmonary fibrosis by a mechanism that may include preventing the accumulation of macrophages in the lung. In addition, it has been shown that miR-29 family has anti-fibrotic properties in cardiac and skin fibrosis [15,24]. In support of a role for miR-29 in fibrosis we found that the expression of members of the miR-29 family is decreased in the lung of patients with IPF compared with normal individuals [15]. Taken together, these findings suggest that administration of the miR-29 family may be a novel anti-fibrotic strategy in the treatment of lung fibrosis.
miR-21
Liu and collaborators [25] have shown that mice with bleomycin-induced pulmonary fibrosis exhibit a significant increase in miR-21 levels and that this increase was associated with increased TGFβ levels. Expression of miR-21 was also increased in IPF lung [25]. Using inhibitors for miR-21 in the model of bleomycin, the authors found that ablation of miR-21 prevented experimental pulmonary fibrosis [25]. In support of these findings, previous studies have shown that miR-21 is not only essential to control of cell survival and proliferation, it can also indirectly regulate the expression of matrix metalloproteinases via RECK (reversion-inducing-cysteine-rich protein with kazal motifs) and TIMP3 (tissue inhibitor of metalloproteinases 3) [26]. It has been suggested that miR-21 participates in TGFβ1-induced myofibroblast differentiation by targeting PDCD4 (programmed cell death protein 4) [27]. Taken together these findings, the inhibition of miR-21 might provide an effective therapy for IPF.
miR-30 family
Zhang et al. [28] have elucidated a role for the miR-30 family as blockers of EMT. Here, the authors demonstrated that Snail, a transcriptional repressor of E-cadherin and a crucial EMT transcription factor [29], is a direct target of miR-30. The authors showed that miR-30 blocked EMT by down-regulating the expression of Snail1 [28]. Other targets of miR-30 include Smad1 and Runx2 that are known to play crucial roles in TGFβ signaling and hence in the cell growth, differentiation, extracellular matrix production and apoptosis [28]. In our data set, the miR-30 family was down-regulated in patients with IPF [5]. Thus the evidence suggests that members of the miR-30 family are potential anti-fibrotic agents. However, in vivo data are lacking.
miR-154
Milosevic et al. [15] have identified a cluster of 24 up-regulated microRNAs in IPF lungs that were localized to chromosome 14q32. Among those microRNAs, they chose to focus on miR-154 and demonstrated that it positively regulated lung fibroblast proliferation and migration, processes that are potentially important to the accumulation of fibroblasts in IPF. In addition, like many of the other microRNAs previously mentioned, miR-154 is a TGFβ target. In vitro inhibition of miR-154 diminished TGFβ-induced fibroblast mobility. miR-154 is also a negative regulator of CDKN2B, a tumor suppressor involved in TGFβ-induced growth inhibition and a positive regulator of the WNT/β-catenin pathway. Further studies may demonstrate that a protective effect of miR-154 inhibition in animal models of lung fibrosis would suggest that miR-154 should also be studied as therapeutic target in IPF.
Other microRNAs in IPF
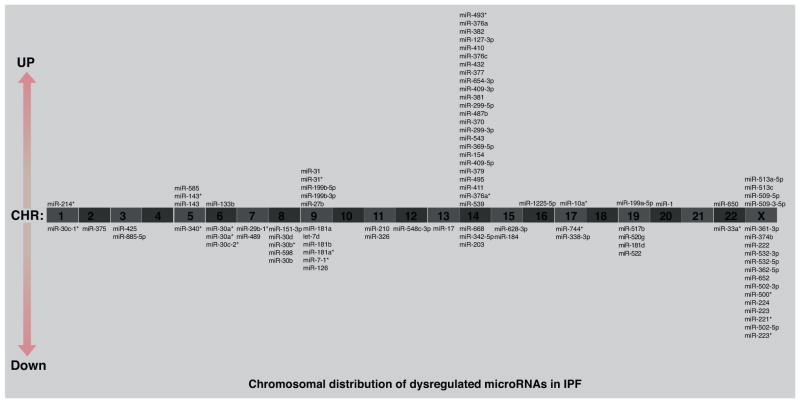
In several of our previous studies we have employed high throughput genomic approaches, and we have found that IPF exhibits a distinct microRNA profile [5,15,16]. Interestingly, we have found that more than 30% of dysregulated micro-RNAs were clustered on the chromosome 14q32 (Fig. 2). This finding suggests that chromosome 14q32 may be a susceptible region in IPF [15]. Further studies are needed to clarify the role of other microRNAs in this chromosomal region. The experimental data and microarray designs have been deposited in the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under series GSE34812. In Table 1, we summarize the microRNAs that have been shown to play a role in pulmonary fibrosis in both animals and humans.
Figure 2.
Chromosomal distribution of up- and down-regulated microRNAs in IPF lungs. Distribution in human chromosomes of the top 100 dysregulated microRNAs from patients with IPF compared with normal individuals.
Table 1.
Summary of microRNAs involved in pulmonary fibrosis
| microRNA | First identified | expression in fibrotic lung | Reference | Suggested therapy |
|---|---|---|---|---|
| Let-7 family | Human | Down | [16] | Reconstitution |
| miR-21 | Human/Mouse | Up | [25] | Inhibition |
| miR-29 family | Mouse | Down | [21] | Reconstitution |
| miR-30 family | Human | Down | [28] | Reconstitution |
| miR-154 | Human | Up | [15] | Inhibition |
| miR-155 | Mouse | Up | [13] | Inhibition |
A conceptual framework for studying microRNAs in pulmonary fibrosis and their potential as biomarkers in IPF: from the mouse back to humans
How can these data be translated back to IPF patients? The first and most basic translational application of microRNA biology is the design of microRNA mimics or antagonists as potential therapies. A very simplistic paradigm conceives of microRNAs as negative regulators of gene expression: increased expression of a microRNA will lead to down-regulation of its target genes, and decreased expression of one microRNA will lead to up-regulation of its target genes. Thus a strategy, for example, to promote let-7d expression, which is down-regulated in IPF, would be predicted to block fibrosis. Targeting microRNA would seem to offer a therapeutic advantage over the targeting of a particular gene because the micro-RNA may regulate multiple pro- or anti-fibrotic genes. While no clinical trials are currently underway in IPF, several pharmaceutical companies are actively engaged in the manipulation of microRNAs with therapeutic intent in other diseases.
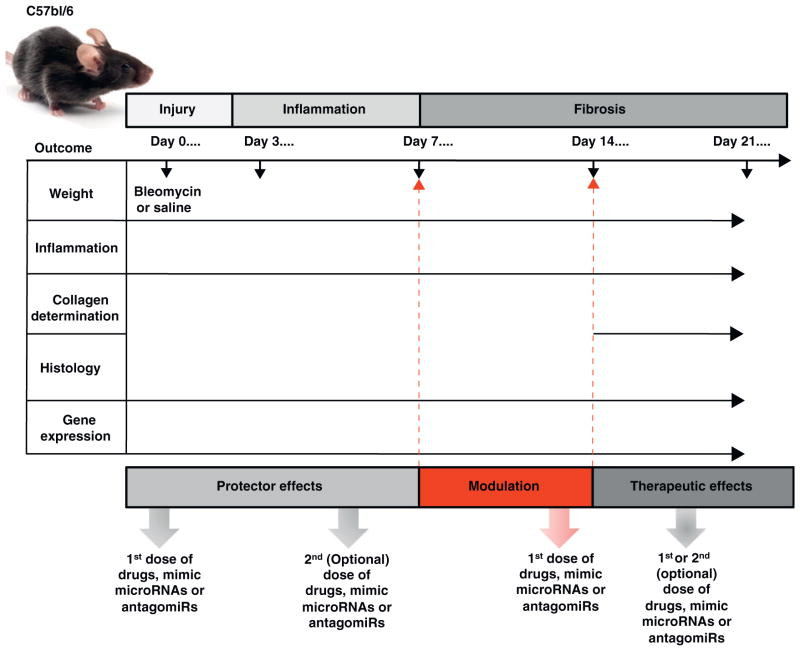
The choice of animal model is based on the knowledge of the unique features of the fibrotic disorder and on the scientific question studied. Several approaches to recapitulating pulmonary fibrosis have been used by investigators and are summarized in Table 2. Thoracic radiation of 11–14 Gy is employed to induce lung fibrosis in C57bl/6 strain by 28 weeks post-exposure [30]. The advantages of this approach are the clinical relevance and differences in susceptibility among potential inbred mouse strains [31]. Intratracheal instillation of crystalline silica has shown to produce fibrotic response in C57bl/6 and CBA/J mice [32]. A disadvantage of this animal model is that up to 30 weeks may be necessary for fibrosis to develop. Pulmonary-specific or adenoviral vector-directed transgenic models have been invaluable for explicating the role of many individual genes. Some examples include overexpression of human TGF-β directed by the surfactant protein C (SP-C) promoter [33] and IL-13 directed by Clara cell 10-kDa (CC10) protein promoter [34]. The advantage of this approach is that expression of these trans-genes can be controlled. Adenoviruses have been used to overexpress a variety of cytokines and chemokines such as TNF-α [35], TGF-β [36], IL-1β [37] and GM-CSF [38]. The well-characterized and the most widely employed model of pulmonary fibrosis is bleomycin injury. Bleomycin is an anti-neoplastic agent which promotes fibrosis as a prominent side effect. Bleomycin-induced lung injury is characterized by inflammation followed by apoptosis of epithelial and endothelial cells, mesenchymal cell proliferation and deposition of extracellular matrix resulting in the destruction of the lung architecture and the progressive the loss of pulmonary function [38]. Direct pulmonary delivery of bleomycin (doses ~ 0.35–0.75 unit/mL in a volume of 80 μl) remains the preferred route to induce pulmonary fibrosis in mice, but intravenous and intraperitoneal routes have been used as well. Some of the histologic features of bleomycin-induced pulmonary fibrosis in mice are comparable to human including collagen deposition and expression of alpha-smooth muscle actin (α-SMA), a hallmark of myofibroblast differentiation. C57bl/6 mice are susceptible to bleomycin injury and are the most characterized murine model. After intratracheal administration of bleomycin in C57bl/6 strain, pulmonary fibrosis can be seen by day 14 with a maximal response around days 21–28 [13]. In Fig. 3, we suggest a strategy for designing microRNA-based therapeutics studies in bleomycin injury [39].
Table 2.
Summary of currently used animal models of pulmonary fibrosis
| Model | Administration | Advantages |
|---|---|---|
| Bleomycin | Intratracheal/pump/inhalation/intravenous (IV)/intraperitoneal (IP) | Most widely used model in literature |
| Silica | Intratracheal/inhalation | Persistent fibrosis and clinically relevant as a model for human silicosis |
| Fluorescein isothiocyanate (FITC) | Intratracheal/IV/IP | Persistent fibrosis, quick development of fibrosis and not mouse strain dependent |
| Radiation | Thoracic exposure | Clinically relevant as a model for pneumonitis and fibrosis |
| Viral vector delivery | Intratracheal/IV | Specific fibrosis-related genes |
| Transgenic models | Genetic engineering: pronuclear injection (PNI)/transgene insertion | Specific fibrosis-related genes |
Figure 3.
A strategy for studying microRNA-based therapeutics in the bleomycin model of pulmonary fibrosis. Bleomycin or saline is administered on day 0 of the experiment. Drugs, mimic microRNAs, or antagomirs may be dosed at various times over the duration of experiment. When therapies are dosed before day 7, the effect is considered ‘protective,’ and when dosed after day 14, the effect is considered ‘therapeutic.’ Dosing between days 7 and 14 after injury may ‘modulate’ the final phenotype of bleomycin injury. The overall phenotype can be measured in several ways. Changes in the weight of the animals, measures of inflammation, histology, and gene expression can be assessed throughout the experiment. Determination of collagen deposition is typically measured after day 14.
Discussion
While there are relatively few studies of microRNAs in IPF, certainly when compared to the cancer literature, several animal studies suggest that manipulation of microRNA may prove to be ‘magic bullets’ for the treatment of IPF. Collaboration between academic centers and pharmaceutical companies will be essential in this regard to test and validate a very limited number of potential candidates. As mentioned above, there are several companies that are already focused on these pathways. Compared to the number of differentially expressed genes in IPF, there are far fewer differentially regulated microRNAs in IPF. And as microRNAs have multiple targets, therapeutic manipulation of microRNA may have multiplicative effects on gene expression and on the fibrotic phenotype in the lung. Animal studies will be indispensable. The ideal vehicle and route for delivery to the lungs have yet to be elucidated. And while no animal model of pulmonary fibrosis is perfect, therapeutic efficacy of microRNA targets in multiple models of pulmonary fibrosis will be necessary to move microRNAs intro clinical testing. In conclusion, in this review we have discussed the latest literature in microRNAs in IPF with a focus on the use of animal models in pulmonary fibrosis.
Footnotes
Conflict of interest statement
CLLC has no financial relationship with a commercial entity that has an interest in the subject of this manuscript. DJK has consulted for Gilead and NK has a patent application for use of microRNAs as therapeutic targets in IPF.
References
- 1.American Thoracic Society Idiopathic Pulmonary Fibrosis. Diagnosis and treatment. International consensus statement American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Raghu G, et al. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 3.Rabinovich EI, et al. Global methylation patterns in idiopathic pulmonary fibrosis. PLoS One. 2012;4:e33770. doi: 10.1371/journal.pone.0033770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Kaminski N. Biomarkers in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:441–446. doi: 10.1097/MCP.0b013e328356d03c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandit KV, et al. MicroRNAs in idiopathic pulmonary fibrosis. Transl Res. 2011;4:191–199. doi: 10.1016/j.trsl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart BJ, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 7.Friedman JM, Jones PA. MicroRNAs: critical mediators of differentiation, development and disease. Swiss Med Wkly. 2009;139:466–472. doi: 10.4414/smw.2009.12794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee Y, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 9.Zeng Y, et al. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi R, et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lund E, et al. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 12.Gregory RI, et al. Micro RNA biogenesis: isolation and characterization of the microprocessor complex. Methods Mol Biol. 2006;342:33–47. doi: 10.1385/1-59745-123-1:33. [DOI] [PubMed] [Google Scholar]
- 13.Pottier N, et al. Identification of keratinocyte growth factor as a target of microRNA-155 in lung fibroblasts: implication in epithelial–mesenchymal interactions. PLoS One. 2009;4:e6718. doi: 10.1371/journal.pone.0006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finch PW, Rubin JS. Keratinocyte growth factor/fibroblast growth factor 7, a homeostatic factor with therapeutic potential for epithelial protection and repair. Adv Cancer Res. 2004;91:69–136. doi: 10.1016/S0065-230X(04)91003-2. [DOI] [PubMed] [Google Scholar]
- 15.Milosevic J, et al. Profibrotic role of mir-154 in pulmonary fibrosis. Am j Respir Cell Mol Biol. 2012;47:879–887. doi: 10.1165/rcmb.2011-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pandit KV, et al. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, et al. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes Dev. 2007;21:1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rock JR, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA. 2011;108:E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapman HA. Epithelial–mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 20.Hwang HW, et al. A hexanucleotide element directs microRNA nuclear import. Science. 2007;315:97–100. doi: 10.1126/science.1136235. [DOI] [PubMed] [Google Scholar]
- 21.Fabbri M, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci USA. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushing L, et al. MIR-29 is a major regulator of genes associated with pulmonary fibrosis. Am J Respir Cell Mol Biol. 2011;45:287–294. doi: 10.1165/rcmb.2010-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao J, et al. miR-29 inhibits bleomycin-induced pulmonary fibrosis in Mice. Mol Ther. 2012;20:1251–1260. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maurer B, et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010;62:1733–1743. doi: 10.1002/art.27443. [DOI] [PubMed] [Google Scholar]
- 25.Liu G, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gabriely G, et al. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol Cell Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Q, et al. Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor–stroma interaction. Int J Cancer. 2011;128:1783–1792. doi: 10.1002/ijc.25506. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, et al. miR-30 inhibits TGF-β1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1. Biochem Biophys Res Commun. 2012;417:1100–1105. doi: 10.1016/j.bbrc.2011.12.121. [DOI] [PubMed] [Google Scholar]
- 29.Chapman HA. Epithelial responses to lung injury: role of the extracellular matrix. Proc Am Thorac Soc. 2012;9:89–95. doi: 10.1513/pats.201112-053AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rube CE, et al. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int J Radiat Oncol Biol Phys. 2000;47:1033–1042. doi: 10.1016/s0360-3016(00)00482-x. [DOI] [PubMed] [Google Scholar]
- 31.O‘Brien TJ, et al. Radiation-induced strain differences in mouse alveolar inflammatory cell apoptosis. Can J Physiol Pharmacol. 2005;83:117–122. doi: 10.1139/y05-005. [DOI] [PubMed] [Google Scholar]
- 32.Ohtsuka Y, et al. Genetic linkage analysis of pulmonary fibrotic response to silica in mice. Eur Respir J. 2006;28:1013–1019. doi: 10.1183/09031936.06.00132505. [DOI] [PubMed] [Google Scholar]
- 33.Lee CG, et al. Transgenic modeling of transforming growth factor-beta(1): role of apoptosis in fibrosis and alveolar remodeling. Proc Am Thorac Soc. 2006;3:418–423. doi: 10.1513/pats.200602-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–788. doi: 10.1172/JCI5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ita M, et al. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29:669–676. doi: 10.1165/rcmb.2002-0046OC. [DOI] [PubMed] [Google Scholar]
- 36.Kolb M, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163:770–777. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 37.Kolb M, et al. Transient expression of IL-1beta induces acute lung injury and chronic repair leading to pulmonary fibrosis. J Clin Invest. 2001;107:1529–1536. doi: 10.1172/JCI12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weiss DJ. Perflubron enhances adenovirus-mediated gene expression in lungs of transgenic mice with chronic alveolar filling. Hum Gene Ther. 1999;10:2287–2293. doi: 10.1089/10430349950016933. [DOI] [PubMed] [Google Scholar]
- 39.Moeller A, et al. The bleomycin animal model: a useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int J Biochem Cell Biol. 2008;40:362–382. doi: 10.1016/j.biocel.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]