Abstract
A role for the cell cycle protein cyclin A2 in regulating progesterone receptor (PR) activity is emerging. This study investigates the role of cyclin A2 in regulating endogenous PR activity in T47D breast cancer cells by depleting cyclin A2 expression and measuring PR target genes using q-RT-PCR. Targets examined included genes induced by the PR-B isoform more strongly than PR-A (SGK1, FKBP5), a gene induced predominantly by PR-A (HEF1), genes induced via PR tethering to other transcription factors (p21, p27), a gene induced in part via extra-nuclear PR signaling mechanisms (cyclin D1) and PR-repressed genes (DST, IL1R1). Progestin induction of target genes was reduced following cyclin A2 depletion. However, cyclin A2 depletion did not diminish progestin target gene repression. Furthermore, inhibition of the associated Cdk2 kinase activity of cyclin A2 also reduced progestin induction of target genes, while Cdk2 enhanced the interaction between PR and cyclin A2. These results demonstrate that cyclin A2 and its associated kinase activity are important for progestin-induced activation of endogenous PR target genes in breast cancer cells.
Keywords: progesterone receptor, cyclin A2, cyclin dependent kinase 2
1. Introduction
The progesterone receptor (PR) is a hormone activated transcription factor, whose activity is regulated not only by its ligand, but also by a number of kinases that phosphorylate the receptor as well as its associated coactivators. We found, previously, that PR interacts with cyclin A2 and that overexpression of cyclin A2 enhances PR activity measured using a transiently transfected reporter [1]. This activity was dependent on its ability to interact with its kinase partner, cyclin dependent kinase 2 (Cdk2), since a mutated cyclin A2 unable to interact with Cdks failed to stimulate PR activity while the general Cdk inhibitor, roscovitine, inhibited PR activity [1]. We subsequently found that both Cdk2 and Cdk1 were required for optimal PR activity [2] and Beato’s group has shown that Cdk2 activates PARP-1 to facilitate PR dependent transcription [3]. This raises the question of whether the observed stimulation by cyclin A2 was due to a general increase in Cdk1/2 activity or whether cyclin A2 bound kinase was itself effective in facilitating PR dependent activity. Our previous studies showing that endogenous PR activity in T47D breast cancer cells varied as a function of cell cycle with optimal activity in S phase, where cyclin A2 expression is highest [4], suggests that cyclin A2 may be particularly important for PR activity.
Cyclin A2 mRNA and protein are overexpressed in many cancers [5], including cancers of PR target tissues. Overexpression of cyclin A2 is associated with poor prognosis in early stage, invasive and metastatic breast cancer [6–11] as well as endometrial cancer [12] and ovarian cancer [13]. In normal breast tissue, PR is expressed in approximately 30–40% of luminal epithelial cells and only in non-proliferating cells with progesterone regulation of proliferation mediated by paracrine signaling pathways [14]. In contrast, PR is expressed in a greater number of proliferating malignant breast epithelial cells indicating a switch to an autocrine mode of regulation [15]. These findings led us to investigate whether cyclin A2 regulates PR function in breast cancer cells.
PR is expressed as two isoforms, PR-B and PR-A, which are translated from unique mRNAs transcribed from alternate estrogen inducible promoters within a single gene [16]. PR-A lacks the first 164 amino acids of PR-B but the isoforms have otherwise identical sequence. PR-B and PR-A can regulate distinct subsets of target genes [17] while studies in knockout mice suggest that PR-B predominantly regulates mammary gland development and PR-A is critical for normal uterine function [18, 19].
In the classical model of gene regulation by PR, hormone binding to the receptor triggers loss of associated heat shock and chaperone proteins, facilitating PR dimerization. The receptor dimer binds directly to progestin response elements in the regulatory regions of its target genes and a multi-component complex is assembled to enable transcription [20]. Additionally, PR can regulate transcription independently of direct DNA binding via tethering to other transcription factors such as specificity protein 1 (Sp1) or activator protein 1 (AP-1) and modulating their transcriptional activity on target genes [21–28]. PR also represses target genes. Direct repression through PR binding to a progestin response element and recruitment of corepressor molecules [29–32], dissociation of PR and Sp4 from Sp1 sites [33] and interference with other transcription factors [34] have been described as mechanisms by which progestins repress transcription. In addition to its transcriptional actions in the nucleus, ligand activated PR can regulate the Src/Ras/MAPK, Src/Jak/Stat3 and PI3 kinase/Akt cytoplasmic signaling pathways [35–42]. These extra-nuclear actions of PR can act to stimulate the activity of other transcription factors, such as the MAPK target Elk-1, to regulate gene expression [43]. In turn, cytoplasmic signaling pathways and kinase cascades strongly influence PR activity in the nucleus [1, 44–46]. The collective outcome of these various PR mechanisms of action directs cell proliferation, differentiation and tissue maintenance and function in progestin target organs [47].
Based on our previous studies using transiently transfected reporter genes [1], we hypothesized that cyclin A2 regulates endogenous PR function in breast cancer cells. Our findings suggest that cyclin A2 and its associated kinase activity are required for maximal induction of progestin responsive target genes regulated via both the nuclear and extra-nuclear mechanisms of PR action, although cyclin A2 does not appear necessary for repression of target genes by progestins. These studies contribute to a better understanding of the integration of cell cycle regulation and progestin signaling in breast cancer.
2. Materials and Methods
2.1. Materials
General chemicals were purchased from Sigma (St Louis, MO) while cell culture reagents were from Invitrogen (Carlsbad, CA). The synthetic progestin, R5020 (Promegestone), was obtained from NEN Life Science (Waltham, MA). Cdk inhibitors (NU6102 and roscovitine) were from Calbiochem (Gibbstown, NJ).
2.2. Cell culture
All cell lines were obtained from the American Type Culture Collection (Manassas, VA). T47D cells were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS) and 5 µg/ml insulin. ZR-75-1 cells were maintained in RPMI 1640 containing 10% FBS. Cells were maintained in a 37°C humidified tissue culture incubator containing 5% CO2.
2.3. Transient transfection
For reporter gene assays, T47D cells in RPMI 1640 medium containing either 10% FBS or charcoal stripped FBS (sFBS) were seeded in 12 well plates at a density of 150,000 cells/well and transiently transfected with a progestin responsive luciferase reporter (GRE2-E1b-luc, 250 ng/well) using lysine-coupled inactivated adenovirus as previously described [48]. Hormone (10 nM R5020) or vehicle was added to the medium 24 hours after transfection and cells were harvested for analysis 18 hours later.
For small interfering RNA (siRNA) experiments, T47D cells were transiently transfected with siRNA using Lipofectamine reagent (Invitrogen) according to the manufacturer’s instructions for five hours in serum free medium before addition of RPMI containing 10% FBS. For RNA and immunoblot experiments, cells were seeded in 12 well plates at a density of 200,000 cells/well and transfected with 25 pmol/well siRNA. For cell cycle analysis experiments, cells were seeded in 6 well plates (400,000 cells per/well) and transfected with 50 pmol/well siRNA. Cyclin A2 siGENOME SMARTpool (Cat. # M-003205-02, Dharmacon, Lafayette, CO) or two individual siRNAs were used (cyclin A2 ON-TARGETplus #11, Cat. # J-003205-11, and #12, Cat. # J-003205-12). siGENOME non-targeting siRNA pool #1 (Cat. # D-001206-13, Dharmacon) was transfected as a control. Hormone (10 nM R5020) or vehicle (0.1% ethanol) was added to the medium 48 hours after transfection and the cells were incubated for the indicated times before harvesting for analysis. Triplicate wells for RNA experiments were analyzed. Protein lysates from triplicate wells were combined for immunoblot assays. Cells from duplicate wells were combined prior to cell cycle analysis.
2.4. Reporter gene analysis
Cells were lysed with 1× reporter lysis buffer (Promega, Madison, WI) at room temperature for 30 minutes. Luciferase activity was measured using Luciferase Assay Reagent (Promega) and a LuminoSkan Ascent luminometer (Thermo Scientific, Waltham, MA). Luciferase activity was normalized to total cellular protein concentration as determined by Bradford assay using Protein Assay Dye Reagent (Bio-Rad, Hercules, CA) and a Multiscan Plus plate reader (Thermo Scientific).
2.5. Real-time reverse transcription (RT)-PCR
RNA was prepared using TRIzol Reagent (Invitrogen) and reverse transcribed using random primers and SuperScript III reverse transcriptase (Invitrogen). Real-time PCR was performed using TaqMan Universal PCR Master Mix or SYBR Green PCR Master Mix on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers and probes used for real-time PCR were purchased from Sigma-Genosys (The Woodlands, TX) and are indicated in Table 1, along with the full name and a brief description of the function of each target gene in the different categories investigated. Relative mRNA levels of each target gene were determined using a standard curve and normalized to the level of 18S rRNA (primer probe mix from Applied Biosystems).
TABLE 1.
Primers, probes and descriptions of the different classes of PR target genes amplified by QPCR.
| Full name (other names) |
Function | Primer and probe sequences |
|---|---|---|
| SGK1 (regulated by PR-B and PR-A via genomic mechanisms) | ||
| serum/glucocorticoid regulated kinase 1 | ion channel activation, kinase signaling pathways and transcription [70] | sense 5’-TGACCCCGAGTTTACCGAAG-3’ antisense: 5’-CCTTGACGCTGGCTGTGAC-3’ probe 5’-FAMCCTGTCCCCAACTCCATTGGCAAGTTAMRA-3’ |
| FKBP5 (regulated by PR-B > PR-A) | ||
| FK506 binding protein 5 | immunophilin with functions in protein folding and steroid receptor trafficking [71] | sense 5’-GGATATACGCCAACATGTTCAA-3’ antisense 5’-CCATTGCTTTATTGGCCTCT-3’ |
| NDRG1 (regulated by PR-B > PR-A) | ||
| n-myc downstream regulated gene 1 | stress responsive gene involved in cell proliferation, differentiation, growth arrest and hypoxia, thought to have tumor suppressor functions [72] | sense 5’-CTACCATGACATCGGCATGAA-3’ antisense 5’-CGTGGCAGACGGCAAAGT-3’ |
| HEF1 (regulated by PR-A > PR-B) | ||
| Enhancer of Filamentation 1 (NEDD9 or Cas-L) | scaffold protein strongly associated with tumor metastasis [73] | sense 5’-GAGAGGAGCTGGATGGATGACT-3’ antisense 5’-AGCTCTTTCTGTTGCCTCTCAAA-3’ |
| cyclin D1 (regulated by classical and extra-nuclear mechanisms) | ||
| key regulator of progression through G1 of the cell cycle and overexpressed in many cancers [74] | sense 5’-GTCCTACTACCGCCTCACACG-3’ antisense 5’-GGGCTTCGATCTGCTCCTG-3’ |
|
| p21 (regulated via PR tethering to Sp1) | ||
| CDKN1A | Cdk inhibitor regulating G1 phase of the cell cycle [75] | sense 5’-CCTGTCACTGTCTTGTACCCTTGT-3’ antisense 5’-GCCGTTTTCGACCCTGAGA-3’ |
| p27 (regulated via PR tethering to Sp1) | ||
| CDKN1B | Cdk inhibitor regulating G1 phase of the cell cycle [75] | sense 5’-AGCAATGCGCAGGAATAAGG-3’ antisense 5’-TCTGTTGGCTCTTTTGTTTTGAGT-3’ |
| DST (PR-repressed gene, regulated by PR-B and PR-A) | ||
| Dystonin (BPAG1) | spectraplakin that interacts with cytoskeletal elements [76] | sense 5’-GATCTTACAGCTCTGCCAGTGTGT-3’ antisense 5’-AGTAGCTTCTTTGGCATCATTGAA-3’ |
| IL1R1 (PR-repressed gene, regulated by PR-B > PR-A) | ||
| interleukin receptor 1, type 1 | membrane receptor that mediates interleukin signaling in immune and inflammatory responses [77] | sense 5’-AATTGATGAAGATGACCCAGTGC-3’ antisense 5’-GCAGGATTTTCCACACTGTAATAGTCT-3’ |
| cyclin A2 (siRNA target) | ||
| Activator of Cdk1 and Cdk2, regulator of G1/S and G2/M cell cycle transitions [78] | sense 5’-CGCTCCAAGAGGACCAGGA-3’ antisense 5’-GGTCCGCGGTTGTTGGAC-3’ probe 5’-FAMAATATCAACCCGGAAAAG-TAMRA-3’ |
|
2.6. Immunoblot assays
Cells were lysed in 1× reporter lysis buffer containing 0.4 M NaCl, 0.2 mM PMSF and 5 µg/ml each of leupeptin, antipain, aprotinin, benzamidine HCl, chymostatin and pepstatin using four freeze-thaw cycles. Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes using a Trans-Blot semi-dry transfer apparatus (Bio-Rad). Primary antibodies used were: cyclin A2 (Santa Cruz Biotechnology, Santa Cruz, CA), actin (Chemicon, Temecula, CA), PR (1294, previously described in [49]) and 6× his tag (1162/F6, previously described in [50]). All proteins were detected using ECL (Enhanced Chemiluminescence) Detection Reagent (GE Healthcare, Buckinghamshire, England). Band intensity was measured using ImageJ software version 1.40g (http://rsb.info.nih.gov/ij/). Following correction for background intensity, the value for each protein was normalized to the value for actin.
2.7. Cell cycle analysis
Cells transfected and treated as above were prepared for fluorescence activated cell sorting (FACS) analysis as previously described [4]. Cell cycle distribution was analyzed using a FACSAria II SORP (BD Biosciences, San Jose, CA) and quantified using ModFit LT version 3.2 (Verity Software House, Topsham, ME).
2.8. GST pull-down assay
Sf9 cells infected with baculovirus encoding GST-cyclin A, GST-Cyclin A/Cdk2 or his-PR-B NTD (amino acids 1–535) were lysed in 250 mM Tris pH 8.0, 0.4 M NaCl, 10% glycerol containing protease inhibitors and subjected to Dounce homogenization. GST-cyclin A and GST-cyclin A/Cdk2 were purified using glutathione sepharose 4B beads (GE Healthcare) as previously described [51]. Purified GST-cyclin A or GST-cyclin A/Cdk2 and crude his-PR-B NTD extracts were then used in GST pull-down assays performed as previously described [1]. PR and cyclin A2 were detected by immunoblot as described above using his-tag or cyclin A2 antibodies, respectively.
2.9. Experiment repetition and statistical analysis
All experiments were performed at least three times. Representative experiments are shown for immunoblot assays. For Fig. 1, bars represent the average +/− standard error of the mean (SEM) of six samples, with a representative experiment shown. For Fig. 2, 7 and 8 representative experiments are shown; data represents the average +/− SEM of triplicate samples. For Fig. 3, 4, 5 and 6 bars represent the average +/− SEM of all nine points from three independent experiments. Experiments performed in parallel are indicated in figure legends. Statistical comparisons between multiple groups were analyzed by two-way ANOVA with Tukey’s post-hoc test. Pairwise comparisons were analyzed with a two-tailed independent samples t-test. Tests were performed using SPSS Statistics 17.0 software (Chicago, IL) and significance was accepted at p < 0.05.
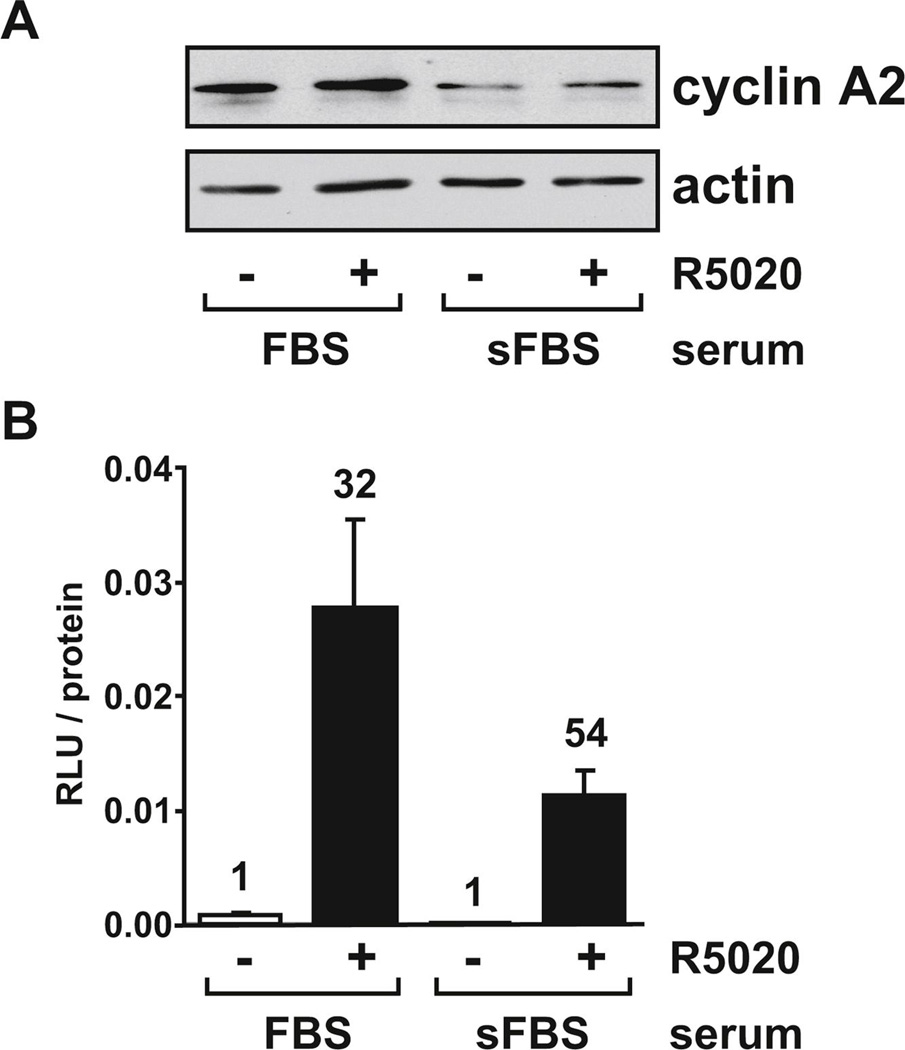
FIG. 1. Cyclin A2 levels and PR activity are higher in cells growing in FBS than sFBS.
(A) T47D cells were seeded in RPMI containing 10% FBS or sFBS. After 72 hours, cells were treated for another four hours with vehicle (0.1% ethanol) or 10 nM R5020 and protein extracts were analyzed for cyclin A2 and actin levels by immunoblot. (B) T47D cells were seeded as described in (A). After 24 hours, cells were transiently transfected with the GRE2-E1b-luc reporter construct and 24 hours later treated with vehicle or 10 nM R5020 for another 18 hours. Cells were lysed and luciferase activity (measured in relative light units, RLU) was measured and normalized for total protein concentration. Numbers above the bars indicate fold-induction relative to vehicle-treated samples under the same serum conditions.
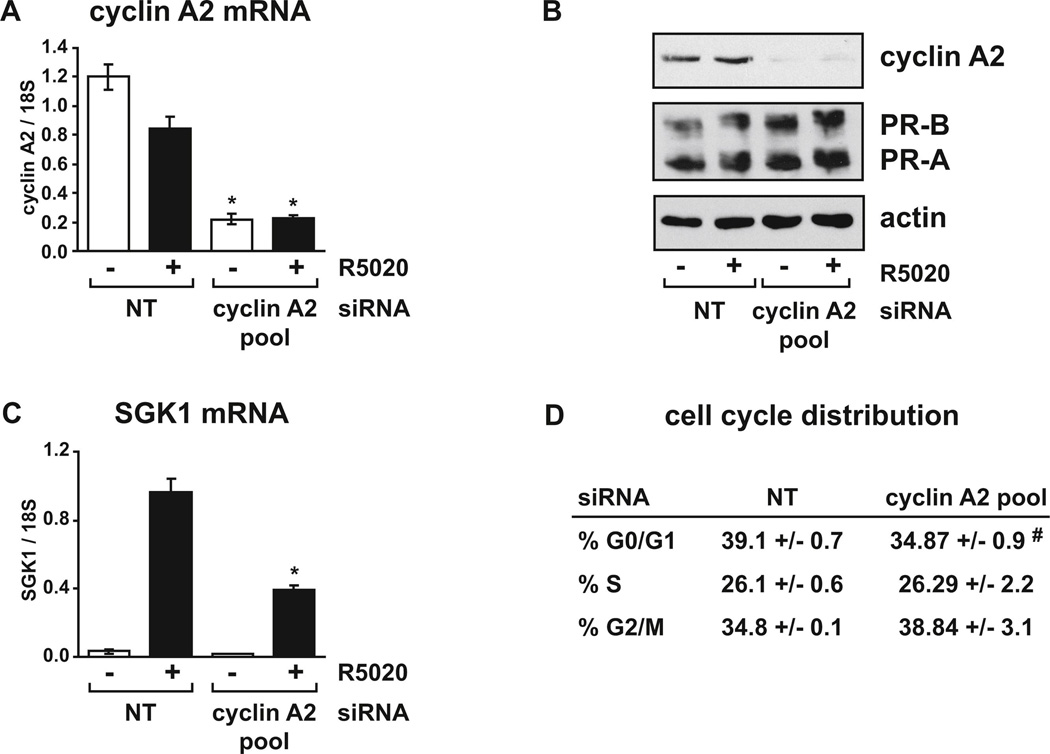
FIG. 2. Cyclin A2 depletion diminishes PR activity without affecting cells in S phase.
T47D cells growing in RPMI with FBS were transfected with control non-targeting (NT) siRNA or cyclin A2 siRNA pool for 48 hours and treated with vehicle or 10 nM R5020 for 4 hours. (A) Relative cyclin A2 mRNA levels were determined using real-time RT-PCR. (B) Cyclin A2, PR and actin protein levels were detected by immunoblot assay. (C) Relative mRNA levels for the progestin responsive target gene SGK1 were determined using real-time RT-PCR. (D) The percentage of cells in each phase of the cell cycle in R5020 treated cells was determined by FACS analysis. Data for (A), (C) and (D) are presented as the average +/− SEM of triplicate samples from representative experiments. Parts (A) and (C) are from one experiment (with confirmation of cyclin A2 protein depletion determined by immunoblot of cells processed in parallel, data not shown) while parts (B) and (D) were from a separate experiment, with cell cycle analysis and immunoblot assay performed in parallel. * p < 0.05 Two-way ANOVA, NT vs. cyclin A2 siRNA. # p < 0.05 Two-tailed t-test, NT vs. cyclin A2 siRNA.
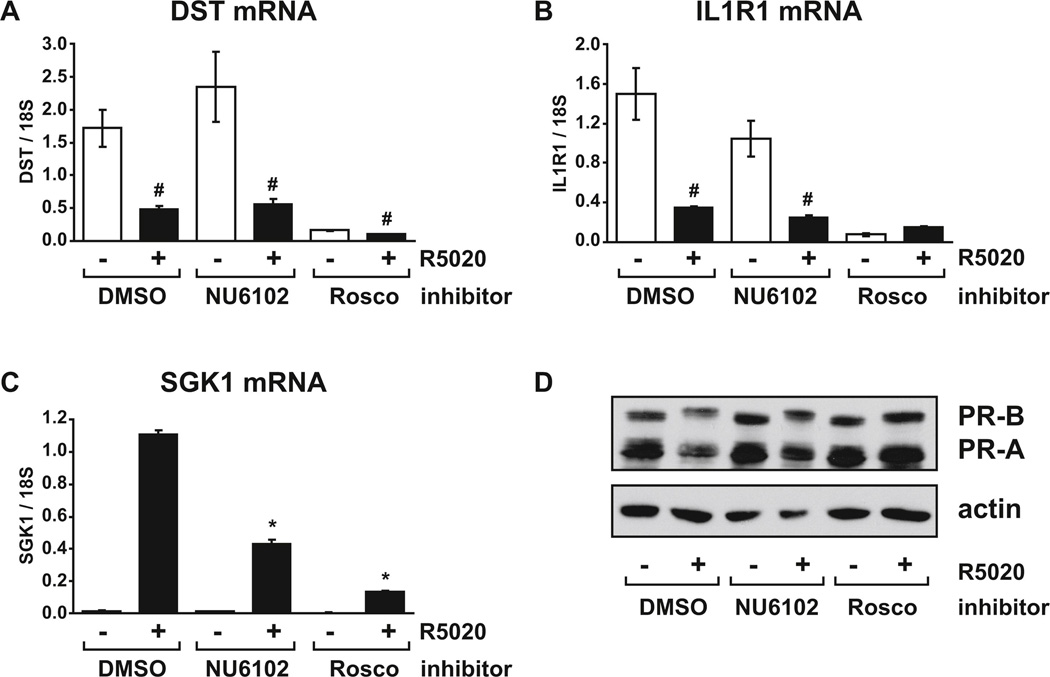
FIG. 7. Repression of target genes by progestin does not require Cdk1/2 activity.
T47D cells growing in RPMI with FBS were pre-treated with vehicle (DMSO), 10 µM NU6102 or 30 µM roscovitine for one hour before addition of vehicle (ethanol) or 10 nM R5020 for a further 16 hours. Relative mRNA levels for (A) DST, (B) IL1R1 and (C) SGK1 were determined using real-time RT-PCR. (D) PR and actin protein levels were determined by immunoblot of cell extracts prepared in parallel. # p < 0.05 Two-way ANOVA, significant repression by R5020 vs. vehicle. * p < 0.05 Two-way ANOVA, significant reduction in inhibitor treated cells compared to DMSO controls in the same hormone group.
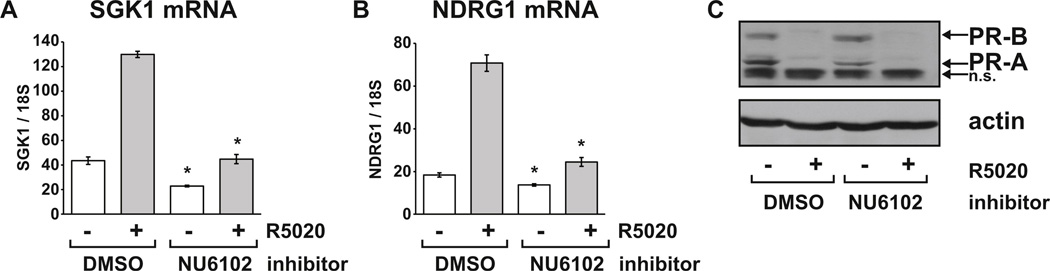
FIG. 8. Cdk1/2 activity is also required for progestin induction of target genes in ZR-75-1 cells.
ZR-75-1 cells growing in RPMI with FBS and 10 nM E2 were pre-treated with vehicle (DMSO) or 10 µM NU6102 for one hour before the addition of vehicle (ethanol) or 10 nM R5020 for a further 24 hours. Relative mRNA levels for (A) SGK1 and (B) NDRG1 were determined using real-time RT-PCR. (C) PR and actin protein levels were determined by immunoblot of cell extracts prepared in parallel. n.s. = non-specific band. * p < 0.05 Two-way ANOVA, significant reduction in inhibitor treated cells compared to DMSO controls in the same hormone group.
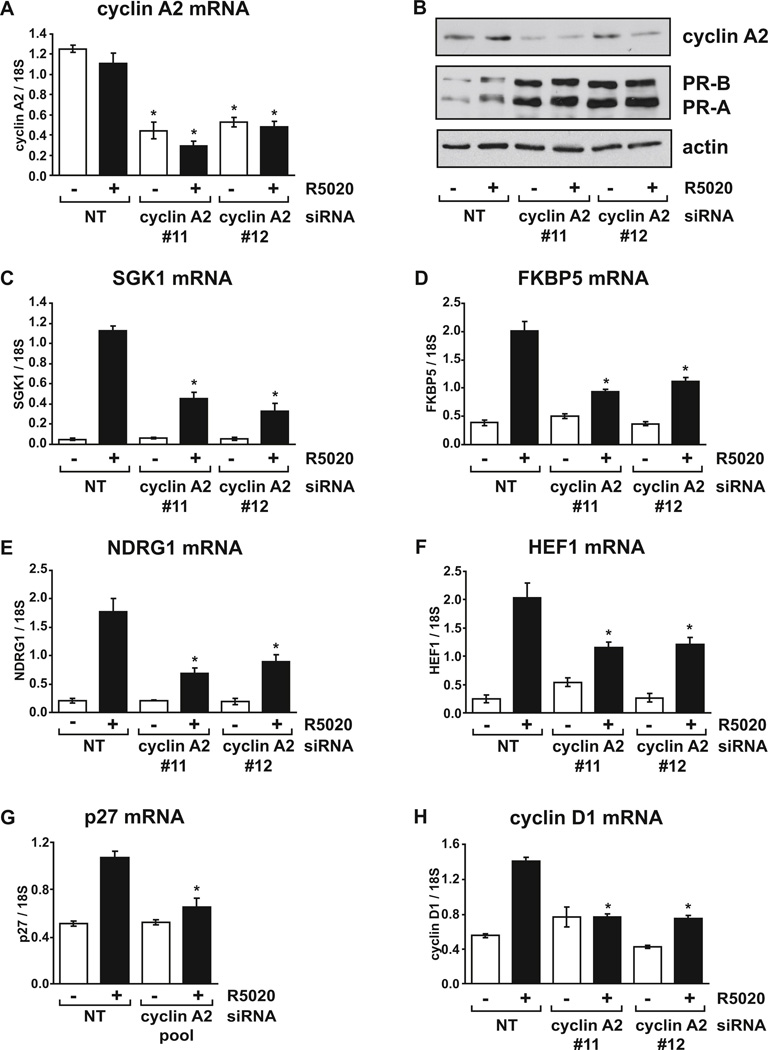
FIG. 3. Cyclin A2 depletion diminishes progestin induction of PR target genes.
T47D cells growing in RPMI with FBS were transfected with control non-targeting siRNA or cyclin A2 individual siRNA #11 or #12 for 48 hours and treated with vehicle or 10 nM R5020 for 4 hours. (A) Relative cyclin A2 mRNA levels were determined using real-time RT-PCR. (B) Cyclin A2, PR and actin protein levels were detected by immunoblot assay. Relative mRNA levels for (C) SGK1, (D) FKBP5, (E) NDRG1 and (F) HEF1 were determined using real-time RT-PCR. (G) T47D cells growing in RPMI with FBS were transfected with control non-targeting siRNA or cyclin A2 siRNA pool for 48 hours and treated with vehicle or 10 nM R5020 for 16 hours. Relative mRNA levels for p27 were determined using real-time RT-PCR. This experiment was performed in parallel with RT-PCRs shown in Fig. 4D, which indicates the extent of cyclin A2 mRNA depletion. (H) Measurement of cyclin D1 mRNA levels, performed in parallel with samples from panels 3A-3F. PCR results are expressed as averages from three separate experiments, each performed in triplicate, while the immunoblot assay confirming cyclin A2 depletion is a representative of three experiments. * p < 0.05 Two-way ANOVA, NT vs. cyclin A2 siRNA.
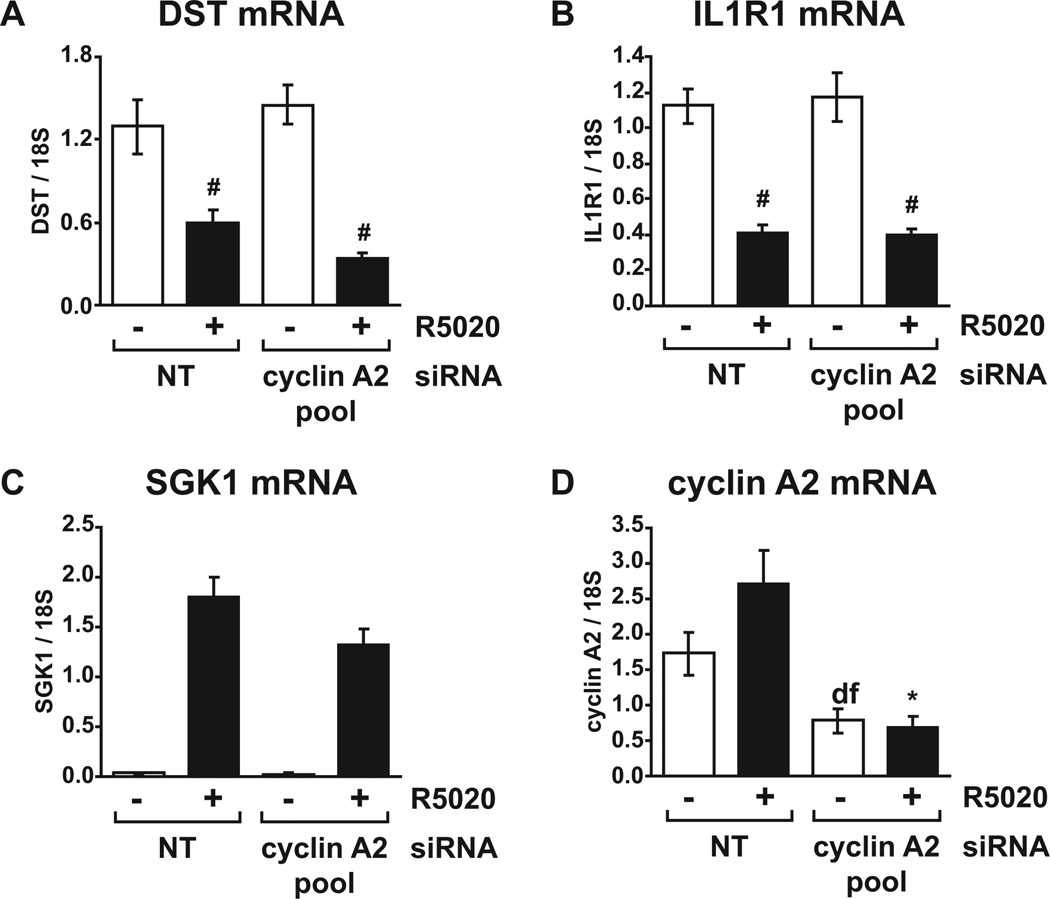
FIG. 4. Repression of gene expression by PR does not require cyclin A2.
T47D cells growing in RPMI with FBS were transfected with control non-targeting siRNA or cyclin A2 siRNA pool for 36 hours and treated with vehicle or 10 nM R5020 for a further 16 hours. Relative mRNA levels for the repressed genes (A) DST and (B) IL1R1 were determined using realtime RT-PCR. (C) Relative SGK1 and (D) cyclin A2 mRNA levels in the same experiments were determined using real-time RT-PCR. # p < 0.05 Two-way ANOVA, significant repression by R5020 vs. vehicle. * p < 0.05 Two-way ANOVA, NT vs. cyclin A2 siRNA.
FIG. 5. Cdk2 enhances the interaction between PR and cyclin A2.
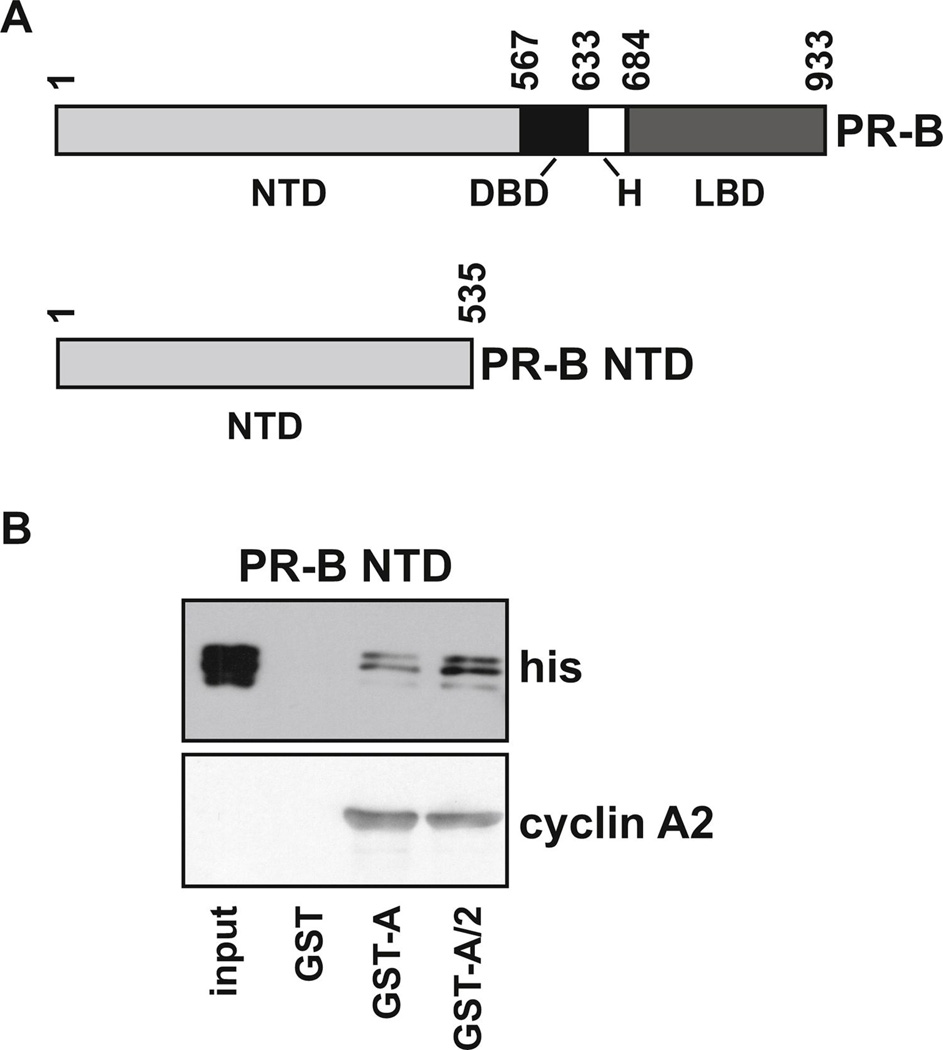
(A) Schematic diagram showing full length PR-B and the PR-B NTD fragment (1–535) used in GST pull-down assays. (B) GST, GST-cyclin A2 or GST-cyclin A/Cdk2 were bound to glutathione sepharose beads then incubated with crude his-PR-B NTD extract. Following washing and elution from the beads, samples were run with 1% input on SDS-PAGE and PR and cyclin A2 were detected by immunoblot assay with his-tag or cyclin A2 antibodies, respectively.
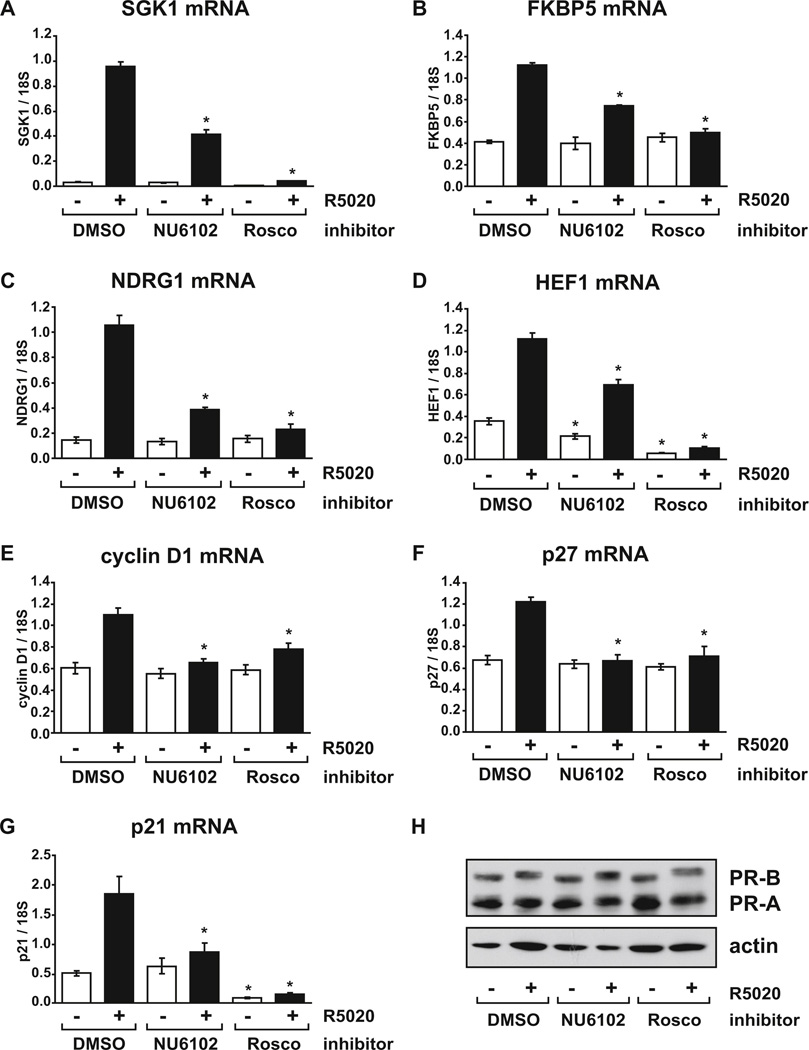
FIG. 6. Cdk1/2 activity is also required for induction of progestin responsive genes.
T47D cells growing in RPMI with FBS were pre-treated with vehicle (DMSO), 10 µM NU6102 or 30 µM roscovitine (Rosco) for one hour before addition of vehicle (ethanol) or 10 nM R5020 for another 4 (A–E, H) or 16 (F, G) hours. Relative mRNA levels for (A) SGK1, (B) FKBP5, (C) NDRG1, (D) HEF1, (E) cyclin D1, (F) p27 and (G) p21 were determined using real-time RT-PCR. (H) PR and actin protein levels were determined by immunoblot of cell extracts prepared in parallel following 4 hours exposure to R5020 (parallel immunoblot data for the 16 hour time point is shown in Fig. 6D). * p < 0.05 Two-way ANOVA, significant reduction in inhibitor treated cells compared to DMSO controls in the same hormone group.
3. Results
3.1. Cyclin A2 levels are higher in T47D cells cultured in the presence of FBS compared to cells growing in sFBS
In this study, we applied a siRNA approach to test whether cyclin A2 is required for transcriptional regulation of target gene expression by endogenous PR in breast cancer cells. We used T47D breast cancer cells because they are a well characterized model of PR function. However, we were concerned with the fact that cyclin A2 levels fluctuate during the cell cycle, peaking during the S phase, while cells growing in charcoal stripped FBS (sFBS) often grow more slowly (due to the stripping procedure removing many growth factors as well as steroids), causing cells to accumulate in the G1 phase of the cell cycle. We hypothesized that cells growing under FBS conditions may have higher cyclin A2 levels and that these conditions may be more suitable for cyclin A2 depletion experiments to examine effects on PR function. Therefore, cyclin A2 protein levels were compared in cells growing in medium containing FBS or sFBS. Cyclin A2 levels were at least 2.5-fold higher in FBS conditions compared to sFBS (Fig. 1A). This suggests that serum culture conditions used for these experiments do indeed influence cyclin A2 levels, and that a greater effect on PR function resulting from depleting cyclin A2 may be observed in FBS. However, as hormone-dependent regulation of target genes is usually studied in cells growing in medium containing sFBS, we wanted to verify that PR activity was sufficiently induced by hormone under FBS conditions. Surprisingly, when measured on a transiently transfected progestin responsive luciferase reporter, higher PR activity was observed in T47D cells under FBS conditions compared to sFBS, although the fold-induction by hormone was greater in sFBS (54-fold) relative to FBS (32-fold) (Fig. 1B). This suggests that endogenous progestin levels in FBS are low enough to permit detection of progestin induction of target genes under these conditions. We therefore chose to perform subsequent experiments investigating the effect of cyclin A2 on PR activity in T47D cells grown in FBS because cyclin A2 is higher than in cells grown in sFBS and any endogenous progestin in FBS is insufficient to mask agonist-dependent induction of target genes by exogenous R5020.
3.2. Cyclin A2 is required for optimal progestin-induction of endogenous PR target genes
Cyclin A2 mRNA was initially depleted in T47D breast cancer cells by transient transfection of a siRNA pool against cyclin A2. A marked reduction in cyclin A2 mRNA and protein levels was observed in cells transfected with the cyclin A2 siRNA pool compared to control non-targeting siRNA transfected cells (Fig. 2A and 2B). Expression of the PR target gene SGK1 was then examined by real-time RT-PCR. SGK1 is a direct target of PR and contains a progestin/glucocorticoid response element in the promoter region which binds PR in response to hormone [43, 52, 53]. Depletion of cyclin A2 significantly reduced expression of SGK1 in R5020 treated cells in comparison to control transfected cells (Fig. 2C), suggesting that cyclin A2 is required for optimal endogenous PR activity in T47D cells. In order to determine whether the reduced expression of SGK1 might be due to lower PR levels in cyclin A2 depleted cells, PR protein levels were examined. Cyclin A2 depletion did not reduce PR-B or PR-A levels and if anything slightly increased PR levels (Fig. 2B). Finally, as cyclin A2 plays a role in regulation of the cell cycle, and because PR activity varies as a function of cell cycle [4], the effect of cyclin A2 depletion on cell cycle distribution for the duration of this experiment was determined. FACS analysis performed on R5020 treated cells indicated that there was no significant change in the number of cells in the S or G2/M phases of the cell cycle in cyclin A2 siRNA transfected cells compared to control cells (Fig. 2D), although a slight but significant decrease in G0/G1 cells was observed. As maximum PR activity is observed during S phase [4], these results suggest that changes in cell cycle were not responsible for reduced progestin induction of SGK1 when cyclin A2 was depleted.
To rule out the possibility of off-target effects of the cyclin A2 siRNA pool, two of the four siRNAs from the pool (#11 and #12) were separately transfected into T47D cells. Fig. 3A demonstrates that significant depletion of cyclin A2 mRNA was obtained with either siRNA compared to control siRNA transfected cells. Both cyclin A2 siRNAs also depleted cyclin A2 protein, while PR levels were again increased as demonstrated for the cyclin A2 pool (Fig. 3B). However, cyclin A2 depletion at the RNA and protein levels was less effective with the individual siRNAs compared to that obtained with the siRNA pool. Despite this, a significant reduction in hormone-induced SGK1 mRNA levels was observed following cyclin A2 depletion with both siRNAs (Fig. 3C).
We also assessed the role of cyclin A2 in progestin induction of other PR target genes that are regulated by a variety of mechanisms. FKBP5 and NDRG1 are other direct PR targets containing PR binding sites in upstream and/or intronic regions [53–56]. Induction of both FKBP5 (Fig. 3D) and NDRG1 (Fig. 3E) mRNA by R5020 was significantly reduced in cells where cyclin A2 was depleted. While FKBP5 and NDRG1 have been reported to be regulated by both PR-B and PR-A isoforms, PR-B is a stronger inducer of these genes; in contrast, HEF1 is regulated more strongly by PR-A [17, 57]. Significantly reduced induction of HEF1 mRNA was also observed when cyclin A2 was depleted (Fig. 3F). p27 is induced by progestin via tethering of PR to the Sp1 transcription factor at Sp1 sites without direct binding of PR to DNA [24, 27]. Unlike the other genes measured so far, no significant induction of p27 was observed with four hour exposure to R5020 (data not shown). We therefore increased the treatment time to 16 hours which resulted in significant induction by R5020. A significant reduction of R5020 dependent p27 levels was observed following cyclin A2 depletion, (Fig. 3G). Finally, cyclin D1, which is induced by progestin via a combination of mechanisms involving direct PR binding to DNA, PR tethering via STAT3, ERBB2 and AP-1 and PR extra-nuclear actions [37, 43, 58–60], was reduced to basal levels upon cyclin A2 depletion (Fig. 3H). Together, these results suggest that cyclin A2 is required for maximum progestin induction of target genes by both PR-B and PR-A isoforms and through mechanisms involving direct PR DNA binding, PR tethering via other transcription factors and extra-nuclear PR signaling.
3.3. Cyclin A2 is not required for optimal repression of endogenous target genes by PR
All tested progestin-induced genes were sensitive to cyclin A2 depletion. We next asked whether cyclin A2 is required for repression of genes by progestin. DST and IL1R1 are two genes that have been shown to be repressed by progestin; DST is repressed by PR-B and PR-A while IL1R1 is repressed by PR-B only [17]. Repression of DST and IL1R1 mRNA levels was not observed with four hour R5020 treatment (data not shown) although significant repression of DST and IL1R1 following 16 hour exposure to R5020 was observed in cells transfected with both control and cyclin A2 siRNA pool (Fig. 4A and 4B). Importantly, depletion of cyclin A2 did not diminish the ability of PR to repress either gene, suggesting that cyclin A2 is not involved in the mechanisms that regulate inhibition of target gene expression by PR signaling. We observed a less robust transcriptional effect of cyclin A2 depletion on some induced genes with the 16 hour R5020 treatment compared to 4 hours (Fig. 2); a reduction in hormone-induced SGK1 mRNA levels was consistently observed with cyclin A2 depletion (Fig. 4C) although the effect did not reach significance. However, significantly reduced cyclin A2 (Fig. 4D) and p27 (Fig. 3G) mRNA levels were observed with the siRNA pool, confirming efficient depletion of cyclin A2 in parallel with an outcome for a subset of target genes in these experiments.
3.4. Depletion of cyclin A2 does not affect general transcription
One potential mechanism by which cyclin A2 depletion may cause reduced hormone-dependent gene induction is through the inhibition of general transcription. We therefore looked more closely at the effect of cyclin A2 depletion on PR target genes in the absence of hormone. While all induced genes showed reduced mRNA levels in R5020 treated cells following cyclin A2 depletion, none of them showed significant reduction in mRNA levels in the absence of hormone. This is especially evident for those genes which are expressed at relatively high basal levels, such as FKBP5 and cyclin D1 (Fig. 2). Similarly, mRNA levels for the repressed genes were unchanged by cyclin A2 depletion in the absence of hormone (Fig. 4). These findings imply that cyclin A2 is not required for general transcription, and that physical and/or functional interaction with PR is important for cyclin A2 effects on progestin-dependent gene expression.
3.5. PR interaction with cyclin A2 is stronger in the presence of Cdk2
Previously, we have shown that cyclin A2 interacts with a truncated PR-B lacking the hormone binding domain (amino acids 1–684, Fig. 5A) and that the interaction between full length PR-B and cyclin A2 is direct [1]. We therefore wanted to investigate the effect of Cdk2 on the interaction between cyclin A2 and PR and to determine whether the PR-B N-terminal domain (NTD) region was sufficient to interact with cyclin A2. In GST pull-down assays, the PR-B NTD interacted with cyclin A2 and this interaction was stronger in the presence of Cdk2 (Fig. 5B). Cyclin A2 input levels were similar in the cyclin A2 and cyclin A/Cdk2 samples, indicating that the increased interaction is due to the presence of Cdk2, rather than altered levels of cyclin A2. These results indicate a direct interaction of cyclin A2 with the PR-B NTD that is enhanced by Cdk2.
3.6. Inhibition of Cdk1/2 kinase activity mimics the effect of depletion of cyclin A2 on PR activity
To determine whether inhibition of Cdk1/2 activity mimics depletion of cyclin A2 or was more effective in inhibiting PR activity we treated cells with two chemical inhibitors of Cdk activity. We have previously used roscovitine to inhibit Cdk2 activity in T47D cells; however, it has a broad specificity and inhibits many other kinases [61]. We have compared the effects of roscovitine with NU6102, a more potent and specific inhibitor of the cyclin A2 partners Cdk1 and Cdk2 [62], on PR regulation of the target genes described above. The concentration of each inhibitor was chosen based on previous studies that investigated their effects on steroid receptor activity and cell growth [1, 62, 63]. NU6102 treatment suppressed R5020-induced expression (at four hours) of SGK1, FKBP5, NDRG1, HEF1 and cyclin D1 compared to DMSO controls (Fig. 6A–E) to levels similar to that achieved with cyclin A2 depletion (Fig. 3). The results for SGK1 are similar to those reported previously [2]. Additionally, when the exposure to hormone was increased to 16 hours, reduced R5020 induction of p27 as well as p21, another gene regulated by Sp1 tethering mechanisms [21, 64], was observed in cells treated with NU6102 (Fig. 6F and 6G). In comparison, roscovitine potently inhibited progestin induction of all target genes tested (Fig. 6), consistent with its ability to block the activity of multiple kinases in addition to Cdk1 and Cdk2. Immunoblot assays confirmed that neither NU6102 nor roscovitine reduced PR levels (Fig. 6H). Similar to cyclin A2 depletion, which did not affect mRNA levels for any target genes in the absence of hormone, inhibition of Cdk1 and Cdk2 did not affect basal mRNA levels for most target genes measured; however, NU6102 did significantly reduce HEF1 in the absence of hormone while roscovitine also reduced HEF1 and p21 (Fig. 6). From these results it appears that inhibiting kinase activity mimics the effects of cyclin A2 depletion on PR activity.
In contrast, NU6102 did not relieve repression of DST or IL1R1 by R5020 (Fig. 7A and 7B). However, under the same conditions significant reduction of R5020-induced SGK1 mRNA levels were observed with NU6102 treatment (Fig. 7C) and this reduction was substantially greater than that caused by cyclin A2 depletion at the same time point (Fig. 4C). While NU6102 did not significantly affect DST or IL1R1 mRNA levels in the absence of hormone, roscovitine potently reduced basal mRNA levels of both genes such that, when compared to mRNA levels in the presence of R5020, repression was lost. This implies that roscovitine-sensitive kinases other than Cdk1 and Cdk2 affect basal levels of these repressed genes. Immunoblot analysis indicates that PR protein levels were not decreased by either NU6102 or roscovitine (Fig. 7D).
To exclude the possibility that the effects of Cdk1 and Cdk2 inhibition on PR activity are specific to T47D cells, we also treated ZR-75-1 breast cancer cells with R5020 in the presence or absence of NU6102. R5020 induction of both SGK1 (Fig. 8A) and NDRG1 (Fig. 8B) was significantly suppressed by NU6102. Although PR expression in ZR-75-1 cells was lower than observed in T47D cells, we still observed the expected R5020-dependent downregulation of PR-B and PR-A. However NU6102 did not affect PR levels relative to control cells (Fig. 8C). These results are consistent with observations in T47D cells and demonstrate that Cdk1/2 activity is important for PR function in multiple breast cancer contexts.
4. Discussion
We have shown that PR requires cyclin A2 and its associated kinase activity for maximal induction of endogenous target genes regulated by a variety of different mechanisms. Our previous studies showed that PR and cyclin A2 are recruited to the stably integrated progestin responsive mouse mammary tumor virus promoter in T47D breast cancer cells and that cyclin dependent kinase activity is required for maximal expression of the associated chloramphenicol acetyltransferase reporter [1]. Therefore, we predicted that at least the endogenous progestin target genes such as SGK1 and FKBP5, which also contain progestin response elements that directly bind PR [52, 53, 55, 56], would require cyclin A2 and Cdk activity for maximal progestin induction. Our results indicate that cyclin A2 and Cdk1/2, at least in part, play an important role in PR dependent transcription of these directly regulated genes. Furthermore, both isoforms of PR function with cyclin A2 and Cdk1/2, which is consistent with previous studies in which cyclin A2 was able to coactivate both PR-A and PR-B in transient transfection assays [1].
In contrast, p21 and p27, and cyclin D1 are regulated, at least in part, by PR tethering to Sp1, STAT3 or AP-1 general transcription factors [24, 27, 59, 60, 64]. Our results suggest that cyclin A2 and Cdk1/2 activity are also involved in this process. Although cyclin A2 has previously been shown to interact directly with Sp1 and enhance its activity [65], Cdk2 has been shown to suppress STAT3 activity [66]. However direct modulation of general transcription factor activity by cyclin A2 may not be responsible for the reduced progestin induction of p21, p27 and cyclin D1 following cyclin A2 depletion or Cdk1/2 inhibition as it might be expected to result in reduced target gene expression in both the absence and presence of hormone, whereas we only observed a difference in mRNA levels for these genes in R5020-treated cells. PR tethering to Sp1 sites also requires phosphorylation of PR serine 345 but not of PR serine 294 [21]. Although neither of these sites are Cdk2 targets [51], it is possible that the interaction between PR and cyclin/Cdk complexes leads to phosphorylation of other sites in PR that may be required for tethering to Sp1 sites and subsequent regulation of p21 and p27 expression.
Because cyclin A2 and its associated kinase activity were shown to be required for optimal induction of all progestin responsive genes examined, we wanted to rule out the possibility that general transcription mechanisms were affected. Our data on mRNA levels for all target genes measured in the absence of hormone suggest that this is not the case and that cyclin A2 is specifically required for transcriptional mechanisms involving PR. Similarly, basal levels of most genes measured were not affected by inhibition of Cdk1/2 activity although we did observe that HEF1 was reduced in cells treated with NU6102; this may result from differential sensitivity of certain genes to this compound and/or more potent inhibition by the inhibitor compared to cyclin A2 ablation. The absence of an effect on general transcription is also consistent with a recent study investigating Cdk2 effects on PARP-1 and PR activity which interestingly showed that a small subset of progestin responsive genes, induced or repressed, were not sensitive to Cdk2 inhibition [3]. While that study investigated global gene expression by microarray analysis rather than only selected genes, it differs from our current work in that it used a different Cdk2 inhibitor which has a higher IC50 than NU6102 and may therefore have weaker inhibitory effects on PR activity in certain contexts. Furthermore, inhibitor and hormone treatment times differed between our work and that of Wright et al. Cdk2 is reported to transiently occupy progestin responsive promoters [67]. At four hours of R5020 treatment, cyclin A2 ablation and Cdk1/2 inhibition resulted in effects of similar magnitude on PR target genes (Fig. 3 and 6). This suggests that cyclin A2 may be the predominant cyclin regulating PR activity, at least at early time points where the primary transcriptional activity of PR is likely to be the predominant mechanism regulating levels of PR target genes. In contrast, at 16 hours of hormone treatment, where post-transcriptional mechanisms of regulation play a larger role, the impact of cyclin A2 depletion, but not Cdk2 inhibition, is diminished (Fig. 4 and 7), suggesting that cyclin A2 and Cdk1/2 activity may have different effects on post-transcriptional regulation of PR target genes. Furthermore, the capacity to recruit the cyclin A2/Cdk1/2 complex may be most important for optimal initiation of transcription, but other Cdk2 and/or Cdk1 complexes, such as cyclin E/Cdk2 or cyclin B/Cdk1, can ultimately compensate for a lack of cyclin A2. These observations highlight the complexity of PR-cyclin A2/Cdk1/2 functional interactions and prompt further investigation into the specific role of cyclin A2 on the PR cistrome and transcriptome in breast cancer cells.
Kinase independent actions of cyclins have previously been reported for estrogen receptor and androgen receptor [68, 69]. However, we observed that both cyclin A2 and its associated kinase activity are required for PR activity, which is consistent with our previous studies showing that a cyclin A2 mutant unable to bind Cdks could not coactivate PR [1] and that PR recruits cyclin A and Cdk2 to target promoters [67]. Other studies from our laboratory suggest that at least one of the mechanisms by which this can occur is via recruitment of PR and cyclin A2 to progestin-induced promoters followed by phosphorylation of steroid receptor coactivator-1 (SRC-1) by the associated Cdk1/2 and a subsequent increase in transcription ([1, 2]). Further evidence for the involvement of SRC-1 in this process comes from our results with the progestin-repressed genes which likely do not require SRC-1 and are not sensitive to cyclin A2/Cdk1/2 ablation. Although the mechanisms governing repression of target gene expression by PR are not well understood, our results suggest that cyclin A2/Cdk1/2 are not critical in this process, at least for these particular targets.
Cyclin A2 protein levels were increased in T47D cells growing in FBS, which proliferate quickly, compared to cells in sFBS, which proliferate more slowly. These results are consistent with clinical studies showing overexpression of cyclin A2 in breast cancer compared to non-malignant tissue and an association with poor prognosis [5–11]. The specific co-expression of cyclin A2 and PR in malignant breast epithelial cells and not in proliferating epithelial cells of the normal breast suggest an important, yet poorly understood, association between cyclin A2 and PR. While the current study has shown that neither cyclin A2 depletion nor inhibition of Cdk1/2 activity completely abolished overall progestin dependent PR activity, significant effects (>50% in most cases) were observed even without complete depletion. Our results suggest that cyclin A2 is a key regulator of progestin action in breast cancer cells, capable of influencing PR function mediated by multiple mechanisms and facilitating the association between PR activity and cell proliferation pathways.
Highlights.
Cyclin A2 depletion compromises progestin induction of PR target genes
Cdk1/2 activity is also required for maximum PR transactivation function
Cyclin A2/Cdk1/2 are not absolutely required for gene repression by PR
Cyclin A2 interaction with PR is stronger in the presence of Cdk2
Acknowledgements
The authors wish to thank the Baylor College of Medicine Department of Molecular and Cellular Biology Tissue Culture Core; Kurt Christensen, Manager of the Proteomic Shared Resource of the NCI designated Dan L. Duncan Cancer Center at Baylor College of Medicine for production of monoclonal antibodies to PR (1294) and 6× his-tag (1162/F6) and Sf9 cell pellets with GST-cyclin A, GST-Cyclin A/Cdk2 and his-PR-B NTD; and William Edward Bingman III and Yanhong Liu for technical assistance.
Grant support: This project was supported by NIH R01 CA57539 (to N.L.W.), NIH R01 DK49030 (to D.P.E), the Proteomic Shared Resource of the NCI designated Dan L. Duncan Cancer Center at Baylor College of Medicine (P30CA125123) and the BCM Cytometry and Cell Sorting Core with funding from the NIH (NCRR grant S10RR024574, NIAID AI036211 and NCI P30CA125123).
Abbreviations
- ANOVA
analysis of variance
- Cdk
cyclin dependent kinase
- DST
dystonin
- ECL
enhanced chemiluminescence
- FACS
fluorescence activated cell sorting
- FBS
fetal bovine serum
- FKBP5
FK506 binding protein 5
- GRE
glucocorticoid responsive element
- HEF1
Enhancer of Filamentation 1
- IL1R1
interleukin receptor 1, type 1
- luc
luciferase
- MAPK
mitogen activated protein kinase
- NDRG1
n-myc downstream regulated gene 1
- NT
non-targeting
- PAGE
polyacrylamide gel electrophoresis
- PMSF
phenylmethylsulfonyl fluoride
- PR
progesterone receptor
- RLU
relative light units
- RT-PCR
reverse transcription-PCR
- SEM
standard error of the mean
- sFBS
charcoal stripped FBS
- SGK1
serum and glucocorticoid regulated kinase-1
- siRNA
small interfering RNA
- Sp1
specificity protein 1
- SRC-1
steroid receptor coactivator-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: N.L.M., and N.L.W. have nothing to disclose. D.P.E. receives royalties for monoclonal antibodies to steroid receptors from DAKO, Upstate, Affinity Bioreagents, Stressgen, Santa Cruz Biotechnology, and PhosphoSolutions.
References
- 1.Narayanan R, Adigun AA, Edwards DP, Weigel NL. Cyclin-dependent kinase activity is required for progesterone receptor function: novel role for cyclin A/Cdk2 as a progesterone receptor coactivator. Mol.Cell Biol. 2005;25:264–277. doi: 10.1128/MCB.25.1.264-277.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore NL, Weigel NL. Regulation of progesterone receptor activity by cyclin dependent kinases 1 and 2 occurs in part by phosphorylation of the SRC-1 carboxyl-terminus. Int.J.Biochem.Cell Biol. 2011;43:1157–1167. doi: 10.1016/j.biocel.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wright RH, Castellano G, Bonet J, Le DF, Font-Mateu J, Ballare C, Nacht AS, Soronellas D, Oliva B, Beato M. CDK2-dependent activation of PARP-1 is required for hormonal gene regulation in breast cancer cells. Genes Dev. 2012;26:1972–1983. doi: 10.1101/gad.193193.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Narayanan R, Edwards DP, Weigel NL. Human progesterone receptor displays cell cycle-dependent changes in transcriptional activity. Mol.Cell Biol. 2005;25:2885–2898. doi: 10.1128/MCB.25.8.2885-2898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol.Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poikonen P, Sjostrom J, Amini RM, Villman K, Ahlgren J, Blomqvist C. Cyclin A as a marker for prognosis and chemotherapy response in advanced breast cancer. Br.J.Cancer. 2005;93:515–519. doi: 10.1038/sj.bjc.6602735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaltonen K, Ahlin C, Amini RM, Salonen L, Fjallskog ML, Heikkila P, Nevanlinna H, Blomqvist C. Reliability of cyclin A assessment on tissue microarrays in breast cancer compared to conventional histological slides. Br.J.Cancer. 2006;94:1697–1702. doi: 10.1038/sj.bjc.6603147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bukholm IR, Bukholm G, Holm R, Nesland JM. Association between histology grade, expression of HsMCM2, and cyclin A in human invasive breast carcinomas. J.Clin.Pathol. 2003;56:368–373. doi: 10.1136/jcp.56.5.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michels JJ, Duigou F, Marnay J, Henry-Amar M, Delozier T, Denoux Y, Chasle J. Flow cytometry and quantitative immunohistochemical study of cell cycle regulation proteins in invasive breast carcinoma: prognostic significance. Cancer. 2003;97:1376–1386. doi: 10.1002/cncr.11209. [DOI] [PubMed] [Google Scholar]
- 10.Bukholm IR, Bukholm G, Nesland JM. Over-expression of cyclin A is highly associated with early relapse and reduced survival in patients with primary breast carcinomas. Int.J.Cancer. 2001;93:283–287. doi: 10.1002/ijc.1311. [DOI] [PubMed] [Google Scholar]
- 11.Michalides R, van TH, Balkenende A, Vermorken JB, Benraadt J, Huldij J, van DP. Cyclin A is a prognostic indicator in early stage breast cancer with and without tamoxifen treatment. Br.J.Cancer. 2002;86:402–408. doi: 10.1038/sj.bjc.6600072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shih HC, Shiozawa T, Kato K, Imai T, Miyamoto T, Uchikawa J, Nikaido T, Konishi I. Immunohistochemical expression of cyclins, cyclin-dependent kinases, tumor-suppressor gene products, Ki-67, and sex steroid receptors in endometrial carcinoma: positive staining for cyclin A as a poor prognostic indicator. Hum.Pathol. 2003;34:471–478. doi: 10.1016/s0046-8177(03)00124-2. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BS, Kim YT, Kim S, Lee CS, Kim JW, Kim JH, Kim SW, Cho NH. Prognostic value of nuclear DNA quantification and cyclin A expression in epithelial ovarian carcinoma. Eur.J.Obstet.Gynecol.Reprod.Biol. 2008;136:110–115. doi: 10.1016/j.ejogrb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Tanos T, Sflomos G, Echeverria PC, Ayyanan A, Gutierrez M, Delaloye JF, Raffoul W, Fiche M, Dougall W, Schneider P, Yalcin-Ozuysal O, Brisken C. Progesterone/RANKL is a major regulatory axis in the human breast. Sci.Transl.Med. 2013;5:182ra55. doi: 10.1126/scitranslmed.3005654. [DOI] [PubMed] [Google Scholar]
- 15.Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150:3318–3326. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 1990;9:1603–1614. doi: 10.1002/j.1460-2075.1990.tb08280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richer JK, Jacobsen BM, Manning NG, Abel MG, Wolf DM, Horwitz KB. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J.Biol.Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 18.Mulac-Jericevic B, Lydon JP, DeMayo FJ, Conneely OM. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc.Natl.Acad.Sci.U.S.A. 2003;100:9744–9749. doi: 10.1073/pnas.1732707100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science. 2000;289:1751–1754. doi: 10.1126/science.289.5485.1751. [DOI] [PubMed] [Google Scholar]
- 20.Li X, O'Malley BW. Unfolding the action of progesterone receptors. J.Biol.Chem. 2003;278:39261–39264. doi: 10.1074/jbc.R300024200. [DOI] [PubMed] [Google Scholar]
- 21.Faivre EJ, Daniel AR, Hillard CJ, Lange CA. Progesterone Receptor Rapid Signaling Mediates Ser345 Phosphorylation and Tethering to Sp1 Transcription Factors. Mol.Endocrinol. 2008 doi: 10.1210/me.2007-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tseng L, Tang M, Wang Z, Mazella J. Progesterone receptor (hPR) upregulates the fibronectin promoter activity in human decidual fibroblasts. DNA Cell Biol. 2003;22:633–640. doi: 10.1089/104454903770238102. [DOI] [PubMed] [Google Scholar]
- 23.Bamberger AM, Bamberger CM, Gellersen B, Schulte HM. Modulation of AP-1 activity by the human progesterone receptor in endometrial adenocarcinoma cells. Proc.Natl.Acad.Sci.U.S.A. 1996;93:6169–6174. doi: 10.1073/pnas.93.12.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen GI, Richer JK, Tung L, Takimoto G, Horwitz KB. Progesterone regulates transcription of the p21(WAF1) cyclin- dependent kinase inhibitor gene through Sp1 and CBP/p300. J.Biol.Chem. 1998;273:10696–10701. doi: 10.1074/jbc.273.17.10696. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Mazella J, Seppala M, Tseng L. Ligand activated hPR modulates the glycodelin promoter activity through the Sp1 sites in human endometrial adenocarcinoma cells. Mol.Cell Endocrinol. 2001;176:97–102. doi: 10.1016/s0303-7207(01)00450-6. [DOI] [PubMed] [Google Scholar]
- 26.Tang M, Mazella J, Gao J, Tseng L. Progesterone receptor activates its promoter activity in human endometrial stromal cells. Mol.Cell Endocrinol. 2002;192:45–53. doi: 10.1016/s0303-7207(02)00111-9. [DOI] [PubMed] [Google Scholar]
- 27.Gizard F, Robillard R, Gervois P, Faucompre A, Revillion F, Peyrat JP, Hum WD, Staels B. Progesterone inhibits human breast cancer cell growth through transcriptional upregulation of the cyclin-dependent kinase inhibitor p27Kip1 gene. FEBS Lett. 2005;579:5535–5541. doi: 10.1016/j.febslet.2005.08.084. [DOI] [PubMed] [Google Scholar]
- 28.Gizard F, Robillard R, Gross B, Barbier O, Revillion F, Peyrat JP, Torpier G, Hum DW, Staels B. TReP-132 is a novel progesterone receptor coactivator required for the inhibition of breast cancer cell growth and enhancement of differentiation by progesterone. Mol.Cell Biol. 2006;26:7632–7644. doi: 10.1128/MCB.00326-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pauklin S, Petersen-Mahrt SK. Progesterone inhibits activation-induced deaminase by binding to the promoter. J.Immunol. 2009;183:1238–1244. doi: 10.4049/jimmunol.0803915. [DOI] [PubMed] [Google Scholar]
- 30.De Amicis F, Zupo S, Panno ML, Malivindi R, Giordano F, Barone I, Mauro L, Fuqua SA, Ando S. Progesterone receptor B recruits a repressor complex to a half-PRE site of the estrogen receptor alpha gene promoter. Mol.Endocrinol. 2009;23:454–465. doi: 10.1210/me.2008-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong X, Yu C, Shynlova O, Challis JR, Rennie PS, Lye SJ. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1) Mol.Endocrinol. 2009;23:1147–1160. doi: 10.1210/me.2008-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buser AC, Obr AE, Kabotyanski EB, Grimm SL, Rosen JM, Edwards DP. Progesterone receptor directly inhibits beta-casein gene transcription in mammary epithelial cells through promoting promoter and enhancer repressive chromatin modifications. Mol.Endocrinol. 2011;25:955–968. doi: 10.1210/me.2011-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman S, Lovett DH, Shalev E. Mechanisms of matrix metalloproteinase-2 (mmp-2) transcriptional repression by progesterone in jar choriocarcinoma cells. Reprod.Biol.Endocrinol. 2009;7:41. doi: 10.1186/1477-7827-7-41. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buser AC, Gass-Handel EK, Wyszomierski SL, Doppler W, Leonhardt SA, Schaack J, Rosen JM, Watkin H, Anderson SM, Edwards DP. Progesterone receptor repression of prolactin/signal transducer and activator of transcription 5-mediated transcription of the beta-casein gene in mammary epithelial cells. Mol.Endocrinol. 2007;21:106–125. doi: 10.1210/me.2006-0297. [DOI] [PubMed] [Google Scholar]
- 35.Skildum A, Faivre E, Lange CA. Progesterone receptors induce cell cycle progression via activation of mitogen-activated protein kinases. Mol.Endocrinol. 2005;19:327–339. doi: 10.1210/me.2004-0306. [DOI] [PubMed] [Google Scholar]
- 36.Migliaccio A, Piccolo D, Castoria G, Di DM, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 1998;17:2008–2018. doi: 10.1093/emboj/17.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol.Cell. 2001;8:269–280. doi: 10.1016/s1097-2765(01)00304-5. [DOI] [PubMed] [Google Scholar]
- 38.Faivre E, Skildum A, Pierson-Mullany L, Lange CA. Integration of progesterone receptor mediated rapid signaling and nuclear actions in breast cancer cell models: role of mitogen-activated protein kinases and cell cycle regulators. Steroids. 2005;70:418–426. doi: 10.1016/j.steroids.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol.Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ballare C, Uhrig M, Bechtold T, Sancho E, Di Domenico M, Migliaccio A, Auricchio F, Beato M. Two domains of the progesterone receptor interact with the estrogen receptor and are required for progesterone activation of the c-Src/Erk pathway in mammalian cells. Mol.Cell Biol. 2003;23:1994–2008. doi: 10.1128/MCB.23.6.1994-2008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proietti C, Salatino M, Rosemblit C, Carnevale R, Pecci A, Kornblihtt AR, Molinolo AA, Frahm I, Charreau EH, Schillaci R, Elizalde PV. Progestins induce transcriptional activation of signal transducer and activator of transcription 3 (Stat3) via a Jak- and Src-dependent mechanism in breast cancer cells. Mol.Cell Biol. 2005;25:4826–4840. doi: 10.1128/MCB.25.12.4826-4840.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carnevale RP, Proietti CJ, Salatino M, Urtreger A, Peluffo G, Edwards DP, Boonyaratanakornkit V, Charreau EH, Bal de Kier JE, Schillaci R, Elizalde PV. Progestin effects on breast cancer cell proliferation, proteases activation, and in vivo development of metastatic phenotype all depend on progesterone receptor capacity to activate cytoplasmic signaling pathways. Mol.Endocrinol. 2007;21:1335–1358. doi: 10.1210/me.2006-0304. [DOI] [PubMed] [Google Scholar]
- 43.Boonyaratanakornkit V, McGowan E, Sherman L, Mancini MA, Cheskis BJ, Edwards DP. The role of extranuclear signaling actions of progesterone receptor in mediating progesterone regulation of gene expression and the cell cycle. Mol.Endocrinol. 2007;21:359–375. doi: 10.1210/me.2006-0337. [DOI] [PubMed] [Google Scholar]
- 44.Trevino LS, Bingman WE, III, Edwards DP, Weigel NL. The requirement for p42/p44 MAPK activity in progesterone receptor-mediated gene regulation is target gene-specific. Steroids. 2013;78:542–547. doi: 10.1016/j.steroids.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hagan CR, Regan TM, Dressing GE, Lange CA. ck2-dependent phosphorylation of progesterone receptors (PR) on Ser81 regulates PR-B isoform-specific target gene expression in breast cancer cells. Mol.Cell Biol. 2011;31:2439–2452. doi: 10.1128/MCB.01246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vicent GP, Ballare C, Nacht AS, Clausell J, Subtil-Rodriguez A, Quiles I, Jordan A, Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol.Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Obr AE, Edwards DP. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol.Cell Endocrinol. 2012;357:4–17. doi: 10.1016/j.mce.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nazareth LV, Weigel NL. Activation of the human androgen receptor through a protein kinase A signaling pathway. J.Biol.Chem. 1996;271:19900–19907. doi: 10.1074/jbc.271.33.19900. [DOI] [PubMed] [Google Scholar]
- 49.Press M, Spaulding B, Groshen S, Kaminsky D, Hagerty M, Sherman L, Christensen K, Edwards DP. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids. 2002;67:799–813. doi: 10.1016/s0039-128x(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 50.Tetel MJ, Giangrande PH, Leonhardt SA, McDonnell DP, Edwards DP. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol.Endocrinol. 1999;13:910–924. doi: 10.1210/mend.13.6.0300. [DOI] [PubMed] [Google Scholar]
- 51.Knotts TA, Orkiszewski RS, Cook RG, Edwards DP, Weigel NL. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J.Biol.Chem. 2001;276:8475–8483. doi: 10.1074/jbc.M009805200. [DOI] [PubMed] [Google Scholar]
- 52.Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extra-nuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Clarke CL, Graham JD. Non-overlapping progesterone receptor cistromes contribute to cell-specific transcriptional outcomes. PLoS.One. 2012;7:e35859. doi: 10.1371/journal.pone.0035859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 55.Hubler TR, Scammell JG. Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress.Chaperones. 2004;9:243–252. doi: 10.1379/CSC-32R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Magklara A, Smith CL. A composite intronic element directs dynamic binding of the progesterone receptor and GATA-2. Mol.Endocrinol. 2009;23:61–73. doi: 10.1210/me.2008-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tung L, bdel-Hafiz H, Shen T, Harvell DM, Nitao LK, Richer JK, Sartorius CA, Takimoto GS, Horwitz KB. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol.Endocrinol. 2006;20:2656–2670. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- 58.Quiles I, Millan-Arino L, Subtil-Rodriguez A, Minana B, Spinedi N, Ballare C, Beato M, Jordan A. Mutational analysis of progesterone receptor functional domains in stable cell lines delineates sets of genes regulated by different mechanisms. Mol.Endocrinol. 2009;23:809–826. doi: 10.1210/me.2008-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beguelin W, az Flaque MC, Proietti CJ, Cayrol F, Rivas MA, Tkach M, Rosemblit C, Tocci JM, Charreau EH, Schillaci R, Elizalde PV. Progesterone receptor induces ErbB-2 nuclear translocation to promote breast cancer growth via a novel transcriptional effect: ErbB-2 function as a coactivator of Stat3. Mol.Cell Biol. 2010;30:5456–5472. doi: 10.1128/MCB.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diaz Flaque MC, Galigniana NM, Beguelin W, Vicario R, Proietti CJ, Russo RC, Rivas MA, Tkach M, Guzman P, Roa JC, Maronna E, Pineda V, Munoz S, Mercogliano MF, Charreau EH, Yankilevich P, Schillaci R, Elizalde PV. Progesterone receptor assembly of a transcriptional complex along with activator protein 1, signal transducer and activator of transcription 3 and ErbB-2 governs breast cancer growth and predicts response to endocrine therapy. Breast Cancer Res. 2013;15:R118. doi: 10.1186/bcr3587. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 61.Bach S, Knockaert M, Reinhardt J, Lozach O, Schmitt S, Baratte B, Koken M, Coburn SP, Tang L, Jiang T, Liang DC, Galons H, Dierick JF, Pinna LA, Meggio F, Totzke F, Schachtele C, Lerman AS, Carnero A, Wan Y, Gray N, Meijer L. Roscovitine targets, protein kinases and pyridoxal kinase. J.Biol.Chem. 2005;280:31208–31219. doi: 10.1074/jbc.M500806200. [DOI] [PubMed] [Google Scholar]
- 62.Davies TG, Bentley J, Arris CE, Boyle FT, Curtin NJ, Endicott JA, Gibson AE, Golding BT, Griffin RJ, Hardcastle IR, Jewsbury P, Johnson LN, Mesguiche V, Newell DR, Noble ME, Tucker JA, Wang L, Whitfield HJ. Structure-based design of a potent purine-based cyclin-dependent kinase inhibitor. Nat.Struct.Biol. 2002;9:745–749. doi: 10.1038/nsb842. [DOI] [PubMed] [Google Scholar]
- 63.Chen S, Xu Y, Yuan X, Bubley GJ, Balk SP. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc.Natl.Acad.Sci.U.S.A. 2006;103:15969–15974. doi: 10.1073/pnas.0604193103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Proietti CJ, Beguelin W, Flaque MC, Cayrol F, Rivas MA, Tkach M, Charreau EH, Schillaci R, Elizalde PV. Novel role of signal transducer and activator of transcription 3 as a progesterone receptor coactivator in breast cancer. Steroids. 2011;76:381–392. doi: 10.1016/j.steroids.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 65.Haidweger E, Novy M, Rotheneder H. Modulation of Sp1 activity by a cyclin A/CDK complex. J.Mol.Biol. 2001;306:201–212. doi: 10.1006/jmbi.2000.4406. [DOI] [PubMed] [Google Scholar]
- 66.Steinman RA, Wentzel A, Lu Y, Stehle C, Grandis JR. Activation of Stat3 by cell confluence reveals negative regulation of Stat3 by cdk2. Oncogene. 2003;22:3608–3615. doi: 10.1038/sj.onc.1206523. [DOI] [PubMed] [Google Scholar]
- 67.Vicent GP, Nacht AS, Font-Mateu J, Castellano G, Gaveglia L, Ballare C, Beato M. Four enzymes cooperate to displace histone H1 during the first minute of hormonal gene activation. Genes Dev. 2011;25:845–862. doi: 10.1101/gad.621811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamamoto A, Hashimoto Y, Kohri K, Ogata E, Kato S, Ikeda K, Nakanishi M. Cyclin E as a coactivator of the androgen receptor. J.Cell Biol. 2000;150:873–880. doi: 10.1083/jcb.150.4.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zwijsen RM, Wientjens E, Klompmaker R, van der SJ, Bernards R, Michalides RJ. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]
- 70.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 71.Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr.Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 72.Ellen TP, Ke Q, Zhang P, Costa M. NDRG1, a growth and cancer related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis. 2008;29:2–8. doi: 10.1093/carcin/bgm200. [DOI] [PubMed] [Google Scholar]
- 73.O'Neill GM, Seo S, Serebriiskii IG, Lessin SR, Golemis EA. A new central scaffold for metastasis: parsing HEF1/Cas-L/NEDD9. Cancer Res. 2007;67:8975–8979. doi: 10.1158/0008-5472.CAN-07-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J.Cell Physiol. 2009;220:292–296. doi: 10.1002/jcp.21791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 76.Roper K, Gregory SL, Brown NH. The 'spectraplakins': cytoskeletal giants with characteristics of both spectrin and plakin families. J.Cell Sci. 2002;115:4215–4225. doi: 10.1242/jcs.00157. [DOI] [PubMed] [Google Scholar]
- 77.Akira S, Takeda K. Toll-like receptor signalling. Nat.Rev.Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 78.Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat.Rev.Mol.Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]