Abstract
Background
Cryptic prophages are genetically defective in their induction and propagation, and are simply regarded as genetic remnants. There are several putative cryptic WO prophages in the sequenced Wolbachia genomes. Whether they are lytic is unclear and their functions are poorly understood. Only three open reading frames (ORFs) in cryptic WO prophages have been reported to be actively transcribed.
Results
In this study, we comprehensively examined the transcription of the only cryptic WO prophage (WOSol) in a Wolbachia strain that infects a fig wasp, Ceratosolen solmsi (Agaonidae, Chalcidoidea). By analyzing the transcriptions of all the ORFs of WOSol in both sexes of C. solmsi, using qualitative and quantitative methods, we demonstrated that i) a high percentage of ORFs are actively transcribed (59%, 17/29); ii) the expression of these ORFs is highly sex-specific, with a strong male bias (three in females and 15 in males); iii) an ank (ankyrin-domain-containing) gene actively transcribed in both wasp sexes is more highly expressed in males.
Conclusions
A large proportion of the genes in the cryptic WO prophage WOSol are expressed, which overturns the concept that cryptic prophages are simply genetically defective. The highly sex-specific expression patterns of these genes in the host suggest that they play important roles in Wolbachia biology and its reproductive manipulation of its insect host, particularly through the males.
Electronic supplementary material
The online version of this article (doi:10.1186/1471-2164-15-893) contains supplementary material, which is available to authorized users.
Keywords: Defective prophage, Bacteriophage WO, Reverse-transcription PCR, Real-time quantitative PCR, Ankyrin-domain-containing protein
Background
Bacteriophages, or phages, are viruses that infect bacteria, and can be either lytic or temperate. Lytic phages are strict pathogens of their bacterial hosts, and their infections culminate in the production of large numbers of new viral particles and lysis of the host cells. Temperate phages, such as the WO phages in Wolbachia, have two different forms. They can be lysogenic, with the viral DNA integrating into the host DNA and replicating as part of the host chromosome, which is referred to as the “prophage” form [1]. However, upon some signals, they can also be induced to produce a lytic form, which generates virions and causes bacterial lysis [2, 3]. For example, some WO prophages have been reported to form virions, including WOCauB2 and WOCauB3 [4], WOVitA1 [5, 6], WOCauB1 [7], and at least one haplotype located in the Wolbachia infecting Drosophila melanogaster [8].
Wolbachia, a cytoplasmically inherited Rickettsiales, causes a number of reproductive anomalies in its arthropod hosts, including cytoplasmic incompatibility (CI) [9], parthenogenesis [10], feminization of genetic males [11], and male killing [12]. These reproductive phenotypes impart a selective advantage on Wolbachia [13, 14], facilitating the spread of Wolbachia infections in the host population. More than 80% of Wolbachia strains contain bacteriophage-WO-related gene fragments [15], so whether the mobile genetic elements of the WO phages contribute to Wolbachia’s reproductive manipulation of their hosts is a hot topic. Based on evidence from G + C content and codon usage analyses of Wolbachia and WO, some scholars indicate that Wolbachia and WO have had a very long evolutionary association and that WO must confer some benefit on Wolbachia [16]. However, some Wolbachia strains without WO can still manipulate the reproduction of their hosts, indicating the dispensability of WO in the function of Wolbachia [15, 17].
Selective pressure can cause the degradation of prophages to genetically defective forms [18]. Prophages may become trapped in the chromosome of the host through recombination and/or deletion, and gradually decay [19], becoming inactive in terms of cell lysis, phage particle production, and plaque formation. These prophage fragments are referred to as cryptic or defective prophages [20]. To date, several putative cryptic WO prophages have been found in the sequenced Wolbachia strains [21, 22]. However, all of these putative cryptic WO prophages occur with at least one other complete WO prophage, carrying the complete head, baseplate, and tail gene modules that are essential for proper phage function [22, 23]. For example, prophages WORiA and WORiB are regarded as cryptic prophages in Wolbachia wRi, which infects D. simulans, but occur with at least one active phage, WORiC [23].
Bacteriophages play many roles in the ecology and genomic evolution of bacteria. For example, they can mediate lateral gene transfer [24], and in some cases provide their hosts with beneficial genes [25, 26]. Bacteriophages can also regulate the numbers of their host bacteria by inhibiting their replication or inducing cell lysis [5]. Furthermore, as mentioned above, some WO phages may contribute to Wolbachia’s reproductive manipulation of their hosts. Cryptic prophages can also benefit their hosts, because they can be involved in the host physiology and biofilm formation [27], and can increase the host’s resistance to general environmental stresses and to antibiotics [28]. Although cryptic prophages may have functions in the host, we still know very little about the mechanisms of these functional processes. The introduction of novel genes by these phages may confer beneficial phenotypes on their hosts [28] and prophage–prophage interactions could also be important pathways through which the potential activities of defective prophages are induced [29].
However, the expression and functions of cryptic WO prophage in Wolbachia are still poorly known. Until now, only two ank genes [30] and a putative DNA adenine methyltransferase gene (met2) [23] located within the cryptic WO prophage WORiB have been reported to be actively transcribed, and may play active roles in Wolbachia biology [20]. This suggests that there is an extreme paucity of data on the active transcription of the genes of cryptic WO prophages. In this study, we confirmed a cryptic WO prophage, WOSol, in Wolbachia strain wSol, which infects the fig wasp Ceratosolen solmsi. This is the only prophage detected in wSol. WOSol is highly degenerate and may lack a tail module. We demonstrated a comprehensive analysis of the transcription of this putative cryptic phage WO. Surprisingly, we found that a high percentage of the genes of this cryptic prophage are actively transcribed and display significantly different expression patterns in female and male fig wasps.
Results
Only one cryptic prophage occurred in C. solmsi
In our previous study [31], we have demonstrated that the fig wasp species C. solmsi is infected by a single Wolbachia strain that contains only one defective prophage WOSol, which lacks a tail module. Here, using real-time quantitative PCR (real-time qPCR), we counted and compared the densities of the Wolbachia genomes (represented by the single-copy groEL gene), and the phage WOSol genomes (represented by the single-copy orf7 gene) to determine whether WOSol was replicated extrachromosomally (the primers are listed in Additional file 1). With a single lysogenic copy of WOSol, the WOSol density should always equal (no lytic activity) or exceed (with lytic activity producing multiple phage virions) the wSol copy number [32]. The correlation between the copy counts of groEL and orf7 can thus reflect the total phage abundance in the female and male individuals of C. solmsi. We calculated the relative copy numbers (orf7:groEL) in 31 female and 35 male wasp individuals. The mean relative densities were consistent with the prediction of a single integrated copy in the Wolbachia genome and indicated no extrachromosomal WOSol (0.88 ± 0.05 in females and 1.15 ± 0.06 in males; all p values >0.05; two-tailed t test; Additional file 2).
The total bacteriophage WOSol abundance correlated strongly with the total bacterial abundance in both females (rho = 0.8756, P <0.0001; Figure 1A) and males (rho = 0.8064, P <0.0001; Figure 1B), as expected for a cryptic prophage with which a lysogenic phage is co-transmitted in the bacterial host.
Figure 1.
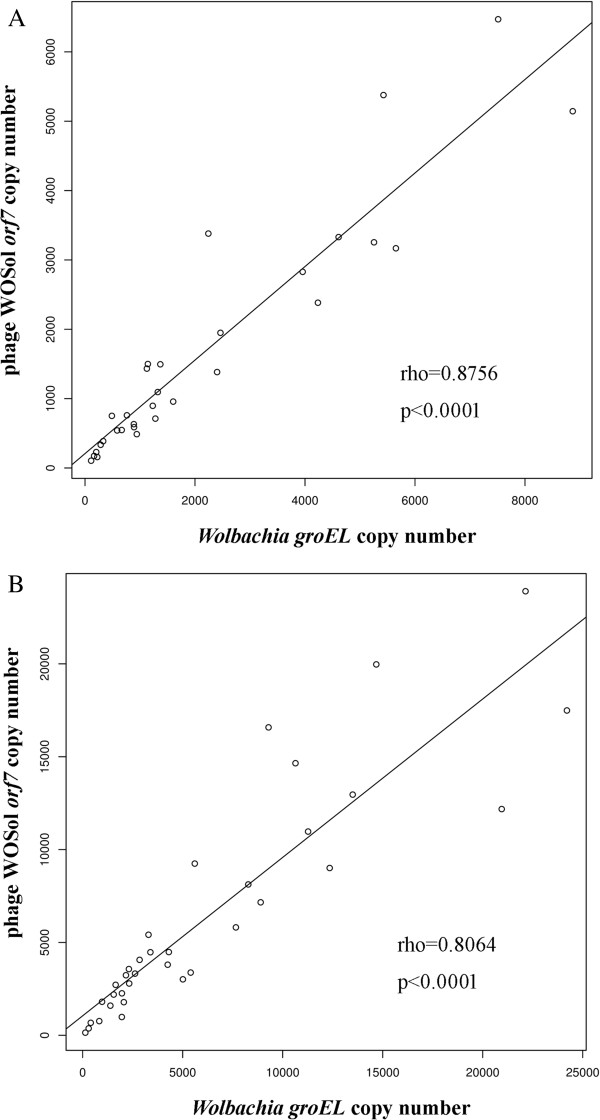
The correlation between the abundance of prophage WOSol and that of Wolbachia w Sol in females (A) and males (B) of C. solmsi . Each circle on the charts denotes the absolute copy number of a single-copy gene (orf7) of prophage WOSol (according to the vertical axis) and a single-copy gene (groEL) of Wolbachia wSol (according to the horizontal axis) in an adult female or male C. solmsi individual infected with Wolbachia. Altogether, 31 female and 35 male wasps were investigated. Correlation coefficients (rho values) and significances (P values) were calculated according to the nonparametric method of Spearman’s rho.
We also designed degenerate PCR primers based on the sequenced phage WO genomes (phage tail tape measure protein, GenBank accession number: CP001391.1|:758319-759499, AB478515.1|:52906-54086, AB478516.1|:48642-49819, CP003883.1|:1115914-1117093, AE017196.1|:553968-555151, CP003884.1|:442584-443767, AM999887.1|:1409653-1410819; phage late control gene protein GpD, GenBank accession number: AB478515.1|:55418-56398, CP001391.1|:755998-756978, AB478516.1|:51163-52143, CP003883.1|:1118431-1119411, HQ906662.1|:38950-39741, AM999887.1|:482503-483235, AM999887.1|:1412374-1413106) to amplify the bacteriophage WO tail genes, and obtained no successful amplification with normal PCR (data not shown). This further suggested that WOSol had no tail module. Moreover, we amplified no WOSol genes from Wolbachia-uninfected fig wasp individuals with normal PCR, by which we could exclude the possibility that prophage WOSol was present in the genome of the fig wasp.
High percentage of genes in the cryptic prophage WOSol genome were actively transcribed
Using reverse transcription PCR (RT–PCR) and nested RT–PCR, which have been commonly used in previous WO phage studies (details in the Methods section), we examined the mRNA expression of all 29 genes of the cryptic prophage WOSol in both female and male fig wasps (the primers are listed in Additional file 3). In females, only three ORFs were actively transcribed, whereas 15 ORFs were actively transcribed in males (Table 1 and Additional file 4).
Table 1.
Sex-specific RNA expression of the cryptic prophage WOSol
| ORF ID | Product | ♀ | ♂ |
|---|---|---|---|
| So0001 | Site-specific recombinase | n- | n- |
| So0002 | Putative phage related protein | n- | + |
| So0003 | Ankyrin repeat-containing prophage LambdaW1 | n- | n+ |
| So0004 | Ankyrin repeat-containing prophage LambdaW1, authentic point mutation; This gene contains a premature stop which is not the result of sequencing error, pseudo | n- | n+ |
| So0005 | Tail I | n- | + |
| So0006 | Prophage LambdaW1, baseplate assembly protein J | n+ | n- |
| So0007 | Prophage LambdaW1, baseplate assembly protein W | n- | + |
| So0008 | Baseplate assembly protein GpV | n- | + |
| So0009 | Conserved hypothetical protein | n- | n- |
| So0010 | Prophage LambdaW5, minor tail protein Z, authentic frameshift; This gene contains a frame shift which is not the result of sequencing error, pseudo | n- | + |
| So0011 | Conserved hypothetical protein | n- | n- |
| So0012 | Major capsid protein, putative | n- | n- |
| So0013 | Conserved hypothetical protein | n- | n- |
| So0014 | Minor capsid protein C, putative | n- | + |
| So0015 | Phage portal protein | + | n- |
| So0016 | Lyzozyme M1 | n- | n- |
| So0017 | Conserved hypothetical protein | n- | n+ |
| So0018 | Phage terminase large subunit GpA, authentic frameshift; This gene contains a frame shift which is not the result of sequencing error, pseudo | n- | n- |
| So0019 | Conserved hypothetical protein | n- | n- |
| So0020 | Prophage LambdaW1, DNA methylase,authentic frameshift; This gene contains a frame shift which is not the result of sequencing error, pseudo | n- | n- |
| So0021 | Putative Holliday junction resolvasome, endonuclease subunit | n- | + |
| So0022 | RepA,fragement; This gene is a fragement which is not the result of sequencing error. Identified by similarity to NZ_CAGB01000010.1:4487..6541, pseudo | n- | n- |
| So0023 | Putative rhoptry protein | n- | n+ |
| So0024 | Regulatory protein RepA, authentic frameshift; This gene contains a frame shift which is not the result of sequencing error, pseudo | n- | n+ |
| So0025 | Helicase, SNF2 family, authentic frameshift; This gene contains a frame shift which is not the result of sequencing error, pseudo | n- | n+ |
| So0026 | Patatin family protein, fragement; This gene is a fragement which is not the result of sequencing error. Identified by similarity to NC_002978.6:549882..550790, pseudo | n- | n- |
| So0027 | Hypothetical protein | n- | n+ |
| So0028 | Ankyrin repeat protein | n- | n- |
| So0029 | Ankyrin repeat protein | n+ | + |
Notes: +, positive using conventional RT-PCR; n+, positive using nested RT-PCR; n-, negative using both conventional and nested RT-PCR. ♂, male adult of C.solmsi; ♀, female adult of C.solmsi.
We then summarized the transcribed genes in the different modules of the WOSol genome. Of the three genes transcribed in females, So0006 and So0015 were from the baseplate and head module, respectively, but So0029 was uncharacterized. However, in males, all the modules, except the virulence module, included actively transcribed ORFs (Figure 2).
Figure 2.
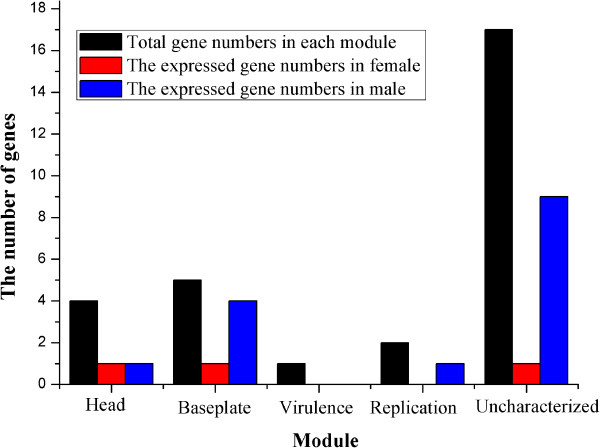
Distributions of sex-specifically expressed ORFs in each module of the cryptic prophage WOSol. In females, So0006 in the baseplate module, So0015 in the head module, and So0029 in the uncharacterized module are actively expressed; in males, So0005, So0007, So0008, and So0010 in the baseplate module, So0014 in the head module, So0024 in the replication module, and So0002, So0003, So0004, So0017, So0023, So0021, So0025, So0027, and So0029 in the uncharacterized module are actively expressed.
The prophage genes showed variable expression levels and sex-specific differences in the fig wasp (Table 1). Of the expressed genes, some were highly expressed and could be detected with conventional RT–PCR, whereas some were expressed at low levels and could only be detected with an additional round of nested PCR. All of the actively transcribed genes were expressed in either the females or males, but not both, except an ank gene (So0029), which was actively transcribed in both females and males.
Real-time qPCR assay of So0029 gene expression in female and male C. solmsi
The results described above showed that an ank gene (So0029), the only actively transcribed gene expressed in both females and males, differed in its expression in the two sexes: low in females and high in males (Table 1). However, these results were based on qualitative RT–PCR, and differences in primer sensitivity and primary template concentrations could affect the levels of amplified product. Therefore, we used real-time qPCR to quantitatively examine the expression of this gene (the primers are listed in Additional file 1). The So0029 gene was expressed with sex-dependent variations after normalization with the expression of the fig wasp’s nuclear genes of RPL13a & UBC, and groEL gene from Wolbachia (Figure 3A and C). However, the Wolbachia gene groEL showed sex-independent expression after normalization to the RPL13a and UBC genes, suggesting that the level of Wolbachia infection was not a major part of the observed variations of the phage gene So0029 (Figure 3B). Altogether, our data showed that the only one actively transcribed gene in both sexes, So0029, was sex-dependently expressed.
Figure 3.
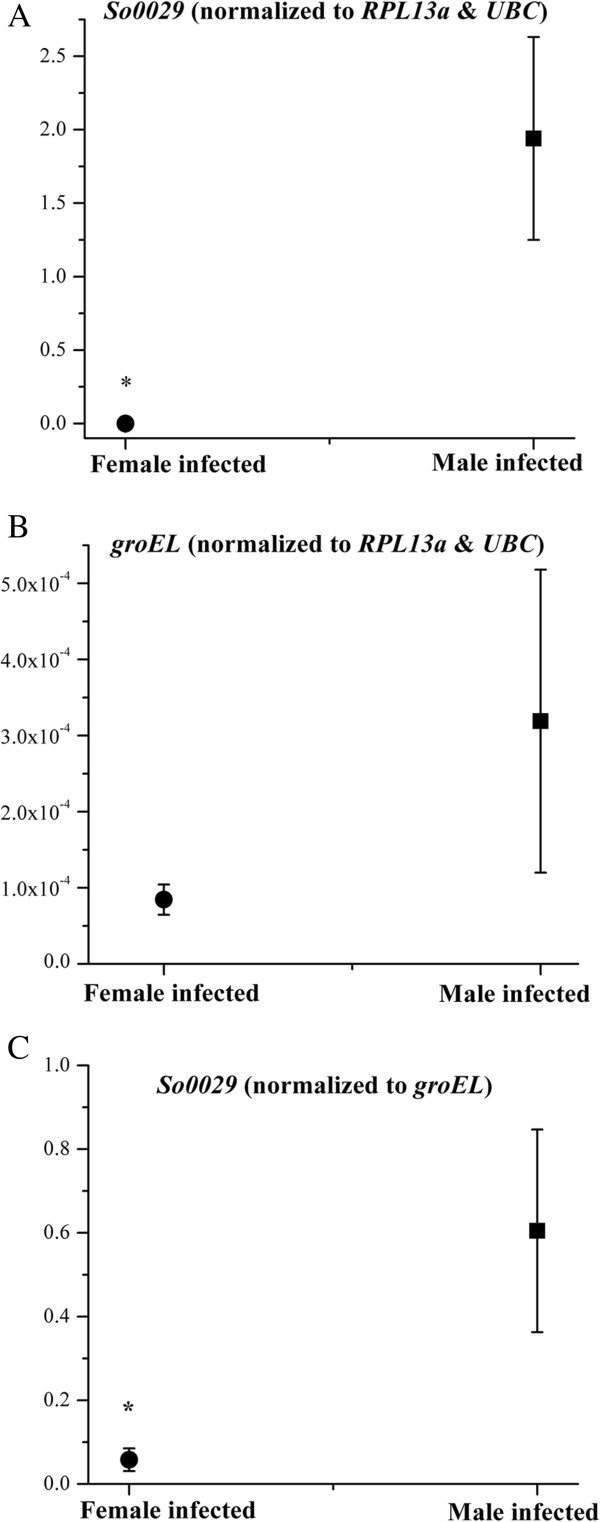
Expressions of the ank gene in female and male individuals of C. solmsi . Expression of So0029 was provided after normalized with fig wasp’s nuclear gene (RPL13a & UBC), and with Wolbachia groEL gene expression (A and C, respectively). Expression of groEL was provided relative to RPL13a & UBC expression (B). Each value represents the average ± SE of six biological replicates for females and males. The asterisk indicates a significant difference between females and males (P <0.05).
Discussion
In this study, we confirmed the presence of a single cryptic prophage WOSol in the only wSol genome in the fig wasp species C. solmsi, and demonstrated that a large proportion of the genes of the cryptic prophage were actively transcribed. Cryptic prophages are genetically defective because of the deletion or disruption of genes essential for their lytic growth and the production of infectious particles. Therefore, they have been regarded as simple genetic remnants, and researchers have tended to ignore their possible functions [29]. Recently, investigators noticed that cryptic prophages can confer multiple benefits on their hosts [27, 28]. However, how the cryptic prophage WO affects its host, Wolbachia, is poorly understood. To our knowledge, WORiA and WORiB are the only two known cryptic WO prophages confirmed by real-time qPCR to have no lytic processes, but have become trapped in the chromosome of wRi [23]. Only three ORFs within the WORiB genome have been shown with RT–PCR to be actively transcribed and may therefore have roles in Wolbachia biology [20, 30]. These actively transcribed ORFs may function in Wolbachia wRi during its infection of D. simulans, through prophage–prophage interactions, because wRi harbors four prophage genomes [30]. Unexpectedly, in this study, we detected that of the 29 ORFs of the only cryptic prophage WOSol genome in C. solmsi, 17 are actively transcribed, which suggests that they may also play essential roles in the biology of Wolbachia wSol. Moreover, some “cryptic” prophage haplotypes, although they have not been finally confirmed, have been reported to transcribe phage-related genes, with potential to affect the host biology. For example, the met2 gene of “cryptic” prophages WOMelA and WOMelB within wMel are actively transcribed in both sexes of D. melanogaster [20]. In the “cryptic” prophages within wPip, the sex-specific expression of ank in Culex quinquefasciatus [33] and the stage-specific expression of orf7 in C. pipiens [34] have also been detected. All these results suggest that in the cryptic prophage WO, there are many genes that play active roles to Wolbachia biology.
The phenomenon of the reproductive manipulation by Wolbachia of its host is compelling, but the molecular basis remains unknown [7, 35, 36]. One potential mechanism is the variable expression and activity of Wolbachia genes in the female and male insect hosts or their infections with different Wolbachia strains [37]. However, variable gene expression in Wolbachia is suggested to occur at a low rate, considering that only a small number of regulatory genes have been identified in the sequenced Wolbachia genomes [37, 38]. Interestingly, in bacteriophage WO, some genes are sex- [33, 34, 37], stage- [34], and strain-specifically expressed in the host [34, 39], which suggests that WO contributes to the manipulation by Wolbachia of its host. In the cryptic prophage WOSol, the expressions of ORFs are highly sex specific (three in female fig wasps, 15 in males, with only one ORF actively transcribed in both females and males), which leads us to make an assumption that these genes may have the possibility to be involved (directly or indirectly) in CI in C. solmsi by wSol, based on the high prevalence of Wolbachia (more than 80% infection, as previously reported; 83.3% (364/437) in the present study) [31, 40] and the highly female-biased sex ratio of this species [40]. However, we need to experimentally confirm the present of CI phenotype in the species first. Furthermore, it is especially interesting that far more genes (15 of the 17 actively transcribed genes) are expressed in males, which further hints that the WO genes function as effectors, causing Wolbachia to exert different effects on the two sexes of the host. Future studies should examine the stage- and tissue-specific transcription of these phage ORFs. Interesting questions to be addressed are whether this sex-specific transcription reflects differences in the expression of the gene products in the ovaries and/or oocytes (in the female) and the testes and/or spermatocytes (in the male), and whether these genes may be involved in reproductive manipulation.
ANK mediates interactions between proteins, and thus acts as a transcription factor to regulate the expression of proteins involved in diverse aspects of cell biology [41, 42]. ANK is commonly found in eukaryote and viral proteins, whereas it is relatively rare in bacteria [41, 43]. There are often only 1–3 ank genes in the α-Proteobacteria, including Rickettsia, Anaplasma, and Ehrlichia [44, 45]. However, notably, some CI- inducing bacteria strains encode the largest number of ANK proteins. For example, there are 60 ank genes in Wolbachia wPip from C. pipiens [46], 35 in Wolbachia strain wRi infecting D. simulans [30], 23 in Wolbachia strain wMel in D. melanogaster [47], and 19 in Cardinium hertigii, cEper1 [48]. However, in mutualist Wolbachia strains, the ank genes are very reduced; for example, only five in wBm [49] and six in wOo [50]. The overrepresentation of ANK proteins in CI-inducing but distantly related Cardinium and Wolbachia strains thus suggests that ANK plays important roles in the process of CI [48]. The sequence variability of ank genes in CI-inducing strain wMel and non-CI-inducing wAu [51], and sex-specific expression patterns of some ank genes in wRi and wPip also suggest that they function directly in the reproductive manipulation by the bacteria of their hosts [30, 33, 37]. We detect an ank gene that is actively transcribed in both the females and males of C. solmsi, and its level of expression is higher in males. Further investigation of all the ank genes in Wolbachia wSol may help to determine whether ANK proteins are responsible for the reproductive manipulation of this fig wasp species by Wolbachia.
Surprisingly, we note that some structural prophage ORFs are actively transcribed in C. solmsi. For example, the ORF So0015 (in the head module) and So0006 (in the baseplate module) are actively transcribed in females, whereas ORF So0014 (in the head module) and ORFs So0005, So0007, and So0008 (all in the baseplate module) are actively transcribed in males. Structural ORFs are often expected to be expressed during the viral replication process and their transcription levels are considered to be evidence of whether bacteriophage WO is a lytic virion or an inactive prophage [34]. However, bacteriophage WOSol is a cryptic prophage and there is no viral replication. Therefore, rather than being actual structural/lytic genes responding to a density signal, these actively transcribed structural ORFs may have evolved some new functions in C. solmsi, distinct from their roles in viral structure formation.
Conclusions
We comprehensively examine the transcription of a cryptic WO prophage in a Wolbachia strain and find that large proportion of the genes are actively expressed, which confirms that cryptic prophages are not nonfunctional fragments. The highly sex-specifically transcribed cryptic WO prophage genes may indicate their important roles in Wolbachia biology and its master manipulation of insect host, which need further study.
Methods
Sample collection
Ceratosolen solmsi, the pollinator species of Ficus hispida (Moraceae), was collected from Danzhou (N19°30′29″, E109°29′6″), Hainan Province, China, in June 2013. All fig fruits were collected at the same developmental stage, several days before becoming ripe. The female and male pollinators removed from the inside of the syconia were adults because the fig wasps are in the adult stage after they emerge from the galls into the fig syconia. They were identified and confirmed according to their morphological traits, under a Nikon SMZ80 microscope. Some specimens were immersed in Sample Protector (TaKaRa, Beijing, China) for RNA extraction and the others were immersed in 95% ethanol for DNA extraction.
In total, eight RNA sample groups (four female and four male samples; because the fig pollinators are very small, we used 10 whole-body individuals for each RNA sample) were collected to qualitatively determine the transcription of the prophage genes by RT–PCR. An additional 12 RNA sample groups (six female and six male samples; each sample contained 10 individual wasps) were collected to quantitatively determine the transcription of the So0029 and groEL genes with real-time qPCR.
DNA was extracted from 31 female and 35 male wasps to determine their infection with Wolbachia, and to compare the gene densities of Wolbachia (wSol) and the WO prophage (WOSol), determined with real-time qPCR.
RNA isolation and cDNA synthesis
Total RNA from each RNA sample was extracted with TRIzol Reagent (Invitrogen) and treated with RNase-free DNaseI (Invitrogen). A NanoDrop-2000 spectrophotometer (Thermo, Madison, WI, USA) was used to measure the RNA purity (A260/A280) and concentration. The key issue related to this method is the “false positives” generated by genomic DNA contamination, so before reverse transcription, all RNA samples were confirmed to contain no genomic DNA contamination by PCR with the universal Wolbachia wsp 81 F/691R primers [52] using TransTaq polymerase High Fidelity (TransGen Biotech, Beijing, China), [20, 34] (Additional file 5). First-stranded cDNA was then synthesized from 1 μg of total RNA with random primers [53] in a 20 μl reaction volume using TransScript II First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). wsp expression was characterized as the positive control to demonstrate the quality of all the cDNA samples (Additional file 5).
DNA extraction
Total genomic DNA was extracted from each wasp using the EasyPure Genomic DNA Extraction Kit (TransGen Biotech, Beijing, China), following the manufacturer’s recommendations, and suspended in 20 μl of double-distilled sterile water. DNA purity and concentration was determined with a NanoDrop-2000 Spectrophotometer (Thermo, Madison, WI, USA). The Wolbachia infection status of these wasps was confirmed by PCR with the wsp 81F/691R primers [52].
RT–PCR and real-time qPCR expression analysis
Only expressed prophage genes can confer benefit on its bacterial host [54]. Therefore we tested the candidate functional genes for transcription. RT–PCR and sometimes nested RT–PCR with inner primer pairs were used to qualitatively determine the expression of all 29 ORFs of the prophage WOSol. Nested RT–PCR was only used when conventional RT–PCR did not detect the targeted fragment. Two samples were tested to represent each gene and sex; the positive results for the expressed genes that are presented in Table 1 show that all the genes were expressed in both samples.The resulting amplicons were run on a 1% TBE agarose gel and photographed under UV illumination. The PCR products were purified with the EasyPure PCR Purification Kit (TransGen Biotech, Beijing, China) and directly sequenced with an ABI3730 capillary autosequencer (Biosune, Beijing, China).
Real-time qPCR was performed with a Stratagene Mx3000p qPCR system (Stratagene, La Jolla, CA) (the primers are listed in Additional file 1). Reaction volumes of 20 μl containing 1 μl of template, 10 μl of TransStart Green qPCR SuperMix UDG (TransGen Biotech, Beijing, China), 0.4 μl of Passive Reference Dye II (50×) (TransGen Biotech, Beijing, China), 0.8 μl of primer mix (0.2 mM), and 7.8 μl of sterile water were prepared. A no-template control was included in each run to check for reagent contamination. A melting curve analysis was performed for each run to confirm the amplification specificity. The same thermal conditions were used for all real-time qPCR reactions: 40 cycles of 95°C for 10 s, 57°C for 15 s, and 72°C for 10 s. Two technical replicate experiments were performed for each real-time qPCR assay.
To quantify the densities of a minor capsid protein gene (orf7) from WOSol and a heat-shock protein 60 gene (groEL) [5] from wSol in the DNA templates, we prepared standard solutions for the real-time qPCR. The PCR amplicons for orf7 or groEL were resolved electrophoretically on TBE 1.0% agarose gel, and then cloned with the pEasy-T5 Zero Cloning Kit (TransGen Biotech, Beijing, China). The plasmids were then prepared with the EasyPure Plasmid MiniPrep Kit (TransGen Biotech, Beijing, China), and quantified with a NanoDrop-2000 spectrophotometer (Thermo, Madison, WI, USA). Standard 10-fold dilution series from 107 to 103 copies were prepared and used to calculate the copy numbers of the genes.
We also calculated the expression of So0003, So0007, So0014, So0015, and So0025 relative to that of reference genes. Several studies have demonstrated that the mean of individual PCR efficiencies (Em) gives a more reliable result than efficiencies derived with a standard curve because interwell differences would lead to the erroneous determination of gene expression, and an assumption of identical efficiency for each well confound the data analysis [55–57]. Therefore, we obtained all the Em values by determining the baseline from the raw real-time qPCR data using LinRegPCR [58, 59]. The quantification cycle (Cq) and Em values obtained from LinRegPCR were then used to calculate the relative expression of the selected genes with respect to the reference genes RPL13a and UBC, with the following equation [60]:
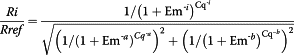 |
Ri is the expression of each selected gene; Rref is the expression of the reference genes; Ri/Rref is the expression of each selected gene normalized to that of the reference genes; Cq-i and Em-i are the quantification cycle value and the mean individual PCR efficiency for each selected gene, respectively; Cq_a and Cq_b are the quantification cycle values for each reference gene; Em_a and Em_b are the mean individual PCR efficiencies for each reference gene. Six biological replicates for females and males were performed in our experiments. Two technical replicate experiments were performed for each real-time qPCR assay.
Statistical analysis
The average copy number of the integrated phage was compared with the expected number and the difference was analyzed statistically with a two-tailed t test (SAS Institute, Cary, NC, USA). In the real-time qPCR experiments, small plate effects (the apparent trends towards slightly elevated or reduced threshold cycle (Ct) values for the same template DNA used for the standard curve between different plates) were common. We normalized the plate effects using a common threshold with a multiple experiment analysis (MxPro QPCR Software, Stratagene, La Jolla, CA). Correlation coefficients were calculated using nonparametric Spearman’s rho (JMP v.5.0, SAS Institute, Cary, NC, USA). One-way ANOVA (SAS Institute, Cary, NC, USA) was used to test for variations in the levels of ank mRNA between female and male C. solmsi. The significance level for all analyses was set at P <0.05.
MIQE guidelines
For the real-time qPCR, we followed the Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines [61] to increase the reliability and integrity of the results, and to promote experimental consistency and transparency between research laboratories. The MIQE checklist is provided in Additional file 6.
Availability of supporting data
The data sets supporting the results of this article are available in the LabArchives repository, DOI:10.6070/H4S46PXM and https://mynotebook.labarchives.com/doi/NzY1NzUuMnw1ODkwNC81ODkwNC9Ob3RlYm9vay8yNzczMDg2MjMxfDE5NDM4My4y/10.6070/H4S46PXM.
Electronic supplementary material
Additional file 1: The primer pairs used for real-time qPCR analysis. Notes: Es(%),PCR reaction efficiency; R 2 , Pearson correlation coefficient. (DOCX 22 KB)
Additional file 2: Summary statistics for the Quantitative PCR. (PDF 87 KB)
Additional file 3: All the primers used for RT-PCR and nested RT-PCR. (DOCX 19 KB)
Additional file 4: The electrophoresis pictures of the RT-PCR and nested RT-PCR for all the studied genes. So0001 ~ So0029, the ORF IDs of prophage WOSol. For the image of each gene: M, 100 bp DNA ladder; For each ORF, the first round of RT-PCR (first lane, for female sample; second lane, for male sample; third lane, positive control with gonomic DNA as template; fourth lane, negative control with distilled water as template); If the first and/or the second lane did not detect the targeted fragment, then the fifth and/or the sixth lane is nested RT-PCR with diluted products of the first and/or the second RT-PCR as template; The following two lanes are nested RT-PCR with diluted products of the third and fourth lane PCR products as template. (PDF 460 KB)
Additional file 5: The electrophoresis pictures of PCR products of wsp gene with wsp 81 F/691R primers. PCR based on template of total RNA with DNaseI treatment but no reverse transcription (A) and first-stranded cDNA samples which were synthesized from 1 μg of total RNA with random primers in a 20 μl reaction volume using TransScript II First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) (B). The comparison between (A) and (B) indicates that the RNA samples are not contaminated by genomic DNA. Lane 1 ~ 19: the results of 19 samples. PC: positive controls with genomic DNA as template. NC: negative controls with distilled water as template. M: 100 bp DNA ladder. (PDF 114 KB)
Additional file 6: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. (DOCX 20 KB)
Acknowledgements
This project was supported by the National Natural Science Foundation of China (NSFC grant nos 31090253, 31172072, 31210103912), partially by a grant (O529YX5105) from the Key Laboratory of the Zoological Systematics and Evolution of the Chinese Academy of Sciences, and the National Science Fund for Fostering Talents in Basic Research (Special Subjects in Animal Taxonomy, NSFC-J0930004). We thank Dr Wen Xin and TransGen Biotech for providing most of the reagents used in this study. Thanks also go to Jia-Jie Yu at Institute of Zoology, Chinese Academy of Sciences on data analysis. We thank the anonymous reviewers for their valuable comments and suggestions.
Abbreviations
- ORFs
Open reading frames
- ank
ankyrin-domain-containing
- CI
Cytoplasmic incompatibility
- met2
DNA adenine methyltransferase gene
- real-time qPCR
real-time quantitative PCR
- RT–PCR
Reverse transcription PCR
- Em
Individual PCR efficiencies
- Cq
Quantification cycle
- Ct
Threshold cycle
- MIQE
Minimum information for publication of quantitative real-time PCR experiments.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GHW, JHX and DWH designed the study. GHW and JHX performed the analyses. LMN and GCM collected the materials. GHW performed experiments. GHW, JHX and DWH wrote the manuscript. All authors revised the manuscript and approved the final version.
Contributor Information
Guan-Hong Wang, Email: wangguanhong@ioz.ac.cn.
Li-Ming Niu, Email: niulm@yahoo.com.cn.
Guang-Chang Ma, Email: mgc53@126.com.
Jin-Hua Xiao, Email: xiaojh@ioz.ac.cn.
Da-Wei Huang, Email: huangdw@ioz.ac.cn.
References
- 1.Lwoff A. Interaction among virus, cell, and organism. Science. 1966;152(3726):1216–1220. doi: 10.1126/science.152.3726.1216. [DOI] [PubMed] [Google Scholar]
- 2.Adams MH. Bacteriophages. New York: Interscience Publishers; 1959. pp. 1–4. [Google Scholar]
- 3.Echols H. Developmental pathways for the temperate phage: lysis vs lysogeny. Annu Rev Genet. 1972;6(1):157–190. doi: 10.1146/annurev.ge.06.120172.001105. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka K, Furukawa S, Nikoh N, Sasaki T, Fukatsu T. Complete WO phage sequences reveal their dynamic evolutionary trajectories and putative functional elements required for integration into the Wolbachia genome. Appl Environ Microbiol. 2009;75(17):5676–5686. doi: 10.1128/AEM.01172-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bordenstein SR, Marshall ML, Fry AJ, Kim U, Wernegreen JJ. The tripartite associations between bacteriophage, Wolbachia, and arthropods. PLoS Pathog. 2006;2(5):e43. doi: 10.1371/journal.ppat.0020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kent BN, Salichos L, Gibbons JG, Rokas A, Newton ILG, Clark ME, Bordenstein SR. Complete bacteriophage transfer in a bacterial endosymbiont (Wolbachia) determined by targeted genome capture. Genome Biol Evol. 2011;3:209. doi: 10.1093/gbe/evr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujii Y, Kubo T, Ishikawa H, Sasaki T. Isolation and characterization of the bacteriophage WO from Wolbachia, an arthropod endosymbiont. Biochem Biophys Res Commun. 2004;317(4):1183–1188. doi: 10.1016/j.bbrc.2004.03.164. [DOI] [PubMed] [Google Scholar]
- 8.Gavotte L, Vavre F, Henri H, Ravallec M, Stouthamer R, Bouletreau M. Diversity, distribution and specificity of WO phage infection in Wolbachia of four insect species. Insect Mol Biol. 2004;13(2):147–153. doi: 10.1111/j.0962-1075.2004.00471.x. [DOI] [PubMed] [Google Scholar]
- 9.Breeuwer JA, Werren JH. Microorganisms associated with chromosome destruction and reproductive isolation between two insect species. Nature. 1990;346(6284):558–560. doi: 10.1038/346558a0. [DOI] [PubMed] [Google Scholar]
- 10.Stouthamer R, Luck RF, Hamilton W. Antibiotics cause parthenogenetic Trichogramma (Hymenoptera/Trichogrammatidae) to revert to sex. Proc Natl Acad Sci USA. 1990;87(7):2424–2427. doi: 10.1073/pnas.87.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legrand J, Legrand Hamelin E, Juchault P. Sex determination in crustacea. Biol Rev. 1987;62(4):439–470. doi: 10.1111/j.1469-185X.1987.tb01637.x. [DOI] [Google Scholar]
- 12.Werren JH, Hurst G, Zhang W, Breeuwer J, Stouthamer R, Majerus M. Rickettsial relative associated with male killing in the ladybird beetle (Adalia bipunctata) J Bacteriol. 1994;176(2):388–394. doi: 10.1128/jb.176.2.388-394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48:1500–1513. doi: 10.2307/2410244. [DOI] [PubMed] [Google Scholar]
- 14.Barreto FS, Burton RS. Evidence for compensatory evolution of ribosomal proteins in response to rapid divergence of mitochondrial rRNA. Mol Biol Evol. 2013;30(2):310–314. doi: 10.1093/molbev/mss228. [DOI] [PubMed] [Google Scholar]
- 15.Gavotte L, Henri H, Stouthamer R, Charif D, Charlat S, Bouletreau M, Vavre F. A survey of the bacteriophage WO in the endosymbiotic bacteria Wolbachia. Mol Biol Evol. 2007;24(2):427–435. doi: 10.1093/molbev/msl171. [DOI] [PubMed] [Google Scholar]
- 16.Masui S, Kamoda S, Sasaki T, Ishikawa H. Distribution and evolution of bacteriophage WO in Wolbachia, the endosymbiont causing sexual alterations in arthropods. J Mol Evol. 2000;51(5):491–497. doi: 10.1007/s002390010112. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf JA, Bordenstein SR. The complexity of virus systems: the case of endosymbionts. Curr Opin Microbiol. 2012;15:1–7. doi: 10.1016/j.mib.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence JG, Hendrix RW, Casjens S. Where are the pseudogenes in bacterial genomes? Trends Microbiol. 2001;9(11):535–540. doi: 10.1016/S0966-842X(01)02198-9. [DOI] [PubMed] [Google Scholar]
- 19.Canchaya C, Proux C, Fournous G, Bruttin A, Brüssow H. Prophage genomics. Microbiol Mol Biol Rev. 2003;67(2):238–276. doi: 10.1128/MMBR.67.2.238-276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saridaki A, Sapountzis P, Harris HL, Batista PD, Biliske JA, Pavlikaki H, Oehler S, Savakis C, Braig HR, Bourtzis K. Wolbachia prophage DNA adenine methyltransferase genes in different Drosophila-Wolbachia associations. PLoS ONE. 2011;6(5):e19708. doi: 10.1371/journal.pone.0019708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furukawa S, Tanaka K, Ikeda T, Fukatsu T, Sasaki T. Quantitative analysis of the lytic cycle of WO phages infecting Wolbachia. Appl Entomol Zoolog. 2012;47(4):449–456. doi: 10.1007/s13355-012-0142-6. [DOI] [Google Scholar]
- 22.Kent BN, Funkhouser LJ, Setia S, Bordenstein SR. Evolutionary genomics of a temperate bacteriophage in an obligate intracellular bacteria (Wolbachia) PLoS ONE. 2011;6(9):e24984. doi: 10.1371/journal.pone.0024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biliske JA, Batista PD, Grant CL, Harris HL. The bacteriophage WORiC is the active phage element in wRi of Drosophila simulans and represents a conserved class of WO phages. BMC Microbiol. 2011;11:251. doi: 10.1186/1471-2180-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wommack KE, Colwell RR. Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev. 2000;64(1):69–114. doi: 10.1128/MMBR.64.1.69-114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brüssow H, Canchaya C, Hardt WD. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol Mol Biol Rev. 2004;68(3):560–602. doi: 10.1128/MMBR.68.3.560-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abedon ST, LeJeune JT. Why bacteriophage encode exotoxins and other virulence factors. Evol Bioinformatics Online. 2005;1:97. [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Kim Y, Wood TK. Control and benefits of CP4-57 prophage excision in Escherichia coli biofilms. ISME J. 2009;3(10):1164–1179. doi: 10.1038/ismej.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. Cryptic prophages help bacteria cope with adverse environments. Nat Commun. 2010;1:147. doi: 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asadulghani M, Ogura Y, Ooka T, Itoh T, Sawaguchi A, Iguchi A, Nakayama K, Hayashi T. The defective prophage pool of Escherichia coli O157: prophage–prophage interactions potentiate horizontal transfer of virulence determinants. PLoS Pathog. 2009;5(5):e1000408. doi: 10.1371/journal.ppat.1000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klasson L, Westberg J, Sapountzis P, Näslund K, Lutnaes Y, Darby AC, Veneti Z, Chen L, Braig HR, Garrett R. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc Natl Acad Sci USA. 2009;106(14):5725–5730. doi: 10.1073/pnas.0810753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang GH, Xiao JH, Xiong TL, Li Z, Murphy RW, Huang DW. High-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR) for determination of a highly degenerated prophage WO genome in a Wolbachia strain infecting a fig wasp species. Appl Environ Microbiol. 2013;79(23):7476–7481. doi: 10.1128/AEM.02261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chauvatcharin N, Ahantarig A, Baimai V, Kittayapong P. Bacteriophage WO-B and Wolbachia in natural mosquito hosts: infection incidence, transmission mode and relative density. Mol Ecol. 2006;15(9):2451–2461. doi: 10.1111/j.1365-294X.2006.02947.x. [DOI] [PubMed] [Google Scholar]
- 33.Sinkins SP, Walker T, Lynd AR, Steven AR, Makepeace BL, Godfray HCJ, Parkhill J. Wolbachia variability and host effects on crossing type in Culex mosquitoes. Nature. 2005;436(7048):257–260. doi: 10.1038/nature03629. [DOI] [PubMed] [Google Scholar]
- 34.Sanogo YO, Dobson SL. WO bacteriophage transcription in Wolbachia-infected Culex pipiens. Insect Biochem Mol Biol. 2006;36(1):80–85. doi: 10.1016/j.ibmb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Werren JH. Biology of Wolbachia. Annu Rev Entomol. 1997;42(1):587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 36.LePage D, Bordenstein S. Wolbachia: can we save lives with a great pandemic? Trends Parasitol. 2013;29:8. doi: 10.1016/j.pt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walker T, Klasson L, Sebaihia M, Sanders MJ, Thomson NR, Parkhill J, Sinkins SP. Ankyrin repeat domain-encoding genes in the wPip strain of Wolbachia from the Culex pipiens group. BMC Biol. 2007;5(1):39. doi: 10.1186/1741-7007-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fenn K, Blaxter M. Wolbachia genomes: revealing the biology of parasitism and mutualism. Trends Parasitol. 2006;22(2):60–65. doi: 10.1016/j.pt.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 39.Pichon S, Bouchon D, Liu C, Chen L, Garrett RA, Greve P. The expression of one ankyrin pk2 allele of the WO prophage is correlated with the Wolbachia feminizing effect in isopods. BMC Microbiol. 2012;12(1):55. doi: 10.1186/1471-2180-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao JH, Wang NX, Murphy RW, Cook J, Jia LY, Huang DW. Wolbachia infection and dramatic intraspecific mitochondrial DNA divergence in a fig wasp. Evolution. 2011;66:1907–1916. doi: 10.1111/j.1558-5646.2011.01561.x. [DOI] [PubMed] [Google Scholar]
- 41.Bork P. Hundreds of ankyrin-like repeats in functionally diverse proteins: mobile modules that cross phyla horizontally? Protein Struct Funct Bioinformatics. 1993;17(4):363–374. doi: 10.1002/prot.340170405. [DOI] [PubMed] [Google Scholar]
- 42.Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13(6):1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J, Mahajan A, Tsai M-D. Ankyrin repeat: a unique motif mediating protein-protein interactions. Biochemistry. 2006;45(51):15168–15178. doi: 10.1021/bi062188q. [DOI] [PubMed] [Google Scholar]
- 44.Andersson SG, Zomorodipour A, Andersson JO, Sicheritz Pontén T, Alsmark UCM, Podowski RM, Näslund AK, Eriksson AS, Winkler HH, Kurland CG. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396(6707):133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 45.Caturegli P, Asanovich KM, Walls JJ, Bakken JS, Madigan JE, Popov VL, Dumler JS. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect Immun. 2000;68(9):5277–5283. doi: 10.1128/IAI.68.9.5277-5283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, Lord A, Sanders S, Earl J, O'Neill SL, Thomson N. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol. 2008;25(9):1877–1887. doi: 10.1093/molbev/msn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol. 2004;2(3):e69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Penz T, Schmitz-Esser S, Kelly SE, Cass BN, Müller A, Woyke T, Malfatti SA, Hunter MS, Horn M. Comparative genomics suggests an independent origin of cytoplasmic incompatibility in Cardinium hertigii. PLoS Genet. 2012;8(10):e1003012. doi: 10.1371/journal.pgen.1003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, Bhattacharyya A, Kapatral V, Kumar S, Posfai J. The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005;3(4):e121. doi: 10.1371/journal.pbio.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Darby AC, Armstrong SD, Bah GS, Kaur G, Hughes MA, Kay SM, Koldkjær P, Radford AD, Blaxter ML, Tanya VN. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iturbe-Ormaetxe I, Burke GR, Riegler M, O'Neill SL. Distribution, expression, and motif variability of ankyrin domain genes in Wolbachia pipientis. J Bacteriol. 2005;187(15):5136–5145. doi: 10.1128/JB.187.15.5136-5145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou W, Rousset F, O'Neill S. Phylogeny and PCR–based classification of Wolbachia strains using wsp gene sequences. Proc R Soc LondB. 1998;265(1395):509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noonan KE, Roninson IB. mRNA phenotyping by enzymatic amplification of randomly primed cDNA. Nucleic Acids Res. 1988;16(21):10366–10366. doi: 10.1093/nar/16.21.10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDaniel L, Breitbart M, Mobberley J, Long A, Haynes M, Rohwer F, Paul JH. Metagenomic analysis of lysogeny in Tampa Bay: implications for prophage gene expression. PLoS ONE. 2008;3(9):e3263. doi: 10.1371/journal.pone.0003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karlen Y, McNair A, Perseguers S, Mazza C, Mermod N. Statistical significance of quantitative PCR. BMC Bioinformatics. 2007;8(1):131. doi: 10.1186/1471-2105-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peirson SN, Butler JN, Foster RG. Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res. 2003;31(14):e73–e73. doi: 10.1093/nar/gng073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression’s C T difference” formula. J Mol Med. 2006;84(11):901–910. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 58.Ruijter J, Ramakers C, Hoogaars W, Karlen Y, Bakker O, Van den Hoff M, Moorman A. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37(6):e45–e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramakers C, Ruijter JM, Deprez RHL, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339(1):62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Xiao JH, Bian SN, Niu LM, Murphy RW, Huang DW. Evolution and expression plasticity of opsin genes in a fig pollinator, Ceratosolen solmsi. PLoS ONE. 2013;8(1):e53907. doi: 10.1371/journal.pone.0053907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55(4):611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: The primer pairs used for real-time qPCR analysis. Notes: Es(%),PCR reaction efficiency; R 2 , Pearson correlation coefficient. (DOCX 22 KB)
Additional file 2: Summary statistics for the Quantitative PCR. (PDF 87 KB)
Additional file 3: All the primers used for RT-PCR and nested RT-PCR. (DOCX 19 KB)
Additional file 4: The electrophoresis pictures of the RT-PCR and nested RT-PCR for all the studied genes. So0001 ~ So0029, the ORF IDs of prophage WOSol. For the image of each gene: M, 100 bp DNA ladder; For each ORF, the first round of RT-PCR (first lane, for female sample; second lane, for male sample; third lane, positive control with gonomic DNA as template; fourth lane, negative control with distilled water as template); If the first and/or the second lane did not detect the targeted fragment, then the fifth and/or the sixth lane is nested RT-PCR with diluted products of the first and/or the second RT-PCR as template; The following two lanes are nested RT-PCR with diluted products of the third and fourth lane PCR products as template. (PDF 460 KB)
Additional file 5: The electrophoresis pictures of PCR products of wsp gene with wsp 81 F/691R primers. PCR based on template of total RNA with DNaseI treatment but no reverse transcription (A) and first-stranded cDNA samples which were synthesized from 1 μg of total RNA with random primers in a 20 μl reaction volume using TransScript II First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) (B). The comparison between (A) and (B) indicates that the RNA samples are not contaminated by genomic DNA. Lane 1 ~ 19: the results of 19 samples. PC: positive controls with genomic DNA as template. NC: negative controls with distilled water as template. M: 100 bp DNA ladder. (PDF 114 KB)
Additional file 6: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. (DOCX 20 KB)


