Abstract
Background
Cell therapy for cardiovascular disease has been limited by low engraftment of administered cells and modest therapeutic effects. Bone marrow (BM) -derived CD31+ cells are a promising cell source owing to their high angiovasculogenic and paracrine activities.
Objective
This study sought to identify culture conditions that could augment the cell adhesion, angiogenic, and anti-inflammatory activities of BM-derived CD31+ cells, and to determine whether these cultured CD31+ cells are effective for cardiac and vascular repair.
Methods
CD31+ cells were isolated from human BM by magnetic-activated cell sorting and cultured for 10 days under hematopoietic stem cell, mesenchymal stem cell, or endothelial cell culture conditions. These cells were characterized by adhesion, angiogenesis, and inflammatory assays. The best of the cultured cells were implanted into myocardial infarction (MI) and hindlimb ischemia (HLI) models to determine therapeutic effects and underlying mechanisms.
Results
The CD31+ cells cultured in endothelial cell medium (EC-CD31+ cells) showed the highest adhesion and angiogenic activities and lowest inflammatory properties in vitro compared with uncultured or other cultured CD31+ cells. When implanted into mouse MI or HLI models, EC-CD31+ cells improved cardiac function and repaired limb ischemia to a greater extent than uncultured CD31+ cells. Histologically, injected EC-CD31+ cells exhibited higher retention, neovascularization, and cardiomyocyte proliferation. Importantly, cell retention and endothelial transdifferentiation was sustained up to 1 year.
Conclusions
Short-term cultured EC-CD31+ cells have higher cell engraftment, vessel-formation, cardiomyocyte proliferation, and anti-inflammatory potential, are highly effective for both cardiac and peripheral vascular repair, and enhance survival of mice with heart failure. These cultured CD31+ cells may be a promising source for treating ischemic cardiovascular diseases.
Keywords: angiogenesis, CD31, engraftment, inflammation, myocardial infarction, peripheral vascular disease
Cell therapy has emerged as a promising new strategy for regenerating damaged ischemic tissue. Experimental studies and pilot clinical trials with various bone marrow (BM) cells, BM-mononuclear cells (MNCs), early endothelial progenitor cells (EPCs), or mesenchymal stem cells (MSCs) have shown favorable effects on cardiac repair after myocardial infarction (MI) (1,2). Mechanistically, paracrine actions are now known to be the main mechanism underlying ischemic tissue repair (3-6).
Recent meta-analyses of clinical trials for cardiac cell therapy with BM cells showed that left ventricular ejection fraction improved only ∼4% (7). Interestingly, selected populations such as CD34+ and CD133+ (also known as prominin 1 [PROM]) cells did not show significant therapeutic advantages over controls; rather, BM-MNCs and EPCs were more effective than controls. These results are not surprising given that paracrine (rather than transdifferentiation) effects are the main mechanism for BM cell therapy, and further suggest that selection of stem or progenitor cells may not be necessary when using BM-derived cells (5,6). We recently reported that BM-derived or peripheral blood-derived MNCs that express CD31 (also known as platelet endothelial cell adhesion molecule 1 [PECAM1]) on the surface are a specific cell population enriched with angiovasculogenic properties (8,9). Although they include a small stem cell population (<2%), the majority of CD31+ cells are lineage-committed and constitute ∼25% of total MNCs. We found that these cells are more effective than BM-MNCs or BM-CD31 cells for repairing limb ischemia.
However, collective data have shown that there is still much room for improvement in therapeutic efficacy. Specifically, low cell retention in vivo is a major limiting factor for cardiac cell therapy (10), and vessel-forming capability also needs improvement. Moreover, despite its importance, the need to reduce inflammation is relatively underestimated and thus underdeveloped (11).
Accordingly, this study was designed to improve the function of newly identified CD31+ cells by cell culture. Specifically, we sought to find culture conditions to induce higher adhesive, angiogenic, and vasculogenic, but lower inflammatory, activities. We also aimed to determine the therapeutic capability of the cultured CD31+ cells in the treatment of ischemic heart and vascular disease. In addition to the well-known paracrine or humoral effects of the cells, we also addressed important and long-debated mechanistic issues: endothelial transdifferentiation and long-term fate of the implanted BM cells in tissues (12,13). The present study demonstrated that CD31+ cells cultured under specific endothelial cell media exhibited the augmented cell biological characteristics mentioned in the preceding text and are effective for repairing experimental MI and limb ischemia.
Methods
An expanded Methods section is available in the Online Appendix.
Isolation and Cultivation of CD31+ Cells
Fresh human BM samples were purchased from Lonza (Walkersville, Maryland). Isolation of CD31+ cells was performed using magnetic activated cell sorting (MACS; Miltenyi Biotec, Auburn, California) with a CD31 antibody, as we previously described (9). CD31+ cells were plated at a density of 1 × 106 cells/cm2 on plates in EBM-2 basal medium supplemented with EGM-2 SingleQuots (Lonza) and 15% fetal bovine serum (FBS) for the endothelial cell (EC) culture conditions. In the MSC culture conditions (MC), cells were grown in Dulbecco’s Modified Eagle’s Medium with low glucose containing 15% FBS. In the hematopoietic stem cell culture conditions (HC), cells were grown in Iscove’s Modifed Dulbecco’s Medium supplemented with 10% FBS, stem cell factor (50 ng/ml), thrombopoietin (20 ng/ml), and Fms-related tyrosine kinase 3 ligand (FLT3LG; 20 ng/ml). All of the subsequent assays were performed with 10-day cultured CD31+ cells.
Cell Adhesion Assay and Matrigel Network Formation Assay
See the Online Appendix.
Flow Cytometry Analysis
See the Online Appendix.
Quantitative Rt-Pcr (Qrt-Pcr) Assay
See the Online Appendix.
Cell Transplantation in Myocardial Infarction and Hindlimb Ischemia Models
We performed MI and cell transplantation as described previously (6,14). See the Online Appendix.
Histological Analysis
See the Online Appendix.
Flow Cytometry Analysis of Digested Tissues
See the Online Appendix.
Statistical Analysis
See the Online Appendix.
Results
Cell Biological Characteristics of CD31+ Cells Cultured Under Various Conditions
To investigate the adhesive, vasculogenic, and proliferative capacity of CD31+ cells, we isolated CD31+ cells from human BM-MNCs using MACS. The purity of CD31+ cells was 97.6 ± 1.2%. First, we compared the expansion profile of CD31+ cells. The same number of CD31+ cells was cultivated under 3 culture conditions, MC, EC, and HC. After 7 days of culture, nonadherent cells were removed in MC and EC. The adherent cells showed heterogeneous morphology, including round and spindle-shaped cells (Figure 1A). MC-CD31+ and EC-CD31+ cells grew quickly from day 7 to 25, showing higher proliferation properties than the HC-CD31+ cells (Figure 1B). CD31+ cells in EC (EC-CD31+) for 10 days expressed endothelial cell proteins von Willebrand factor (VWF), kinase insert domain receptor (KDR), CDH5 (VE-cadherin), and CD31, but MC-CD31+ and HC-CD31+ did not express CDH5 or VWF by immunocy-tochemistry (Figure 1C).
Figure 1. In Vitro Cell Biological Characteristics of Cultured and Uncultured CD31+ Cells.
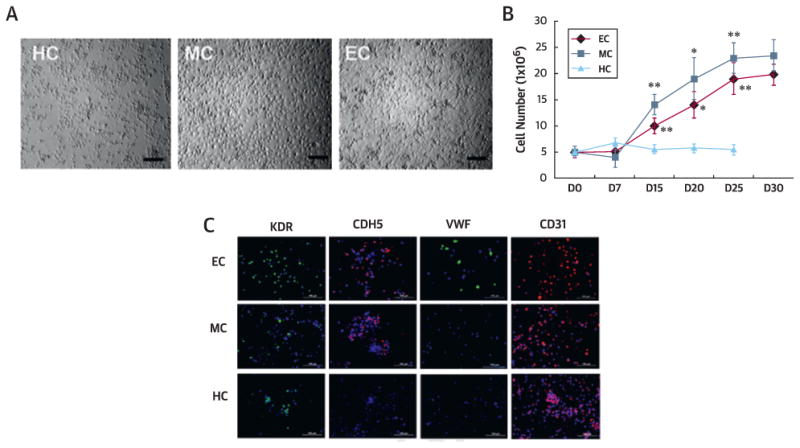
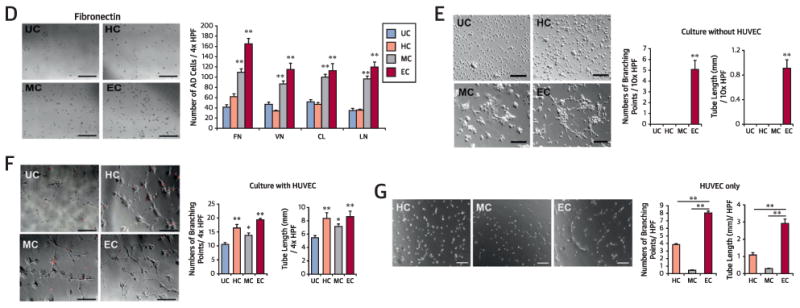
(A) Morphologies of CD31+ cells cultured under hematopoietic stem cell (HC), mesenchymal stem cell (MC), and endothelial cell (EC) conditions at day 10. Bars = 200 µm. (B) Growth curves of HC, MC, and EC. n = 5 per group. **p < 0.01, *p < 0.05 vs. HC. (C) Endothelial and mesenchymal culture conditions induced CD31+ cells to express endothelial proteins kinase insert domain receptor (KDR), CDH5, von Willebrand factor (VWF), and CD31 in culture. (D) Cell adhesion assays showed higher adhesion of EC and MC to all tested extracellular matrix proteins (fibronectin [FN], vitronectin [VN], type I collagen [CL], laminin [LN]) compared with uncultured cells (UC). n = 5 per group. Bars = 500 µm. (E) Tube formation potential. Tube lengths and the number of branching points were measured 24 h after seeding of cultured or uncultured CD31+ cells in Matrigel coated plates. n = 5 per group. Bars: 500 µm. (F) Tube formation by coculture with human umbilical vein endothelial cells (HUVEC). Tube lengths and the number of branching points were measured 12 hours after seeding of 1,1∼-dioctadecyl-3,3,3∼,3∼-tetramethylindocarbocyanine perchlorate-labeled cultured or uncultured CD31+ cells in Matrigel-coated plates. n = 5 per group. Bars: 500 µm. (G) Tube formation assay using HUVEC only. Tube lengths and the number of branching points were measured 5 hours after seeding of HUVEC in Matrigel-coated plates. n = 5 per group. **p < 0.01. Bars = 200 µim. (D-F) **p < 0.01 vs. UC, *p < 0.05 vs. UC. AD = adherent; HPF = high-powered field; UC = uncoated.
As adhesion capacity is an important indicator of cell engraftment and survival in vivo, we performed an adhesion assay. The number of cells adherent to extracellular matrix proteins fibronectin, vitronectin, collagen I, and laminin was significantly higher in the MC and EC groups than the uncultured CD31+ cells (UC) (all p < 0.01) (Figure 1D). We performed Matrigel tube formation assays with cultured or uncultured CD31+ cells alone or cocultured with human umbilical vein endothelial cells (HUVECs). Without coculture with HUVECs, only EC-CD31+J cells showed meaningful tube formation. With coculture with HUVECs, all cultured CD31+ cells showed higher branching points and tube lengths compared with the UC (Figure 1E and 1F). When HUVECs were cultured alone on Matrigel under these conditions, the HUVECS cultured under EC conditions (EC-HUVECs) exhibited higher branching points and tube lengths compared with the HC-HUVECs and MC-HUVECs (Figure 1G). These data indicate that CD31+ cells cultured in EC have the highest adhesion and independent tube-forming capability.
Surface Protein Expression of Cultured and Uncultured CD31+ Cells
To characterize cultured CD31+ cells, we performed flow cytometry analyses. Regardless of cell culture, CD31+ cells maintained expression of the pan-hematopoietic marker PTPRC (protein tyrosine phosphatase, receptor type, C, also known as CD45) as well as CD31 (Figure 2). Cells expressing lineage-committed markers CD3 (T cell) or CD19 (B cell) were decreased during culture. Cells exhibiting stem cell markers CD34, PROM1 (CD133), and KIT (proto-oncogene c-Kit, or CD117) were maintained in HC but generally decreased in the other conditions. Under EC, the cell populations expressing hematopoietic-endothelial markers KDR and TEK (tyrosine kinase, endothelial, or TIE2), monocyte-macrophage markers ITGAM (integrin alpha M, or CD11b) and CD14, and a dendritic cell marker, THBD (thrombomodulin, or CD141), were significantly increased, whereas those expressing ENG (end-oglin, or CD105) and MCAM (melanoma cell adhesion molecule, or CD146) were not significantly changed. Although MC cells showed similar trends to EC with less prominent increases in TEK, KDR, THBD, ITGAM, and CD14, HC cells did not change or reduced their expression. These data suggest that CD31+ cells cultured under 3 different conditions have distinct characteristics and the EC conditions in general induced the highest endothelial-monocytic pheno-type shift of CD31+ cells.
Figure 2. Flow Cytometric Analyses of Cultured and Uncultured CD31+ Cells.
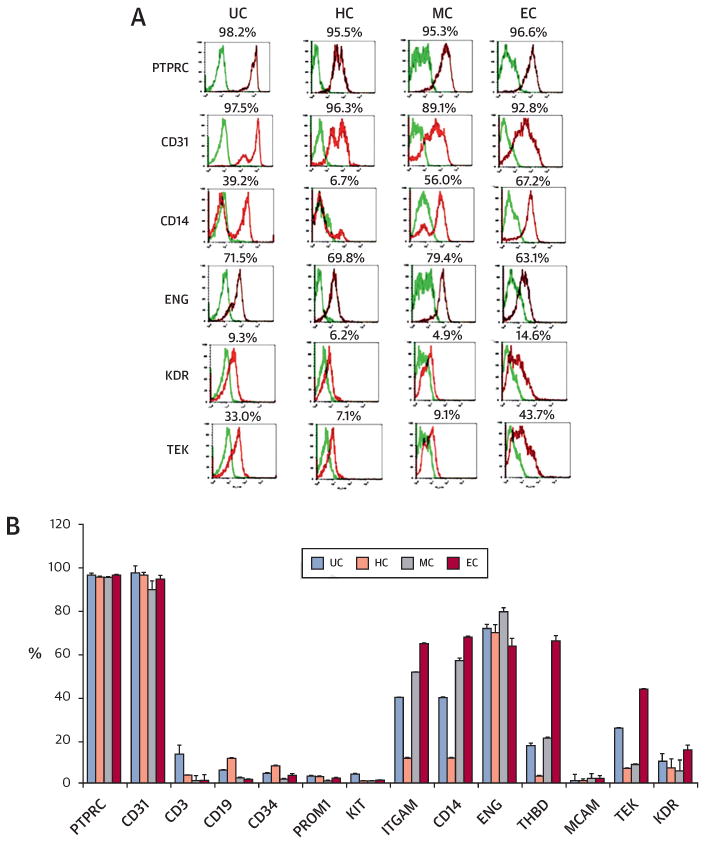
(A) Representative histograms of flow cytometry data. The CD31+ cells cultured under the 3 conditions for 10 days were labeled with phycoerythrin-conjugated (red spectra) or fluorescein isothiocyanate-conjugated (green spectra) antibodies and analyzed by flow cytometry for hematopoietic and endothelial markers. (B) Quantitative analyses (n = 4 each). ENG = endoglin; ITGAM = integrin alpha M; KIT = proto-oncogene c-Kit; MCAM = melanoma cell adhesion molecule; PROM1 = prominin 1; PTPRC = protein tyrosine phosphatase, receptor type, C; TEK = tyrosine kinase, endothelial; THBD = thrombomodulin. Other abbreviations as in Figure 1.
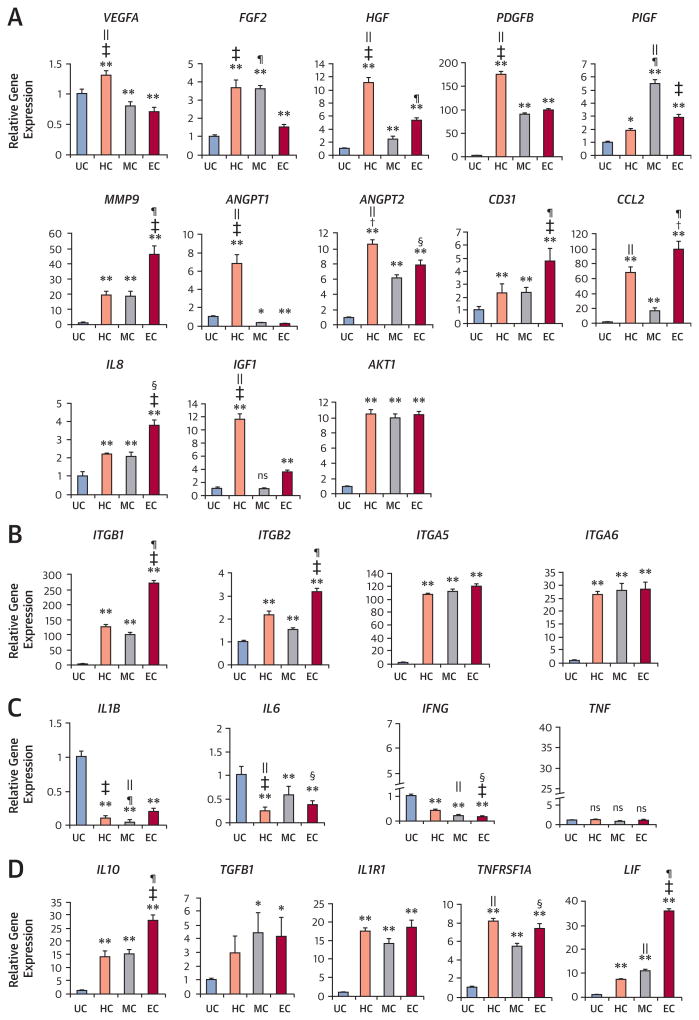
Expression of Angiogenic, Integrin, and Inflammatory Genes
To measure mRNA expression, we conducted qRT-PCR. Expression of angiogenic factors such as basic fibroblast growth factor (FGF2), hepatocyte growth factor (HGF), platelet-derived growth factor beta (PDGFB), placental growth factor (PlGF), and matrix metallopeptidase 9 (MMP9) were significantly increased in all culture conditions compared with the UC (Figure 3A). of note, chemo-kine (C-C motif) ligand 2 (CCL2) and interleukin 8 (IL8), which play important roles in arteriogenesis, were also increased in all conditions (Figure 3A). Expression of cell survival factors insulin-like growth factor 1 (IGF1) and Ak strain transforming oncogene (AKT1) were generally increased after culture. We also measured expression of integrin beta-1 (ITGB1), integrin beta-2 (ITGB2), integrin alpha-5 (ITGA5), and integrin alpha-6 (ITGA6), as these molecules mediate cell-to-cell and cell-to-extracellular matrix interactions, regulating adhesion and angiogenesis (15,16). These integrin levels were increased in all culture conditions and were particularly higher in EC compared with UC (Figure 3B). Expression of representative inflammatory genes IL1B, IL6, and interferon gamma (IFNG) was decreased in all conditions compared with UC (Figure 3C). However, anti-inflammatory factors IL10 (17), transforming growth factor beta-1 (TGFB1) (18), interleukin 1 receptor, type 1 (IL1R1), TNFRSF1A (tumor necrosis factor receptor 1A), and leukemia inhibitory factor (LIF) were 3-fold to 30-fold increased in the cultured CD31+ cells, notably in EC (Figure 3D). Collectively, these findings indicate that cultured CD31+ cells, particularly in EC, are enriched with vessel-formation, cell-survival, and anti-inflammatory factors.
Figure 3. Expression of Angiogenic, Integrin, and Inflammatory Factors.
Quantitative reverse transcriptase polymerase chain reaction was performed to measure the level of gene expression from cultured and uncultured CD31+ cells. Various angiogenic (A), cell adhesion (integrin) (B), proinflammatory (C), and anti-inflammatory (D) factors were up-regulated or down-regulated in the cultured groups compared with the uncultured group (n = 4 each; *p < 0.05; **p < 0.01). Individual values were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (+p < 0.05, HC vs. EC; ‡p < 0.01, HC vs. EC; §p < 0.05, EC vs. MC; ¶p < 0.01, EC vs. MC; ‖p < 0.01, MC vs. HC). AKT1 = Ak strain transforming oncogene; ANGPT1/2 = angiopoietin 1/2;CCL2 = chemokine (C-C motif) ligand 2; FGF2 = basic fibroblast growth factor; HGF = hepatocyte growth factor; IFNG = interferon gamma; IGF1 = insulin-like growth factor 1; IL(n) = interleukin (n); IL1R1 = interleukin 1 receptor, type1; ITGA5/6 = integrin alpha-5/-6; ITGB1/2 = integrin beta-1/-2; LIF = leukemia inhibitory factor; MMP9 = matrix metallopeptidase 9; PDGFB = platelet-derived growth factor beta; PlGF = placental growth factor; TGFB1 = transforming growth factor beta-1; TNF = tumor necrosis factor; TNFRSF1A = tumor necrosis factor receptor 1A; VEGFA = vascular endothelial growth factor A. Other abbreviations as in Figure 1.
Favorable Effects of Cultured CD31+ Cells on Repair of Cardiac And Hindlimb Ischemia
We next investigated the therapeutic effects of cultured CD31+ cells on infarct repair. On the basis of the in vitro data, we selected EC-CD31+ cells cultured for 10 days and compared the effects to UC-CD31+ cells and phosphate-buffered saline (PBS). After induction of MI in nude mice, we injected 1 × 106 cells or the same volume of PBS directly into the peri-infarct area. Echocardiography at 3 weeks after cell transplantation demonstrated that left ventricular end-diastolic dimension and left ventricular end-systolic dimension were the lowest in the EC group, and left ventricular fractional shortening was the highest in the EC group followed by the UC and PBS groups (Figure 4A and 4B). Compared with the PBS group, both UC and EC groups showed significantly lower circumferential fibrosis area examined at 4 weeks, with the smallest in the EC group (Figure 4C and 4D). These results indicate that intracardiac implantation of uncultured or cultured CD31+ cells can attenuate cardiac dysfunction and remodeling, with the EC-CD31+ cells having higher potency.
Figure 4. Endothelial Cell Transplantation Is Effective for Infarct Repair.
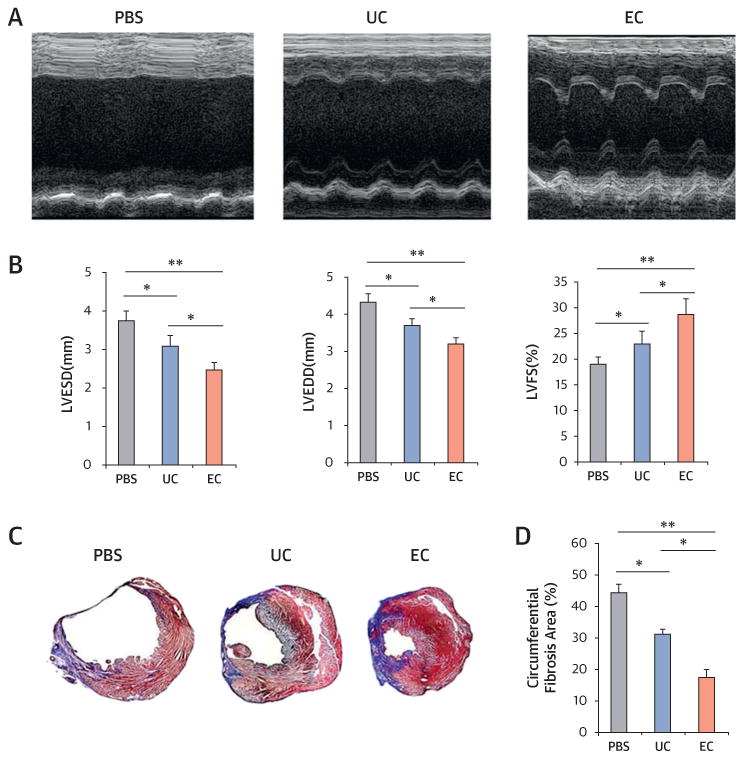
(A) Representative M-mode echocardiograms. (B) Left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD) were lowest in the endothelial cell (EC) group. Left ventricular fractional shortening (LVFS) was better in EC-injected hearts compared with uncultured cell (UC)-injected or phosphate-buffered saline (PBS)-injected hearts at 3 weeks after myocardial infarction (n = 7 each; *p < 0.05). (C) Representative cross-sectional images of hearts stained with Masson’s trichrome at 4 weeks after cell transplantation. The blue color represents fibrosis. (D) EC transplantation offered the highest reduction in fibrosis area (n = 7; *p < 0.05; **p < 0.01).
We next evaluated the therapeutic potential of cultured CD31+ cells in HLI. After creating HLI in nude mice, we injected 10-day cultured EC-CD31+ cells, MC-CD31+ cells, or HC-CD31+ cells, UC-CD31+ cells, or PBS into the ischemic hindlimbs (1 × 106 cells per mouse). Laser Doppler perfusion imaging was performed weekly to monitor the ischemic hindlimb’s blood flow. Laser Doppler perfusion imaging analysis revealed a greater recovery of blood perfusion in the EC group compared with each of the other 4 groups (Online Figure 1A and 1B). Although limb loss was frequently observed in the PBS control group, the EC group showed a significantly lower limb loss score than the PBS, UC, or MC groups (Online Figure 1C and 1D). Capillary density in the hindlimb muscle was significantly higher in the EC group compared with that of the PBS, UC, and MC groups (p < 0.01 vs. PBS; p < 0.05 vs. UC or MC) (Online Figure 2A). These results suggest that among all the groups, EC-CD31+ cells have better therapeutic potential for ischemic limb recovery.
Neovascularization, Cardiac Proliferative and Protective Effects, and Anti-Inflammatory Effects of Cultured CD31+ Cells
In the MI model, the capillary density at the peri-infarct area at 4 weeks was also highest in the EC group, followed by the UC and PBS groups (Figure 5A). We determined cardiomyocyte proliferation and apoptosis with cardiac samples harvested at 2 weeks using double staining for Ki67 and sarcomeric α-actinin or double staining for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and sarcomeric α-actinin, respectively. The EC group showed significantly higher numbers of Ki67+ car-diomyocytes compared with the UC and PBS groups (Figure 5B). Apoptotic cardiomyocytes at the peri-infarct area measured by TUNEL assay were lowest in the EC group, followed by the UC and PBS groups (Figure 5C). These results suggest that EC-CD31+ cells have robust cardiac proliferative and protective effects.
Figure 5. Endothelial Cell Transplantation Increased Neovascularization and Cardiomyocyte Proliferation and Protection, and Suppressed Inflammation In Vivo.
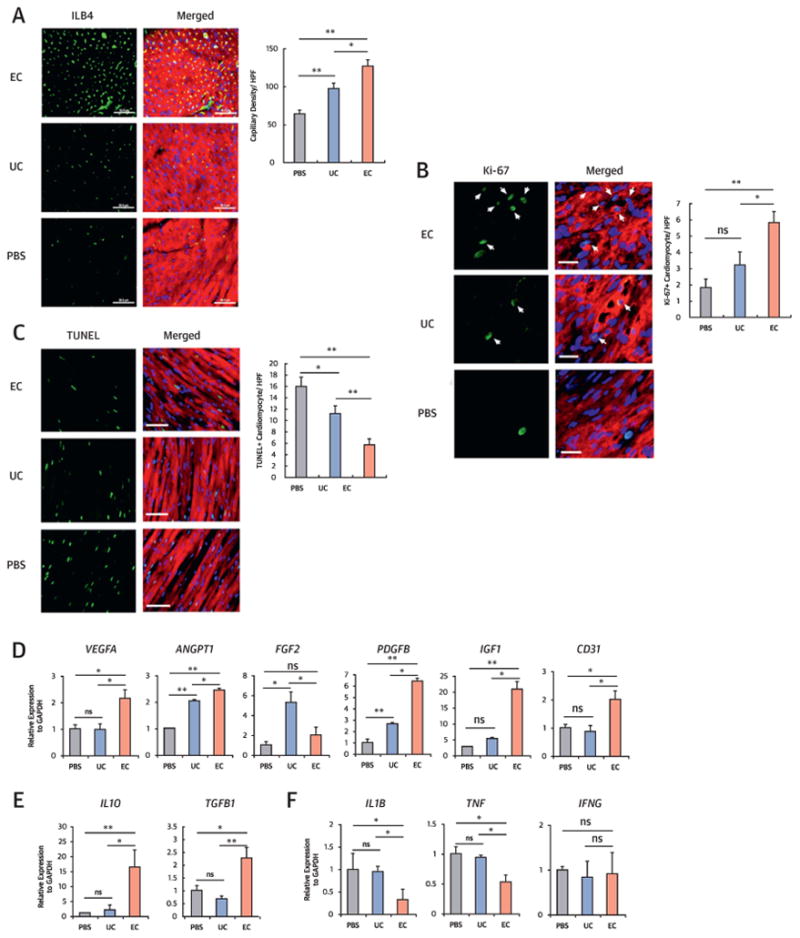
(A) Isolectin B4 (ILB4) staining for the peri-infarct regions and quantitative analysis of capillary density (n = 7 each; *p < 0.05, **p < 0.01). Bars = 50 μm; sarcomeric α-actinin staining (red). (B) Double immunohistochemistry with Ki-67 and sarcomeric α-actinin antibodies for the peri-infarct regions and quantitative analysis (n = 7 each; *p < 0.05, **p < 0.01). Arrows indicate Ki-67-positive cardiomyocytes. Bars = 20 μm. (C) Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay with sarcomeric α-actinin staining for the peri-infarct regions and quantitative analysis (n = 7 each; *p < 0.05, **p < 0.01). Bars = 50µm. (D-E) Quantitative reverse transcriptase polymerase chain reaction analyses with peri-infarct tissues injected with PBS, UC, or EC. EC transplantation showed highest expression of most angiogenic factors (D) and anti-inflammatory factors (E) and lowest expression of pro-inflammatory factors (F). Data are presented as fold differences from the PBS group (n = 5 each; *p < 0.05; **p < 0.01). Other abbreviations as in Figures 1, 3, and 4.
To determine other humoral effects of cell transplantation on ischemic hearts, MI mice were sacrificed and cardiac tissues were collected at 1 week. The expression levels of vascular endothelial growth factor A (VEGFA), angiopoietin 1 (ANGPT1), PDGFB, IGF1, and CD31 were significantly increased in the EC group compared with the PBS or UC groups (Figure 5D). FGF2 was the only factor more highly expressed in the UC group compared with the EC or PBS groups. Compared with the other groups, the expression of anti-inflammatory factors IL10 and TGFB1 was significantly higher and that of the proinflammatory factors IL1B and tumor necrosis factor (TNF) was lower in the EC group (Figure 5E and 5F). These data indicate that direct cardiac injection of EC-CD31+ cells augmented multiple biological factors associated with vascularization and anti-inflammation and reduced proinflammatory factors.
Cultured CD31+ Cells Showed Higher Engraft-Ment And Endothelial Transdifferentiation Potential
We determined engraftment and trans-differentiation potential in tissue samples harvested from the heart and the limb muscles at 4 weeks. Histologic analyses demonstrated that injected 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil)-labeled EC-CD31+ cells or UC-CD31+ cells were engrafted in both tissues and mainly localized in the pericytic or perivascular areas of the hearts (Figure 6A) and limb muscles (Online Figure 3A). We also found that a portion of CD31+ cells was incorporated into vessels and exhibited endothelial marker isolectin B4 (ILB4) (Figure 6B, Online Figure 3B). By histomorphometric analyses, the numbers of engrafted cells (Dil+ cells) and endothelially transdifferentiated cells (Dil+, ILB4+ cells) were significantly higher in the EC group compared with the UC group (Figure 6B and Online Figure 3B, right panels). To more precisely quantify the rate of engraftment and transdifferentiation of CD31+ cells, we conducted flow cytometric analysis on enzymatically digested hearts after systemic injection of ILB4 as described (8). This analysis again demonstrated that the EC group exhibited ∼ 2-fold higher engraftment and ∼ 3-fold higher endothelial transdifferentiation rates compared with the UC group (Figure 6C and 6D). Similar results were found in the histologic and flow cytometric analyses of the HLI model (Online Figure 3C and 3D). Collectively, these findings suggest that, compared with uncultured CD31+ cells, EC-CD31+ cells have superior potency for engraftment and endothelial trans-differentiation.
Figure 6. Cultured CD31+ Cells Showed Higher Engraftment and Endothelial Transdifferentiation Capacity in the Ischemic Heart.
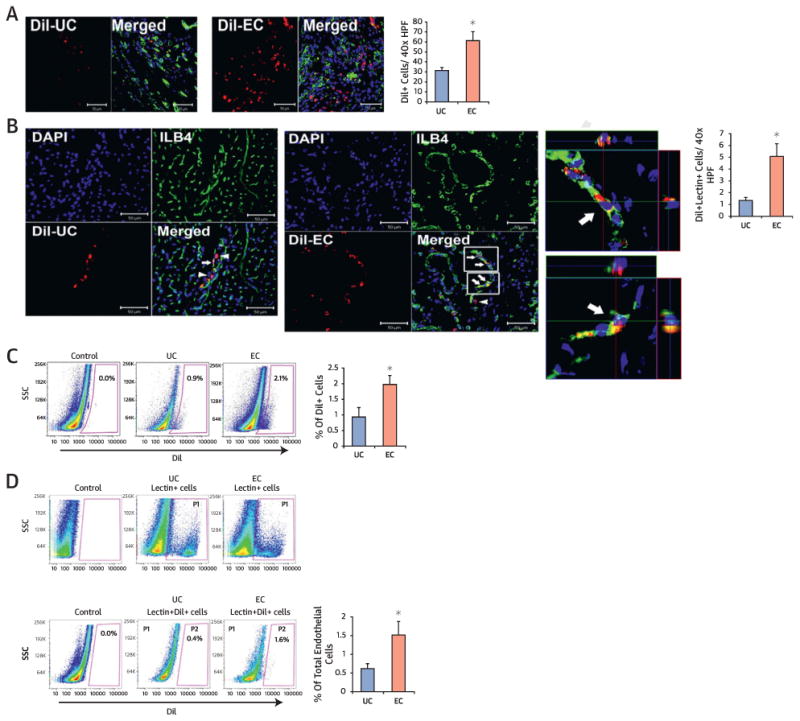
(A-B) Histological analyses of engrafted cells in the heart sections 4 weeks after EC or UC (1,1∼-dioctadecyl-3,3,3∼,3∼-tetramethylindocarbocyanine perchlorate [Dil]-labeled, red fluorescence) injection. (A) EC had higher retention compared with UC. n = 5 each. *p < 0.05. (B) Confocal microscopic examination demonstrated that injected UC-CD31+ cells (upper panel) or EC-CD31+ cells (lower panel) were colocalized with ILB4 (white arrows) using 3-dimensional z-stacked orthogonal and mul-tipanel images. Arrows indicate colocalized cells and arrowheads, engrafted cells in the perivascular areas. Quantifications of Dil and ILB4-double positive cells are shown on the right. n = 5 each. *p < 0.05. (C-D) Flow cytometry analyses of digested cardiac tissues at 4 weeks. (C) Higher engraftment of EC compared with UC occurred, n = 5 each. *p < 0.05. (D) Quantification of Dil and ILB4 double-positive cells showing a higher rate of endothelial transdifferentiation in EC compared with UC-injected hearts. n = 5 each. *p < 0.05. P1 gating represents ILB4+ fraction and P2 gating double-positive population for Dil and ILB4. DAPI = 4∼,6-diamidino-2-phenylindole. Other abbreviations as in Figures 1 and 5.
Long-Term Engraftment and Endothelial Transdifferentiation Potential of CD31+ Cells
We further quantified the long-term engraftment and endothelial transdifferentiation of cultured CD31+ cells using tissues harvested 1 year after cell transplantation. Contrary to the prevailing notion that injected BM cells in tissues survive only for a short term, histological analyses of the heart and hindlimb muscles showed engrafted CD31+ cells, as well as those expressing ILB4, within the vascular structure (Figure 7A and Online Figure 4A). Quantitative analyses with flow cytometry after perfusion of ILB4 and enzymatic digestion of heart and hindlimb muscle revealed an ∼0.6% engraft-ment rate (DiI+ cells per total cells) (Figure 7B) and ∼2.1% endothelially transdifferentiated cells (DiI+ILB4+ cells) among total endothelial cells in heart tissues (Figure 7C), and w0.4% engrafted cells (Online Figure 4B) and w3.7% endothelially trans-differentiated cells in hindlimb muscle (Online Figure 4C). We also confirmed these results with fluorescence in situ hybridization. Y chromosome signals derived from human CD31+ cells were detected in nuclei of endothelial cells as well as in other myocardial cells (Online Figure 5A and 5B). These findings confirmed the long-term survival and endothelial cell transdifferentiation capabilities of EC-CD31+ cells in vivo.
Figure 7. Engraftment and Endothelial Transdifferentiation Capacity at 1 Year in the Ischemic Heart.
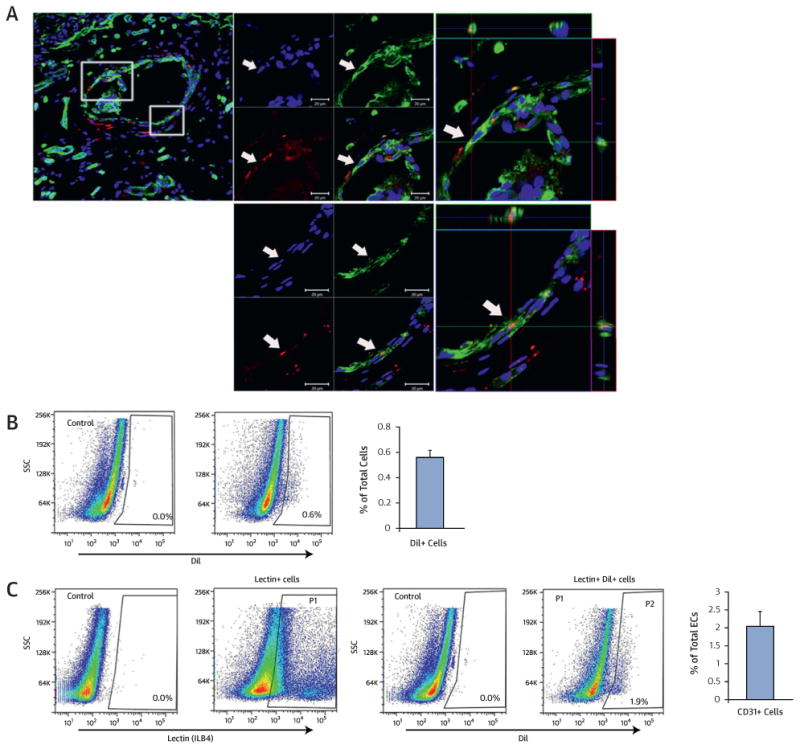
(A) Cardiac tissues were harvested at 1 year after injection with Dil-labeled EC. Confocal microscopic examination showed perivascular localization of injected EC as well as colocalized Dil-labeled EC (red) with ILB4-stained endothelial cells (green) in vessels in z-stacked orthogonal and multipanel images. Blue, DAPI. (B) Flow cytometry analysis showing presence of Dil-labeled EC in hearts at 1 year after EC injection (n = 5 each). Heart tissues were harvested at 1 year and digested with an enzyme cocktail. (C) Flow cytometry analysis demonstrating Dil and ILB4 double-positive cells showing endothelial transdifferentiation of injected Dil-labeled EC. P1 gating represents the ILB4+ fraction and P2 gating represents the cell population double positive for both Dil and ILB4. Other abbreviations as in Figures 1, 5, and 6.
Discussion
To improve cardiovascular cell therapy, continuous efforts have been made to identify more potent cells and to improve their function. This study demonstrated that specific culture conditions enhance the tissue reparative function of BM-derived hematopoietic CD31+ cells. In modified endothelial culture conditions, CD31+ cells expressed the highest levels of adhesion, pro-angiogenic, and anti-inflammatory factors, and the lowest levels of inflammatory factors compared with uncultured CD31+ cells or CD31+ cells that were cultured under other conditions. These EC-CD31+ cells, when implanted in vivo, showed robust neovascularization and cell retention, and induced cardiac and ischemic limb repair to a greater extent than uncultured CD31+ cells. Of note, mice receiving EC-CD31+ cells after MI had a significantly higher 7-month survival rate, implying additional clinical benefits of EC-CD31+ cell therapy for heart failure. Although low, cell survival and endothelial trans-differentiation capacity was confirmed for up to a year. This is the first report to demonstrate such long-term engraftment of any injected BM cells and maintenance of the endothelially transdifferentiated phenotype.
The original goals of culturing CD31+ cells were to enhance their adhesion and angiogenic-paracrine capacities. As expected, cultures in the EC conditions augmented both activities in CD31+ cells. The most notably increased factors were integrins, which are cell adhesion receptors that interact with extracellular matrices (19) and play a critical role in cell adhesion, cell survival, endothelial cell migration and proliferation, and matrix metalloproteinase activation. Integrins also interact with receptors for 2 main angiogenic factors, VEGF and FGF, to facilitate angiogenesis (16,20). Under EC conditions, multiple angiogenic, arteriogenic, antiapoptotic, and chemoattractant factors essential for vessel formation, cell survival, and tissue repair were significantly increased. These higher cell adhesion and angiogenic activities in the EC-CD31+ cells synergistically functioned to enhance cell survival, neovascularization, and tissue regeneration in vivo. They further increased cardiomyocyte proliferation and reduced cardiomyocyte apoptosis. It is also likely that the enriched paracrine factors, such as IGF1 and HGF, could have activated resident cardiac stem cells to induce myocardial regeneration (21,22).
To our surprise, key proinflammatory cytokines were suppressed and anti-inflammatory cytokines were increased in the EC-CD31+ cells. These anti-inflammatory properties are beneficial for acute cardiovascular tissue repair. After MI, inflammatory cells infiltrate the ischemic heart tissue and secrete inflammatory cytokines (23). Excessive or prolonged inflammation in MI leads to cardiac dysfunction. Studies also reported that unselected BM cell transplantation caused calcification in hearts or ulceration in limbs, which are associated with proinflammatory activities of the injected cells (24,25). The EC-CD31+ cells highly expressed anti-inflammatory factors IL10 and TGFB1. IL10 is a well-known anti-inflammatory cytokine, a representative suppressor of proinflammatory mediators, and has auspicious effects on cardiac repair (17). TGFB1 is another major regulator controlling inflammation (18). Anti-inflammation factors, such as IL1R1, TNFRSF1A, and LIF, were also more highly expressed compared with uncultured CD31+ cells (26,27).
We also observed improved cell engraftment in the cultured CD31+ cells. At 4 weeks, there were twice as many surviving EC-CD31+ cells as uncultured CD31+ cells in both the MI and HLI models. Given our prior studies showing 4.5 times higher survival of uncultured CD31+ cells compared with uncultured CD31 cells (8,9), EC-CD31+ cells have more than 9 times higher cell engraftment potential than the uncultured CD31 cells that comprise 75% of the most commonly used MNCs for cell therapy. Another important observation is the endothelial transdifferentiation or vasculogenic potential of EC-CD31+ cells. Although the transdifferentiation potential of BM-derived cells has been under debate, this study showed that EC-CD31+ cells retained even higher endothelial transdifferentiation potential than uncultured CD31+ cells (8,9). Compared with uncultured CD31+ cells, EC-CD31+ cells had a 3-fold (MI model) to 4-fold (HLI model) higher transdifferentiation rate at 1 month. The hindlimb muscle showed a substantially higher rate of transdifferentiated ECs than myocardium (8% vs. 1.5%). These engraftment and transdifferentiation analyses were extended up to a year. To date, no studies have addressed the long-term fate of direct tissue-injected BM cells in vivo. Histologic examination of >1000 sections and flow cytometry analyses demonstrated durable survival and endothelial. transdifferentiation of EC-CD31+ cells in both heart land limb muscle at 1 year.
Although EC-CD31+ cells were the most effective for ischemic limb recovery, CD31+ cells cultured under other conditions also were more effective than control cells. Compared with uncultured CD31+ cells, all cultured CD31+ cells displayed increased angiogenic and decreased inflammatory properties. However, EC-CD31+ or HC-CD31+ cells have higher proangiogenic factors and activities than MC-CD31+ cells. The proinflammatory and anti-inflammatory profiles are similar between HC-CD31+ cells and MC-CD31+ cells, but are more favorable in EC-CD31+ cells. However, it remains to be determined whether these cultured cells have different effects on the repair of acute MI according to the culture conditions.
Study Limitations
Although meta-analyses demonstrated relatively small effects in improvement of ejection fraction overall for BM cell therapy (7), recent clinical trials with MSCs for ischemic cardiomyopathy demonstrated various beneficial effects, including enhanced heart failure score and reduction of scar size and cardiac volume indices (28,29). The current study also attempted long-term follow-up of cardiac function for a year, but all animals in the UC and PBS groups died by 7 months, whereas those treated with the EC-CD31+ cell group all survived. Together, these studies suggested that adult cell therapy remains a viable therapeutic option, and various parameters other than left ventricular ejection fraction are needed to appropriately address the advantages of clinical cell therapy.
Conclusions
In conclusion, we demonstrated for the first time that culture-expanded CD31+ cells induced enhanced adhesion, vessel formation, cardiomyocyte proliferation, and anti-inflammatory effects, and are effective for repairing cardiovascular damage (Central Illustration). These cells may be a novel and advanced therapeutic option for treating ischemic cardiovascular disease.
Central Illustration. Enhanced therapeutic effects of BM-derived CD31+ cells for cardiac and vascular repair.
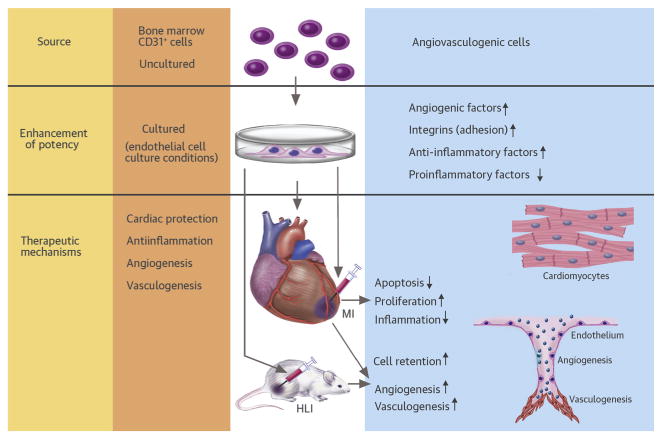
The bone marrow-derived CD31+ cells cultured in endothelial cell medium (EC-CD31+ cells) showed higher adhesion and angiogenic activities and low inflammatory properties in vitro compared with uncultured or other cultured CD31+ cells. When implanted into mouse myocardial infarction (MI) or hindlimb ischemia (HLI) models, EC-CD31+ cells improved cardiac function and repaired limb ischemia to a greater extent than uncultured CD31+ cells. Mechanistically, injected EC-CD31+ cells induced higher retention, neovascularization, cardiac protection, and cardiomyocyte proliferation, and lower inflammatory reactions.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Cell therapy holds promise as a treatment strategy to regenerate damaged ischemic tissue, but low engraftment of administered cells and modest therapeutic effects are important limitations. Short-term cultured bone marrow-derived CD31+ cells yielded higher cell engraftment, vessel formation, cardiomyocyte proliferation, and anti-inflammatory effects in mouse models of ischemic cardiac and vascular diseases.
Translational Outlook
The clinical efficacy and safety of bone marrow-derived CD31+ cells for tissue salvage and recovery in patients with ischemic limbs and for restoration of ventricular function and survival after myocardial infarction should be investigated in future studies.
Acknowledgments
This work was supported in part by NIH grants DP3DK094346, UL1 RR025008, and NIH contract HHSN268201000043C; by NSF-EBICS grant, CBET-0939511 to Dr. Yoon; and by Basic Science Research Program and National Research Foundation (NRF) grants funded by the Korean Ministry of Education (2013059998) and Korean government (MSIP, 2013 041811) to Dr. Kim. The authors have reported that they have no relevant relationships to the contents of this paper to disclose.
Abbreviations and Acronyms
- BM
bone marrow
- Dil
1,1′ - dioctadecyl-3,3,3′,3′ tetramethylindocarbocyanine perchlorate
- EC
endothelial cell culture conditions
- EPCs
endothelial progenitor cells
- FBS
fetal bovine serum
- HC
hematopoietic stem cell culture conditions
- HLI
hindlimb ischemia
- HUVECs
human umbilical vein endothelial cells
- MACS
magnetic activated cell sorting
- MC
MSC culture conditions
- MI
myocardial infarction
- MNCs
mononuclear cells
- MSCs
mesenchymal stem cells
- PBS
phosphate-buffered saline
- UC
uncultured cells
- VWF
von Willebrand factor
Footnotes
Appendix For supplemental tables, figures, and an expanded Methods, please see the online version of this article.
References
- 1.Assmus B, Schachinger V, Teupe C, et al. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–17. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 2.Amado LC, Saliaris AP, Schuleri KH, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci U S A. 2005;102:11474–9. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon YS, Wecker A, Heyd L, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. J Clin Invest. 2005;115:326–38. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 5.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 6.Cho HJ, Lee N, Lee JY, et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–69. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–68. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived CD31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H, Cho HJ, Kim SW, et al. CD31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: novel role of nonendothelial CD31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–14. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musialek P, Tekieli L, Kostkiewicz M, et al. Randomized transcoronary delivery of CD34(+) cells with perfusion versus stop-flow method in patients with recent myocardial infarction: early cardiac retention of 99(m)Tc-labeled cells activity. J Nucl Cardiol. 2011;18:104–16. doi: 10.1007/s12350-010-9326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziegelhoeffer T, Fernandez B, Kostin S, et al. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–8. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 13.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic stem cells do not trans-differentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 14.Jeong JO, Han JW, Kim JM, et al. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108:1340–7. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNally AK, Anderson JM. Beta1 and beta2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am J Pathol. 2002;160:621–30. doi: 10.1016/s0002-9440(10)64882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch W, Forsberg E, Lentini S, et al. Beta 1 integrin is essential for teratoma growth and angiogenesis. J Cell Biol. 1997;139:265–78. doi: 10.1083/jcb.139.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frangogiannis NG, Mendoza LH, Lindsey ML, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- 18.Topper JN. TGF-beta in the cardiovascular system: molecular mechanisms of a context-specific growth factor. Trends Cardiovasc Med. 2000;10:132–7. doi: 10.1016/s1050-1738(00)00061-x. [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Juliano R. Mitogenic signal transduction by integrin- and growth factor receptor-mediated pathways. Mol Cells. 2004;17:188–202. [PubMed] [Google Scholar]
- 20.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 21.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbanek K, Rota M, Cascapera S, et al. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97:663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 23.Frangogiannis NG. Targeting the inflammatory response in healing myocardial infarcts. Curr Med Chem. 2006;13:1877–93. doi: 10.2174/092986706777585086. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto K, Nishigami K, Nagaya N, et al. Unblinded pilot study of autologous transplantation of bone marrow mononuclear cells in patients with thromboangiitis obliterans. Circulation. 2006;114:2679–84. doi: 10.1161/CIRCULATIONAHA.106.644203. [DOI] [PubMed] [Google Scholar]
- 25.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–7. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 26.Carter DB, Deibel MR, Jr, Dunn CJ, et al. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990;344:633–8. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- 27.Hendriks JJ, Slaets H, Carmans S, et al. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol. 2008;204:52–7. doi: 10.1016/j.jneuroim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 28.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heldman AW, DiFede DL, Fishman JE, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.