Abstract
Bile acids are possible candidate agents in newly identified pathways through which energy expenditure may be regulated. Preclinical studies suggest that bile acids activate the enzyme type 2 iodothyronine deiodinase, which deiodinates thyroxine (T4) to the biologically active triiodothyronine (T3). We aimed to evaluate the influence of bile acid exposure and incretin hormones on thyroid function parameters in patients with type 2 diabetes. Thyroid-stimulating hormone (TSH) and thyroid hormones (total T3 and free T4) were measured in plasma from two human studies: i) 75 g-oral glucose tolerance test (OGTT) and three isocaloric (500 kcal) and isovolaemic (350 ml) liquid meals with increasing fat content with concomitant ultrasonographic evaluation of gallbladder emptying in 15 patients with type 2 diabetes and 15 healthy age, gender and BMI-matched controls (meal-study) and ii) 50 g-OGTT and isoglycaemic intravenous glucose infusions (IIGI) alone or in combination with glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide 1 (GLP1) and/or GLP2, in ten patients with type 2 diabetes (IIGI-study). In both studies, TSH levels declined (P<0.01) similarly following all meal and infusion stimuli. T3 and T4 concentrations did not change in response to any of the applied stimuli. TSH levels declined independently of the degree of gallbladder emptying (meal-study), route of nutrient administration and infusion of gut hormones. In conclusion, intestinal bile flow and i.v. infusions of the gut hormones, GIP, GLP1 and/or GLP2, do not seem to affect thyroid function parameters. Thus, the presence of a ‘gut–thyroid–pituitary’ axis seems questionable.
Keywords: bile acids, gallbladder, glucagon-like peptide 1, GLP1, thyroid, thyroid-stimulating hormone, TSH, TGR5, type 2 diabetes
Introduction
Bile acids are water soluble, amphipathic molecules synthesised in the liver from cholesterol. Upon meal ingestion, the gallbladder contracts, whereby bile acids from the liver and highly concentrated bile acids from the gallbladder are released into to the intestinal lumen. Here they interact with dietary lipids, lipid-soluble vitamins and cholesterol, forming mixed micelles, and thereby facilitating the transport and uptake of these molecules (1). Nowadays, bile acids are no longer labelled as detergents necessary for lipid digestion and absorption, but are increasingly recognised as metabolic integrators, capable of regulating glucose homeostasis, lipid metabolism and energy expenditure through nuclear receptors and the G protein-coupled receptor TGR5 (2, 3, 4). A study by Watanabe et al. (4) carried out in mice showed that cholic acid supplementation augmented energy expenditure in brown adipose tissue, caused weight reduction and improved insulin sensitivity. The effects were mediated through TGR5-induced intracellular cAMP formation and activation of the intracellular type 2 iodothyronine deiodinase (D2), which converts thyroxine (T4) into the active thyroid hormone triiodothyronine (T3).
Up to date, human studies have yielded conflicting results regarding the association between circulating bile acids and thyroid function parameters. Patti et al. (5) have found that bile acids in serum correlated inversely with thyroid-stimulating hormone (TSH) in non-diabetic patients who had undergone Roux-en-Y gastric bypass surgery, and recent reports have demonstrated similar associations of bile acids with TSH in type 2 diabetes (6, 7, 8). In contrast, Brufau et al. (9) could not demonstrate any effect of bile acids on energy expenditure in patients with type 2 diabetes. Recently, Ockenga et al. (8) showed that TSH concentrations decreased post-prandially in both patients with liver cirrhosis and healthy controls, and the result furthermore indicated that post-prandial bile acids are capable of increasing D2 activity, which in turn converts T4 into T3 presumably suppressing TSH upon meal intake. Such inhibitory effect of nutrient ingestion on TSH secretion in humans has been sparsely reported in the literature (10, 11). As meal-stimulated gallbladder emptying leads to prompt elevations of plasma bile acids concentrations (12, 13), we speculated whether various degrees of gallbladder emptying – induced by meals with a wide range of fat content – would modulate the thyroid hormone axis accordingly in both type 2 diabetes patients and controls. Moreover, in a second study in type 2 diabetes patients, using oral glucose tolerance test (OGTT) and isoglycaemic intravenous glucose infusions (IIGI), we analysed i) whether i.v. glucose per se could affect thyroid function parameters and ii) whether any changes could be elicited by infusion of the gastrointestinal hormones glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide 1 (GLP1) and GLP2, which are known to be secreted after nutrient ingestion.
Subjects and methods
Meal-study
Detailed description of the experimental procedures and subjects was provided previously (14). In short, measurement of TSH, total T3 and free T4 was carried out with chemiluminescence immunoassays in the plasma from 15 patients with type 2 diabetes (mean duration of diabetes, 7.5 years (range 6–20); age, 59.4±9.6 years (mean±s.d.); BMI, 28.0±2.2 kg/m2 and HbA1c, 7.5±1.4%) and 15 healthy age, gender and BMI-matched control subjects (age, 59.7±10.0 years; BMI, 27.9±2.0 kg/m2 and HbA1c, 5.2±0.2%) undergoing four separate ‘meal’ tests: a 75 g-OGTT and three isocaloric (500 kcal) and isovolaemic (350 ml) liquid meals (low fat: 2.5 g fat, 107 g carbohydrate and 13 g protein; medium fat: 10 g fat, 93 g carbohydrate and 11 g protein and high fat: 40 g fat, 32 g carbohydrate and 3 g protein). Four type 2 diabetes patients were treated with diet alone, eight were also treated with metformin and three with sulphonylurea (any oral antidiabetic therapy was omitted for a period of no less than a week before each study day). Gallbladder emptying was evaluated by calculating gallbladder ejection fraction using ultrasound measurements as previously described (14).
IIGI-study
Detailed experimental procedures and patient characteristics were reported previously (15). In short, measurement of TSH, total T3 and free T4 was carried out with chemiluminescence immunoassays in plasma from ten patients with type 2 diabetes (mean duration of diabetes, 4.8 years (range 0.5–14); age, 50.8±10.7 years (mean±s.d.); BMI, 33.2±4.9 kg/m2 and HbA1c, 6.5±0.7%) undergoing six separate test days: 50 g-OGTT, IIGI+saline (placebo), IIGI+GIP, IIGI+GLP1, IIGI+GLP2 and IIGI+GIP+GLP1+GLP2 (saline and peptides were infused intravenously). Nine patients were treated with metformin alone and one with metformin and sulphonylurea in combination (any oral antidiabetic therapy was omitted for a period of not less than a week before each study day).
All participants gave their informed consent to the studies, which were performed in accordance with the Declaration of Helsinki and Danish legislation.
Calculations and statistical analyses
The results are reported as means with 95% CIs unless otherwise stated. For evaluation of TSH suppression within groups, repeated measures two-way ANOVA with Dunnet's post hoc test was used (mean baseline concentrations were chosen as control mean). For between-group comparison of TSH responses, area under the curve (AUC) was calculated by applying the trapezoid rule for TSH. For analysis of variations and differences between AUC values, repeated measures ANOVA with Sidak's post hoc test was used. The data were transformed logarithmically (log10) to approximate a Gaussian distribution. A two-sided P<0.05 was used to indicate significant differences.
Results
Meal-study
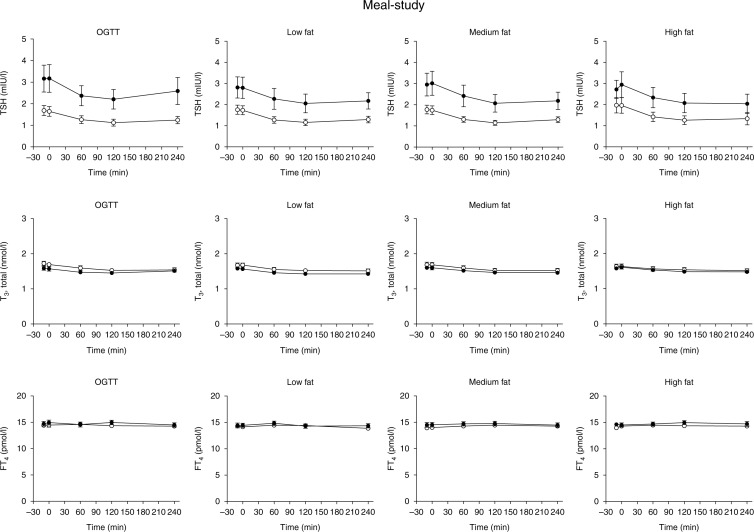
As previously reported, gallbladder ejection fraction increased similarly in the two groups with increasing meal fat content (with similar ejection fraction following the medium fat meal and the high fat meal nevertheless) (14). Baseline TSH levels were within normal range (0.3–4.0 mIU/l) in all participants. As shown in Fig. 1, AUC levels and basal TSH concentrations were higher in controls vs type 2 diabetes patients during oral glucose (P<0.05, Fig. 1); a tendency to this was also seen during the meal stimuli (P=0.05–0.09). TSH levels declined significantly after the applied stimuli in both groups with maximum changes from baseline amounting to −0.54 UI/l (−0.75, −0.33) (OGTT), −0.59 (−0.78, −0.41) (low fat), −0.62 (−0.84, −0.39) (medium fat) and −0.70 (−1.10, −0.34) (high fat) in the type 2 diabetic group and −0.96 (−1.40, −0.53) (OGTT), −0.75 (−1.06, −0.45) (low fat), −0.92 (−1.33, −0.51) (medium fat) and −0.76 (−0.99, −0.52) (high fat) in controls, with no statistical significant differences within or between groups. Neither, incremental AUC values differed within or between groups. The concentrations of T3 and T4, respectively, were similar in the two groups and did not change after any of the oral stimuli.
Figure 1.
Meal-study. Plasma levels of thyroid-stimulating hormone (TSH), thyroxine (T4) and thyroid hormone triiodothyronine (T3) during a 75 g-oral glucose tolerance test (OGTT) and three isocaloric (500 kcal) and isovolaemic (350 ml) liquid meals with low fat, medium fat and high fat, in healthy control subjects (n=15, closed symbols) and patients with type 2 diabetes (n=15, open symbols). Mean±s.e.m. values are shown.
IIGI-study
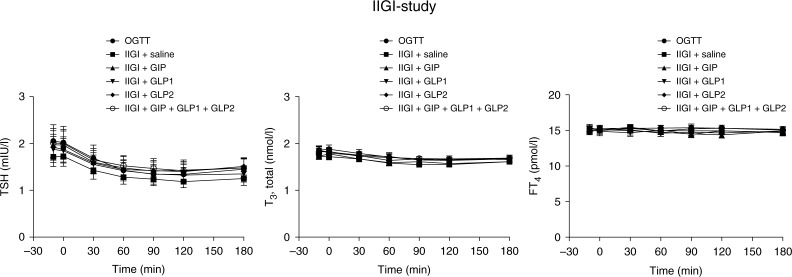
TSH declined similarly on all study days following oral glucose intake and all of the i.v. stimuli respectively. Accordingly, maximum changes from baseline amounted to −0.76 (−1.15, −0.37) (OGTT), −0.56 (−0.79, −0.33) (IIGI+saline), −0.64 (−0.89, −0.38) (IIGI+GIP), −0.57 (−0.82, −0.32) (IIGI+GLP1), −0.55 (−0.82, −0.28) (IIGI+GLP2) and −0.63 (−0.92, −0.34) (IIGI+GIP+GLP1+GLP2), with no statistical significant differences between the different study days. Also, values of AUCs were similar during the different study days (Fig. 2). The concentrations of T3 and T4, respectively, were comparable along the entire time course on all study days. Neither suppression nor elevation of T3 or T4 was demonstrated.
Figure 2.
IIGI-study. Plasma levels of thyroid-stimulating hormone (TSH), thyroxine (T4) and thyroid hormone triiodothyronine (T3) during 50 g-oral glucose tolerance test (OGTT) and isoglycaemic intravenous glucose infusions (IIGI) with concomitant infusions of saline, glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide 1 (GLP1), GLP2 or a combination of the three hormones in patients with type 2 diabetes (n=10). Mean±s.e.m. values are shown.
Discussion
This study demonstrates a similar degree of TSH suppression in type 2 diabetes patients and healthy controls to oral glucose and isocaloric and isovolaemic meals with increasing fat content (giving rise to different gallbladder emptying rates), and suggests that the oral route of nutrient administration and subsequent release of the gut hormones, GIP, GLP1 and GLP2 do not seem to play a major role in post-absorptive TSH suppression. To our knowledge, no previous study has compared thyroid function parameters following both OGTT and various meal tests (isocaloric and isovolaemic) affecting gallbladder emptying differentially in type 2 diabetes patients and matched healthy controls, or thyroid function parameters after oral and i.v. glucose in type 2 diabetes.
In both studies, basal and stimulated TSH concentrations were within normal range, but tended to be lower in type 2 diabetes patients vs controls (meal-study). This contrasts to the common observation of subclinical hypothyroidism in type 2 diabetes patients (16, 17). Interestingly, recent data indicates that metformin may suppress TSH concentrations in type 2 diabetes patients, thus providing a plausible explanation of the low(er) TSH levels in our type 2 diabetic patients (18). However, contrasting results have also been reported (19). In spite of the observed TSH suppression, concentrations of T3 and T4 did not exhibit post-prandial changes and were similar in type 2 diabetes patients vs controls. This is supported by a previous study in hypothyroid patients, showing that T3 and T4 were relatively insensitive to small alterations in T4 (administered daily) compared with TSH, which was very sensitive to fine adjustments of T4 dosage (20). The notion of oral glucose eliciting a similar degree of TSH suppression as compared with IIGI suggests that gastrointestinal factors are less likely to play a role in nutrient-induced TSH suppression. Supporting this notion, possible candidate ‘gut factors’, such as GIP, GLP1 and GLP2, infused intravenously during IIGI neither altered the suppression pattern of TSH or thyroid hormones. By contrast, a previous study indicated that peripheral T3 formation was stimulated by oral, but not by i.v., glucose suggesting that the intestine or liver might involve in the regulation of T3 formation (21).
As plasma bile acid concentrations reflect intestinal bile acid absorption, as the fractional hepatic uptake of bile acids is constant (13), the degree of gallbladder contraction can be considered an ‘indirect’ measure of the amount of bile acids that reaches the systemic circulation (‘spill-over’ from liver). On the basis of this assumption, our results do not support a role for post-prandial bile acids in modulating bile acid–TGR5-D2 activation in peripheral tissues (skeletal muscle and brown adipose tissue). However, we acknowledge that the study design does not allow drawing causal inferences in this regard. Indeed, gallbladder emptying and even bile acids provide only surrogate information of the complexity of the enterohepatic circulation of bile acids even though both are positively correlated with portal venous concentrations (22).
A prompt inhibitory effect of nutrient ingestion on TSH secretion in humans has been sparsely reported in the literature (8, 10, 11) and the mechanism underlying the suppression of TSH after ingestion of meals or glucose remains to be established. In fact, TSH concentrations are generally believed to decrease following long-term fasting and increase upon refeeding (23, 24, 25). Considering the role of gallbladder emptying and systemic bile acid fluctuations, bile acid–TGR5 signalling might, as hypothesised by Ockenga et al., acutely activate D2 in pituitary thyrotropes, which are known to express D2 (26) and possibly TGR5 (8), resulting in feedback on TSH upon meal challenge. Importantly, such activation of D2 may also be potentiated from the skeletal muscle where D2 activity is sufficiently high that it can provide significant quantities of circulating T3 (27). Intriguingly, in rodents TGR5 expression has been described in the paraventricular nucleus and in the supraoptic nucleus of the hypothalamus, suggesting that bile acid–TGR5 signalling may regulate the thyroid axis at the level of thyrotropin-releasing hormone (8, 28). In support of this, Ockenga et al. (8) demonstrated an association between bile acid and TSH concentrations after a nutrient challenge (0–60 min) and found a close correlation between meal-stimulated bile acid levels and energy expenditure after 60 min (most evident in patients with liver cirrhosis). However, D2 activation might also simply be enhanced by nutrients – especially glucose (29, 30) – reaching the systemic circulation either via the oral route or during i.v. stimulation. Interestingly, this is corroborated well in another human study, which showed that 62 h of fasting reduced D2 mRNA expression in skeletal muscle, whereas 5 h of insulin infusion increased D2 mRNA expression (at 62 h) (31). However, D2 activities were very low and were not influenced by hypothyroidism, fasting or insulin.
Another explanation for the acute post-prandial TSH decline observed in this study could be somatostatin being released concomitantly from the intestine and/or hypothalamus upon nutrient stimulation (10, 11, 32). However, the physiological role of somatostatin for the inhibition of TSH secretion in man has been questioned (33). Accordingly, our observation of i.v. glucose infusions suppressing TSH levels to similar levels as observed during oral glucose argues against gut-derived somatostatin playing a role in post-prandial inhibition of TSH release. In contrast, hypothalamic somatostatin secretion could have been stimulated during both oral and i.v. glucose and thereby contribute to the observed TSH suppression patterns (11). Also, as GIP and GLP1 increase somatostatin secretion, at least in animals (34), suppression of TSH during incretin infusion might have been augmented, which, however, was not demonstrated in this study. Moreover, hypoglycaemia, which is known to suppress somatostatin levels, has also been shown to reduce TSH concentrations (35), indicating that other factors apart from somatostatin are responsible for TSH suppression under normal physiological circumstances.
Finally, TSH concentrations display pronounced circadian changes – typically with nocturnal increases and daytime nadirs, which might also offer an explanation for the decline observed after all stimuli (36). However, a recent study in 475 000 outpatients has indicated that TSH concentrations are constant from 0600 to 1900 h (37). Accordingly, TSH baseline concentrations in this study were similar, and suppression was noticeable only after the nutrient stimuli were applied. Nonetheless, it remains an obvious limitation to this study, as well as the study by Ockenga et al. (8), that TSH concentrations were not evaluated following non-nutrient stimuli (negative control).
In conclusion, patients with type 2 diabetes and healthy controls display significant post-prandial TSH suppression to a series of meal-related stimuli, but independently of the degree of gallbladder emptying and of the gut hormones GIP, GLP1 and GLP2. Obviously, further studies are warranted to clarify whether bile acids affect energy expenditure and diet-induced thermogenesis (via brown adipose tissue) in humans, but the present findings do not support the presence of a ‘gut–thyroid–pituitary’ axis.
Author contribution statement
D P Sonne: study design, clinical experiments (meal-study), data research, statistical analysis and drafting of manuscript; A Lund: clinical experiments (IIGI-study) and review and editing of the manuscript; J Faber, J J Holst and T Vilsbøll: review and editing of the manuscript and F K Knop: study design, and review and editing of the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgements
Clinical trial registration number (ClinicalTrails.gov identifier) NCT01374594. The authors are grateful to our volunteers whose availability made this work possible and to J Purtoft and N Kjeldsen for their expert technical assistance.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This work has been supported by an unrestricted grant from the Novo Nordisk Foundation.
References
- 1.Russell DW. Fifty years of advances in bile acid synthesis and metabolism. Journal of Lipid Research. 2009;50(Suppl):S120–S125. doi: 10.1194/jlr.R800026-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maruyama T, Miyamoto Y, Nakamura T, Tamai Y, Okada H, Sugiyama E, Nakamura T, Itadani H, Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochemical and Biophysical Research Communications. 2002;298:714–719. doi: 10.1016/S0006-291X(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 3.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, Fukusumi S, Habata Y, Itoh T, Shintani Y, et al. A G protein-coupled receptor responsive to bile acids. Journal of Biological Chemistry. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 5.Patti M-E, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, et al. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity. 2009;17:1671–1677. doi: 10.1038/oby.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonen M, Dali-Youcef N, Kaminska D, Venesmaa S, Käkelä P, Pääkkönen M, Hallikainen M, Kolehmainen M, Uusitupa M, Moilanen L, et al. Conjugated bile acids associate with altered rates of glucose and lipid oxidation after Roux-en-Y gastric bypass. Obesity Surgery. 2012;22:1473–1480. doi: 10.1007/s11695-012-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism: Clinical and Experimental. 2009;58:1400–1407. doi: 10.1016/j.metabol.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Ockenga J, Valentini L, Schuetz T, Wohlgemuth F, Glaeser S, Omar A, Kasim E, duPlessis D, Featherstone K, Davis JR, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. Journal of Clinical Endocrinology and Metabolism. 2012;97:535–542. doi: 10.1210/jc.2011-2329. [DOI] [PubMed] [Google Scholar]
- 9.Brufau G, Bahr MJ, Staels B, Claudel T, Ockenga J, Böker KH, Murphy EJ, Prado K, Stellaard F, Manns MP, et al. Plasma bile acids are not associated with energy metabolism in humans. Nutrition & Metabolism. 2010;7:73. doi: 10.1186/1743-7075-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamat V, Hecht WL, Rubin RT. Influence of meal composition on the postprandial response of the pituitary–thyroid axis. European Journal of Endocrinology. 1995;133:75–79. doi: 10.1530/eje.0.1330075. [DOI] [PubMed] [Google Scholar]
- 11.Shibasaki T, Masuda A, Hotta M, Yamauchi N, Hizuka N, Takano K, Demura H, Shizume K. Effects of ingestion of glucose on GH and TSH secretion: evidence for stimulation of somatostatin release from the hypothalamus by acute hyperglycemia in normal man and its impairment in acromegalic patients. Life Sciences. 1989;44:431–438. doi: 10.1016/0024-3205(89)90268-3. [DOI] [PubMed] [Google Scholar]
- 12.Sonne DP, Hare KJ, Martens P, Rehfeld JF, Holst JJ, Vilsbøll T, Knop FK. Postprandial gut hormone responses and glucose metabolism in cholecystectomized patients. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2013;304:G413–G419. doi: 10.1152/ajpgi.00435.2012. [DOI] [PubMed] [Google Scholar]
- 13.LaRusso NF, Hoffman NE, Korman MG, Hofmann AF, Cowen AE. Determinants of fasting and postprandial serum bile acid levels in healthy man. American Journal of Digestive Diseases. 1978;23:385–391. doi: 10.1007/BF01072919. [DOI] [PubMed] [Google Scholar]
- 14.Sonne DP, Rehfeld J, Holst JJ, Vilsbøll T, Knop FK. Postprandial gallbladder emptying in patients with type 2 diabetes: potential implications for bile-induced secretion of glucagon-like peptide-1. European Journal of Endocrinology. 2014;171:407–419. doi: 10.1530/EJE-14-0309. [DOI] [PubMed] [Google Scholar]
- 15.Lund A, Vilsbøll T, Bagger JI, Holst JJ, Knop FK. The separate and combined impact of the intestinal hormones, GIP, GLP-1, and GLP-2, on glucagon secretion in type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2011;300:E1038–E1046. doi: 10.1152/ajpendo.00665.2010. [DOI] [PubMed] [Google Scholar]
- 16.Perros P, McCrimmon RJ, Shaw G, Frier BM. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Diabetic Medicine. 1995;12:622–627. doi: 10.1111/j.1464-5491.1995.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 17.Chubb SA, Davis WA, Inman Z, Davis TM. Prevalence and progression of subclinical hypothyroidism in women with type 2 diabetes: the Fremantle Diabetes Study. Clinical Endocrinology. 2005;62:480–486. doi: 10.1111/j.1365-2265.2005.02246.x. [DOI] [PubMed] [Google Scholar]
- 18.Cappelli C, Rotondi M, Pirola I, Agosti B, Gandossi E, Valentini U, De Martino E, Cimino A, Chiovato L, Agabiti-Rosei E, et al. TSH-lowering effect of metformin in type 2 diabetic patients: differences between euthyroid, untreated hypothyroid, and euthyroid on l-T4 therapy patients. Diabetes Care. 2009;32:1589–1590. doi: 10.2337/dc09-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lupoli R, Di Minno A, Tortora A, Ambrosino P, Arianna Lupoli G, Di Minno MN. Effects of treatment with metformin on TSH levels: a meta-analysis of literature studies. Journal of Clinical Endocrinology and Metabolism. 2014;99:E143–E148. doi: 10.1210/jc.2013-2965. [DOI] [PubMed] [Google Scholar]
- 20.Carr D, McLeod DT, Parry G, Thornes HM. Fine adjustment of thyroxine replacement dosage: comparison of the thyrotrophin releasing hormone test using a sensitive thyrotrophin assay with measurement of free thyroid hormones and clinical assessment. Clinical Endocrinology. 1988;28:325–333. doi: 10.1111/j.1365-2265.1988.tb01219.x. [DOI] [PubMed] [Google Scholar]
- 21.Westgren U, Ahrén B, Burger A, Melander A. Stimulation of peripheral T3 formation by oral but not by intravenous glucose administration in fasted subjects. Acta Endocrinologica. 1977;85:526–530. doi: 10.1530/acta.0.0850526. [DOI] [PubMed] [Google Scholar]
- 22.Angelin B, Björkhem I, Einarsson K, Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. Journal of Clinical Investigation. 1982;70:724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azizi F. Effect of dietary composition on fasting-induced changes in serum thyroid hormones and thyrotropin. Metabolism: Clinical and Experimental. 1978;27:935–942. doi: 10.1016/0026-0495(78)90137-3. [DOI] [PubMed] [Google Scholar]
- 24.Croxson MS, Hall TD, Kletzky OA, Jaramillo JE, Nicoloff JT. Decreased serum thyrotropin induced by fasting. Journal of Clinical Endocrinology and Metabolism. 1977;45:560–568. doi: 10.1210/jcem-45-3-560. [DOI] [PubMed] [Google Scholar]
- 25.Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus–pituitary–thyroid axis. Thyroid. 2008;18:123–129. doi: 10.1089/thy.2007.0253. [DOI] [PubMed] [Google Scholar]
- 26.Baur A, Buchfelder M, Kohrle J. Expression of 5′-deiodinase enzymes in normal pituitaries and in various human pituitary adenomas. European Journal of Endocrinology. 2002;147:263–268. doi: 10.1530/eje.0.1470263. [DOI] [PubMed] [Google Scholar]
- 27.Salvatore D, Bartha T, Harney JW, Larsen PR. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology. 1996;137:3308–3315. doi: 10.1210/endo.137.8.8754756. [DOI] [PubMed] [Google Scholar]
- 28.Doignon I, Julien B, Serrière-Lanneau V, Garcin I, Alonso G, Nicou A, Monnet F, Gigou M, Humbert L, Rainteau D, et al. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. Journal of Hepatology. 2011;54:481–488. doi: 10.1016/j.jhep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Gavin LA, Moeller M, McMahon FA, Castle JN, Gulli R, Cavalieri RR. Carbohydrate feeding increases total body and specific tissue 3,5,3′-triiodothyronine neogenesis in the rat. Endocrinology. 1988;123:1075–1081. doi: 10.1210/endo-123-2-1075. [DOI] [PubMed] [Google Scholar]
- 30.Otten MH, Hennemann G, Docter R, Visser TJ. The role of dietary fat in peripheral thyroid hormone metabolism. Metabolism: Clinical and Experimental. 1980;29:930–935. doi: 10.1016/0026-0495(80)90035-9. [DOI] [PubMed] [Google Scholar]
- 31.Heemstra KA. Type 2 iodothyronine deiodinase in skeletal muscle: effects of hypothyroidism and fasting. Journal of Clinical Endocrinology and Metabolism. 2009;94:2144–2150. doi: 10.1210/jc.2008-2520. [DOI] [PubMed] [Google Scholar]
- 32.Siler TM, Yen SC, Vale W, Guillemin R. Inhibition by somatostatin on the release of TSH induced in man by thyrotropin-releasing factor. Journal of Clinical Endocrinology and Metabolism. 1974;38:742–745. doi: 10.1210/jcem-38-5-742. [DOI] [PubMed] [Google Scholar]
- 33.Williams TC, Kelijman M, Crelin WC, Downs TR, Frohman LA. Differential effects of somatostatin (SRIH) and a SRIH analog, SMS 201-995, on the secretion of growth hormone and thyroid-stimulating hormone in man. Journal of Clinical Endocrinology and Metabolism. 1988;66:39–45. doi: 10.1210/jcem-66-1-39. [DOI] [PubMed] [Google Scholar]
- 34.Orskov C, Holst JJ, Nielsen OV. Effect of truncated glucagon-like peptide-1 [proglucagon-(78–107) amide] on endocrine secretion from pig pancreas, antrum, and nonantral stomach. Endocrinology. 1988;123:2009–2013. doi: 10.1210/endo-123-4-2009. [DOI] [PubMed] [Google Scholar]
- 35.Schultes B, Oltmanns KM, Kern W, Born J, Fehm HL, Peters A. Acute and prolonged effects of insulin-induced hypoglycemia on the pituitary–thyroid axis in humans. Metabolism: Clinical and Experimental. 2002;51:1370–1374. doi: 10.1053/meta.2002.35193. [DOI] [PubMed] [Google Scholar]
- 36.Brabant G, Ranft U, Ocran K, Hesch RD, von zur Mühlen A. Thyrotropin – an episodically secreted hormone. Acta Endocrinologica. 1986;112:315–322. doi: 10.1530/acta.0.1120315. [DOI] [PubMed] [Google Scholar]
- 37.Ehrenkranz J, Bach PR, Benvenga S. Thyroid hormone circadian and circannual variation in 475.000 outpatients (Abstract). 83rd Annual Meeting of the American Thyroid Association Meeting Abstracts & Program. Thyroid. 2013;23(S1):A-80–A-81. doi: 10.1089/thy.2013.2310.abs. [DOI] [Google Scholar]



 This work is licensed under a
This work is licensed under a