Abstract
There are limited numbers of studies which have evaluated the sexual dysfunction (SD) in patients with alcohol and opioids dependence. This article reviews the existing literature. Electronic searches were carried out using the PubMed, Google Scholar, and ScienceDirect to locate the relevant literature. Subjects addicted to heroin or on methadone maintenance treatment (MMT) or buprenorphine maintenance treatment (BMT) show higher rates of SD in comparison to the general population. SD rates have ranged 34-85% for heroin addicts, 14-81% for MMT, 36-83% for BMT, and 90% for naltrexone maintenance. The rates of SD in alcohol-dependent population have ranged 40-95.2%, with rates being consistently much higher in alcohol-dependent population than in the healthy controls or social drinkers. The common SDs reported have been erectile dysfunction followed by premature ejaculation, retarded ejaculation and decreased sexual desire among men, and dyspareunia and vaginal dryness among women. This review suggests that long-term use of alcohol and opioids are associated with SD in almost all domains of sexual functioning. There is a need to increase the awareness of clinicians about this association as many times SD in patients with substance abuse lead to poor treatment compliance and relapse. Further, there is a need to carry out more number of studies to understand the relationship in a better way.
Keywords: Alcohol, opioids, sexual dysfunction
INTRODUCTION
Sexual dysfunction (SD) is quite common in the community population. Large epidemiological community survey from the United States report >40% of women and 30% of men as suffering from some form of SD, with low sexual desire in women (22%) and premature ejaculation in men (21%) being the most common.[1] These figures are not very different from those of 34% women and 15% men from eight European countries reporting low sexual desire.[2]
Substance abuse is widely prevalent in the community. World Health Report in 2002 reported that 8.9% of the total burden of disease worldwide in 2000 came from the use of psychoactive substances.[3]
People may use alcohol and other substances to tackle sexual performance anxiety, enhance sexual performance, or overcome SD. A World Health Organization cross-cultural study for alcohol and high-risk sexual behavior across eight countries reported that 12% males in the general population consumed alcohol prior to first sexual intercourse due to perceived positive effect of alcohol to improve sexual pleasure.[4] Furthermore, alcohol was commonly used prior to intercourse with commercial sex worker.[4] However, in the long run, substance abuse impacts on sexual functioning negatively and may lead to the onset of sexual disorders.[5] Opioids have also been used as aphrodisiac and to delay ejaculation.[6] However, there is evidence that in the long run, substance abuse including for tobacco smoking impacts on sexual functioning negatively and may lead to the onset of SD or disorders,[5,7] Recovering substance abusers may face continuation or recurrence of SD, while the therapists may be perplexed about addressing the addiction and sexual problems of their clients.[8]
However, very few studies have systematically evaluated the relationship of SD and substances such as alcohol and opioids. This review aims to summarize the available literature on the effect of alcohol and opioids on the reproductive system and the prevalence of SD in males/dependent on alcohol and opioids.
For this review, an Internet search was carried out using the search engines of PubMed, Google Scholar, and ScienceDirect to locate the relevant literature. The keywords used in various combinations were: Alcohol, opioids, sexual function/dysfunction, alcohol dependence, opioid dependence, methadone, buprenorphine, heroin, erectile dysfunction (ED), premature ejaculation, libido, and reproductive hormones etc. Abstract of all the articles was screened, and the relevant articles were selected with a specific focus being effect of alcohol/opioid on the sexual hormones and prevalence of SD in patients with alcohol/opioid dependence. Full text articles were evaluated, and the relevant data were extracted. Cross references from these full-text articles also provided few more relevant articles.
PHYSIOLOGICAL SEXUAL FUNCTIONING: HOW IS IT INFLUENCED BY OPIOIDS AND ALCOHOL?
Endogenous opioids play an important role in the physiological sexual functioning. They act at specific opioid receptors, and contribute to control the release of gonadotropin-releasing hormone and thus, the sex hormones follicle-stimulating hormone (FSH) and luteinizing hormone (LH). Both FSH and LH are secreted by the anterior pituitary and act directly on the testes to stimulate the somatic cells that contribute to spermatogenesis. While FSH stimulates the proliferation of Sertoli cells during puberty, the LH regulates the synthesis of testosterone in the adult testes.[9] Morphine administration suppresses LH release and reduces the levels of testosterone and estradiol, which effects testicular function.[10,11] This is corroborated by an array of evidence. Opioid abuse is linked to the development of hypogonadism, decreased libido, ED, and infertility.[12,13,14] Opioid antagonists like naltrexone can improve symptoms of hypogonadism and erectile function without increasing testosterone or LH levels, suggesting regulation at the central rather than the peripheral level.[15] Opioids also exert a negative influence on adrenal androgen production. The adrenal hormones dehydroepiandrosterone (DHEA), DHEA sulfate (DHEAS), and androstenedione are weakly androgenic, and they are precursors of testosterone. Serum DHEAS levels are used to determine adrenal function in general and adrenal androgen production in particular. Daily use of opioids decreases adrenal androgen production as measured by DHEAS levels.[16]
Studies on endocrine and other biological effects of alcohol report that long-term use of alcohol leads to inhibition of hypothalamic-pituitary-adrenal axis and reduces the release of gonadotropins from the pituitary. Chronic alcohol abuse has been recorded to cause testicular atrophy, inhibition of testosterone production, and inhibition of spermatogenesis, apart from its direct oxidative toxicity.[17,18]
Animal studies have shown that acute and chronic alcohol exposure affects sex hormones in terms of profound testosterone suppression accompanied by lower or normal LH and FSH levels, when actually elevated levels of these are expected.[19,20] Human studies have shown lower levels of hypothalamic LH-releasing hormone and pituitary LH in adults[21,22] and inhibition of testosterone secretion by the testes[23] by alcohol.
Testicular opioids are messenger molecules similar to morphine within the testes, which suppress testosterone synthesis. An opioid, beta-endorphin has been shown to increase with acute and chronic alcohol consumption and may be a link between alcohol use and testicular damage.[24] Increased level of testicular opioids has been implicated to increase the rate of apoptosis.[25,26] Apoptosis at gonadal level may result in the death of both Leydig and seminiferous cells, which are involved in sperm cell formation and maturation; thus, leading to low testosterone levels and diminished sperm production. Another mechanism postulated for alcohol's harmful effect on testosterone production is the reduced level of nitric oxide (NO) that acts as a local vasodilator.[27] Oxidation of alcohol, part of alcohol metabolism, generates oxidants that can contribute to cell damage and play a role in alcohol-induced tissue damage in the testes. An imbalance between oxidants and antioxidants can create oxidative stress. Alcohol consumption may induce oxidative damage either by enhancing the production of toxic free radicals or by decreasing the levels of antioxidants. Certain oxidants produced by alcohol metabolism are known as reactive oxygen species (ROS). These include anion superoxide, hydrogen peroxide, hydroxyl radicals, and nitrogen reactive species like NO. The metabolism of alcohol and acetaldehyde, which is the principle product of alcohol metabolism, produces highly toxic ROSs. Increased oxidative stress is a well-accepted mechanism of alcohol-induced tissue injury, particularly in the liver,[28,29] heart, and central nervous system, and this also occurs in the testes.[30] There is some suggestion that acetaldehyde is more toxic than alcohol to the production of testosterone, altering the process of testosterone production by inhibiting protein kinase C, a key enzyme in testosterone synthesis.[31] There is research to show that men with chronic alcoholism and hypogonadism actually eliminate alcohol more rapidly, building up less acetaldehyde. Because the build-up of acetaldehyde in the body is nauseating, enhanced clearance of these by-products could lead to reduced gastrointestinal side-effects from drinking (e.g., abdominal discomfort and vomiting) in men with low testosterone levels. This may increase the risk of developing a drinking problem, because a person who does not experience the negative gastrointestinal side-effects of drinking will be more likely to continue to drink, often in larger amounts.[32] Lipid rich testicular membranes being prone to oxidative injury it is reasonable to consider that lipid peroxidation (i.e., damage to the cell membranes) may contribute to the gonadal dysfunction that occurs as a result of chronic alcohol use. Other explanations for alcohol-related gonadal suppression invoke the metabolic cascade of alcohol to toxins lok acetoacetate to salsolinol and others.[33] In alcohol fed animals and chronic alcoholics, when testosterone levels decrease the expected increase in LH levels is not seen. This inability of the pituitary gland to respond appropriately to testosterone decline implies that alcohol has a central effect on the interaction between the nervous system and the endocrine system.[19,20] Studies in alcohol-fed rats have established that the decrease in LH levels results from impairment in both LH production and LH secretion[22] and alcohol's deleterious effects on LH function appear to be both qualitative as well as quantitative. Secretion of FSH also appears to be reduced by alcohol; though, alcohol does not appear to affect FSH synthesis.
SEXUAL DYSFUNCTION ASSOCIATED WITH OPIOIDS AND ALCOHOL
Prevalence of sexual dysfunction in patients with opioid dependence
We identified 24 studies [Table 1] that specifically evaluated prevalence of SD in opioid-dependent patients taking illicit opioids like heroin or opioid substitution therapy like methadone maintenance treatment (MMT) or buprenorphine maintenance treatment (BMT); a few studies included those receiving opioid antagonist naltrexone.
Table 1.
Studies evaluating SD in males receiving/taking opioids
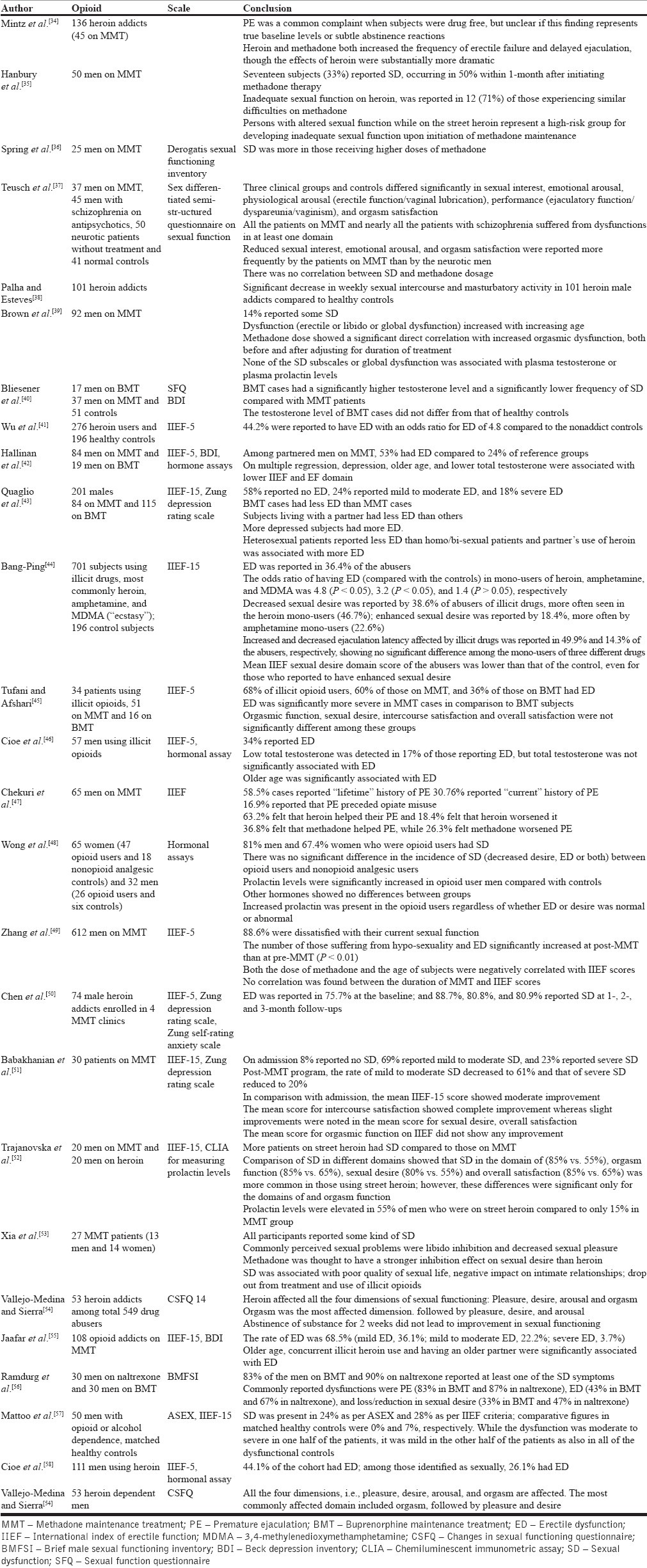
Heroin addiction or MMT or BMT subjects show higher rates of SD in comparison to the general population.[38,41,42,49,52,53] SD rates have ranged 34-85% for heroin addicts,[41,45,46,52] 14-81% for MMT,[35,39,45,51,52,55,56] 36-83% for BMT,[42,43,45,56] and 90% for naltrexone maintenance.[45,56]
Most of the quoted studies did not evaluate SD comprehensively; some focused only on premature ejaculation[34,47] or ED,[41,49,52,55] some evaluated only the frequency of sexual intercourse and masturbatory activity,[38,53] and some did not use a standardized instrument for assessment of SD.[34,35,38,53] Studies that assessed more than one functioning domain suggest the most common dysfunction to be any of the following: ED,[45,49,50,52,55] premature ejaculation,[47,56] orgasmic dysfunction,[51,52,54] and low libido.[38,49,53] However, dysfunction across all the domains has been reported more often.[37,39,52,53,54,56]
Prevalence of sexual dysfunction in patients with alcohol dependence
In clinical population, the relationship between alcohol and SD has been studied from the following point of view: Prevalence and correlates of SD in patients seeking treatment for alcohol problems, prevalence of alcohol use/abuse/dependence in patients seeking treatment for SD, and effect of alcohol on various mechanisms involved in proper sexual functioning.
In one of the first observations, Lemere and Smith[59] reported the prevalence of SD in alcohol-dependent population to be 8%, the dysfunction persisting in 50% patients even into long-term abstinence from alcohol. They postulated the persistent SD to the neurogenic damage caused by alcohol. The research since then is summarized in Table 2. With sample sizes of 18-816 covering men, women, or both, the rates of SD have ranged 40-95.2%, with rates being consistently much higher in alcohol-dependent population than in the healthy controls or social drinkers. The common SDs reported have been ED followed by premature ejaculation, retarded ejaculation and decreased sexual desire among men, and dyspareunia and vaginal dryness among women. Association between long-term and high amount of alcohol consumption and SD has been widely reported[60,61] and men with SD are frequently noted to be chronic alcohol dependent.[78] A review of clinical and experimental studies concluded that in male alcoholics, the greater quantity, frequency, and duration of drinking are associated with ED, inhibited libido, and retarded ejaculation.[79] A major limitation of these data has been the lack of standard instruments to assess SD; only four recent studies using International Index of Erectile Function (IIEF), of which two used full form of IIEF to assess SD.
Table 2.
Prevalence of SD in patients with alcohol dependence
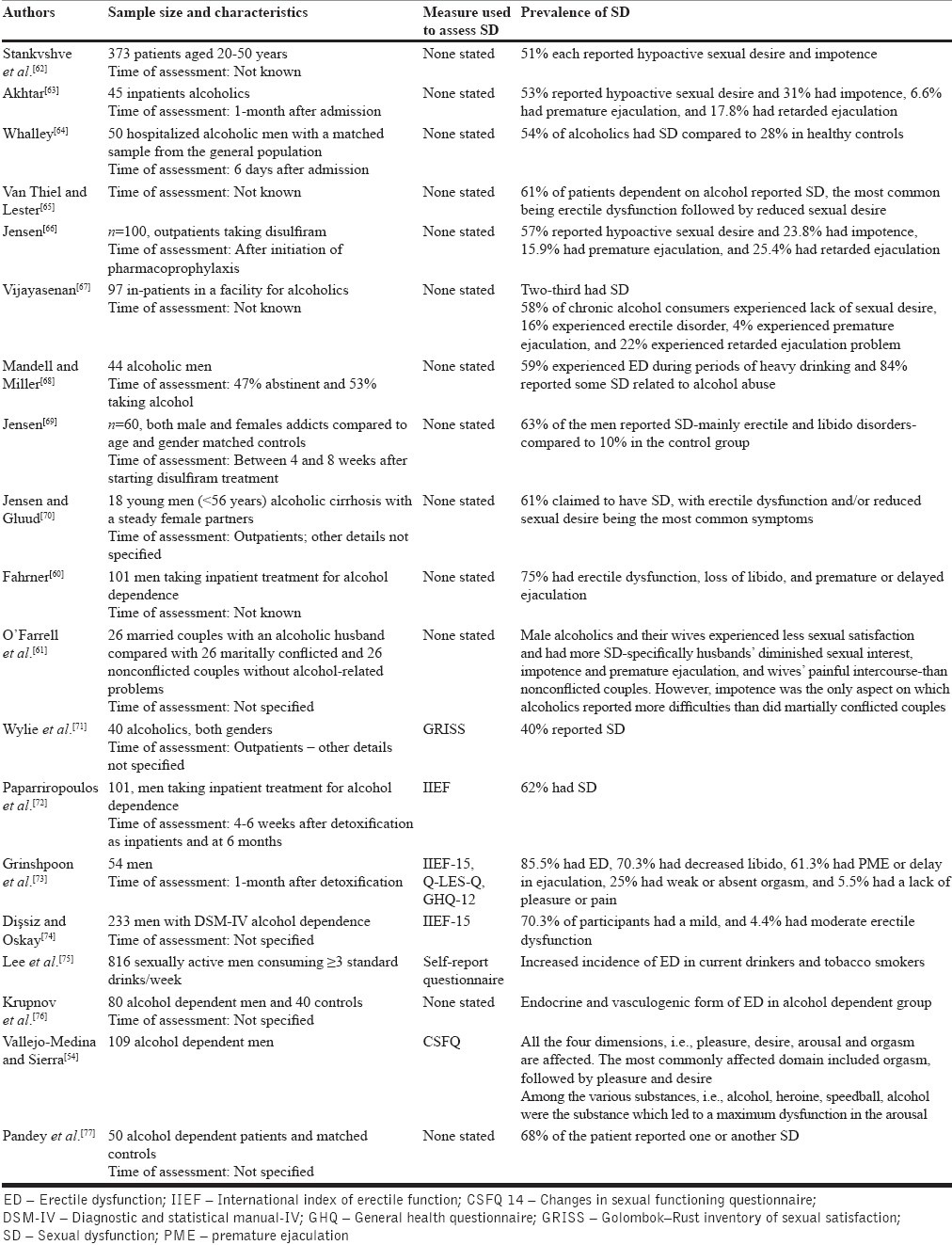
One consistent correlate that emerges in these studies is advancing age; other correlates include age of onset for alcohol use, duration of chronic alcoholism, presence of liver disease, cigarette use, education level, and unemployment.
However, some of the recent studies refute the link between SD and alcohol. One study which evaluated the effect of alcohol abuse, panic disorder, and depression on ED did not report any increase in the risk of ED with alcohol abuse.[80] Another study with HIV-positive men reported IIEF assessed ED to be related to nonalcohol drinking status.[81] A more recent population-based cross-sectional postal survey of men's health which assessed the association between usual alcohol consumption and IIEF assessed ED reported that compared to never-drinkers, the age-adjusted odds of having ED were lower among current, weekend, and binge drinkers and higher among ex-drinkers. Among current drinkers, the odds were the lowest for consumption of 1-20 standard drinks a week. After adjustment for cardiovascular disease or cigarette smoking, age-adjusted odds of ED were reduced by 25-30%.[82] A meta-analysis of population-based cross-sectional studies to assess association of alcohol consumption and ED yielded a protective association of alcohol on ED.[83] Studies on patients presenting with SD have reported a variable percentage of alcohol use. Fagan et al.[84] reported 29% of 145 consecutive patients with sexual problems to score on the probable alcoholism range on the Michigan Alcohol Screening Test, of which only six were diagnosed with alcoholism. Masters and Johnson[85] reported that in 35 out of 213 men with secondary impotence, the ED occurred as a direct result of acute alcohol intake; they did not detail out the chronicity of alcohol intake. One study from China reported alcohol as one of the important risk factors for low sexual function among urban women; the odds ratio of 2.67 was more than that for age, depression, low education level, chronic medical disease, and living apart from the partner.[86] Snyder and Karacan[87] measured nocturnal penile tumescence in 26 alcoholic men going through detoxification and found that their nocturnal erections were fewer, slower, and less rigid than in a nonalcoholic comparison group; they speculated peripheral neuropathology as the explanatory factor. Another double-blind study[88] reported significantly decreased frequency and duration of full erections in abstinent alcoholics on disulfiram. This finding is of particular importance due to the possible confounding effect of disulfiram on the reported frequency of sexual disorders.
Correlates of sexual dysfunction
Other studies have correlated ED with older age[4,9,13] and lower total testosterone.[4,20] Studies which have tried to establish a correlation between altered hormonal levels (testosterone, prolactin, and LH) and SD have in general come up with negative findings.[6,9,18,20] Some studies suggest that there is no correlation between methadone dose and SD[10,21,22] whereas others suggest that ED is higher in those taking higher doses of methadone.[5,18,23] Studies have also reported that higher methadone dose correlates negatively with ejaculation frequency[22] and positively with orgasmic dysfunction in men on MMT.[11] Other studies suggest that the rates of SD are affected by co-morbid depression[15] and number of psychological symptoms[23] whereas some studies suggest no correlation between SD and depression.[12,13,18]
A study in patients on MMT reported that those with altered sexual function while on street heroin represented a high-risk group for developing inadequate sexual function upon initiation of MMT.[35] Other studies have correlated ED with older age[42,46,55] and lower total testosterone.[42,48] Some others failed to establish a correlation between altered hormonal levels (testosterone, prolactin, LH) and SD.[46,48,50,52] While some studies suggest no correlation between methadone dose and SD[35,37,89] others suggest that ED is higher in those taking higher doses of methadone.[36,49,50] In contrast, some studies have also reported that higher methadone dose correlates negatively with ejaculation frequency[89] and positively with orgasmic dysfunction in men on MMT.[39] Other studies suggest that the rates of SD are affected by co-morbid depression[43] and number of psychological symptoms[36] whereas some studies suggest no correlation between SD and depression in patients with opioid dependence.[50,51,55]
A longitudinal study evaluated the effect of short-term and long-term abstinence from alcohol, opioids, speedball, cocaine, and cocaine plus alcohol on SD; the authors recorded the SD to persist after 3 weeks as also 1-year despite continued abstinence from substance abuse.[54]
DISCUSSION
In substance dependence, SD is of high clinical relevance as it often leads to treatment nonadherence and sexual or marital disharmony. Yet, it is often neglected and unexplored in routine clinical care. This is also reflected by the limited research in this area. This review suggests that long-term use of alcohol and opioids are associated with SD in almost all domains of sexual functioning. Studies in patients with either heroin addiction or on MMT or BMT have demonstrated higher rates of SD than in the general population, the rates ranging 34-85% for heroin addicts, 14-81% for MMT, 36-83% for BMT, and 90% for naltrexone maintenance. In contrast, in case of alcohol dependence, the SD rates have varied from 51% to 58% for low sexual desire, 16-59% for erectile impotence, 4-15.9% for premature ejaculation, and 17.8-25.4% for retarded ejaculation. There is evidence to suggest that endogenous opioids play a role in alcohol-related SD too.
However, the available studies suffer from many limitations. Some assessed SD by either spontaneous self-reporting (which might give lower rates) or by open questions (which may be interpreted differently by different patients). Some used inconsistent and nonvalidated measures of SD. Others took mixed groups of subjects (i.e., single and married subjects) or did not evaluate for contextual factors which could contribute to SD. Still others lacked consecutive or random samples and matched controls, or did not evaluate the impact of other opioids like dextropropoxyphene, codeine, etc. Furthermore, some studies did not try to distinguish the SD due to harmful effects of alcohol/opioid per se and SD due to other co-morbidities like use of other substances, effects of co-administered medications, the presence of a primary sexual disorder, presence of a medical condition affecting sexual function, psychosocial factors such as relationship conflict, presence of co-morbid psychiatric disorder, hepatocellular dysfunction, etc. Many co-morbid disorders are known to influence the prevalence of SD. Among the major predictors of ED observed in the Massachusetts Male Aging Study, diabetes mellitus, heart disease, hypertension, and decreased high-density lipoprotein levels were all associated with increased risk for ED. Medications for diabetes, hypertension, and cardiovascular disease also increase the risk of ED.[90] A prospective study[91] showed obesity and smoking to be additional risk factors for ED. In addition, there is a higher prevalence of ED among men who have undergone radiation or surgery for prostate cancer, or who have a lower spinal cord injury. Delayed or absent ejaculation may be associated with a variety of medical or surgical conditions (e.g., multiple sclerosis, spinal cord injury, surgical prostatectomy), or the use of anti-adrenergic or neuroleptic medications. A recent study that assessed the bio-psychosocial determinants of sexual desire in men concluded that cognitive factors (sexual beliefs and automatic thoughts during sexual activity) were the best predictors of sexual desire in men.[92] Various beliefs related to restrictive attitudes toward sexuality, erection concerns, and lack of erotic thoughts in a sexual context had a significant direct effect on reduced sexual desire. This set of cognitive-emotional factors also mediated the relationship between medical problems, age, and sexual desire. Another similar study[93] also highlighted the impact of early maladaptive schemas, helpless, and incompetence schemas impacting sexual function in men. A major limitation of existing research is that most of the studies have focused only on men, and the data for women are very limited.
Sexual side effects are not spontaneously reported by patients due to associated feelings of inadequacy; hence, direct inquiry is required. A careful enquiry can establish the patient's baseline levels of desire, arousal, and orgasmic function and determine whether a plausible chronological relationship exists between the onset of substance dependence and the beginning of SD. The most valuable asset is the patient's own history and description. SD is most obviously a side effect when it is reported as a new symptom after onset of substance dependence. In such a situation, clinicians should take measures to keep the patient abstinent and evaluate the SD longitudinally and treat it with appropriate measures.
There is thus, a need to study the multiple dimensions of association of substance abuse and SD. Future research should attempt to overcome the limitations of the existing literature. There is a need for studies with larger sample size using standardized instruments. These studies should attempt to evaluate the SDs in various phases of drug dependence, especially during the stable abstinence phase. Although studies have evaluated efficacy of various pharmacological agents in patients with SD, there is a need to evaluate these agents in patients with drug dependence using double-blind randomized control trials.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:1174–5. doi: 10.1001/jama.281.6.537. Erratum in: JAMA 1999;281:1174-5. [DOI] [PubMed] [Google Scholar]
- 2.Moreira ED, Glasser DB, Nicolosi A, Duarte FG, Gingell C GSSAB Investigators’ Group. Sexual problems and help-seeking behaviour in adults in the United Kingdom and continental Europe. BJU Int. 2008;101:1005–11. doi: 10.1111/j.1464-410X.2008.07453.x. [DOI] [PubMed] [Google Scholar]
- 3.Obot IS, Poznyak V, Monteiro M. From basic research to public health policy: WHO report on the neuroscience of substance dependence. Addict Behav. 2004;29:1497–502. doi: 10.1016/j.addbeh.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Geneva: World Health Organization; 2005. Alcohol Use and Sexual Risk Behaviour: A Cross-Cultural Study in Eight Countries. [Google Scholar]
- 5.Peugh J, Belenko S. Alcohol, drugs and sexual function: A review. J Psychoactive Drugs. 2001;33:223–32. doi: 10.1080/02791072.2001.10400569. [DOI] [PubMed] [Google Scholar]
- 6.James G. Opioids and sexual behaviour. Neurosci Biobehav Rev. 1986;11:1–34. doi: 10.1016/s0149-7634(87)80002-7. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan ME, Keoghane SR, Miller MA. Vascular risk factors and erectile dysfunction. BJU Int. 2001;87:838–45. doi: 10.1046/j.1464-410x.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 8.Zaazaa A, Bella AJ, Shamloul R. Drug addiction and sexual dysfunction. Endocrinol Metab Clin North Am. 2013;42:585–92. doi: 10.1016/j.ecl.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Leinonen P, Bolton N, Vihko R. Human testicular LH receptors: Correlations with circulating gonadotrophins and testicular steroid secretion. Int J Androl. 1982;5:145–57. doi: 10.1111/j.1365-2605.1982.tb00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Azizi F, Vagenakis AG, Longcope C, Ingbar SH, Braverman LE. Decreased serum testosterone concentration in male heroin and methadone addicts. Steroids. 1973;22:467–72. doi: 10.1016/0039-128x(73)90002-0. [DOI] [PubMed] [Google Scholar]
- 11.Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafisca S, Bolelli G, Franceschetti F, Danieli A, Tagliaro F, Marigo M, et al. Free and bound testosterone in male heroin addicts. Arch Toxicol Suppl. 1985;8:394–7. doi: 10.1007/978-3-642-69928-3_83. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Chan V, Yeung RT. The effect of heroin addiction on pituitary-testicular function. Clin Endocrinol (Oxf) 1978;9:455–61. doi: 10.1111/j.1365-2265.1978.tb03585.x. [DOI] [PubMed] [Google Scholar]
- 14.Ragni G, De Lauretis L, Bestetti O, Sghedoni D, Gambaro V. Gonadal function in male heroin and methadone addicts. Int J Androl. 1988;11:93–100. doi: 10.1111/j.1365-2605.1988.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 15.Fabbri A, Jannini EA, Gnessi L, Moretti C, Ulisse S, Franzese A, et al. Endorphins in male impotence: Evidence for naltrexone stimulation of erectile activity in patient therapy. Psychoneuroendocrinology. 1989;14:103–11. doi: 10.1016/0306-4530(89)90059-0. [DOI] [PubMed] [Google Scholar]
- 16.Daniell HW. DHEAS deficiency during consumption of sustained-action prescribed opioids: Evidence for opioid-induced inhibition of adrenal androgen production. J Pain. 2006;7:901–7. doi: 10.1016/j.jpain.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Nordmann R, Ribière C, Rouach H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol Alcohol. 1990;25:231–7. doi: 10.1093/oxfordjournals.alcalc.a044996. [DOI] [PubMed] [Google Scholar]
- 18.Rivier C, Rivest S, Vale W. Alcohol-induced inhibition of LH secretion in intact and gonadectomized male and female rats: Possible mechanisms. Alcohol Clin Exp Res. 1992;16:935–41. doi: 10.1111/j.1530-0277.1992.tb01896.x. [DOI] [PubMed] [Google Scholar]
- 19.Hadley ME. Englewoods Cliff, New Jersey: Prentice Hall; 1988. Endocrinology. [Google Scholar]
- 20.Yen SS, Jaffe RB. Philadelphia: WB Saunders Company; 1991. Reproductive Endocrinology. [Google Scholar]
- 21.Cicero TJ. Alcohol-induced deficits in the hypothalamic-pituitary-luteinizing hormone axis in the male. Alcohol Clin Exp Res. 1982;6:207–15. doi: 10.1111/j.1530-0277.1982.tb04964.x. [DOI] [PubMed] [Google Scholar]
- 22.Salonen I, Pakarinen P, Huhtaniemi I. Effect of chronic ethanol diet on expression of gonadotropin genes in the male rat. J Pharmacol Exp Ther. 1992;260:463–7. [PubMed] [Google Scholar]
- 23.Little PJ, Adams ML, Cicero TJ. Effects of alcohol on the hypothalamic-pituitary-gonadal axis in the developing male rat. J Pharmacol Exp Ther. 1992;263:1056–61. [PubMed] [Google Scholar]
- 24.Gianoulakis C. Characterization of the effects of acute ethanol administration on the release of beta-endorphin peptides by the rat hypothalamus. Eur J Pharmacol. 1990;180:21–9. doi: 10.1016/0014-2999(90)90588-w. [DOI] [PubMed] [Google Scholar]
- 25.Yin D, Mufson RA, Wang R, Shi Y. Fas-mediated cell death promoted by opioids. Nature. 1999;397:218. doi: 10.1038/16612. [DOI] [PubMed] [Google Scholar]
- 26.Nanji AA, Hiller-Sturmhöfel S. Apoptosis and necrosis: Two types of cell death in alcoholic liver disease. Alcohol Health Res World. 1997;21:325–30. [PMC free article] [PubMed] [Google Scholar]
- 27.Adams ML, Nock B, Truong R, Cicero TJ. Nitric oxide control of steroidogenesis: Endocrine effects of NG-nitro-L-arginine and comparisons to alcohol. Life Sci. 1992;50:PL35–40. doi: 10.1016/0024-3205(92)90384-2. [DOI] [PubMed] [Google Scholar]
- 28.Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- 29.Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–6. [PubMed] [Google Scholar]
- 30.Emanuele NV, LaPagli N, Steiner J, Colantoni A, Van Thiel DH, Emanuele MA. Peripubertal paternal EtOH exposure. Endocrine. 2001;14:213–9. doi: 10.1385/endo:14:2:213. [DOI] [PubMed] [Google Scholar]
- 31.Anderson RA, Jr, Quigg JM, Oswald C, Zaneveld LJ. Demonstration of a functional blood-testis barrier to acetaldehyde. Evidence for lack of acetaldehyde effect on ethanol-induced depression of testosterone in vivo. Biochem Pharmacol. 1985;34:685–95. doi: 10.1016/0006-2952(85)90265-5. [DOI] [PubMed] [Google Scholar]
- 32.Vaubourdolle M, Guechot J, Chazouilleres O, Poupon RE, Giboudeau J. Effect of dihydrotestosterone on the rate of ethanol elimination in healthy men. Alcohol Clin Exp Res. 1991;15:238–40. doi: 10.1111/j.1530-0277.1991.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 33.Stammel W, Thomas H, Staib W, Kühn-Velten WN. Tetrahydroisoquinoline alkaloids mimic direct but not receptor-mediated inhibitory effects of estrogens and phytoestrogens on testicular endocrine function. Possible significance for Leydig cell insufficiency in alcohol addiction. Life Sci. 1991;49:1319–29. doi: 10.1016/0024-3205(91)90196-i. [DOI] [PubMed] [Google Scholar]
- 34.Mintz J, O’Hare K, O’Brien CP, Goldschmidt J. Sexual problems of heroin addicts. Arch Gen Psychiatry. 1974;31:700–3. doi: 10.1001/archpsyc.1974.01760170088014. [DOI] [PubMed] [Google Scholar]
- 35.Hanbury R, Cohen M, Stimmel B. Adequacy of sexual performance in men maintained on methadone. Am J Drug Alcohol Abuse. 1977;4:13–20. doi: 10.3109/00952997709002743. [DOI] [PubMed] [Google Scholar]
- 36.Spring WD, Jr, Willenbring ML, Maddux TL. Sexual dysfunction and psychological distress in methadone maintenance. Int J Addict. 1992;27:1325–34. doi: 10.3109/10826089209047354. [DOI] [PubMed] [Google Scholar]
- 37.Teusch L, Scherbaum N, Böhme H, Bender S, Eschmann-Mehl G, Gastpar M. Different patterns of sexual dysfunctions associated with psychiatric disorders and psychopharmacological treatment. Results of an investigation by semistructured interview of schizophrenic and neurotic patients and methadone-substituted opiate addicts. Pharmacopsychiatry. 1995;28:84–92. doi: 10.1055/s-2007-979596. [DOI] [PubMed] [Google Scholar]
- 38.Palha AP, Esteves M. A study of the sexuality of opiate addicts. J Sex Marital Ther. 2002;28:427–37. doi: 10.1080/00926230290001547. [DOI] [PubMed] [Google Scholar]
- 39.Brown R, Balousek S, Mundt M, Fleming M. Methadone maintenance and male sexual dysfunction. J Addict Dis. 2005;24:91–106. doi: 10.1300/J069v24n02_08. [DOI] [PubMed] [Google Scholar]
- 40.Bliesener N, Albrecht S, Schwager A, Weckbecker K, Lichtermann D, Klingmüller D. Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J Clin Endocrinol Metab. 2005;90:203–6. doi: 10.1210/jc.2004-0929. [DOI] [PubMed] [Google Scholar]
- 41.Wu CJ, Hsieh JT, Lin JS, Hwang TI, Jiann BP, Huang ST, et al. Comparison of prevalence between self-reported erectile dysfunction and erectile dysfunction as defined by five-item International Index of Erectile Function in Taiwanese men older than 40 years. Urology. 2007;69:743–7. doi: 10.1016/j.urology.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 42.Hallinan R, Byrne A, Agho K, McMahon C, Tynan P, Attia J. Erectile dysfunction in men receiving methadone and buprenorphine maintenance treatment. J Sex Med. 2008;5:684–92. doi: 10.1111/j.1743-6109.2007.00702.x. [DOI] [PubMed] [Google Scholar]
- 43.Quaglio G, Lugoboni F, Pattaro C, Melara B, G.I.C.S. Mezzelani P, et al. Erectile dysfunction in male heroin users, receiving methadone and buprenorphine maintenance treatment. Drug Alcohol Depend. 2008;94:12–8. doi: 10.1016/j.drugalcdep.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Bang-Ping J. Sexual dysfunction in men who abuse illicit drugs: A preliminary report. J Sex Med. 2009;6:1072–80. doi: 10.1111/j.1743-6109.2007.00707.x. [DOI] [PubMed] [Google Scholar]
- 45.Tufani KH, Afshari R. Proceeding of 8th Annual Congress of the Asia Pacific of Medical Toxicology. Beijing, China: 2009. Opioid induced erectile dysfunction. [Google Scholar]
- 46.Cioe PA, Friedmann PD, Stein MD. Erectile dysfunction in opioid users: Lack of association with serum testosterone. J Addict Dis. 2010;29:455–60. doi: 10.1080/10550887.2010.509279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chekuri V, Gerber D, Brodie A, Krishnadas R. Premature ejaculation and other sexual dysfunctions in opiate dependent men receiving methadone substitution treatment. Addict Behav. 2012;37:124–6. doi: 10.1016/j.addbeh.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Wong D, Gray DP, Simmonds M, Rashiq S, Sobolev I, Morrish DW. Opioid analgesics suppress male gonadal function but opioid use in males and females does not correlate with symptoms of sexual dysfunction. Pain Res Manag. 2011;16:311–6. doi: 10.1155/2011/807123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, Wang P, Ma Z, Xu Z, Li Y. Sexual function of 612 male addicts treated by methadone. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2011;36:739–43. doi: 10.3969/j.issn.1672-7347.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, Li X, Li X, Ling L, Xia Y, Chen J, et al. Erectile dysfunction among male heroin addicts receiving methadone maintenance treatment in Guangdong, China. J Addict Med. 2012;6:212–8. doi: 10.1097/ADM.0b013e318259b2c4. [DOI] [PubMed] [Google Scholar]
- 51.Babakhanian M, Alam Mehrjerdi Z, Shenaiy Y. Sexual dysfunction in male crystalline heroin dependents before and after MMT: A pilot study. Arch Iran Med. 2012;15:751–5. [PubMed] [Google Scholar]
- 52.Trajanovska AS, Vujovic V, Ignjatova L, Janicevic-Ivanovska D, Cibisev A. Sexual dysfunction as a side effect of hyperprolactinemia in methadone maintenance therapy. Med Arch. 2013;67:48–50. doi: 10.5455/medarh.2013.67.48-50. [DOI] [PubMed] [Google Scholar]
- 53.Xia Y, Zhang D, Li X, Chen W, He Q, Jahn HJ, et al. Sexual dysfunction during methadone maintenance treatment and its influence on patient's life and treatment: A qualitative study in South China. Psychol Health Med. 2013;18:321–9. doi: 10.1080/13548506.2012.729845. [DOI] [PubMed] [Google Scholar]
- 54.Vallejo-Medina P, Sierra JC. Effect of drug use and influence of abstinence on sexual functioning in a Spanish male drug-dependent sample: A multisite study. J Sex Med. 2013;10:333–41. doi: 10.1111/j.1743-6109.2012.02977.x. [DOI] [PubMed] [Google Scholar]
- 55.Nik Jaafar NR, Mislan N, Abdul Aziz S, Baharudin A, Ibrahim N, Midin M, et al. Risk factors of erectile dysfunction in patients receiving methadone maintenance therapy. J Sex Med. 2013;10:2069–76. doi: 10.1111/jsm.12105. [DOI] [PubMed] [Google Scholar]
- 56.Ramdurg S, Ambekar A, Lal R. Sexual dysfunction among male patients receiving buprenorphine and naltrexone maintenance therapy for opioid dependence. J Sex Med. 2011;10:1743–61. doi: 10.1111/j.1743-6109.2011.02219.x. [DOI] [PubMed] [Google Scholar]
- 57.Mattoo SK, Bhardawaj V, Aarya K, Basu D. Sexual functioning in substance dependent men attending a de-addiction centre. Paper Presented at the Annual National Conference of the Indian Psychiatric Society. 2008-2009 [Google Scholar]
- 58.Cioe PA, Anderson BJ, Stein MD. Change in symptoms of erectile dysfunction in depressed men initiating buprenorphine therapy. J Subst Abuse Treat. 2013;45:451–6. doi: 10.1016/j.jsat.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lemere F, Smith JW. Alcohol-induced sexual impotence. Am J Psychiatry. 1973;130:212–3. doi: 10.1176/ajp.130.2.212. [DOI] [PubMed] [Google Scholar]
- 60.Fahrner EM. Sexual dysfunction in male alcohol addicts: Prevalence and treatment. Arch Sex Behav. 1987;16:247–57. doi: 10.1007/BF01541612. [DOI] [PubMed] [Google Scholar]
- 61.O’Farrell TJ, Choquette KA, Birchler GR. Sexual satisfaction and dissatisfaction in the marital relationships of male alcoholics seeking marital therapy. J Stud Alcohol. 1991;52:441–7. doi: 10.15288/jsa.1991.52.441. [DOI] [PubMed] [Google Scholar]
- 62.Stankvshve T, Protic M, Shishkova Disturbances of sexual function in alcoholics (in Bulgarian) Nevro Psikhiat Nevrokhir Sofiya. 1974;13:409–15. [Google Scholar]
- 63.Akhtar MJ. Sexual disorders in male alcoholics. In: Madden J, Walker I, Kenyoon LM, editors. Alcoholism and Drug Dependence. New York: Plenum Press; 1977. pp. 3–13. [Google Scholar]
- 64.Whalley LJ. Sexual adjustment of male alcoholics. Acta Psychiatr Scand. 1978;58:281–98. doi: 10.1111/j.1600-0447.1978.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 65.van Thiel DH, Lester R. Hypothalamic-pituitary gonadal dysfunction in patients with alcoholic liver disease. In: Davidson CS, editor. Problems in Liver Disease. New York: Stratton Intercontinental Medical Book Corp; 1979. pp. 289–98. [Google Scholar]
- 66.Jensen SB. Sexual customs and dysfunction in alcoholics. Br J Sex Med. 1979;6:30–4. [Google Scholar]
- 67.Vijayasenan ME. Alcohol and sex. N Z Med J. 1981;93:18–20. [PubMed] [Google Scholar]
- 68.Mandell W, Miller CM. Male sexual dysfunction as related to alcohol consumption: A pilot study. Alcohol Clin Exp Res. 1983;7:65–9. doi: 10.1111/j.1530-0277.1983.tb05413.x. [DOI] [PubMed] [Google Scholar]
- 69.Jensen SB. Sexual function and dysfunction in younger married alcoholics. A comparative study. Acta Psychiatr Scand. 1984;69:543–9. doi: 10.1111/j.1600-0447.1984.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 70.Jensen SB, Gluud C. Sexual dysfunction in men with alcoholic liver cirrhosis. A comparative study. Liver. 1985;5:94–100. doi: 10.1111/j.1600-0676.1985.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 71.Wylie KR, Steward D, Seivewright N, Smith D. Prevalence of sexual dysfunction in three psychiatric outpatient settings: A drug misuse service, an alcohol misuse service and a general adult psychiatry clinic. Sex Relat Ther. 2002;17:149–60. [Google Scholar]
- 72.Paparriropoulos T, Zabelis T, Tzavellas E, Karaiskos D, Zaloni R, Stachtea1 X, et al. Sexual dysfunction and alcohol abuse. Eur Neuropsychopharmacol. 2009;19:S6–7. [Google Scholar]
- 73.Grinshpoon A, Margolis A, Weizman A, Ponizovsky AM. Sildenafil citrate in the treatment of sexual dysfunction and its effect on quality of life in alcohol dependent men: Preliminary findings. Alcohol Alcohol. 2007;42:340–6. doi: 10.1093/alcalc/agm041. [DOI] [PubMed] [Google Scholar]
- 74.Dissiz M, Oskay ÜY. Evaluation of sexual functions in Turkish alcohol-dependent males. J Sex Med. 2011;8:3181–7. doi: 10.1111/j.1743-6109.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 75.Lee AC, Ho LM, Yip AW, Fan S, Lam TH. The effect of alcohol drinking on erectile dysfunction in Chinese men. Int J Impot Res. 2010;22:272–8. doi: 10.1038/ijir.2010.15. [DOI] [PubMed] [Google Scholar]
- 76.Krupnov AN, Shustov DI, Novikov SA, Kiselev DN. Peculiarities of erectile dysfunction in men with alcohol dependence. Zh Nevrol Psikhiatr Im S S Korsakova. 2011;111:55–7. [PubMed] [Google Scholar]
- 77.Pandey AK, Sapkota N, Tambi A, Shyangwa PM. Clinico-demographic profile, sexual dysfunction and readiness to change in male alcohol dependence syndrome inpatients in a tertiary hospital. Nepal Med Coll J. 2012;14:35–40. [PubMed] [Google Scholar]
- 78.McCambridge J, Mitcheson L, Hunt N, Winstock A. The rise of Viagra among British illicit drug users: 5-year survey data. Drug Alcohol Rev. 2006;25:111–3. doi: 10.1080/09595230500537167. [DOI] [PubMed] [Google Scholar]
- 79.Rosen RC. Alcohol and drug effects on sexual response: Human experimental and clinical studies. Annu Rev Sex Res. 1991;2:119–79. [Google Scholar]
- 80.Okulate G, Olayinka O, Dogunro AS. Erectile dysfunction: Prevalence and relationship to depression, alcohol abuse and panic disorder. Gen Hosp Psychiatry. 2003;25:209–13. doi: 10.1016/s0163-8343(03)00015-x. [DOI] [PubMed] [Google Scholar]
- 81.Asboe D, Catalan J, Mandalia S, Dedes N, Florence E, Schrooten W, et al. Sexual dysfunction in HIV-positive men is multi-factorial: A study of prevalence and associated factors. AIDS Care. 2007;19:955–65. doi: 10.1080/09540120701209847. [DOI] [PubMed] [Google Scholar]
- 82.Chew KK, Bremner A, Stuckey B, Earle C, Jamrozik K. Alcohol consumption and male erectile dysfunction: An unfounded reputation for risk? J Sex Med. 2009;6:1386–94. doi: 10.1111/j.1743-6109.2008.01115.x. [DOI] [PubMed] [Google Scholar]
- 83.Cheng JY, Ng EM, Chen RY, Ko JS. Alcohol consumption and erectile dysfunction: Meta-analysis of population-based studies. Int J Impot Res. 2007;19:343–52. doi: 10.1038/sj.ijir.3901556. [DOI] [PubMed] [Google Scholar]
- 84.Fagan PJ, Schmidt CW, Jr, Wise TN, Derogatis LR. Alcoholism in patients with sexual disorders. J Sex Marital Ther. 1988;14:245–52. doi: 10.1080/00926238808403806. [DOI] [PubMed] [Google Scholar]
- 85.Masters W, Johnson VE. Human Sexual Inadequacy. Boston: Little Brown; 1970. [Google Scholar]
- 86.Lianjun P, Aixia Z, Zhong W, Feng P, Li B, Xiaona Y. Risk factors for low sexual function among urban Chinese women: A hospital-based investigation. J Sex Med. 2011;8:2299–304. doi: 10.1111/j.1743-6109.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 87.Snyder S, Karacan I. Effects of chronic alcoholism on nocturnal penile tumescence. Psychosom Med. 1981;43:423–9. doi: 10.1097/00006842-198110000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Snyder S, Karacan I, Salis PJ. Disulfiram and nocturnal penile tumescence in the chronic alcoholic. Biol Psychiatry. 1981;16:399–406. [PubMed] [Google Scholar]
- 89.Crowley TJ, Simpson R. Methadone dose and human sexual behavior. Int J Addict. 1978;13:285–95. doi: 10.3109/10826087809039281. [DOI] [PubMed] [Google Scholar]
- 90.Jackson G. The importance of risk factor reduction in erectile dysfunction. Curr Urol Rep. 2007;8:463–6. doi: 10.1007/s11934-007-0049-x. [DOI] [PubMed] [Google Scholar]
- 91.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. A prospective study of risk factors for erectile dysfunction. J Urol. 2006;176:217–21. doi: 10.1016/S0022-5347(06)00589-1. [DOI] [PubMed] [Google Scholar]
- 92.Carvalho J, Nobre P. Biopsychosocial determinants of men's sexual desire: Testing an integrative model. J Sex Med. 2011;8:754–63. doi: 10.1111/j.1743-6109.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- 93.Quinta Gomes AL, Nobre P. Early maladaptive schemas and sexual dysfunction in men. Arch Sex Behav. 2012;41:311–20. doi: 10.1007/s10508-011-9853-y. [DOI] [PubMed] [Google Scholar]


