Abstract
Conditioned stimuli (CS) can modulate reward-seeking behavior. This modulatory effect can be maladaptive and has been implicated in excessive reward seeking and relapse to drug addiction. We previously demonstrated that exposure to an appetitive CS causes an increase in the activation of extracellular signal-regulated kinase (ERK) and cyclic-AMP response-element binding protein (CREB) in the nucleus accumbens (NAc) of rats, and that CS-evoked ERK activation is critical for CS control over reward seeking. To elucidate the mechanism that mediates CS-driven ERK activation in the NAc, we selectively blocked NMDA glutamate or D1 dopamine receptors in the NAc. To determine whether CS-driven ERK and CREB activation are linked, we selectively blocked ERK signaling in the NAc. We found that both NMDA and D1 receptors are critical for CS-driven ERK signaling in the NAc, and that this recruitment of the ERK cascade is responsible for increased CREB activation in the presence of the CS. Our findings suggest that activation of the NMDAR-D1R/ERK/CREB signal transduction pathway plays a critical role in the control of reward-seeking behavior by reward-predictive cues.
Environmental cues that have been paired repeatedly with reward come to predict the reward. These so-called conditioned stimuli (CSs) can have powerful control over behavior: For instance, exposure to a CS enhances an organism's seeking, or working for, the reward predicted by the CS (Estes 1948; Lovibond 1983; Cardinal et al. 2002; Everitt and Robbins 2005). The potentiating effect of a CS on reward-seeking has obvious beneficial consequences by enhancing an organism's ability to procure valuable resources in its environment (Everitt et al. 1999; Day and Carelli 2007). However, it can also be maladaptive and has also been implicated in excessive reward intake and addiction, including relapse to drug use (Grimm et al. 2002; Corbit and Janak 2007; LeBlanc et al. 2012; Gipson et al. 2013). Therefore, it is important to gain understanding of the mechanisms that underlie CS modulation of reward-seeking behavior.
A brain region critical for CS control over instrumental behavior is the nucleus accumbens (NAc) (Everitt et al. 1999; Zahm 2000; Cardinal et al. 2002; Kelley et al. 2005). This ventral striatal structure receives broad glutamatergic input from the prefrontal cortex, amygdala, subiculum, and thalamus, and dopaminergic input from the ventral tegmental area (Groenewegen et al. 1999; Zahm 2000). In turn, it sends projections to motor areas, such as the ventral pallidum and the substantia nigra (Mogenson et al. 1980; Groenewegen et al. 1999; Zahm 2000). We previously showed that exposure to a food-predicting CS evokes an increase in activation of extracellular signal-regulated kinase (ERK) in the NAc (Shiflett et al. 2008; Remus and Thiels 2013). Importantly, activation of the ERK pathway is necessary for CS modulation of reward-seeking behavior: in animals that received intra-accumbal infusions of an inhibitor of ERK activation, the CS failed to potentiate reward seeking (Shiflett et al. 2008). Here, we investigated the signaling events up- and downstream of CS-evoked ERK activation in the NAc.
Studies have identified a critical role for glutamatergic transmission, particularly through N-methyl-D-aspartate receptors (NMDARs), in striatal ERK activation (Vincent et al. 1998; Schwarzschild et al. 1999; Valjent et al. 2000; Mao et al. 2004; Jenab et al. 2005). Thus, CS-induced ERK activation in the NAc may be mediated through an NMDAR-dependent mechanism. On the other hand, D1 dopamine receptors (D1Rs) also were found to be involved in striatal ERK activation (Valjent et al. 2004, 2005; Bertran-Gonzalez et al. 2008; Borgkvist et al. 2008; Fricks-Gleason and Marshall 2011). Accordingly, both NMDARs and D1Rs may be targets for manipulating accumbal ERK signaling in the presence of a CS. The first goal of our study therefore was to determine the role of these two receptor types in accumbal ERK activation evoked by an appetitive CS.
Our previous work showed that exposure to a food-predicting CS increases not only ERK activation but also phosphorylation of the transcriptional regulator cyclic-AMP response element-binding protein (CREB) in the NAc (Shiflett et al. 2009). These two signaling events may be linked. CREB is one of the well-known downstream targets of the ERK signaling cascade (Xing et al. 1998) (for review, see Shaywitz and Greenberg 1999; Thomas and Huganir 2004; Carlezon et al. 2005). However, CREB phosphorylation alternatively can be mediated by other signaling pathways, including an ERK-independent protein kinase A (PKA) pathway or a calcium/calmodulin-dependent kinase IV (CaMK IV) pathway (Gonzalez and Montminy 1989; Gonzalez et al. 1989; Dash et al. 1991; Sheng et al. 1991; Matthews et al. 1994). Therefore, the second goal of our study was to determine whether the increase in CREB phosphorylation caused by exposure to an appetitive CS is dependent on CS-evoked ERK signaling in the NAc. Our experiments show that both NMDARs and D1Rs are critical for triggering increased ERK signaling in the NAc in the presence of an appetitive CS, and that this recruitment of the ERK cascade leads to increased CREB phosphorylation in the presence of a reward-predictive cue.
Results
Conditioned cue-evoked increases in ERK signaling in the NAc are mediated by NMDA and D1 receptor activation
To determine the receptor pharmacology of the CS-evoked increase in ERK activation in the NAc we observed previously (Shiflett et al. 2008; Remus and Thiels 2013), we infused the specific NMDAR antagonist D-(2R)-amino-5-phosphonovaleric acid (APV; 1 µg in 0.5 µL) into the NAc on one side and vehicle solution (150 mM NaCl; 0.5 µL) into the NAc on the other side shortly before the tone test used for examination of CS-evoked ERK activation (see Fig. 1). Discriminative food cup-approach behavior (rate of food cup approaches during the first 30 sec of the tone minus rate of food cup approaches during 30 sec preCS) of the Tone + Food (T + F) group and the Tone-only (TO) control group during training is shown in Figure 2A. Whereas the two groups did not differ from one another at the beginning of training, only rats in the T + F group (n = 9) but not rats in the TO group (n = 10) developed preferential food cup approaches during the CS over the course of training [group × day interaction: F(4,68) = 5.38, P = 0.001]. Discriminative food cup approach of the two groups on the test day, shortly after intra-NAc drug infusion, is depicted in Figure 2B. As was the case during the later phases of training, the discrimination rates of rats in the T + F group were significantly higher than those by rats in the TO group [t(17) = 5.65, P < 0.001, two-tailed]. These results indicate that unilateral NMDAR blockade in the NAc does not interfere with discriminative responding to the CS.
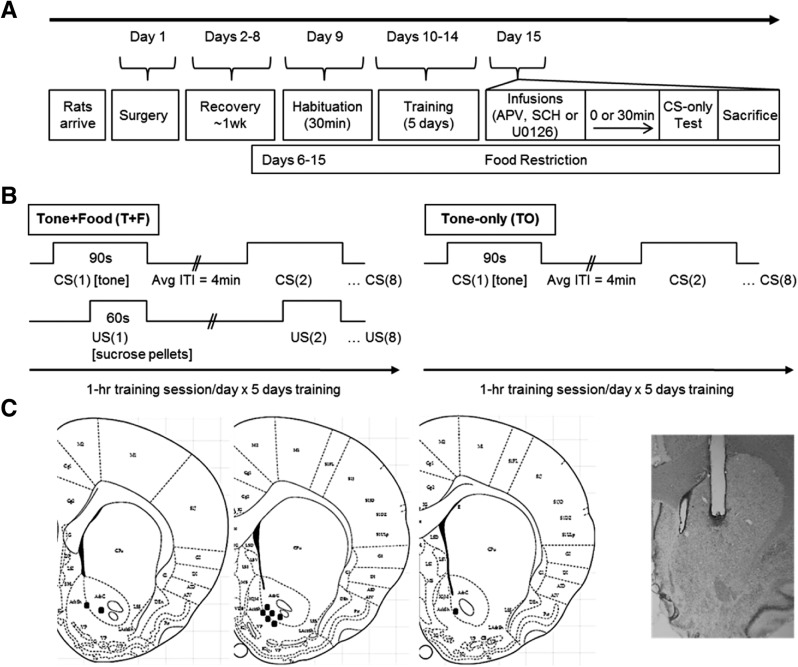
Figure 1.
Experimental details. (A) Experimental time line. After 1 d of habituation and 5 d of training, rats were tested under one of three drug conditions: (1) immediately after infusion of APV/vehicle, (2) immediately after infusion of SCH/vehicle, or (3) 30 min after infusion of U0126/vehicle. (B) Trial time lines for the group that received paired Tone + Food presentations (T + F group) and the group that received Tone-only presentations (TO control group) during training. (C) Cannulae placements for immunohistochemical experiments. Placements spanned from +1.0 to +1.7 mm relative to bregma. Representative track end points are indicated by circles on plate images shown on the left (modified from Paxinos and Watson 2007 with permission from Elsevier © 2007) and a Nissl stain image of a representative guide cannula placement is shown on the right. Comparable placements were observed for Western blot experiments.
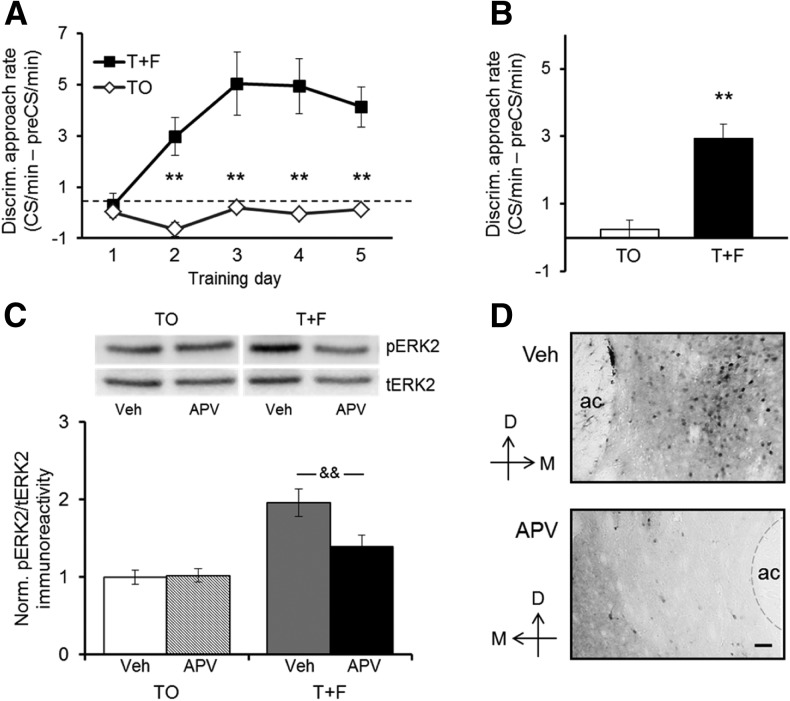
Figure 2.
NMDA receptor blockade in the NAc disrupts conditioned cue-evoked ERK activation. (A) Discriminative food cup approach during daily training sessions for rats in the Tone + Food group (T + F; n = 9) and the Tone-only control group (TO; n = 10). T + F rats preferentially approached the food cup during the tone (CS) compared with the preCS period, whereas TO controls did not exhibit discriminative approach behavior. Values show group means ± SEMs. (**) P < 0.01 for between-group comparisons. (B) Discriminative approach behavior during the CS-only test, immediately after infusion of APV (1 µg/0.5 µL) into one NAc and vehicle solution (150 mM NaCl, 0.5 µL) into the other NAc. Unilateral intra-NAc NMDA receptor blockade had no effect on discriminative approach behavior by the T + F group. Values show group means ± SEMs. (**) P < 0.01. (C) ERK2 activation (pERK2 immunoreactivity relative to tERK2 immunoreactivity in the same sample) in NAc samples harvested from rats of the T + F group and the TO control group shortly after the CS-only test. Ratios of pERK/tERK immunoreactivity are expressed relative to the same ratio observed in vehicle-infused NAc samples from TO control rats included on the same membrane. Values show group means ± SEMs. (&&) P < 0.01 for within-group comparisons. Representative Western blots of the vehicle- and the APV-infused sides of a rat of the T + F group and a rat of the TO group are shown above. (D) Representative immunohistochemical image of pERK-immunoreactive cells in the NAc of a rat from the T + F group. (Top panel) NAc side infused with vehicle solution; (bottom panel) NAc side infused with APV. Similar differences in staining between the vehicle- and APV-infused sides were observed in other T + F rats (n = 3). Scale bar, 50 µm. (D) Dorsal, (M) medial, (ac) anterior commissure. To help discern the border of the ac, a thin gray dashed line was added in some of the images.
The effect of unilateral NMDAR blockade on NAc ERK2 activation evoked by exposure to the tone-CS is shown in Figure 2C. We examined ERK2 (p42 isoform) because the activation state of specifically this isoform was shown to be altered during learning and reward-motivated behavior (Mazzucchelli et al. 2002; Ferguson et al. 2006; Girault et al. 2007; Shiflett et al. 2008). Focusing first on the vehicle-infused side of the NAc, we found that pERK2 immunoreactivity relative to tERK2 immunoreactivity, i.e., ERK2 activation, was about twofold higher in the NAc of rats in the T + F group than in the NAc of rats in the TO group. This finding is in agreement with previous observations of increased ERK2 activation in the NAc upon exposure to an appetitive CS (Shiflett et al. 2008; Remus and Thiels 2013). Importantly, this increase in ERK2 activation in rats of the T + F group was markedly reduced in the presence of APV; in contrast, the NMDAR antagonist had no effect on ERK2 activation in the NAc of the TO control group [group × drug interaction: F(1,17) = 6.19, P = 0.024]. Post hoc comparisons showed that the ERK2 signal was significantly higher in the vehicle-infused side compared with APV-infused side only in rats of the T + F group (P = 0.004). Figure 2D depicts a representative immunohistochemical image of pERK-immunoreactive cells in the vehicle-infused NAc (top) and the APV-infused NAc (bottom) of a T + F rat, and Supplemental Table 1 shows quantification of pERK-staining examined in three T + F rats. The density of pERK-immunoreactive (pERK+) cells on the vehicle-infused side was similar to levels we observed previously in T + F trained rats whereas that on the APV-infused side was similar to levels we observed previously in TO controls (Remus and Thiels 2013). Furthermore, similar to the subregional patterns we described in our earlier work comparing T + F trained rats and TO controls, the number of pERK+ cells was higher in the shell subregion of the NAc than in the core subregion, but the proportional difference between the vehicle- and the APV-infused side was comparable across subregions. Taken together, these findings show that NMDAR activation critically contributes to CS-evoked increases in ERK2 signaling but does not play a role in the regulation of basal ERK2 signaling.
To determine whether the CS-evoked increases in ERK signaling also require D1Rs, we repeated essentially the same experiment as described above with a new set of animals, except that we infused the specific D1-like receptor antagonist SCH23390 (SCH; 0.3 µg in 0.5 µL) into the NAc on one side and vehicle solution (150 mM NaCl; 0.5 µL) into the NAc on the other side shortly before the tone test. Discriminative food cup-approach behavior of the two groups during training is shown in Figure 3A. Similar to our previous observations, discriminative food cup-approach behavior developed only in rats of the T + F group (n = 6) but not in rats of the TO group (n = 6) [group × day interaction: F(4,40) = 3.48, P = 0.016]. On the test day, immediately after unilateral infusion of SCH, the group differences were maintained (Fig. 3B). Discriminative food cup approach by rats in the T + F group was significantly higher than that of rats in the TO group [t(10) = 5.15, P < 0.001]. Thus, unilateral D1R blockade leaves discriminative responding to the CS intact.
Figure 3.
D1 receptor blockade in the NAc disrupts conditioned cue-evoked ERK activation. (A) Discriminative food cup approach during daily training sessions for rats in the Tone + Food group (T + F; n = 6) and the Tone-only control group (TO; n = 6). T + F rats preferentially approached the food cup during the CS compared with the preCS period, whereas TO controls did not exhibit discriminative approach behavior. Values show group means ± SEMs. (**) P < 0.01 for between-group comparison. (B) Discriminative approach behavior during the CS-only test, immediately after infusion of SCH (0.3 µg/0.5 µL) into one NAc and vehicle solution (150 mM NaCl, 0.5 µL) into the other NAc. Unilateral intra-NAc D1 receptor blockade had no effect on discriminative approach behavior by the T + F group. Values show group means ± SEMs. (**) P < 0.01. (C) ERK2 activation (pERK2 immunoreactivity relative to tERK2 immunoreactivity in the same sample) in NAc samples harvested from rats of the T + F group and the TO control group shortly after the CS-only test. Ratios of pERK/tERK immunoreactivity are expressed relative to the same ratio observed in vehicle-infused NAc samples from TO control rats included on the same membrane. Values show group means ± SEMs. (&&) P < 0.01 for within-group comparisons. Representative Western blots of the vehicle- and SCH-infused sides of a rat of the T + F group and a rat of the TO group are shown above. (D) Representative immunohistochemical image of pERK-immunoreactive cells in the NAc of a rat from the T + F group. (Top panel) NAc side infused with vehicle solution; (bottom panel) NAc side infused with SCH. Similar differences in staining between the vehicle- and SCH-infused sides were observed in other T + F rats (n = 3). Scale bar, 50 µm. (D) Dorsal, (M) medial, (ac) anterior commissure. To help discern the border of the ac, a thin gray dashed line was added in some of the images.
The effect of unilateral D1R blockade on NAc ERK2 activation evoked by exposure to the tone-CS is shown in Figure 3C. Once again, ERK2 activation on the vehicle-infused side of the NAc was about twofold higher in rats in the T + F group than rats in the TO group. This increase in ERK2 activation in the NAc of rats in the T + F group was reduced in the presence of SCH, whereas the D1R antagonist had no effect on ERK2 activation in the NAc of TO rats [group × drug interaction: F(1,10) = 10.73, P = 0.008]. Post hoc tests confirmed that ERK2 activation was significantly higher in the vehicle-infused compared with the SCH-infused NAc of rats from the T + F group (P = 0.003), but did not differ between drug conditions in rats from the TO group. Figure 3D depicts a representative immunohistochemical image of pERK-immunoreactive cells in the vehicle-infused NAc (top) and the SCH-infused NAc (bottom) of a T + F rat, and Supplemental Table 1 shows quantification of pERK+ cells in vehicle-infused NAc versus SCH-infused NAc of three T + F rats. The differential pattern of density of pERK+ cells between the vehicle-infused and the drug-infused side and between the NAc core and the NAc shell was similar to the pattern we found after unilateral APV infusion (see above) and observed previously in T + F trained and TO control rats, respectively (Remus and Thiels 2013). The findings with the D1R antagonist show that basal ERK2 activation is not regulated in a D1R-dependent fashion but that D1R activation is necessary for increased ERK2 signaling after exposure to an appetitive CS. Taken together, our results indicate that both NMDARs and D1Rs are critical for the regulation of NAc ERK signaling by reward-predictive cues.
Conditioned cue effects on ERK signaling are responsible for cue-evoked increases in CREB phosphorylation in the NAc
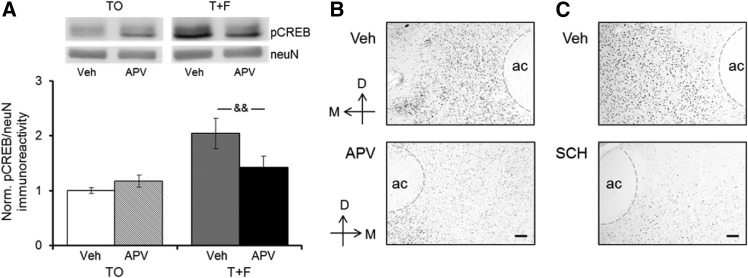
A major downstream target of activated ERK is the plasticity-relevant transcription factor, CREB. We previously found that exposure to a reward-predictive cue increases not only ERK activation but also phosphorylation at the transcriptionally relevant Ser-133 site of CREB in the NAc (Shiflett et al. 2009). As a first step to determine whether these two signaling events are linked, we probed NAc samples of the animals that received APV infusions immediately before the tone test (see Fig. 2) for Ser-133-phosphorylated CREB (pCREB). We reasoned that if increased phosphorylation of CREB upon exposure to a reward-predictive cue lies downstream of the cue-evoked increase in ERK activation, then disruption of the CS effect on ERK should also interfere with the CS effect on CREB. Figure 4A shows that in the presence of vehicle solution, pCREB immunoreactivity relative to neuN immunoreactivity measured in the same samples (i.e., pCREB) is markedly higher in the NAc of T + F rats than the NAc of TO rats, similar to the CS-evoked increase in CREB phosphorylation we reported previously (Shiflett et al. 2009). In the presence of APV, the CS-evoked increase in pCREB was greatly reduced, whereas basal levels of pCREB were unaffected [group × drug interaction: F(1,16) = 11.31, P = 0.004]. Post hoc comparisons confirmed a significant difference between the drug- and vehicle-infused sides in the T + F group (P = 0.002) but not the TO group. Figure 4B shows representative immunohistochemical images of pCREB-immunoreactive cells in the vehicle-infused NAc (top) and the drug-infused NAc (bottom) of a T + F rat infused with the NMDAR antagonist APV. Whereas the density of pCREB staining on the vehicle-infused side is similar to what we observed previously in the NAc of T + F rats, the distinctly lighter staining pattern on the APV-infused side resembles pCREB staining levels we observed in the NAc of TO rats (Shiflett et al. 2009). Figure 4C depicts similar immunohistochemical images of pCREB-immunoreactive cells from a representative T + F rat infused with vehicle into the NAc on one side (top) and the D1R antagonist SCH into the NAc on the other side (bottom). Again, the density of pCREB immunostaining is reduced on the drug-infused compared with the vehicle-infused side and values are similar to those we previously observed. Overall, more immunopositive cells were detected with the pCREB than the pERK antibody (Figs. 2,3), most likely because the pCREB antibody is known to recognize ATF1 and CREM as well (Mattson et al. 2005). Supplemental Table 2 shows quantification of pCREB density examined in three T + F rats treated with APV, and three T + F rats treated with SCH. In both APV and SCH immunohistochemical analyses, the proportional difference of pCREB density between the vehicle- and drug-infused NAc was greater in the core than in the shell, similar to the subregional difference in pCREB staining between TO controls and T + F rats we observed previously as well (Shiflett et al. 2009). Taken together, these results parallel the respective effects of the receptor blockers on ERK activation, and suggest that an effector of CS-evoked ERK signaling is CREB.
Figure 4.
NMDA or D1 receptor blockade in the NAc interferes with conditioned cue-evoked CREB phosphorylation. (A) Levels of phosphorylated CREB (pCREB immunoreactivity relative to neuN immunoreactivity in the same sample) were quantified from the same NAc samples harvested from nine T + F and nine TO rats receiving APV/vehicle infusions and quantified for pERK (shown in Fig. 2). Ratios of pCREB/neuN immunoreactivity are expressed relative to the same ratio observed in vehicle-infused NAc samples from TO control rats included on the same membrane. Values show group means ± SEMs. (&&) P < 0.01 for within-group comparisons. Representative Western blots of the vehicle- and APV-infused side of a T + F rat and a TO rat are shown above. (B) Representative immunohistochemical image of pCREB-immunoreactive cells in the NAc of a T + F rat infused with APV/vehicle on the test day. (Top panel) NAc side infused with vehicle solution; (bottom panel) NAc side infused with APV. (C) Representative immunohistochemical image of pCREB-immunoreactive cells in the NAc of a T + F rat infused with SCH/vehicle on the test day. (Top panel) NAc side infused with vehicle solution; (bottom panel) NAc side infused with SCH. Scale bar, 50 µm. (D) Dorsal, (M) medial, (ac) anterior commissure. To help discern the border of the ac, a thin gray dashed line was added in some of the images.
To test this idea directly, we trained rats as described above, except that animals received an infusion of the specific mitogen-activated protein kinase kinase/ERK inhibitor U0126 (1 µg in 0.5 µL) into the NAc on one side and vehicle solution (50% DMSO in 150 mM NaCl; 0.5 µL) into the NAc on the other side 30 min prior to the tone test for assessment of CS-driven CREB phosphorylation. Consistent with our previous findings, discriminative food cup-approach behavior developed in rats of the T + F group (n = 8) but not in rats of the TO group (n = 7) [group × day interaction: F(4,52) = 5.72, P = 0.001] (data not shown). Discriminative food cup-approach behavior of the two groups on the test day, after infusion of U0126 into the NAc on one side and vehicle solution into the NAc on the other side, is depicted in Figure 5A. As was the case during the later phases of training, the discrimination rates of rats in the T + F group were significantly higher than that of rats in the TO group [t(13) = 2.92, P = 0.014, two tailed]. These results indicate that unilateral inhibition of ERK activation in the NAc does not interfere with discriminative responding to the CS.
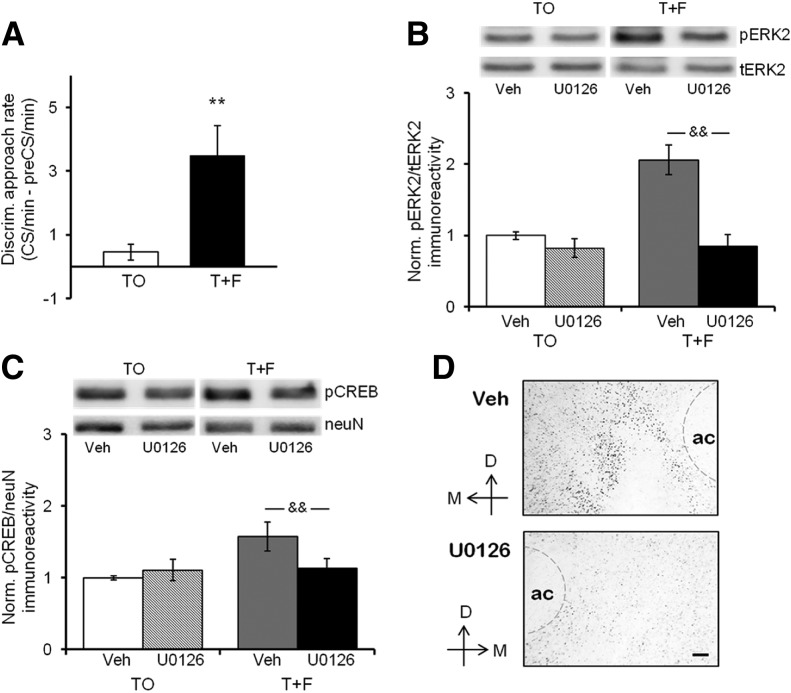
Figure 5.
Blockade of ERK activation in the NAc prevents conditioned cue-evoked CREB phosphorylation. (A) Discriminative approach behavior in T + F (n = 8) and TO (n = 7) rats during the CS-only test, 30 min after infusion of U0126 (1 µg/0.5 µL) into one NAc and vehicle solution (50% DMSO, 0.5 µL) into the other NAc. Unilateral intra-NAc blockade of ERK activation had no effect on discriminative approach behavior by the T + F group. Values show group means ± SEMs. (**) P < 0.01 for between-group comparisons. (B) ERK2 activation (pERK2 immunoreactivity relative to tERK2 immunoreactivity in the same sample) in NAc samples harvested shortly after the CS-only test from rats of the T + F group and the TO control group shown in A. Ratios of pERK/tERK immunoreactivity are expressed relative to the same ratio observed in vehicle-infused NAc samples from TO control rats included on the same membrane. Values show group means ± SEMs. (&&) P < 0.01 for within-group comparisons. Representative Western blots of the vehicle- and U0126-infused sides of a rat of the T + F group and a rat of the TO group are shown above. (C) CREB phosphorylation (pCREB immunoreactivity relative to neuN immunoreactivity in the same sample) from the same rats and NAc samples depicted in A and B, respectively. Ratios of pCREB/neuN immunoreactivity are expressed relative to the same ratio observed in vehicle-infused NAc samples from TO control rats included on the same membrane. Values show group means ± SEMs. (&&) P < 0.01 for within-group comparisons. Representative Western blots of the vehicle- and U0126-infused sides of a rat of the T + F group and a rat of the TO group are shown above. (D) Representative immunohistochemical image of pCREB-immunoreactive cells in the NAc of a T + F rat. (Top panel) NAc side infused with vehicle solution; (bottom panel) NAc side infused with U0126. Similar differences in staining between the vehicle- and U0126-infused sides were observed in other T + F rats (n = 3). Scale bar, 50 µm. (D) Dorsal, (M) medial, (ac) anterior commissure. To help discern the border of the ac, a thin gray dashed line was added in some of the images.
Figure 5B shows the effect of the MEK/ERK inhibitor on ERK activation. Whereas in the presence of vehicle solution ERK2 activation in the NAc of rats from the T + F group was markedly elevated, consistent with our previous observations (Shiflett et al. 2008; this study), this effect was completely abolished in the presence of U0126. At the same time, the MEK/ERK inhibitor had no effect on basal ERK2 activation [group × drug interaction: F(1,13) = 10.85, P = 0.006]. Post hoc comparisons confirmed that ERK2 activation was significantly higher in the vehicle- compared with U0126-infused NAc only for the T + F group (P < 0.001) but not the TO control group. Thus, our manipulation interfered selectively with CS-evoked ERK2 activation. The effect of ERK inhibition on the level of pCREB in the same rats is displayed in Figure 5C. The increase in the pCREB level we observed in the vehicle-infused NAc of rats in the T + F group was not observed in the U0126-infused NAc. Basal pCREB levels, on the other hand, were unaffected by the presence of the MEK/ERK inhibitor [group × drug interaction: F(1,13) = 10.39, P = 0.007]. Post hoc comparisons confirmed that levels of pCREB were significantly higher in the vehicle-infused NAc compared with the U0126-infused NAc among rats of the T + F group (P = 0.002) but did not differ between drug conditions among rats of the TO control group. Figure 5D depicts a representative immunohistochemical image of pCREB-immunoreactive cells in the vehicle-infused NAc (top) and the U0126-infused NAc (bottom) of a T + F rat, and Supplemental Table 2 shows quantification of pCREB density examined in three T + F rats. Similar to our findings with APV and SCH, the density of pCREB immunostaining was reduced on the drug-infused compared with the vehicle-infused side. Taken together, our results indicate that the increase in pCREB in the NAc upon exposure to a reward-predictive cue requires ERK signaling. Furthermore, they strongly suggest a direct link between cue-evoked ERK activation and cue-evoked CREB phosphorylation in the NAc.
Discussion
We previously showed that exposure to an appetitive cue causes an increase in ERK signaling and CREB phosphorylation in the NAc (Shiflett et al. 2008, 2009; Remus and Thiels 2013), and that recruitment of the ERK pathway in the NAc is necessary for CS modulation of reward-seeking behavior (Shiflett et al. 2008). Furthermore, we showed that the CS-driven increase in ERK activation is specific to the ventral striatum and does not occur in the dorsal striatum (Shiflett et al. 2008; Remus and Thiels 2013). The goal of the present experiments was to identify the synaptic signal mediating CS-evoked ERK activation in the NAc, and to establish whether CS-evoked ERK signaling and CREB phosphorylation (Shiflett et al. 2009) are linked. We found that both NMDARs and D1Rs are critical for driving ERK activation by an appetitive CS, and that the NMDAR-D1R/ERK signal transduction pathway is responsible for increased accumbal CREB phosphorylation in the presence of an appetitive CS.
NMDARs have been linked with ERK activation in the NAc (for review, see Girault et al. 2007), and are a reasonable synaptic candidate involved in CS-evoked ERK activation. First, exposure to cues signaling availability of cocaine was found to increase, and cues signaling unavailability to decrease, extracellular glutamate in the NAc (Suto et al. 2013). Second, stimulation of glutamatergic afferents in striatal slices (Sgambato et al. 1998a, b; Vanhoutte et al. 1999) or bath-application of glutamate or NMDA to striatal neurons in culture (Vincent et al. 1998; Schwarzschild et al. 1999; Fuller et al. 2001; Perkinton et al. 2002; Mao et al. 2004) caused increases in striatal ERK activation. Third, NMDAR antagonists were found to interfere with glutamate-, D1R-, or drug-evoked ERK activation in the striatum (Vincent et al. 1998; Valjent et al. 2000; Mazzucchelli et al. 2002; Haberny and Carr 2005; Jenab et al. 2005; Jiao et al. 2007; Fasano et al. 2009; Pascoli et al. 2011). Our data showing that NMDAR blockade interferes with CS-evoked ERK activation in the NAc extend these findings by demonstrating a role for NMDAR activation in accumbal ERK signaling by behaviorally relevant cues as well. Our studies do not rule out a possible enabling role of α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate receptors (AMPARs) in the observed ERK signal. However, selective AMPAR activation causes no or only comparatively small increases in striatal ERK signaling (Fuller et al. 2001; Mao et al. 2004). Our findings, combined with the aforementioned body of work, therefore lead us to conclude that NMDARs are the primary glutamatergic mechanism responsible for the regulation of accumbal ERK signaling by appetitive CSs.
D1Rs also have positive coupling with ERK activation in the NAc (for review, see Girault et al. 2007), and are another reasonable synaptic candidate involved in CS-driven ERK signaling. First, exposure to appetitive CSs was shown to increase extracellular dopamine in the NAc (Bassareo and Di Chiara 1999; Cheng et al. 2003; Phillips et al. 2003; Roitman 2004; Day et al. 2007). Second, ERK activation evoked by exposure to drug-paired contextual cues was shown to be prevented by D1R blockade and found to occur in D1R- but not D2 receptor (D2R)-expressing medium spiny neurons (MSNs) of the NAc (Borgkvist et al. 2008; Fricks-Gleason and Marshall 2011). Stimulation of D2Rs, on the other hand, tends to have no effect or decreases ERK activation (Gerfen et al. 2002), whereas D2R “blockade” can cause an increase in ERK activation in the NAc (Valjent et al. 2004; Bertran-Gonzalez et al. 2008; Fricks-Gleason and Marshall 2011). Taking the foregoing studies into account, we conclude from our present findings that D1Rs are the principal dopaminergic mechanism responsible for the regulation of accumbal ERK signaling by appetitive CSs.
Much evidence indicates that glutamatergic and dopaminergic signals converge at the level of the NAc. Glutamatergic NAc afferents make synaptic contacts onto the same MSN dendritic spines that receive dopaminergic input (Totterdell and Smith 1989; Sesack and Pickel 1990), and NMDAR and D1R are coexpressed in MSNs (Fiorentini 2003; Hara and Pickel 2005). MSN NMDAR activation was shown to recruit functional D1Rs to the membrane surface (Scott et al. 2002); and, conversely, D1R activation can induce NMDAR trafficking (Dunah and Standaert 2001). Finally, NMDAR antagonism blocks D1R-induced stimulation of striatal cells (Konradi et al. 1996), and drug-induced increases in ERK signaling in the NAc requires both NMDARs and D1Rs (Valjent et al. 2000, 2004, 2005). Together, these findings point to ERK as a convergence point between glutamatergic and dopaminergic signals in the NAc; our data suggest that this aspect of ERK also applies to the enzyme's activation by conditioned cues.
Our findings complement numerous electrophysiological studies showing that natural reward- or drug-paired cues can change neuronal firing in the NAc (Carelli 2002; Yun 2004; Roitman et al. 2005; Day et al. 2006; Wan and Peoples 2006), and that disruption of dopaminergic input to the NAc prevents cue-induced changes in NAc cell firing (Yun 2004). It therefore is tempting to speculate that the same population of cells that exhibit increases in the firing rate in response to an appetitive CS also exhibit an increase in ERK activation. Thus, pERK immunoreactivity may prove to be a tool for identification of the specific NAc cells that respond to an appetitive CS in an excitatory manner.
CREB is an important transcription factor involved in regulating many processes, including synaptic plasticity, memory, and addiction (for review, see Nestler 2004; Carlezon et al. 2005). Phosphorylation on serine-133 activates CREB and allows for gene transcription (Gonzalez and Montminy 1989; Sheng et al. 1991; Impey et al. 2004), (for review, see Carlezon et al. 2005). In the NAc, CREB was shown to be activated after exposure to emotionally salient stimuli, drugs of abuse, and drug-associated cues (Miller and Marshall 2005; Kuo et al. 2007; Tropea et al. 2008; Muschamp et al. 2011). We previously showed that CREB is also phosphorylated after exposure to appetitive cues (Shiflett et al. 2009; this work). Here, we demonstrate that CS-evoked CREB activation is mediated through the NMDAR-D1R/ERK signal transduction pathway. Our findings align with studies showing increased striatal CREB phosphorylation in response to application of NMDA (Das et al. 1997; Liu and Graybiel 1998) or activation of D1Rs (Das et al. 1997; Liu and Graybiel 1998; Haberny and Carr 2005), and observations that ERK inhibition prevents D1R-stimulated increases in pCREB (Haberny and Carr 2005). Interestingly, cocaine-induced increases in CREB phosphorylation were found to be associated with increases in ERK but not CaMKIV activation in the NAc (Mattson et al. 2005). Our findings suggest that reward-predictive stimuli trigger a similar signaling pathway in NAc cells as do rewards.
Not only can CSs elicit conditioned responses, they can also modulate reward-seeking behavior. In the case of appetitive CSs, the incentive motivational effect has been implicated in relapse to drug taking (Grimm et al. 2002; Corbit and Janak 2007; LeBlanc et al. 2012; Gipson et al. 2013). We previously showed that NAc ERK activation is critical for CS modulation of reward seeking (Shiflett et al. 2008). D1R blockade within the NAc was also shown to interfere with CS modulation of reward seeking (Dickinson et al. 2000; Lex and Hauber 2008). Our current findings suggest that disruption of the incentive motivational effect of a CS on reward seeking by D1R blockade may be attributable to the absence of CS-evoked ERK activation in the presence of the receptor antagonist. Furthermore, our findings lead to the prediction that NMDAR blockade in the NAc interferes with CS control of reward seeking. Findings of blunted responding for cue-predicted reward in the presence of accumbal NMDAR blockade are consistent with this prediction (Bespalov and Zvartau 1996; Giertler et al. 2003).
Conclusions
We have identified upstream mediators and a downstream effector of CS-driven ERK signaling in the NAc. CSs can cause relapse to drug use. Knowledge of the signaling pathways evoked by CSs and implicated in their behavioral consequences may prove useful in the development of strategies for curbing excessive cue control of reward seeking.
Materials and Methods
Animals
A total of 55 male rats (Sprague–Dawley; 275–300 g at arrival; Hilltop Lab Animals, Scottdale, PA) were used. Animals were housed singly under 12/12-h conditions (lights on at 7:00 a.m.). Until recovery from surgery (see below), rats had ad libitum access to standard rat chow (LabDiet IsoPro Rodent Chow) and tap water. Five to 7 d after surgery, rats were placed on a restricted diet of 15–20 g rat chow per day to maintain their body weight at ∼85% of similar-aged free-feeding rats. A schema of the experimental phases described in detail below is depicted in Figure 1A. All procedures were approved by the University of Pittsburgh's Institutional Animal Care and Use Committee, and were in accordance with the Guide for the Care and Use of Laboratory Animals commissioned by the National Institutes of Health.
Surgical procedures
Within 2–3 d after arrival, rats were implanted bilaterally with cannulae aimed at the NAc, as described previously (Shiflett et al. 2008). Briefly, rats were anesthetized with ketamine (Ketaset, 85 mg/kg intraperitoneal, i.p.; Fort Dodge Animal Health) and xylazine (AnaSed, 8 mg/kg i.p.; Lloyd Laboratories), and positioned in a stereotaxic apparatus. An incision was made to expose the skull, and two small holes were drilled (coordinates relative to bregma: 1.3 mm anterior, ±1.7 mm lateral) (Paxinos and Watson 2007). A 26-gauge stainless steel guide cannula (6 mm long; Plastics One) was lowered into each hole 5.4 mm ventral relative to dural surface, and fixed to the skull with cyanoacrylate glue and dental cement before the skin was sutured closed. Rats were given acetaminophen orally (300 mg/kg/day) for 2 d after surgery.
Behavioral procedures
Behavioral experiments were conducted in the Rodent Behavior Analysis Core of the University of Pittsburgh Schools of Health Sciences. Procedures were as described previously (Shiflett et al. 2008, 2009; Remus and Thiels 2013). Training took place in operant chambers (30 × 23 × 23 cm; Med Associates) equipped with a house light, a floor with metal bars, a loudspeaker that delivered a 3-kHz/80-dB tone when activated, and a food cup attached to a pellet dispenser. When initiated, the pellet dispenser released a single 45-mg dustless food pellet (BioServe) into the food cup. An infrared photo beam, emitted and detected via a source and a sensor placed immediately to the left and the right side of the food cup, was used to measure head insertions into the cup (i.e., food cup approaches). Each operant chamber was housed in a sound-attenuating cubicle with a background noise-generating fan. Chambers were controlled by and data were recorded with Med-PC software (Med Associates).
About 4 d after onset of food-restricted diet, rats were habituated to the conditioning chamber in a single 30-min session with the house light illuminated. Beginning the next day, rats received a daily session of appetitive Pavlovian conditioning for five consecutive days. Sessions began with illumination of the house light and lasted 45–50 min. A 90-sec, 3-kHz/80-dB tone served as the CS, and was presented a total of eight times with a variable inter-trial interval (ITI; tone off → tone on, mean = 4 min). For rats in the experimental condition (Tone + Food group; T + F), three food pellets were delivered on a random VT20 sec schedule during the last 60 sec of each CS (Fig. 1B, left). For rats in the control group (Tone-only group; TO), which served to assess basal ERK activation, the delivery of food pellets was omitted (Fig. 1B, right). Testing occurred 24 h after the last Pavlovian conditioning session. The test session began with the illumination of the house light and lasted ∼15 min. A total of four tone presentations occurred at a variable ITI (mean = 4 min). During training and testing, food cup approaches were recorded during the first 30 sec of the tone (when no pellets were delivered) and during 30 sec preceding tone onset (i.e., during the ITI; preCS). Immediately after the test session, rats were anesthetized with chloral hydrate (300 mg/kg, i.p.; Sigma-Aldrich; dissolved in 150 mM NaCl) and either decapitated or transcardially perfused with 150 mM NaCl, followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). In the case of decapitation (n = 46 rats), brains were removed rapidly, briefly immersed in isopentane (Acros Organics) on dry ice, and stored at −80°C until Western blot analysis (see below). In the case of transcardial perfusion (n = 9 rats), brains were removed, placed in a 20% sucrose solution (dissolved in PB) until they sank, frozen, sectioned at a thickness of 40 μm on a cryostat at −16°C, and stored in cryopreservative at −20°C until immunohistochemical staining (see below).
Intra-NAc microinfusions
To investigate the role of specific receptors in CS-evoked ERK activation and the role of ERK in CS-evoked CREB phosphorylation, rats received intra-NAc infusions of specific reagents shortly before testing as described below (see Fig. 1C for representative cannula placements). Unilateral drug infusions were combined with unilateral vehicle infusions in the same animal, to allow for intra-individual comparisons of NAc tissue (drug versus vehicle). To familiarize rats with the infusion procedure, rats received a mock infusion 2–3 d prior to the test day. Wire stylets were removed from the guide cannulae, short infusion cannulae (33 gauge, projecting 0.5 mm past the guide, Plastics One; attached via PVC tubing to a Hamilton syringe) were inserted, and 0.5 µL of 150 mM NaCl solution was infused bilaterally at a rate of 0.5 µL/min. Infusion cannulae were left in place for an additional minute. Microinfusions on the test day were conducted in the same manner, except that (1) the infusion cannulae extended 2.2 mm past the tip of the guide and into the NAc and (2) the infusates were as follows: To examine the contribution of NMDARs, rats (n = 9 T + F, 10 TO) received immediately before testing the NMDAR antagonist APV (Tocris Bioscience; 2 mg/mL dissolved in 150 mM NaCl; final dose: 1 µg) in one NAc and an equal volume (0.5 µL) of vehicle in the other. To examine the contribution of D1-type dopamine receptors, rats (n = 6 T + F, 6 TO) received immediately before testing the D1R antagonist R(+)-SCH-23390 (SCH; Sigma-Aldrich; 0.6 mg/mL dissolved in 150 mM NaCl; final dose 0.3 µg) in one NAc and an equal volume (0.5 µL) of vehicle in the other. To determine whether CS-evoked increases in ERK and CREB phosphorylation are linked, rats (n = 8 T + F, 7 TO) received 30 min before testing the specific mitogen-activated protein kinase kinase/ERK inhibitor U0126 (Millipore; 2 mg/mL dissolved in 50% dimethyl sulphoxide [DMSO; Sigma-Aldrich]/50% 150 mM NaCl [50% DMSO]; final dose: 1 µg) in one NAc and an equal volume (0.5 µL) of vehicle in the other. The side receiving drug was counterbalanced across subjects. Correct cannulae placements were verified visually when excising tissue samples for Western blot analysis or with immunohistochemical staining (see below). Examples are depicted in Figure 1C.
Western blot analysis
As described previously (Shiflett et al. 2008, 2009; Remus and Thiels 2013), NAc tissue samples were excised from three consecutive 400-µm-thick sections using a tissue punch (diameter = 2 mm; Fine Science Tools) placed over the NAc so as to include about equal parts of the NAc core and shell. Tissue samples were homogenized in a buffer containing 150 mM NaCl, 1 mM EDTA, 50 mM Tris (pH 7.4), 0.05% SDS, 1% Triton X-100, 1 mM dithiothreitol (DTT), 2 mM sodium fluoride (NaF), 1 mM orthovanadate, 2 mM sodium pyrophosphate, 100× protease inhibitor cocktail, and 1 mg/mL pepstatin (Sigma-Aldrich, Millipore, Bio-Rad, Fisher Scientific). Homogenate was centrifuged for 15 min at 14,000 rpm, the supernatant was collected, and protein concentration was determined in triplicates using a bicinchoninic acid assay (Pierce BCA Protein Assay, Fisher Scientific and 4% Copper(II) sulfate solution, Sigma-Aldrich). Samples were diluted to a uniform protein concentration with homogenization buffer and sample buffer containing 2.5 M Tris (pH 6.8), 40% glycerol, 8% SDS, 0.05% bromophenol blue, and 30 mg/mL DTT (Sigma-Aldrich, Bio-Rad, Fisher Scientific), and heated to 95°C for 5 min. Fifty micrograms of protein/sample were loaded for resolution by SDS-PAGE and electrophoretically transferred to Immobilon membranes (Fisher Scientific). For pERK analyses, membranes were incubated for 1 h at room temperature (RT) in a Tris-buffered saline (TBS) (0.05 M Tris [pH 7.9], 0.15 M NaCl) plus 0.1% Tween 20 (Sigma-Aldrich) solution (TBST) containing 5% dried nonfat milk, and then incubated overnight with an antibody that specifically recognizes T-202/183- and Y-204/185-phosphorylated, i.e., active p44/42 MAPK (pERK, 1:2500 dilution in 5% bovine serum albumin [BSA; dissolved in TBST]). Antigen binding was visualized with an HRP-linked secondary antibody (anti-rabbit, 1:5000 dilution in 5% milk) and enhanced chemiluminescence reagent (Lumiglo, Cell Signaling). Blot images were captured with a CCD camera (Hamamatsu Photonics) and analyzed using densitometry software (UVP Labworks). Membranes were stripped by incubation at 50°C for 45 min in a solution containing 62.5 mM Tris (pH 6.7), 2% SDS, and 0.62% β-mercaptoethanol, blocked in 5% milk, and reprobed with an antibody that specifically recognizes phosphorylated and unphosphorylated, i.e., total p44/42 MAPK (tERK, 1:2500 dilution in 5% BSA). Antigen binding (secondary antibody: anti-rabbit, 1:5000 dilution in 5% milk) was visualized and blot images captured as described above. For pCREB analyses, membranes were cut horizontally at 50 kDa, and both halves were incubated for 1 h at RT in TBST containing 5% milk. The top half of each membrane was incubated overnight with an antibody that specifically recognizes neuN (protein loading control; 1:5000 dilution in 5% BSA), and the bottom half with an antibody that specifically recognizes S-133 pCREB (1:1000 dilution in 5% BSA). Antigen binding was visualized (secondary antibody for neuN: anti-mouse, 1:5000 dilution in 5% milk; for pCREB: anti-rabbit, 1:5000 dilution in 5% milk) and blot images captured as described above. Antibodies were obtained from Cell Signaling (pERK, tERK, anti-rabbit, and anti-mouse) and Millipore (pCREB and neuN).
Immunohistochemistry
Nine additional T + F rats were trained, infused, and tested as described above, to conduct immunohistochemical analyses. Three rats were infused with vehicle/APV, three rats with vehicle/SCH, and three rats with vehicle/U0126. We probed coronal tissue sections for pERK1/2 or pCREB immunoreactivity as described previously (Shiflett et al. 2008, 2009; Remus and Thiels 2013). Briefly, free-floating sections were brought to RT over 30 min, washed with sequential rinses in 50 mM TBS (pH 7.6) on a rocker table, pretreated with an antigen retrieval Tris–Tween buffer at 80°C for 20 min, rinsed again several times in 50 mM TBS, and then bathed in 50 mM TBS containing 0.4% Triton X-100 and 10% normal goat serum at RT for 1 h. Consecutive serial sections were incubated with an antibody that selectively recognizes either T-202/183- and Y-204/185-phosphorylated ERK1/2 (pERK, 1:1000 dilution; Cell Signaling) or S-133 pCREB (1:1000 dilution; Millipore) in 50 mM TBS containing 0.4% Triton X-100 and 10% normal goat serum at 4°C for 48–72 h. All perfusion solutions, rinses, and incubation solutions before and during incubation with the primary antibody contained 1 mM NaF and 1 mM orthovanadate, to prevent dephosphorylation of antigen. After washing in 50 mM TBS over 45 min, sections were incubated in biotinylated goat anti-rabbit (Jackson ImmunoResearch Laboratories) in 50 mM TBS containing 0.4% Triton X-100 for 90 min, followed by incubation in avidin–biotin conjugate (ABC; 1:500 for each A and B reagent; Vector Laboratories) in 50 mM TBS containing 0.4% Triton X-100 for 90 min at RT on a rotator. After washing sections in 50 mM TBS, immunostaining was visualized with a substrate solution containing diaminobenzidine 0.6 mg/mL, 0.03% H2O2, and 4 mM NiCl2 in TBS. Sections were washed several times and then mounted on gelatin-coated slides, dried at RT, dehydrated in ethanol, cleared in xylene, and coverslipped with Cytoseal 60 (Fisher Scientific). Images of sections stained for pERK1/2 or pCREB were captured using a 10× objective and a digital camera (Micrometrics 3.2 MP) mounted on a light microscope (Leitz Orthoplan 2). The number of pERK-immunopositive cells or density of pCREB-immunopositive nuclei in the NAc were estimated from digital images using NIH ImageJ software.
Statistical analyses
For Pavlovian approach behavior, the number of photo beam breaks during the CS and preCS intervals was summed for each session. A difference score was calculated for each animal by subtracting CS from preCS beam-break rates for that session. Difference scores, a measure of discriminated approach, were compared using analyses of variance (ANOVAs) with group as between-subject factor and training day as within-subject factor, followed by post hoc pairwise comparisons using t-tests with Bonferroni's correction. For Western blot analysis, ratios of pERK immunoreactivity to tERK immunoreactivity or pCREB immunoreactivity to neuN immunoreactivity were calculated for each sample and then normalized to a loading control included on each membrane. Comparisons were made using ANOVAs with group as between-subject factor and drug condition as within-subject factor, and followed by post hoc t-tests with Bonferroni's correction (Shiflett et al. 2008, 2009; Remus and Thiels 2013). Statistical analyses were performed using the SPSS software package version 19.0 (SPSS). Significance level was set to α ≤ 0.05.
Supplementary Material
Acknowledgments
Support for this work is from National Institutes of Health grants NS0464023, DA027679, and NCRR-UL1RR024153; a grant from the Office of the Senior Vice Chancellor, Health Sciences, University of Pittsburgh (to E.T.); and National Institutes of Health grant 1T32DA031111 (to E.K.Z.K.). We thank Dr. Jessica N. Porter for fruitful discussions and John M. Roberts for technical assistance with the studies described here.
Footnotes
[Supplemental material is available for this article.]
Article is online at http://www.learnmem.org/cgi/doi/10.1101/lm.035113.114.
References
- Bassareo V, Di Chiara G. 1999. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience 89: 637–641 [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Herve D, Valjent E, Girault JA. 2008. Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28: 5671–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov AY, Zvartau EE. 1996. Intraaccumbens administration of NMDA receptor antagonist (+/−)-CPP prevents locomotor activation conditioned by morphine and amphetamine in rats. Pharmacol Biochem Behav 55: 203–207 [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Valjent E, Santini E, Hervé D, Girault J-A, Fisone G. 2008. Delayed, context- and dopamine D1 receptor-dependent activation of ERK in morphine-sensitized mice. Neuropharmacology 55: 230–237 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. 2002. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321–352 [DOI] [PubMed] [Google Scholar]
- Carelli RM. 2002. Nucleus accumbens cell firing during goal-directed behaviors for cocaine vs. ‘Natural’ reinforcement. Physiol Behav 76: 379–387 [DOI] [PubMed] [Google Scholar]
- Carlezon W Jr, Duman R, Nestler E. 2005. The many faces of CREB. Trends Neurosci 28: 436–445 [DOI] [PubMed] [Google Scholar]
- Cheng JJ, De Bruin JPC, Feenstra MGP. 2003. Dopamine efflux in nucleus accumbens shell and core in response to appetitive classical conditioning. Eur J Neurosci 18: 1306–1314 [DOI] [PubMed] [Google Scholar]
- Corbit LH, Janak PH. 2007. Ethanol-associated cues produce general Pavlovian-instrumental transfer. Alcohol Clin Exp Res 31: 766–774 [DOI] [PubMed] [Google Scholar]
- Das S, Grunert M, Williams L, Vincent SR. 1997. NMDA and D1 receptors regulate the phosphorylation of CREB and the induction of c-Fos in striatal neurons in primary culture. Synapse 25: 227–233 [DOI] [PubMed] [Google Scholar]
- Dash PK, Karl KA, Colicos MA, Prywes R, Kandel ER. 1991. Camp response element-binding protein is activated by Ca2+/calmodulin- as well as camp-dependent protein kinase. Proc Natl Acad Sci 88: 5061–5065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Carelli RM. 2007. The nucleus accumbens and Pavlovian reward learning. Neuroscientist 13: 148–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Wheeler RA, Roitman MF, Carelli RM. 2006. Nucleus accumbens neurons encode Pavlovian approach behaviors: evidence from an autoshaping paradigm. Eur J Neurosci 23: 1341–1351 [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. 2007. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10: 1020–1028 [DOI] [PubMed] [Google Scholar]
- Dickinson A, Smith J, Mirenowicz J. 2000. Dissociation of Pavlovian and instrumental incentive learning under dopamine antagonists. Behav Neurosci 114: 468–483 [DOI] [PubMed] [Google Scholar]
- Dunah AW, Standaert DG. 2001. Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J Neurosci 21: 5546–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes WK. 1948. Discriminative conditioning. II. Effects of a Pavlovian conditioned stimulus upon a subsequently established operant response. J Exp Psychol 38: 173–177 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. 2005. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW. 1999. Associative processes in addiction and reward: the role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877: 412–438 [DOI] [PubMed] [Google Scholar]
- Fasano S, D'antoni A, Orban PC, Valjent E, Putignano E, Vara H, Pizzorusso T, Giustetto M, Yoon B, Soloway P, et al. 2009. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry 66: 758–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. 2006. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology 31: 2660–2668 [DOI] [PubMed] [Google Scholar]
- Fiorentini C. 2003. Regulation of dopamine D1 receptor trafficking and desensitization by oligomerization with glutamate N-methyl-D-aspartate receptors. J Biol Chem 278: 20196–20202 [DOI] [PubMed] [Google Scholar]
- Fricks-Gleason AN, Marshall JF. 2011. Role of dopamine D1 receptors in the activation of nucleus accumbens extracellular signal-regulated kinase (ERK) by cocaine-paired contextual cues. Neuropsychopharmacology 36: 434–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G, Veitch K, Ho LK, Cruise L, Morris BJ. 2001. Activation of p44/p42 MAP kinase in striatal neurons via kainate receptors and PI3 kinase. Brain Res Mol Brain Res 89: 126–132 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Miyachi S, Paletzki R, Brown P. 2002. D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J Neurosci 22: 5042–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giertler C, Bohn I, Hauber W. 2003. The rat nucleus accumbens is involved in guiding of instrumental responses by stimuli predicting reward magnitude. Eur J Neurosci 18: 1993–1996 [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. 2013. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron 77: 867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J, Valjent E, Caboche J, Herve D. 2007. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol 7: 77–85 [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Montminy MR. 1989. Cylic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell 59: 675–680 [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Yamamoto KK, Fischer WH, Karr D, Menzel P, Biggs W III, Vale W, Montminy MR. 1989. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature 337: 749–752 [DOI] [PubMed] [Google Scholar]
- Grimm JW, Shaham Y, Hope BT. 2002. Effect of cocaine and sucrose withdrawal period on extinction behavior, cue-induced reinstatement, and protein levels of the dopamine transporter and tyrosine hydroxylase in limbic and cortical areas in rats. Behav Pharmacol 13: 379–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Wright CI, Beijer AV, Voorn P. 1999. Convergence and segregation of ventral striatal inputs and outputs. Ann N Y Acad Sci 877: 49–63 [DOI] [PubMed] [Google Scholar]
- Haberny SL, Carr KD. 2005. Food restriction increases NMDA receptor-mediated calcium-calmodulin kinase II and NMDA receptor/extracellular signal-regulated kinase 1/2-mediated cyclic amp response element-binding protein phosphorylation in nucleus accumbens upon D-1 dopamine receptor stimulation in rats. Neuroscience 132: 1035–1043 [DOI] [PubMed] [Google Scholar]
- Hara Y, Pickel VM. 2005. Overlapping intracellular and differential synaptic distributions of dopamine D1 and glutamate N-methyl-D-aspartate receptors in rat nucleus accumbens. J Comp Neurol 492: 442–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Mccorkle SR, Cha-Molstad H, Dwyer JM, Yochum GS, Boss JM, Mcweeney S, Dunn JJ, Mandel G, Goodman RH. 2004. Defining the CREB regulon: a genome-wide analysis of transcription factor regulatory regions. Cell 119: 1041–1054 [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HBK, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. 2005. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Mol Brain Res 142: 134–138 [DOI] [PubMed] [Google Scholar]
- Jiao H, Zhang L, Gao F, Lou D, Zhang J, Xu M. 2007. Dopamine D(1) and D(3) receptors oppositely regulate NMDA- and cocaine-induced MAPK signaling via NMDA receptor phosphorylation. J Neurochem 103: 840–848 [DOI] [PubMed] [Google Scholar]
- Kelley A, Baldo B, Pratt W, Will M. 2005. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 86: 773–795 [DOI] [PubMed] [Google Scholar]
- Konradi C, Leveque JC, Hyman SE. 1996. Amphetamine and dopamine-induced immediate early gene expression in striatal neurons depends on postsynaptic NMDA receptors and calcium. J Neurosci 16: 4231–4239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y-M, Liang KC, Chen H-H, Cherng CG, Lee H-T, Lin Y, Huang AM, Liao R-M, Yu L. 2007. Cocaine-but not methamphetamine-associated memory requires de novo protein synthesis. Neurobiol Learn Mem 87: 93–100 [DOI] [PubMed] [Google Scholar]
- LeBlanc KH, Ostlund SB, Maidment NT. 2012. Pavlovian-to-instrumental transfer in cocaine seeking rats. Behav Neurosci 126: 681–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex A, Hauber W. 2008. Dopamine D1 and D2 receptors in the nucleus accumbens core and shell mediate Pavlovian-instrumental transfer. Learn Mem 15: 483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FC, Graybiel AM. 1998. Region-dependent dynamics of cAMP response element-binding protein phosphorylation in the basal ganglia. Proc Natl Acad Sci 95: 4708–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond P. 1983. Facilitation of instrumental behavior by a Pavlovian appetitive conditioned stimulus. J Exp Psychol Anim Behav Process 9: 225–247 [PubMed] [Google Scholar]
- Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. 2004. Regulation of MAPK/ERK phosphorylation via ionotropic glutamate receptors in cultured rat striatal neurons. Eur J Neurosci 19: 1207–1216 [DOI] [PubMed] [Google Scholar]
- Matthews RP, Guthrie CR, Wailes LM, Zhao X, Means AR, Mcknight GS. 1994. Calcium/calmodulin-dependent protein kinase types II and IV differentially regulate CREB-dependent gene expression. Mol Cell Biol 14: 6107–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. 2005. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem 95: 1481–1494 [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, et al. 2002. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron 34: 807–820 [DOI] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. 2005. Altered Fos expression in neural pathways underlying cue-elicited drug seeking in the rat. Eur J Neurosci 21: 1385–1393 [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY. 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97 [DOI] [PubMed] [Google Scholar]
- Muschamp JW, Van't Veer A, Parsegian A, Gallo MS, Chen M, Neve RL, Meloni EG, Carlezon WA. 2011. Activation of CREB in the nucleus accumbens shell produces anhedonia and resistance to extinction of fear in rats. J Neurosci 31: 3095–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. 2004. Molecular mechanisms of drug addiction. Neuropharmacology 47: 24–32 [DOI] [PubMed] [Google Scholar]
- Pascoli V, Besnard A, Hervé D, Pagès C, Heck N, Girault J-A, Caboche J, Vanhoutte P. 2011. Cyclic adenosine monophosphate-independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol Psychiatry 69: 218–227 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates, 6th ed. Academic Press/Elsevier, San Diego, CA [Google Scholar]
- Perkinton MS, Ip JK, Wood GL, Crossthwaite AJ, Williams RJ. 2002. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signaling to MAP kinase (Erk 1/2), Akt/PKB and CREB in striatal neurons. J Neurochem 80: 239–254 [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. 2003. Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618 [DOI] [PubMed] [Google Scholar]
- Remus ML, Thiels E. 2013. Stimulus-specific and differential distribution of activated extracellular signal-regulated kinase in the nucleus accumbens core and shell during Pavlovian-instrumental transfer. Brain Struct Funct 218: 913–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF. 2004. Dopamine operates as a subsecond modulator of food seeking. J Neurosci 24: 1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Carelli RM. 2005. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron 45: 587–597 [DOI] [PubMed] [Google Scholar]
- Schwarzschild MA, Cole RL, Meyers MA, Hyman SE. 1999. Contrasting calcium dependencies of SAPK and ERK activations by glutamate in cultured striatal neurons. J Neurochem 72: 2248–2255 [DOI] [PubMed] [Google Scholar]
- Scott L, Kruse MS, Forssberg H, Brismar H, Greengard P, Aperia A. 2002. Selective up-regulation of dopamine D1 receptors in dendritic spines by NMDA receptor activation. Proc Natl Acad Sci 99: 1661–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. 1990. In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527: 266–279 [DOI] [PubMed] [Google Scholar]
- Sgambato V, Pages C, Rogard M, Besson MJ, Caboche J. 1998a. Extracellular signal-regulated kinase (ERK) controls immediate early gene induction on corticostriatal stimulation. J Neurosci 18: 8814–8825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato V, Vanhoutte P, Pages C, Rogard M, Hipskind R, Besson MJ, Caboche J. 1998b. In vivo expression and regulation of Elk-1, a target of the extracellular-regulated kinase signaling pathway, in the adult rat brain. Journal of Neuroscience 18: 214–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz AJ, Greenberg ME. 1999. CREB: a stimulus-induced transcription factor activated by a diverse array of extracellular signals. Annu Rev Biochem 68: 821–861 [DOI] [PubMed] [Google Scholar]
- Sheng M, Thompson MA, Greenberg ME. 1991. CREB: a Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 252: 1427–1430 [DOI] [PubMed] [Google Scholar]
- Shiflett MW, Martini RP, Mauna JC, Foster RL, Peet E, Thiels E. 2008. Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. J Neurosci 28: 1434–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Mauna JC, Chipman AM, Peet E, Thiels E. 2009. Appetitive Pavlovian conditioned stimuli increase CREB phosphorylation in the nucleus accumbens. Neurobiol Learn Mem 92: 451–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto N, Elmer GI, Wang B, You ZB, Wise RA. 2013. Bidirectional modulation of cocaine expectancy by phasic glutamate fluctuations in the nucleus accumbens. J Neurosci 33: 9050–9055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GM, Huganir RL. 2004. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5: 173–183 [DOI] [PubMed] [Google Scholar]
- Totterdell S, Smith AD. 1989. Convergence of hippocampal and dopaminergic input onto identified neurons in the nucleus accumbens of the rat. J Chem Neuroanat 2: 285–298 [PubMed] [Google Scholar]
- Tropea TF, Kosofsky BE, Rajadhyaksha AM. 2008. Enhanced CREB and DARPP-32 phosphorylation in the nucleus accumbens and CREB, ERK, and GluR1 phosphorylation in the dorsal hippocampus is associated with cocaine-conditioned place preference behavior. J Neurochem 106: 1780–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. 2000. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci 20: 8701–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. 2004. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci 19: 1826–1836 [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, et al. 2005. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci 102: 491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte P, Barnier JV, Guibert B, Pages C, Besson MJ, Hipskind RA, Caboche J. 1999. Glutamate induces phosphorylation of Elk-1 and CREB, along with c-fos activation, via an extracellular signal-regulated kinase-dependent pathway in brain slices. Mol Cell Biol 19: 136–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR, Sebben M, Dumuis A, Bockaert J. 1998. Neurotransmitter regulation of MAP kinase signaling in striatal neurons in primary culture. Synapse 29: 29–36 [DOI] [PubMed] [Google Scholar]
- Wan X, Peoples LL. 2006. Firing patterns of accumbal neurons during a Pavlovian-conditioned approach task. J Neurophysiol 96: 652–660 [DOI] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. 1998. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol 18: 1946–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA. 2004. The ventral tegmental area is required for the behavioral and nucleus accumbens neuronal firing responses to incentive cues. J Neurosci 24: 2923–2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS. 2000. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci Biobehav Rev 24: 85–105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.