Abstract
Purpose of review
With important effects on neuronal lipid composition, neurochemical signaling and cerebrovascular pathobiology, docosahexaenoic acid (DHA), a n-3 polyunsaturated fatty acid, may emerge as a neuroprotective agent against cerebrovascular disease. This paper examines pathways for DHA accretion in brain and evidence for possible roles of DHA in prophylactic and therapeutic approaches for cerebrovascular disease.
Recent findings
DHA is a major n-3 fatty acid in the mammalian central nervous system and enhances synaptic activities in neuronal cells. DHA can be obtained through diet or to a limited extent via conversion from its precursor, α-linolenic acid (α-LNA). DHA attenuates brain necrosis after hypoxic ischemic injury, principally by modulating membrane biophysical properties and maintaining integrity in functions between pre-and post-synaptic areas, resulting in better stabilizing intracellular ion balance in hypoxic-ischemic insult. Additionally, DHA alleviates brain apoptosis, by inducing anti-apoptotic activities such as decreasing responses to reactive oxygen species, up-regulating anti-apoptotic protein expression, down-regulating apoptotic protein expression, and maintaining mitochondrial integrity and function.
Summary
DHA in brain relates to a number of efficient delivery and accretion pathways. In animal models DHA renders neuroprotection after hypoxic-ischemic injury by regulating multiple molecular pathways and gene expression.
Keywords: Docosahexaenoic acid, omega 3, polyunsaturated fatty acid, neuroprotection, hypoxic-ischemic brain
Introduction
Cerebrovascular disease encompasses diseases of the blood vessels supplying the brain, and is associated with stroke. In the US, stroke represents the third major cause of death in adults, affecting over 800,000 persons/year [1]. Ischemic stroke, the predominant stroke subtype in many populations, has defined risk factors that can, in part, be effectively reduced through preventive regimens [1–2]. Diet intervention is one example that may impact upon risk factors for stroke [3].
Some but not all human prospective studies indicate that frequent fish consumption may ameliorate the risk of stroke, especially ischemic stroke [4–7]. Long-chain n-3 polyunsaturated fatty acids (n-3 FA), especially eicosapentaenoic acid (EPA, C20:5 n-3) and docosahexaenoic acid (DHA, C22:6 n-3) which are found in high concentration in marine foods, are suggested to be the nutrients underlying neuroprotection [8–9]. Evidence from experimental studies both in humans and animal models demonstrates that n-3 FA modulate mechanisms of development and progression of stroke, with effects on membrane fluidity, cell signaling and gene transcription [10–12]. n-3 FA also decrease other stroke-related risk factors such as high blood pressure, arrhythmias, abnormal lipid profiles, platelet aggregation, endothelial function and atherosclerotic processes [13–16]. Of note, n-3 FA are catabolized to produce promisingly neuroprotective catabolites such as neuroprotectin D1 (NPD1)[17].
Studies on DHA-mediated neuroprotection have prompted the following questions: In the absence of dietary DHA, can cerebral DHA levels be maintained? By what mechanisms does DHA provide neuroprotection? The aim of the present review is to highlight recent findings on accretion of n-3 FA and how this might relate to protective effects after hypoxic ischemic injury (H/I) in brain. These findings might help guide the potential use of n-3 FA in prophylactic and therapeutic treatment of cerebrovascular disease.
Stroke outcomes and n-3 FA intakes in human
Table 1 summarizes six epidemiological studies, one meta-analysis and one interventional study on fish consumption and n-3 FA in humans. Some of these studies provide evidence for benefit of n-3 FA in affecting stroke outcomes [5, 8, 18]. Bouzan et al.[19] evaluated a dose-response relationship between fish consumption and stroke risk from their meta-analysis. Results indicated a small relative risk reduction after fish consumption compared to no fish consumption. The Japan EPA Lipid Intervention Study (JELIS) on cardiac events [20] also evaluated the effect of EPA on the primary and secondary prevention of stroke in hypercholesterolemic patients receiving statin only or statin with EPA. The results showed that in the primary prevention subgroup, EPA had no preventive effect on total stroke. Interestingly, in the secondary prevention subgroup, a significant 20% reduction in the recurrence of stroke in the EPA group was observed. Some studies do not demonstrate a beneficial association between fish consumption and stroke risk [7, 21–22]. Some of these studies were performed in Japanese and Swedish populations with high baseline intakes of fish and n-3 FA so that additional n-3 FA intakes might not be helpful. Studies of increased n-3 FA intakes in US, and some other populations appear to provide mild to moderate protection. Thus, evidence is mixed as to the possible benefits of n-3 FA rich diets or n-3 FA supplements on stroke outcomes in humans.
Table 1.
Summary of studies reporting n-3 FA or fish intake and stroke outcome in human
| Author | Study design, sample size | Results (RR, HR, OR;CI) |
|---|---|---|
| Mozaffarian et al.[5] | Prospective cohort study in US of 4,775 adults aged 65 years or older and free of cerebrovascular disease at baseline, 12 years of follow up. | Inverse correlation between consumption of tuna/other kinds of fish and total stroke (p = 0.04) and ischemic stroke (p = 0.02), with 27% lower risk of ischemic stroke with intakes of 1 to 4 times per week (HR, 0.73; 0.55 – 0.98) and 30% lower risk with intakes of 5 or more times per week (HR, 0.70; 0.50 – 0.99) compared with an intake of less than once per month. |
| Yamagishi et al.[7] | Prospective cohort study in Japan of 57,972 men and women aged 40 to 79 years, 12.7 years of follow up. | Neither fish nor n-3 FA dietary intake was associated with differences in mortality from stroke. |
| He et al. [8] | Prospective cohort study in US of 43,671 men aged 40 to 75 years, 12 years of follow up. | Inverse association between fish consumption intake at least 1/mo and risk of ischemic stroke (RR 0.56; 0.38 – 0.83). No significant associations between fish or n-3 FA intake and risk of hemorrhagic stroke. |
| Sauvaget et al. [18] | Prospective cohort study in Japan of 15,350 men (mean age 54 years) and 24,999 women (mean age 58 years), 16 years of follow up. | Inverse association between fish consumption intake at least 2 time/week and risk of hemorrhagic stroke (RR 0.85; 0.75 – 0.97). No significant associations with fish consumption and risk of ischemic stroke. |
| Bouzan et al. [19] | Meta-analysis from five prospective cohort studies and one case-control study from US and Spanish population. | Fish consumption was associated with 12% relative risk reduction of stroke compared to no fish consumption. Incremental fish intake conferred no additional benefits. |
| Tanaka et al. [20] | Intervention study in Japan (subanalysis of the JELIS Trial) of 18,645 hypercholesterolemia adults (mean aged 60 years). Paticipants received statin only (no EPA) or statin with EPA for 5 years. | No significant difference in total stroke incidence (HR, 1.08; 0.95 – 1.22) was observed between the no EPA and the EPA groups. In the secondary prevention subgroup, EPA supplements were associated with 20% relative reduction in recurrent stroke (HR, 0.80; 0.64 – 0.997). |
| Nakamura et al.[21] | Retrospective cohort study in Japan of 3,945 men and 4,934 women aged 30 years or more, 19 years of follow up. | No significant associations between fish consumption and risk of stroke. |
| Wennberg et al.[22] | Case–control study in northern Sweden of 369 stroke cases and 738 matched controls (mean age 55 years). | No significant associations between either EPA or DHA and risk for strokes in men (OR 1.08; 0.92–1.28) and women (OR 0.98; 0.81– 1.17). |
HR, hazard ratio; RR, relative risk; OR, odds ratio
Polyunsaturated fatty acids in brain
Brain contains the highest concentration of lipids of all organs except adipose tissue. More than one third of these lipids are very long chain polyunsaturated acids such as DHA and arachidonic acid (AA, 20:4 n-6) [23]. DHA is the most abundant n-3 FA in the mammalian central nervous system, specifically concentrating in lipid bilayer membranes of brain grey matter and retinal membranes [24–25]. The incorporation of n-3 FA into neuronal membranes leads to increased membrane fluidity which can increase the number and affinity of receptors in synaptic regions and improve neurotransmission [26]. The levels of DHA in the brain increase during development [27] and decrease with aging [24]. When n-3- and n-6 FA-containing foods are consumed, n-3 and n-6 FA interact and may compete with each other in fatty acid metabolic pathways and in pathways of incorporation into cells, leading to varying levels in brain tissues and other organs [25]. In animal models and perhaps humans, low or very low dietary consumption of n-3 FA or low plasma DHA concentration is associated with impaired neurogenesis, altered metabolism of several neurotransmitters, membrane receptors, gene expression and impaired learning performance in the developing brain and in aging [25, 28–31].
n-3 FA levels in brain
DHA, and also AA, must be obtained through the diet or via conversion from their respective shorter-chain essential fatty acid precursors, α-linolenic acid (α-LNA, 18:3 n-3) and linoleic acid (LA, 18:2 n-6), respectively (Fig. 1). These precursors cannot be synthesized de novo in vertebrate tissue due to the absence of the required 12- and 15-desaturase enzymes [34••]. In mammals both α-LNA and LA can be physiologically metabolized into longer carbon chains via desaturation and elongation pathways by using common enzymes (Fig. 1). The conversion of dietary α-LNA into EPA and DHA is inefficient [10, 35]. For example, supplementing α-LNA during pregnancy does not increase circulating DHA levels in maternal or newborn infant blood lipids [35], while dietary intakes of DHA positively relate to blood circulating DHA levels [36]. Thus, if an individual’s intake of DHA-containing food is low, how does the brain conserve DHA at levels that allow the brain to have normal function? Also, how does brain DHA accretion depend upon dietary FA composition and the liver’s ability to convert α-LNA to DHA? In animal models high intakes of LA impede desaturation of α-LNA into EPA and further into DHA, and increase docosatetraenoic acid (22:4 n-6) and docosapentaenoic acid (22:5 n-6) levels, which leads to reduced accretion of DHA in the brain, retina and other organs [25]. Conversely, increasing α-LNA consumption appears to result in enhancement of DHA content [34••]. Liou et al. [37] demonstrate that decreasing LA consumption (to the ratio 4:1 of LA: α-LNA) results in higher EPA levels in plasma phospholipids and lower AA levels when compared to a higher LA diet. Thus, a number of papers suggest that decreasing amounts and ratios of LA intakes compared to α-LNA, EPA, and DHA intakes will lead to higher accretion of EPA and DHA [31, 38–39]. Evidence in animals demonstrates that high proportions of DHA are still present in brain phospholipids even in herbivores that consume no DHA and have low levels of plasma and liver DHA [25]. When intake of DHA is limited as shown by low circulatory levels of DHA, DHA can still be found at normal levels in brain tissue [25].
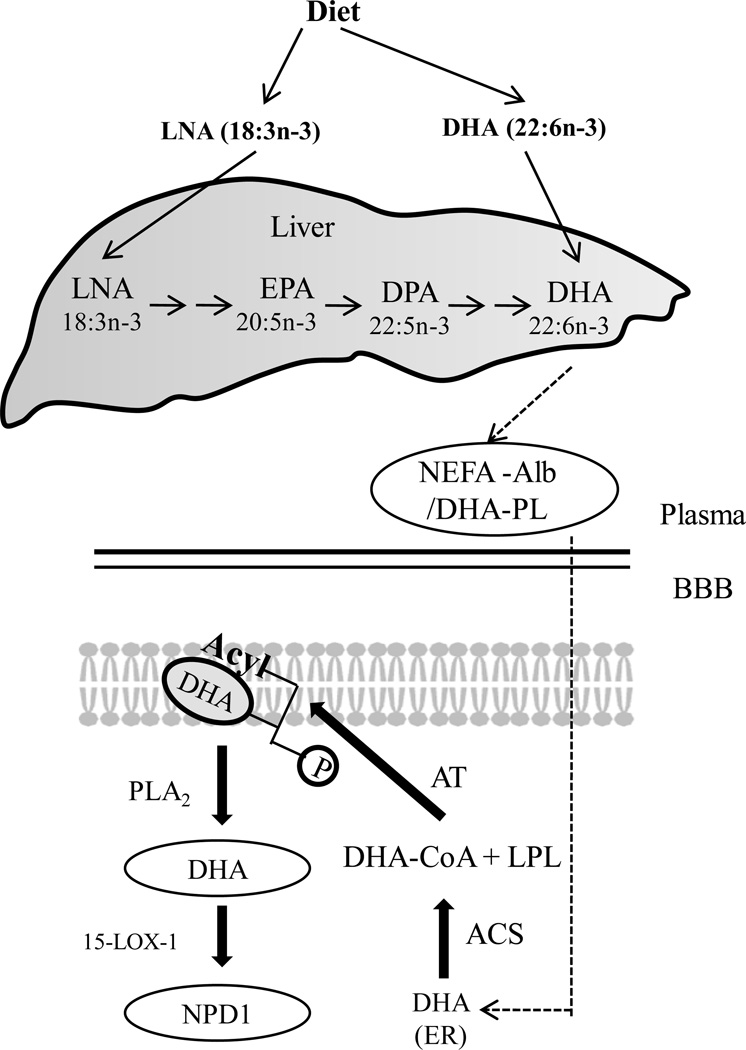
Figure 1. Extracellular trafficking of DHA.
The liver takes up DHA and α-linolenic acid (α-LNA) supplied by diet, and elongates and desaturates α-LNA to DHA, which is then delivered to blood as non-esterified fatty acid bound to albumin, or DHA-esterified phospholipids. After DHA is delivered to brain endoplasmic reticulum, it is activated to docosahexaenoyl-CoA by an acyl-CoA synthetase (ACS), then esterified into membrane lysophospholipid (LPL) by an acyltransferase (AT). After hypoxic-ischemic insult, free DHA is derived from membrane DHA stores, which are liberated via PLA2 cleavage and may be converted into the 10,17S docosatriene, NPD1, through an enzyme-mediated lipoxygenation via 15-LOX, or 15-LOX-like enzymes[32–33].
Can brain DHA levels be maintained in the absence of dietary DHA?
Because of the importance of DHA to brain metabolism and function, and the low conversion (<1%) of circulating α-LNA into DHA, humans should consume more DHA-containing marine foods [40–42]. β-oxidation of most dietary α-LNA for energy occurs before quantitatively desaturating to DHA [36]. DHA brain concentrations relate to diet and to liver synthesis [34••]. Rapoport et al.[34••] have concluded from 15-week dietary studies in rats that normal brain DHA levels can be maintained by hepatic conversion and secretion of DHA derived from circulating α-LNA if sufficient amounts of α-LNA are provided in the diet. The brain’s capacity for conversion is much less [43]. Compared to a diet high in DHA a DHA-free diet together with very low levels of α-LNA reduced DHA concentrations in brain phospholipids by 56%, and a DHA-free diet in combination with high α-LNA reduced the brain phospholipid DHA concentration by only 32%. This result is analogous to human studies in which plasma DHA in subjects who ate meat and fish was proportionately 31% and 59 % higher than in the vegetarians (who consumed no meat or fish) and vegans (who did not eat any animal-origin foods), respectively [43]. Potential underlying reasons why brain DHA concentration may be maintained even in prolonged periods of DHA-deprived settings include: (a) higher liver (but not brain) conversion of α-LNA via (increased expression of liver elongases 2, 5, Δ5 and Δ6 desaturases) [44–46], and (b) the half-life of brain DHA is prolonged by downregulating expression of DHA-metabolizing enzymes, namely Ca2+-independent phospholipase A2 (iPLA2) and cyclooxygenase-1[38, 47–48]. Also, n-3 FA deprivation leads to upregulating AA metabolism as shown by increased brain mRNA, protein and activity levels of AA-selective phospholipase A2 and COX-2- enzymes that are directly involved in brain AA metabolism [49]. These pathways can contribute to neuronal damage [38]. Still, some do not agree that DHA synthesis from α-LNA is insufficient to maintain adequate DHA levels [47, 50]. Therefore, it is possible that α-LNA will emerge as an alternative source of dietary n-3 FA in settings where dietary DHA is low.
Mechanisms of neuronal cell death following hypoxia-ischemia
There are multiple interrelated mechanisms that contribute to progressive brain damage during the initial hours after ischemic stroke. Ischemia leads to a reduction of up to 80% in cerebral blood flow in “core” tissue, leading to a disruption of delivery of glucose and oxygen to cells for ATP generation [51–52•]. The mechanisms of neuronal cell death in animals and humans after H/I can be categorized into two major phases, neuronal necrosis during acute insult, and neuronal apoptosis during a recovery period following circulatory restoration, often termed reperfusion injury [51, 53]. Necrosis relates to a passive process of cell swelling resulting from an imbalance of intracellular ions, especially Na+, K+, Ca2+ ions, with a disruption of cytoplasmic organelles, loss of membrane integrity resulting from overloads of Ca2+ influx to cells, and an activation of inflammatory enzymes such as cyclooxygenases. Processes leading to necrosis occur within in first few minutes after ischemia and continue for hours. Apoptosis is an active process, and includes cell shrinkage, nuclear pyknosis, chromatin condensation and genomic fragmentation [51]. These apoptotic processes occur within several hours to days/weeks after H/I. Neuroprotective studies in cell and animal models have been directed toward prevention of both mechanisms.
Neuroprotective properties of DHA
Exact mechanisms by which DHA exerts neuroprotective effects are not fully defined. DHA is believed to affect multiple pathways. It is generally assumed that the transport of fatty acids to brain occurs from the non-esterified fatty acid pool (NEFA) bound to serum albumin (Fig. 1). However, recent finding suggests that lipoproteins with DHA-acylated phospholipids may also selectively deliver DHA to brain and other tissues [32]. Also, lysophosphatidylcholine (LysoPC), produced by lecithin cholesterol acyl transferase (LCAT) may be another form of targeted transport of DHA to brain [54•].
Our understanding of the potential role of DHA in stroke originates from in vitro cell systems, and in vivo, animal models of hypoxic-ischemic brain [10, 17, 32–34••, 36, 39, 55–64]. The pathways and mechanisms presented below are summarized in Fig.2 and in Table 2. “Beneficial” effects of DHA in preventing and ameliorating stroke have been attributed to their stereospecific derivative, NPD1 [17, 32, 55–56]. “Positive” regulatory actions of DHA and NPD1 likely occur via several interdependent mechanisms (Fig. 2). Under basal conditions in brain, the amount of free DHA is low or negligible and its level is tightly controlled by the action of phospholipase A2 and DHA removal [55, 65]. During brain ischemia and reperfusion (I/R), in human brain cells [61, 66–67], DHA is cleaved from membrane phospholipids to free (unesterified) DHA by phopholipase A2, and free DHA is then converted into NPD1 by lipoxygenation catalyzed by 15-lipoxygenase-1 (15-LOX-1) [17]. NPD1 forms in the ipsilateral side of ischemic brain and peaks at 8 hours of reperfusion, and then decreases, and is still detected at 25 hours after reperfusion [66]. While DHA in brain can produce NPD1, additional DHA from acute administration is likely beneficial. For instance, one study indicated that cerebroventricular-infused NPD1 (400 ng over 48 h) in adult mice elicited substantial protection against ischemia and leukocyte infiltration compared to control mice [66]. Hence, it is possible that endogenously generated NPD1, released from cerebral membrane phospholipids, is not sufficient to counteract ischemic injury especially when the injury is severe [66]. In in vivo experiment-induced liver injury, dietary supplementation of mice with DHA increased hepatic formation of NPD1 [68]. However, it is not known if cerebral NPD1 can be supplied from DHA conversion in liver after brain ischemia.
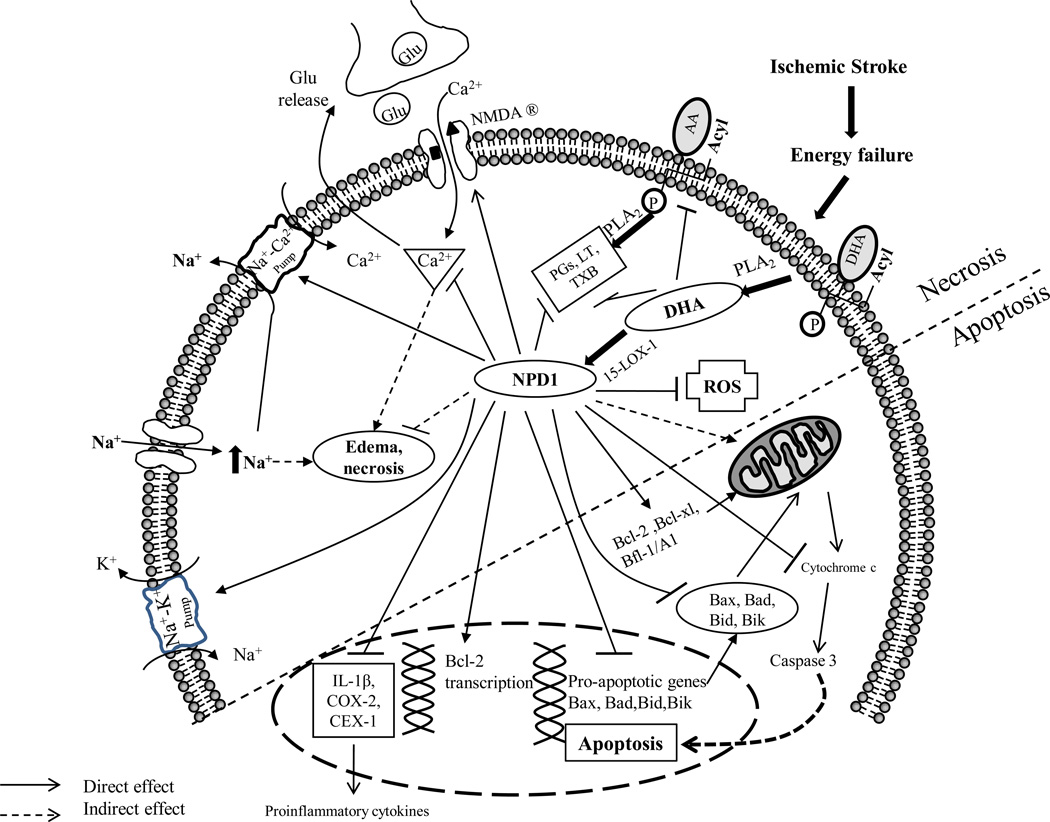
Figure 2. Neurochemical changes and mechanistic actions of DHA contributing to neuroprotection after cerebral ischemia.
After ischemic insult, DHA cleaved by phospholipase A2 synthesizes NPD1, a stereospecific derivative of DHA through an action of a lipoxygenase enzyme (15-LOX-1). NPD1 provides anti-necrotic and anti-apoptotic activities. In “anti-necrosis”, NPD1 and/or DHA enhances membrane integrity by mediating Na+-K+ pumps, Na+-Ca2+ pumps and N-methyl-D-aspartate (NMDA) receptors, leading to less influx of Na+, Ca2+, and improved glutamatergic synaptic activity. These properties prevent water from flowing into the cells due to osmotic gradients, contributing to less edema and anti-necrosis. In anti-apoptosis, NPD1 and/or DHA improves mitochondrial function for coping with reactive oxygen species (ROS) by increasing antioxidant activities, upregulates the Bcl-2 family of anti-apoptotic proteins including the members Bcl-2, Bcl-xL, Bcl-w, Bfl-1(A1), downregulates cytochrome c, caspase-3 in DHA-cultured neurons, and pro-apoptotic signaling proteins including Bax, Bad, Bid and Bik. In addition, the production of proinflammatory eicosanoids such as IL-1β, COX-2, CEX-1 is also impeded (references in text).
Table 2.
Pathways involved in stroke and possible roles of DHA. Relevant references are provided in the text.
| Pathway | Effect of DHA |
|---|---|
| Production of arachidonic acid (AA) | ↓ |
| • AA liberation from membrane phospholipids | ↓ |
| • Free AA levels | ↓ |
| • Pro-inflammatory eicosanoids | ↓ |
| Ionic homeostasis | ↑ |
| • Na+-K+ ATPase activity | ↑ |
| • Ionic imbalance (Na+, K+) | ↓ |
| • Increased intracellular Ca2+ | ↓ |
| • Cell edema | ↓ |
| Glutamate receptor and synaptic activity | ↓ |
| • Glutamate release | ↓ |
| • Impaired glutamate reuptake at presynaptic nerve endings | ↓ |
| • Glutamate receptors stimulation | ↓ |
| • Influx of Ca2+ into cells | ↓ |
| Apoptosis | ↓ |
| • Pro-apoptotic Bcl-2 gene induction | ↓ |
| • Anti-apoptotic Bcl-2 gene induction | ↑ |
| • Expression of anti-apoptotic Bcl-2 protein | ↑ |
| Mitochondrial function | ↑ |
| • Intracellular Ca2+ balance | ↑ |
| • Cytochrome c release | ↓ |
| • Pro-apoptotic caspases | ↓ |
| Formation of free radicals | ↓ |
| • ROS production | ↓ |
| • Anti-oxidant molecules | ↑ |
| Inflammatory mediators | ↓ |
| • Leukocyte infiltration | ↓ |
| • IL-1β expression | ↓ |
| • COX-2-induced prostaglandins | ↓ |
| • NF-kB activity | ↓ |
| • Microglial activation | ↓ |
| Cerebral infarction volume | ↓ |
Formation of NPD1 from DHA is upregulated by many factors such as increased oxidative stress, mediated by H2O2, tumor necrosis factor α [69], I/R[66], Ca2+ ionophore A23187[69], and interleukin (IL)-1β activity[56, 69]. Neuroprotective properties may be facilitated through anti-necrotic pathways such as decreasing the production of n-6 FA [58], enhancing function of Na+/K+ channels [39], enhancing affinity of receptors and improving neurotransmission [59, 70]. Furthermore, they may be mediated through anti-apoptotic functions via inducing anti-apoptotic proteins and inhibiting apoptotic proteins[32, 63, 71–72], improving mitochondrial function [56], diminishing formation of free radicals [63], and decreasing cell inflammation [63, 66], all resulting in the reduction of brain infarction [39, 57–58, 63, 73– 74].
Production of AA
AA competes with DHA for incorporation into cell membrane phospholipids. Under physiological conditions, PLA2 liberates AA from membrane phospholipids at a rate that balances the rate of AA reincorporation into the membranes, resulting in low levels of free AA [58]. AA is oxidized by lipooxygenases and further by cyclooxygenases producing prostaglandins (PG), leukotrienes (LT) thromboxanes (TX) and other bioactive eicosanoids, several of which have been implicated in brain damage following I/R [17, 75]. n-6 FA may negatively affect outcomes after ischemic insult or injury [58, 76]. Modification of dietary fatty acid composition by consuming DHA-containing diets or via DHA supplementation may increase DHA esterification into cellular phospholipids. In support of this latter finding, Cao et al. [58] found that in an animal model a 10-week supplement of ethyl-DHA significantly decreased the content of brain lipid AA; thereby diminishing postischemic AA-derived eicosanoid production, which was associated with preserved cerebral blood flow and attenuated cerebral edema.
Ionic homeostasis
With ischemic insult, there is an interruption of blood supply (followed by decreasing glucose and oxygen levels) to the brain that leads to ATP depletion. AA requires ATP for its metabolism after liberation from cell membranes. Hence, during ischemia, free AA and its metabolites accumulate in the cytoplasm, which induce pathophysiological events such as impairment of ion channels, and other membrane-associated enzymes, particularly Na+-K+ ATPase activity [75]. The fall in Na+-K+ ATPase activity also results from other causes, such as a decline of ATP content itself and increased production of other enzymatic inhibitors. Free AA and PG are examples of potent Na+-K+ ATPase inhibitors [60, 77]. The decline of Na+-K+ ATPase activity can also lead to ionic imbalance, causing cellular edema due to a rapid loss of K+ and influx of Na+ from neurons and neuronal depolarization. Increased intracellular Na+ causes cells to internalize Ca2+, contributing to intracellular Ca2+ overload [78]. Cao et al. [39] suggest that dietary DHA reduces postischemic cerebral edema by maintaining Na+-K+ ATPase function from declining after ischemia, as shown by lower intracellular Na+, but higher K+ content than those in vehicle-treated animals (Fig. 2).
Glutamate and glutamate receptors
Glutamate functions as a major neurotransmitter which is abundant in the central nervous system (CNS). Glutamate is involved in learning/memory, brain development and aging [70, 79]. However, increased amounts of glutamate in the synaptic cleft can cause neurotoxicity due to overstimulation of glutamate receptors [70, 80]. Glutamate levels are mediated by a number of receptors, such as N-methyl-d-aspartate (NMDA) receptors, which lead to both agonistic and antagonistic properties for glutamate. Following the rise in intracellular Ca2+ concentration after ischemic insult, excitatory amino acids including glutamate, are released from axon terminals. Impaired reuptake at presynaptic nerve endings into the extracellular space together with the continuous stimulation of the NMDA receptors of the agonist-operated Ca2+channel by glutamate also leads to accumulation of Ca2+ in cell cytoplasm. Ca2+ is also released into cytoplasm from mitochondria by the Na+/H+ -dependent antiport system and other organelles [78], which leads to irreversible brain damage [81]. Moreover, ischemia-induced glutamate release causes the activation of PLA2, which results in further membrane-bound phospholipid degradation and AA liberation [82]. Within the cytoplasm these free fatty acids undergo peroxidation by ROS produced from ischemic insult. In selected neurons, intracellular Ca2+ induces the production of nitric oxide (NO), a free radical that diffuses to adjacent cells susceptible to NO toxicity, and enhances glutamate release. All of these mechanisms can contribute to the cycle of cell necrosis and further the process of apoptosis [51].
Moreira et al.[70] reported that in DHA-treated rats glutamate equilibrium is better preserved than in DHA-deprived rats after ischemia. Cao et al.[59] demonstrated in chronic (but not acute) DHA-supplemented hippocampal neurons, spontaneous synaptic activity is significantly increased due to increased synapsins, and glutamate receptor subunit (NR and GluR) levels [59], suggesting a unique role of DHA in promoting active synapse formation and/or pre-synaptic neurotransmitter release. The mechanisms by which DHA can block ionotropic glutamate receptors are also supported by other studies [83–84]. As the synaptic activity is activated by chronic intakes of DHA, supplements of DHA in immature brain and stroke-related risk individuals may be an important aspect of ultimately enhancing excitatory synaptic activity for prevention of stroke.
Pro- and anti- apoptotic proteins
Key pro-apoptotic and anti-apoptotic members of Bcl-2 gene family regulate the fate of brain cells. These pro-apoptotic molecules induce, but anti-apoptotic molecules inhibit the release of mitochondrial-bound cytochrome c into cytosol. Cytochrome c activates pro-apoptotic caspases, such as caspase-3 and caspases-9, leading to apoptosis and cellular death [72]. DHA or NPD1 upregulates the Bcl-2 family of anti-apoptotic proteins including the members Bcl-2, Bcl-xL, Bcl-w, Bfl-1(A1), but downregulates caspase-3 and 9, apoptotic proteins in DHA cultured neurons, and pro-apoptotic signaling proteins including Bax, Bad, Bid and Bik [32, 67, 71]. Pan et al. [63] have found that both acute and chronic DHA pretreatment before ischemia insult attenuate cerebral-I/R-induced caspase-3 activity as well as oxidative burden as shown by the reduction of lipid peroxidation products that are capable of generating reactive oxygen free radicals. Surprisingly, acute DHA administration had little effect on expression of Bcl-2 proteins, but chronic administration showed higher levels of Bcl-2 proteins. In parallel with these benefits on Bcl-2 family gene expression, NPD1 impedes pro-inflammatory, COX-mediated production of eicosanoids such as PG, which can further prevent neuronal damage [71–72].
Mitochondrial function
Mitochondrial dysfunction likely plays a vital role in the pathophysiology of ischemic and traumatic brain injury [85–87•]. After stroke, mitochondrial function deteriorates as a direct result of impaired delivery of glucose and oxygen to the tissue and is further modified by changes in its properties that develop during I/R [52•]. Mitochondria are the principal buffers of intracellular Ca2+ and can become overloaded by cytoplasmic Ca2+ flooding secondary to the opening of N-methyl-D-aspartate (NMDA) and voltage Ca2+ channels. In I/R mitochondria lose their ability to modulate excessive Ca2+ from cytoplasm, which further contributes to mitochondrial impairment. In addition, electron transport chain activity at the mitochondrial membrane deteriorates due to the release of cytochrome c through the outer mitochondrial membrane into the cytosol, and due to inhibition of mitochondrial enzymes such as pyruvate dehydrogenase. This is associated with loss of the metabolic cofactor NAD+ through the permeability transition pore (PTP) in the mitochondrial membrane [87•]. Cytochrome c release is also induced by ROS associated with the release of Bax, Bak pro-apoptotic proteins [88], which leads to activation of pro-apoptotic caspases, particularly caspase-3, resulting in chromatin condensation and DNA fragmentation [52•, 89]. ROS themselves are directly involved in oxidative damage such as lipid peroxidation, protein oxidation, protein nitrosylation/nitration, and nucleic acid damage in ischemic tissues, leading to cell death [52•].
Mitochondria regulate proapoptotic proteins such as cytochrome c, caspase-3 and -9, and apoptosis-inducing factor (AIF) [32, 90]. It is assumed that mechanisms by which NPD1/DHA offers neuroprotection for mitochondria involve induction of anti-apoptotic Bcl-2 proteins and inhibition of apoptotic Bcl-2 proteins. These actions lead to downward release of cytochrome c from mitochondria, thereafter caspase-3 levels decrease [56]. Moreover, high intracellular Ca2+ concentration and oxidative stress result in efflux of NAD+ to cell cytoplasm through the PTP, thereby reducing mitochondrial function [87•, 91]. Consequently, the ability to control intracellular Ca2+ via DHA may indirectly provide an addition protective effect from this pathway. AIF and perhaps other proteins, which act independently of caspases, are released from mitochondria to the nucleus not long after I/R, and induce chromatin condensation and DNA fragmentation. These proteins have been implicated in focal ischemic damage as well. But little is known about the effects of DHA on these proteins [39, 56].
Formation of free radicals
Under physiological conditions reactive oxygen species (ROS), produced within cytoplasm and mitochondria are demolished rapidly by endogenous antioxidants. However, during H/I, the generation of ROS found in cytoplasm is increased in excess of the protective ability of the cells. These oxygen free radicals contribute to tissue injury by activation of lipid peroxidation (producing malondialdehyde; MDA), inflammatory mediators and apoptosis [51, 81]. ROS could also result from depletion of antioxidant molecules such as reduced glutathione (GSH) and superoxide dismutase (SOD) [63]. Pan et al. [63] have shown that chronically (but not acutely) administered DHA could reduce MDA levels while enhancing SOD activity and increasing brain GSH levels after ischemic insult with a net result of reducing the oxidative burden in rat brain.
Inflammatory mediators
Leukocyte infiltration and products of pro-inflammatory gene expression are mediators of ischemic stroke damage [66]. Recently, it has been reported that in experimental stroke, DHA or NPD1 treatment is capable of decreasing polymorphonuclear leukocyte infiltration, and pro-inflammatory cytokines, such as interleukin 1 beta (IL-1β), IL-1 alpha (IL-1α), IL-6, inducible nitric oxide (NO) synthase and COX-2-induced PG, most of which are associated with AA liberation after I/R injury [63, 66, 92–93]. In addition, in vivo NPD1 potently inhibits nuclear factor kappa B (NF-kB), a transcription factor that plays an important role in inflammatory signaling pathways, controls several cytokines (e.g. IL-1, IL-2, IL-6, TNF-α), and inducible effector enzymes (e.g. inducible NO synthase and COX-2) [92–93]. NF-kB is activated in I/R conditions similar to high COX-2 expression, which in turn generates PGH2, the substrate for PG synthetases and a contributor to oxidative stress [66].
Cerebral infarction an DHA
The mechanisms described above suggest how acute or chronic administration of DHA may contribute to ameliorating brain infarction. Neuroprotective effects of DHA can be documented after chronic supplementation prior to cerebral I/R, and involve improvement of hemodynamics, blockade of glutamatergic transmission, reduction of eicosanoid production, and enhancement of antioxidative capacity [39, 57–58, 63, 73]. Pan et al. [63] have found that increases in DHA intake reduces post ischemic brain infarction and edema, whether from a single intraperitoneal injection of DHA (100 or 500 nmol/kg) 1 hour or 3 days prior to H/I, or from a daily intraperitoneal administration of DHA for 6 weeks prior to H/I. Likewise, other studies also confirm that repeated administration of n-3 FA exerts a beneficial effect on the ischemic brain under different protocols (eg. 6-week supplement [73], or 2- to 10-week gavages [39, 57–58, 74]).
The timing of DHA administration is likely important. In several animal models, DHA was given before and shortly after H/I insult; nevertheless, it may be difficult to apply these treatments in the context of acute stroke patients with a short therapeutic window. [62–63]. Of interest, other focal ischemia models have shown that benefit from DHA occurs if treatment takes place within 1–3 hours after ischemia [61– 63].
Conclusion
Maintaining ‘optimal’ DHA levels in brain phospholipids relates to efficient regulation of brain synthetic, delivery, and catabolic pathways. Mechanisms of action by which DHA protects against cerebrovascular disease involve multiple mechanisms ranging from ion channels, to nuclear receptors. DHA, and in part through NPD1, its derivative docosanoid, provide neuroprotection both by anti-necrotic and by anti-apoptotic mechanisms (Table 2). Most anti-necrotic mechanisms of DHA involving neuroprotection were defined using protocols of acute or short-term administration, while anti-apoptotic activities of DHA are based on those using chronic or longer-term applications. We might predict from the present review that there are promising indications that supplementation with DHA, and perhaps other n-3 FA such as EPA may provide neuroprotective benefits at different ages from low birth weight infants to the elderly. Use of DHA as a supplement from both before and after an ischemic insult may help in primary prevention and secondary prevention.
A number of issues deserve additional investigation. For instance, it is still unclear whether increased dietary intake of n-3 FA in the absence of n-3 FA deficiency provides additional neuroprotective actions. In addition, before recommendations for clinical use, the choice of n-3 FA, EPA vs. DHA for example and doses must be defined and optimized. Will EPA have similar neuroprotective effects as DHA? Still, a large body of data suggests that the efficacy of n-3 FA, especially DHA, in inhibiting a large number of adverse pathways involved in cerebrovascular disease may lead to its use as a bioactive therapeutic agent for ischemic stroke.
Acknowledgments
Supported in part by NIH grants HL 40404 (RJD), NS 056146 (VST)
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue.
- 1.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups. Circulation. 2007;115:e478–e534. doi: 10.1161/CIRCULATIONAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 2.Wei JW, Wang JG, Huang Y, et al. Secondary prevention of ischemic stroke in urban China. Stroke. 2010;41:967–974. doi: 10.1161/STROKEAHA.109.571463. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.He K, Song Y, Daviglus ML, et al. Fish consumption and incidence of stroke: a meta-analysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Longstreth WT, Jr, Lemaitre RN, et al. Fish consumption and stroke risk in elderly individuals: the cardiovascular health study. Arch Intern Med. 2005;165:200–206. doi: 10.1001/archinte.165.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 7.Yamagishi K, Iso H, Date C, et al. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 8.He K, Rimm EB, Merchant A, et al. Fish consumption and risk of stroke in men. JAMA. 2002;288:3130–3136. doi: 10.1001/jama.288.24.3130. [DOI] [PubMed] [Google Scholar]
- 9.Johnson EJ, Schaefer EJ. Potential role of dietary n-3 fatty acids in the prevention of dementia and macular degeneration. Am J Clin Nutr. 2006;83:1494S–1498S. doi: 10.1093/ajcn/83.6.1494S. [DOI] [PubMed] [Google Scholar]
- 10.Innis SM. The role of dietary n-6 and n-3 fatty acids in the developing brain. Dev Neurosci. 2000;22:474–480. doi: 10.1159/000017478. [DOI] [PubMed] [Google Scholar]
- 11.Youdim KA, Martin A, Joseph JA. Essential fatty acids and the brain: possible health implications. Int J Dev Neurosci. 2000;18:383–399. doi: 10.1016/s0736-5748(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 12.Salem N, Jr, Litman B, Kim HY, et al. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 13.Robinson JG, Stone NJ. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:39i–49i. doi: 10.1016/j.amjcard.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 14.Jung UJ, Torrejon C, Tighe AP, et al. n-3 Fatty acids and cardiovascular disease: mechanisms underlying beneficial effects. Am J Clin Nutr. 2008;87:2003S–2009S. doi: 10.1093/ajcn/87.6.2003S. [DOI] [PubMed] [Google Scholar]
- 15.Zuliani G, Galvani M, Leitersdorf E, et al. The role of polyunsaturated fatty acids (PUFA) in the treatment of dyslipidemias. Curr Pharm Des. 2009;15:4087–4093. doi: 10.2174/138161209789909773. [DOI] [PubMed] [Google Scholar]
- 16.Manerba A, Vizzardi E, Metra M, et al. n-3 PUFAs and cardiovascular disease prevention. Future Cardiol. 2010;6:343–350. doi: 10.2217/fca.10.19. [DOI] [PubMed] [Google Scholar]
- 17.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathol. 2005;15:159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sauvaget C, Nagano J, Allen N, et al. Intake of animal products and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Int J Epidemiol. 2003;32:536–543. doi: 10.1093/ije/dyg151. [DOI] [PubMed] [Google Scholar]
- 19.Bouzan C, Cohen JT, Connor WE, et al. A quantitative analysis of fish consumption and stroke risk. Am J Prev Med. 2005;29:347–352. doi: 10.1016/j.amepre.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Ishikawa Y, Yokoyama M, et al. Reduction in the recurrence of stroke by eicosapentaenoic acid for hypercholesterolemic patients: subanalysis of the JELIS trial. Stroke. 2008;39:2052–2058. doi: 10.1161/STROKEAHA.107.509455. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura Y, Ueshima H, Okamura T, et al. Association between fish consumption and all-cause and cause-specific mortality in Japan: NIPPON DATA80, 1980–99. Am J Med. 2005;118:239–245. doi: 10.1016/j.amjmed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Wennberg M, Bergdahl IA, Stegmayr B, et al. Fish intake, mercury, long-chain n-3 polyunsaturated fatty acids and risk of stroke in northern Sweden. Br J Nutr. 2007;98:1038–1045. doi: 10.1017/S0007114507756519. [DOI] [PubMed] [Google Scholar]
- 23.Haag M. Essential fatty acids and the brain. Can J Psychiatry. 2003;48:195–203. doi: 10.1177/070674370304800308. [DOI] [PubMed] [Google Scholar]
- 24.Giusto NM, Salvador GA, Castagnet PI, et al. Age-associated changes in central nervous system glycerolipid composition and metabolism. Neurochem Res. 2002;27:1513–1523. doi: 10.1023/a:1021604623208. [DOI] [PubMed] [Google Scholar]
- 25.Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008;1237:35–43. doi: 10.1016/j.brainres.2008.08.078. [DOI] [PubMed] [Google Scholar]
- 26.Wassall SR, Brzustowicz MR, Shaikh SR, et al. Order from disorder, corralling cholesterol with chaotic lipids. The role of polyunsaturated lipids in membrane raft formation. Chem Phys Lipids. 2004;132:79–88. doi: 10.1016/j.chemphyslip.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Martinez M. Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr. 1992;120:S129–S138. doi: 10.1016/s0022-3476(05)81247-8. [DOI] [PubMed] [Google Scholar]
- 28.Kitajka K, Sinclair AJ, Weisinger RS, et al. Effects of dietary omega-3 polyunsaturated fatty acids on brain gene expression. Proc Natl Acad Sci U S A. 2004;101:10931–10936. doi: 10.1073/pnas.0402342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalon S. Omega-3 fatty acids and monoamine neurotransmission. Prostaglandins Leukot Essent Fatty Acids. 2006;75:259–269. doi: 10.1016/j.plefa.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Coti Bertrand P, O'Kusky JR, Innis SM. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J Nutr. 2006;136:1570–1575. doi: 10.1093/jn/136.6.1570. [DOI] [PubMed] [Google Scholar]
- 31.Heinrichs SC. Dietary omega-3 fatty acid supplementation for optimizing neuronal structure and function. Mol Nutr Food Res. 2010;54:447–456. doi: 10.1002/mnfr.200900201. [DOI] [PubMed] [Google Scholar]
- 32.Bazan NG. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer's disease. J Lipid Res. 2009;50(Suppl):S400–S405. doi: 10.1194/jlr.R800068-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins Leukot Essent Fatty Acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins Leukot Essent Fatty Acids. 2009;81:119–123. doi: 10.1016/j.plefa.2009.05.021. The authors report that DHA synthesis from alpha linolenic acid in the liver is 5–10 folds higher than that in brain, when both liver metabolic conversion pathways are intact and the diet has a high α-LNA content.
- 35.de Groot RH, Hornstra G, van Houwelingen AC, et al. Effect of alpha-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am J Clin Nutr. 2004;79:251–260. doi: 10.1093/ajcn/79.2.251. [DOI] [PubMed] [Google Scholar]
- 36.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–S75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Liou YA, King DJ, Zibrik D, et al. Decreasing linoleic acid with constant alpha-linolenic acid in dietary fats increases (n-3) eicosapentaenoic acid in plasma phospholipids in healthy men. J Nutr. 2007;137:945–952. doi: 10.1093/jn/137.4.945. [DOI] [PubMed] [Google Scholar]
- 38.Rao JS, Ertley RN, DeMar JC, Jr, et al. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Mol Psychiatry. 2007;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- 39.Cao D, Yang B, Hou L, et al. Chronic daily administration of ethyl docosahexaenoate protects against gerbil brain ischemic damage through reduction of arachidonic acid liberation and accumulation. J Nutr Biochem. 2007;18:297–304. doi: 10.1016/j.jnutbio.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Goyens PL, Spilker ME, Zock PL, et al. Compartmental modeling to quantify alpha-linolenic acid conversion after longer term intake of multiple tracer boluses. J Lipid Res. 2005;46:1474–1483. doi: 10.1194/jlr.M400514-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc. 2006;65:42–50. doi: 10.1079/pns2005473. [DOI] [PubMed] [Google Scholar]
- 42.Carnielli VP, Simonato M, Verlato G, et al. Synthesis of long-chain polyunsaturated fatty acids in preterm newborns fed formula with long-chain polyunsaturated fatty acids. Am J Clin Nutr. 2007;86:1323–1330. doi: 10.1093/ajcn/86.5.1323. [DOI] [PubMed] [Google Scholar]
- 43.Rosell MS, Lloyd-Wright Z, Appleby PN, et al. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. Am J Clin Nutr. 2005;82:327–334. doi: 10.1093/ajcn.82.2.327. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins Leukot Essent Fatty Acids. 2003;68:145–150. doi: 10.1016/s0952-3278(02)00264-8. [DOI] [PubMed] [Google Scholar]
- 45.Jump DB, Botolin D, Wang Y, et al. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–2506. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- 46.Igarashi M, Ma K, Chang L, et al. Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48:2463–2470. doi: 10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 47.Strokin M, Sergeeva M, Reiser G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+ Br J Pharmacol. 2003;139:1014–1022. doi: 10.1038/sj.bjp.0705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DeMar JC, Jr, Ma K, Bell JM, et al. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- 49.Bosetti F, Weerasinghe GR. The expression of brain cyclooxygenase-2 is down-regulated in the cytosolic phospholipase A2 knockout mouse. J Neurochem. 2003;87:1471–1477. doi: 10.1046/j.1471-4159.2003.02118.x. [DOI] [PubMed] [Google Scholar]
- 50.Matsuzaka T, Shimano H, Yahagi N, et al. Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res. 2002;43:107–114. [PubMed] [Google Scholar]
- 51.Perlman J. Pathogenesis of hypoxic-ischemic brain injury. J Perinatol. 2007;27:S39–S46. [Google Scholar]
- 52. Sims NR, Muyderman H. Mitochondria, oxidative metabolism and cell death in stroke. Biochim Biophys Acta. 2010;1802:80–91. doi: 10.1016/j.bbadis.2009.09.003. This review paper summarizes current understanding of mitochondrial responses to focal ischemic brain and describes how this organelle contributes to tissue damage in core and penumbra areas.
- 53.Northington FJ, Ferriero DM, Graham EM, et al. Early neurodegeneration after hypoxia-ischemia in neonatal rat is necrosis while delayed neuronal death is apoptosis. Neurobiol Dis. 2001;8:207–219. doi: 10.1006/nbdi.2000.0371. [DOI] [PubMed] [Google Scholar]
- 54. Picq M, Chen P, Perz M, et al. DHA metabolism: Targeting the brain and lipoxygenation. Mol Neurobiol. 2010;42:48–51. doi: 10.1007/s12035-010-8131-7. This paper reviews the possible pathways of how DHA accumulates in brain phospholipids by specific uptake of DHA-containing lysophosphatidylcholine. The authors suggest that esterified DHA be more efficient to supply DHA to brain than non-esterified DHA.
- 55.Bazan NG. Synaptic lipid signaling: significance of polyunsaturated fatty acids and platelet-activating factor. J Lipid Res. 2003;44:2221–2233. doi: 10.1194/jlr.R300013-JLR200. [DOI] [PubMed] [Google Scholar]
- 56.Bazan NG. Cell survival matters: docosahexaenoic acid signaling, neuroprotection and photoreceptors. Trends Neurosci. 2006;29:263–271. doi: 10.1016/j.tins.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Cao DH, Xu JF, Xue RH, et al. Protective effect of chronic ethyl docosahexaenoate administration on brain injury in ischemic gerbils. Pharmacol Biochem Behav. 2004;79:651–659. doi: 10.1016/j.pbb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 58.Cao D, Zhou C, Sun L, et al. Chronic administration of ethyl docosahexaenoate reduces gerbil brain eicosanoid productions following ischemia and reperfusion. J Nutr Biochem. 2006;17:234–241. doi: 10.1016/j.jnutbio.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Cao D, Kevala K, Kim J, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem. 2009;111:510–521. doi: 10.1111/j.1471-4159.2009.06335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rapoport SI. In vivo approaches and rationale for quantifying kinetics and imaging brain lipid metabolic pathways. Prostaglandins Other Lipid Mediat. 2005;77:185–196. doi: 10.1016/j.prostaglandins.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Belayev L, Marcheselli VL, Khoutorova L, et al. Docosahexaenoic acid complexed to albumin elicits highgrade ischemic neuroprotection. Stroke. 2005;36:118–123. doi: 10.1161/01.STR.0000149620.74770.2e. [DOI] [PubMed] [Google Scholar]
- 62.Belayev L, Khoutorova L, Atkins KD, et al. Robust docosahexaenoic acid-mediated neuroprotection in a rat model of transient, focal cerebral ischemia. Stroke. 2009;40:3121–3126. doi: 10.1161/STROKEAHA.109.555979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan HC, Kao TK, Ou YC, et al. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J Nutr Biochem. 2009;20:715–725. doi: 10.1016/j.jnutbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Innis SM. Perinatal biochemistry and physiology of long-chain polyunsaturated fatty acids. J Pediatr. 2003;143:S1–S8. doi: 10.1067/s0022-3476(03)00396-2. [DOI] [PubMed] [Google Scholar]
- 65.Sun GY, Xu J, Jensen MD, et al. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 67.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonzalez-Periz A, Planaguma A, Gronert K, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 69.Mukherjee PK, Marcheselli VL, Serhan CN, et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreira JD, Knorr L, Thomazi AP, et al. Dietary omega-3 fatty acids attenuate cellular damage after a hippocampal ischemic insult in adult rats. J Nutr Biochem. 2010;21:351–356. doi: 10.1016/j.jnutbio.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Lukiw WJ, Bazan NG. Docosahexaenoic acid and the aging brain. J Nutr. 2008;138:2510–2514. doi: 10.3945/jn.108.096016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lukiw WJ, Bazan NG. Inflammatory, apoptotic, and survival gene signaling in Alzheimer's disease : a review on the bioactivity of Neuroprotectin D1 and apoptosis. Mol Neurobiol. 2010;42:10–16. doi: 10.1007/s12035-010-8126-4. [DOI] [PubMed] [Google Scholar]
- 73.Choi-Kwon S, Park KA, Lee HJ, et al. Temporal changes in cerebral antioxidant enzyme activities after ischemia and reperfusion in a rat focal brain ischemia model: effect of dietary fish oil. Brain Res Dev Brain Res. 2004;152:11–18. doi: 10.1016/j.devbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 74.Bas O, Songur A, Sahin O, et al. The protective effect of fish n-3 fatty acids on cerebral ischemia in rat hippocampus. Neurochem Int. 2007;50:548–554. doi: 10.1016/j.neuint.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 75.Mrsic-Pelcic J, Zupan G, Maysinger D, et al. The influence of MK-801 on the hippocampal free arachidonic acid level and Na+-K+-ATPase activity in global cerebral ischemia-exposed rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1319–1326. doi: 10.1016/s0278-5846(02)00296-8. [DOI] [PubMed] [Google Scholar]
- 76.King VR, Huang WL, Dyall SC, et al. Omega-3 fatty acids improve recovery, whereas omega-6 fatty acids worsen outcome, after spinal cord injury in the adult rat. J Neurosci. 2006;26:4672–4680. doi: 10.1523/JNEUROSCI.5539-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pilitsis JG, Diaz FG, O'Regan MH, et al. Differential effects of phospholipase inhibitors on free fatty acid efflux in rat cerebral cortex during ischemia-reperfusion injury. Brain Res. 2002;951:96–106. doi: 10.1016/s0006-8993(02)03142-6. [DOI] [PubMed] [Google Scholar]
- 78.Callaway JK. Investigation of AM-36: a novel neuroprotective agent. Clin Exp Pharmacol Physiol. 2001;28:913–918. doi: 10.1046/j.1440-1681.2001.03547.x. [DOI] [PubMed] [Google Scholar]
- 79.Maragakis NJ, Rothstein JD. Glutamate transporters: animal models to neurologic disease. Neurobiol Dis. 2004;15:461–473. doi: 10.1016/j.nbd.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 80.Sheldon AL, Robinson MB. The role of glutamate transporters in neurodegenerative diseases and potential opportunities for intervention. Neurochem Int. 2007;51:333–355. doi: 10.1016/j.neuint.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnston MV, Trescher WH, Ishida A, et al. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Umemura A, Mabe H, Nagai H, et al. Action of phospholipases A2 and C on free fatty acid release during complete ischemia in rat neocortex. Effect of phospholipase C inhibitor and N-methyl-D-aspartate antagonist. J Neurosurg. 1992;76:648–651. doi: 10.3171/jns.1992.76.4.0648. [DOI] [PubMed] [Google Scholar]
- 83.Mehta SL, Manhas N,Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev. 2007;54:34–66. doi: 10.1016/j.brainresrev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Besancon E, Guo S, Lok J, et al. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–275. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 85.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 86.Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 87. Robertson CL, Scafidi S, McKenna MC, et al. Mitochondrial mechanisms of cell death and neuroprotection in pediatric ischemic and traumatic brain injury. Exp Neurol. 2009;218:371–380. doi: 10.1016/j.expneurol.2009.04.030. These investigators review how H/I affects mitochondrial function and impairs cerebral energy metabolism, contributing to brain cell death.
- 88.Castino R, Bellio N, Nicotra G, et al. Cathepsin D-Bax death pathway in oxidative stressed neuroblastoma cells. Free Radic Biol Med. 2007;42:1305–1316. doi: 10.1016/j.freeradbiomed.2006.12.030. [DOI] [PubMed] [Google Scholar]
- 89.Galluzzi L, Morselli E, Kepp O, et al. Targeting post-mitochondrial effectors of apoptosis for neuroprotection. Biochim Biophys Acta. 2009;1787:402–413. doi: 10.1016/j.bbabio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Zhu C, Qiu L, Wang X, et al. Involvement of apoptosis-inducing factor in neuronal death after hypoxiaischemia in the neonatal rat brain. J Neurochem. 2003;86:306–317. doi: 10.1046/j.1471-4159.2003.01832.x. [DOI] [PubMed] [Google Scholar]
- 91.Ying W, Wei G, Wang D, et al. Intranasal administration with NAD+ profoundly decreases brain injury in a rat model of transient focal ischemia. Front Biosci. 2007;12:2728–2734. doi: 10.2741/2267. [DOI] [PubMed] [Google Scholar]
- 92.Wall R, Ross RP, Fitzgerald GF, et al. Fatty acids from fish: the anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;68:280–289. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 93.Zhang W, Hu X, Yang W, et al. Omega-3 polyunsaturated fatty acid supplementation confers long-term neuroprotection against neonatal hypoxic-ischemic brain injury through anti-inflammatory actions. Stroke. 2010;41:2341–2347. doi: 10.1161/STROKEAHA.110.586081. [DOI] [PMC free article] [PubMed] [Google Scholar]