Abstract
Lymphoseek is a receptor-binding radiopharmaceutical specifically designed for sentinel lymph node (SLN) mapping. We conducted a clinical trial which measured the injection site clearance and sentinel lymph node accumulation after a single intradermal injection of Lymphoseek or unfiltered [99mTc]sulfur colloid (TcSC) using a “2-day” protocol for SLN mapping of breast cancer. Eleven patients with breast cancer participated in this study. Five patients received an intradermal administration of 1.0 nmol of 99mTc-labeled Lymphoseek; SLN mapping was performed on four subjects within 19 to 27 h. Six subjects received an intradermal administration of TcSC; SLN mapping was performed on five subjects within 18 to 26 h. Lymphoseek exhibited a significantly (P<.001) faster injection site clearance than TcSC. The mean Lymphoseek clearance half-time was 2.18±1.09 h compared to 57.4±92.8 h for TcSC. The mean sentinel lymph node uptake of Lymphoseek (1.5±1.7%) and TcSC (3.5±3.1%) was statistically equivalent (P=.213). When an intradermal injection is employed, Lymphoseek demonstrated faster injection site clearance than unfiltered [99mTc]sulfur colloid and persistent SLN accumulation for at least 24 h.
Keywords: Sentinel lymph node biopsy, Radiopharmaceutical, [99mTc]DTPA-mannosyl-dextran, Lymphoseek, Molecular imaging, Breast cancer, “2-Day” protocol
1. Introduction
Lymphoseek is a receptor-binding radiopharmaceutical specifically designed for the mapping of sentinel lymph nodes (SLNs). Based on a multivalent strategy [1], Lymphoseek exhibits a high affinity for a lymphoid-specific receptor [2], which provides sustained SLN uptake without distal lymph node accumulation [1,3]. In women with breast cancer, Lymphoseek demonstrated significantly faster injection site clearance and equivalent SLN accumulation than filtered technetium99-labeled sulfur colloid, with no adverse events or clinically significant changes in clinical and laboratory values [4]. These findings were confirmed at the 5-nmol dose level [5] in patients with breast cancer and melanoma [6].
In this article, we compared the injection site clearance and sentinel lymph node accumulation after a single intradermal injection of Lymphoseek or unfiltered [99mTc]sulfur colloid for SLN mapping of breast cancer. This study was designed to determine the ability of Lymphoseek to remain within the sentinel lymph node for the time period required for a “2-day” mapping protocol [7], which is typically 15 to 24 h after administration and utilizes unfiltered [99mTc]sulfur colloid.
2. Materials and methods
2.1. Patient enrollment
Eleven female patients with breast cancer who would normally be offered sentinel lymph node biopsy as per University of California, San Diego guidelines participated in this study. We entered women with at least one of the following criteria: (1) over 60 years old, (2) having a nonpalpable lesion or (3) having an upper-outer quadrant lesion. The need to have completion axillary node dissection was determined by pathologic outcome of the sentinel node and did not affect this study. Consenting subjects were randomly assigned to one of two groups: Lymphoseek or unfiltered [99mTc]sulfur colloid. Both groups employed a 1.0-mCi dose and a 2-day mapping procedure. Pregnant and lactating females, patients with known metastatic disease and patients currently enrolled in another protocol were excluded from this study. The subjects ranged in age from 58 to 74 years (Table 1). Lesion size ranged from 0.1 to 4.5 cm. Four sentinel lymph nodes from three subjects were positive on frozen section.
Table 1.
Subject and radiopharmaceutical summary
| Subject and pathology | SN Statusa no. | Radiopharmaceutical | ||||||
|---|---|---|---|---|---|---|---|---|
| Subject no. | Age (years) | Diagnosis | Size (cm) | Stage | Site | RP | Dose (mCi) | |
| 1 | 58 | Infiltrating ductal | 1.3 | T1NmiM0 | RUIQ | 1/1/19 | TcSC | 0.95 |
| 2 | 61 | Invasive DCIS | 3.5 | T1N0MX | RUIQ | ST | TcSC | 0.64 |
| 3 | 74 | Invasive mixed ductal and lobular | 4.5/0.4b | T1aN0MX | LUOQ | 0/8 | Lymphoseek | 0.98 |
| 4 | 51 | Multifocal DCIS | 2.0 | T1cN0MX | LLIQ | 0/1 | TcSC | 0.90 |
| 5 | 69 | DCIS | 0.1 | T1N0MX | LUOQ | 0/1 | Lymphoseek | 1.00 |
| 6 | 71 | Invasive ductal and focal DCIS | 1.0 | T1bN0MX | LUOQ | ST | Lymphoseek | 1.00 |
| 7 | 66 | Invasive lobular | 2.5 | T2N0(i)MX | LLOQ | 0/1 | TcSC | 1.00 |
| 8 | 79 | Involving mixed ductal and lobular and DCIS | 2.3 | T2N1MX | LO | 2/5/23 | TcSC | 1.20 |
| 9 | 58 | Mixed ductal and lobular | 2.3 | T2N1aMX | LUOQ | 1/6/c | Lymphoseek | 0.93 |
| 10 | 46 | Invasive ductal | 1.6 | T1cN0MX | LUIQ | 0/4 | Lymphoseek | 0.93 |
| 11 | 64 | DCIS | 0.8 | TisN0MX | RUOQ | 0/3 | TcSC | 1.00 |
DCIS, Ductal carcinoma in situ; TcSC; ST, study terminated.
Positive/SLN/axillary dissection.
Post-neoadjuvant therapy.
Full axillary dissection declined.
The protocol received the consent of the Division of Medical Imaging and Radiopharmaceutical Drug Products of the US Food and Drug Administration as an Investigational New Drug under the sponsorship of the Neoprobe Corporation (Dublin, OH, USA). The protocol and the informed consent form were approved by the UC San Diego, Office for Human Research Protection, the Moores UC San Diego Cancer Center Protocol Review Monitoring Committee and the UCSD Human Exposure Review Committee.
2.2. Agent preparation
Lymphoseek was synthesized [1] and radiolabeled [8] as previously described. This study used the same Lymphoseek preparation as our Phase I breast cancer [4,5] and melanoma trials [6]. The mean molecular diameter was 7.0 nm and the average molecular weight was 28,200 g mol−1. The average DTPA and mannose densities were 2.1 and 42 mol mol−1 of dextran, respectively. The mean Lymphoseek radiochemical purity was 97%. Technetium-99m-labeled sulfur colloid (CIS-US, Bedford, MA, USA) was prepared by a commercial radiopharmacy. All radiopharmaceutical preparations were administered within 2 h of preparation.
2.3. Nuclear imaging
Each patient received a single injection (0.1 ml) of Lymphoseek (1 nmol) or unfiltered [99mTc]sulfur colloid above the lesion using an intradermal technique [9–11]. The administration site was massaged for several minutes. Each subject was monitored for any sign of an allergic reaction such as the occurrence of rash, hives, edema or other cutaneous manifestations.
Nuclear imaging of the Lymphoseek or unfiltered [99mTc] sulfur colloid employed the same imaging protocol. Images of the injection site were acquired immediately after the injection and at 15-min intervals for 2 h. An imaging standard of a known dilution and volume of injectate was placed within the field-of-view. All images were acquired (256×256×16) for 3 min and stored on an image processing computer. The injection site clearance rate constant kc and half-life Tc of Lymphoseek and unfiltered [99mTc]sulfur colloid were calculated using decay-corrected counts obtained from the nuclear images of the injection site [4]. Regression lines that resulted in positive slopes were assigned a kc of 0 h−1. The purpose of the scintigraphy was to measure the injection site clearance rate and not to identify the sentinel lymph node or guide the intraoperative search.
2.4. Sentinel lymph node detection and measurement
Sentinel lymph node biopsy was performed utilizing standard technique. At the start of the surgical procedure, isosulfan blue (Lymphazurin 1%, US Surgical Corp., Norwalk, CT, USA) was injected at the 3-, 6-, 9- and 12-o’clock positions (1 ml per position) surrounding the lesion. The administration site was massaged for several minutes. Also, during this time a handheld gamma probe (Neoprobe 2000, Neoprobe Corp., Dublin, OH, USA) was used to localize the sentinel lymph node. Scintigrams were used to guide the search. After marking the skin at the highest count rate, the patient was prepped and an incision was made at the marked location. With the aid of the gamma probe the dissection was carried out to the lymph nodes that were radioactive or blue. The lymph node was isolated, removed and placed on the tip of the gamma probe for radioactive counting. A background measurement was made by placing the tip of the gamma probe perpendicular to the skin surface at least 20 cm from the administration site. Finally, the gamma probe was placed back within the nodal basin to ensure no significant residual radioactivity remained. Sentinel lymph nodes were defined by having a node-to-background ratio of at least 10:1. Verification that the node contained radioactivity at least 10 times background was performed before sending it to pathology. Frozen section analysis was then performed to identify metastases. If the sentinel node was histologically positive, the lymph node dissection was completed.
All lymph nodes and injection standards were assayed for radioactivity using a dose calibrator located adjacent to the operating room. The percent-of-injected dose (%ID) in each lymph node was calculated as previously described [4].
2.5. Subject monitoring
Vital signs and EKGs were obtained before Lymphoseek and unfiltered TcSC administration and at 15, 30, 45, 60 and 120 min post-administration. Samples for urinalysis, CBC with differential and platelet counts, and a blood chemistry panel were acquired at the preoperative anesthesia appointment (baseline) and again just prior to surgery.
2.6. Statistical methods
For the measures kc, Tc and %IDSN, statistical significance was evaluated by planned comparisons of each molar dose of Lymphoseek to the unfiltered [99mTc]sulfur colloid control group using the nonpaired Students t test (JMP software, SAS Institute, Cary, NC, USA). Changes in clinical laboratory tests before and after administration of Lymphoseek or [99mTc]sulfur colloid were conducted using the Wilcoxon rank sum. Our analysis of the clinical laboratory tests combined the 11 subjects reported here and the 10 subjects reported in an earlier study [12]. A two-tailed P value of less than .05 was considered statistically significant, with no correction made for multiple comparisons.
3. Results
Table 1 lists the subject number and age, tumor diagnosis, size, stage and location. Also listed in this table are the agent and the amount of radioactivity administered. Table 2 lists the subject number, radiopharmaceutical, the injection site clearance rate constant and half-life, as well as the time interval from injection to SLN excision. Also listed is the probe count rate after excision of each SLN. The entry for Subject 3, injected with Lymphoseek, contains a zero, indicating that the second lymph node was detected by blue dye and not by radioactivity.
Table 2.
Injection site clearance and sentinel lymph node uptake
| Subject no. | Radiopharmaceutical | Injection site clearancea | Sentinel lymph node uptakeb | |||||
|---|---|---|---|---|---|---|---|---|
| Rate constant kc (h−1) |
Half-life Tc (h) |
Excision time (h) |
%IDSN | |||||
| 1st (%) | 2nd (%) | 3rd (%) | 4th (%) | |||||
| 1 | TcSC | 0.000 | 18.0 | 1.10 | ||||
| 2 | TcSC | 0.000 | ST | |||||
| 3 | Lymphoseek | 0.239±0.045 | 2.90±0.55 | 20.8 | 0.15 | 0.0d | ||
| 4 | TcSC | 0.045±0.006 | 15.4±1.9 | 23.6 | <0.05 | |||
| 5 | Lymphoseek | 0.599±0.053 | 1.16±0.10 | 27.1 | 1.85 | |||
| 6 | Lymphoseek | 0.290±0.022 | 2.29±0.18 | ST | ||||
| 7 | TcSC | 0.027±0.020 | 25.3±18.8 | 25.9 | 3.38 | |||
| 8 | TcSC | 0.000 | 25.6 | 7.52 | 0.71 | 0.76 | 1.39c | |
| 9 | Lymphoseek | 0.243±0.061 | 2.85±0.71 | 24.7 | 0.21 | |||
| 10 | Lymphoseek | 0.220±0.081 | 3.16±0.26 | 19.4 | 3.69 | 0.06c | 0.62c | |
| 11 | TcSC | 0.000 | 22.4 | 5.44 | 0.98 | |||
Mean±S.D.
Hot or blue.
Hot or blue.
Not hot.
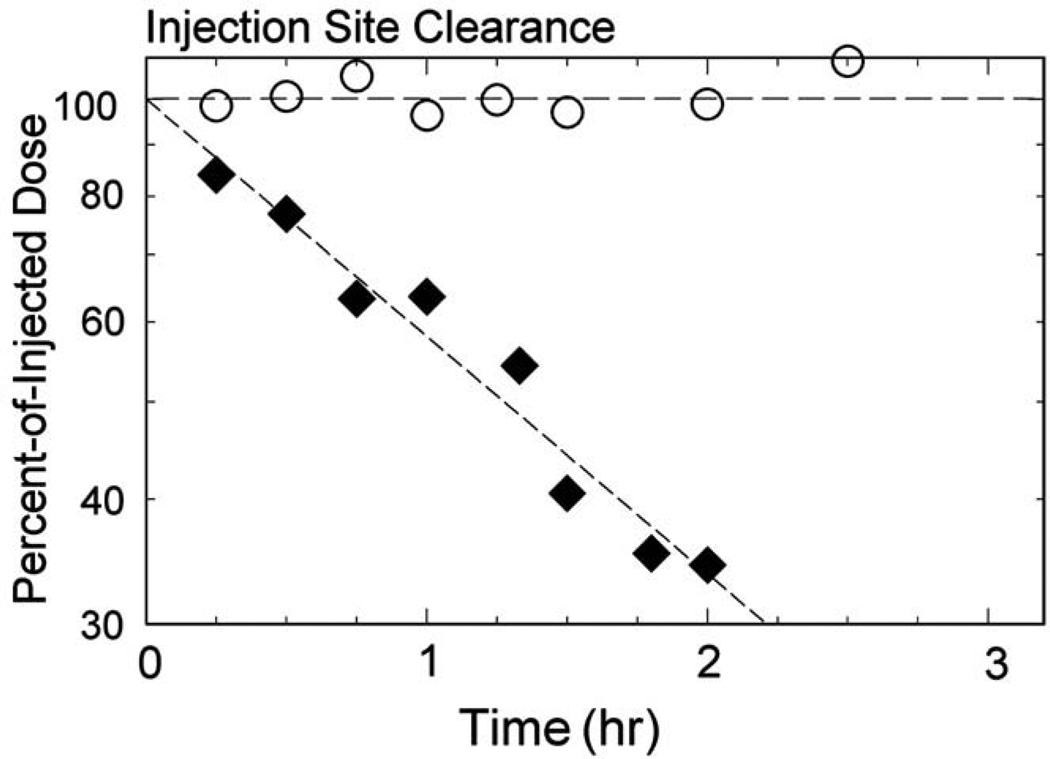
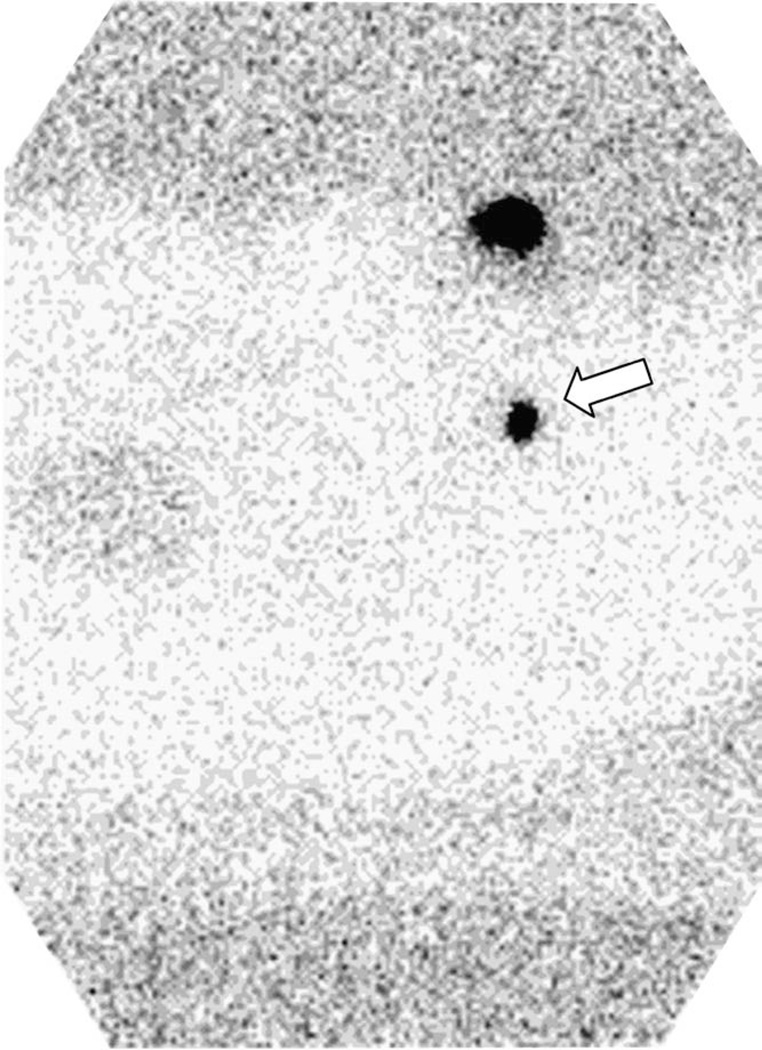
Lymphoseek exhibited a significantly (P<.001) faster injection site clearance than unfiltered TcSC. The Lymphoseek rate constant kc ranged from 0.220 to 0.599 h−1, and the TcSC rate constant ranged from 0.000 to 0.045 h−1. The mean Lymphoseek clearance half-life (Table 3) was 2.18±1.09 hr (n=5) compared to 57.4±98.7 hr for unfiltered TcSC (n=6). The mean sentinel lymph node uptake for Lymphoseek (1.5±1.7%) and unfiltered TcSC (3.5±3.1%) was statistically equivalent (P=.277). The number of Lymphoseek and unfiltered TcSC subjects used to make this comparison was 4 and 5, respectively. One subject in the Lymphoseek group was injected with filtered TcSC when the lymphoscintigraphy of the following morning did not detect a sentinel lymph node. One subject’s (no. 2) surgery was cancelled the day of surgery due to a worsening upper respiratory infection. Fig. 1 demonstrates the clearance of radioactivity from the injection site of two sentinel node cases: Lymphoseek (diamonds, Subject 5) exhibited a half-life of 1.16±.10 h; unfiltered [99mTc]sulfur colloid (circles, Subject 8) exhibited no discernable clearance from the injection site. Fig. 2 is a lateral image acquired 21 h after a 1-nmol (1.0 mCi) injection of Lymphoseek (Subject 5). The sentinel node (arrow) was excised 27 h postadministration; it accumulated 1.9% of the dose. The gamma probe registered 1011 cps. Fig. 3 is an anterior image, acquired 22.3 h after a 1.2-mCi injection of unfiltered [Tc99m]sulfur colloid (Subject 8) detected three sentinel node (arrows). Four SLNs were detected with the handheld gamma probe and removed 24.7 h postadministration. The primary SLN (lowest arrow) registered 7100 cps on the gamma probe and accumulated 7.52% of the dose; it was positive for metastatic disease.
Table 3.
Injection site clearance and sentinel lymph node uptake
| Group | Radiopharmaceutical | Injection site clearance | Sentinel lymph node | |||
|---|---|---|---|---|---|---|
| Studies (no.) | Rate constant kca (h−1) | Half-life Tcb (h) | Studies (no.) | %IDSNc (%) | ||
| 1 | Lymphoseek | 5 | 0.318±0.159 | 2.18±1.09 | 4 | 1.5±1.7 |
| 2 | [99mTc]sulfur colloid | 6 | 0.012±0.020 | 57.4±92.8 | 5 | 3.5±3.1 |
| P value | <.001 | <.001 | .277 | |||
Mean±S.D.
Based on the rate constant kc.
Primary sentinel node only.
Fig. 1.
Clearance of radioactivity from the injection site of two sentinel node cases: Lymphoseek (diamonds, Subject 5) exhibited a half-life of 1.16±0.10 h, and unfiltered [99mTc]sulfur colloid (circles, Subject 8) exhibited a clearance curve with no discernable slope.
Fig. 2.
An image (lateral projection) acquired 21 h after a 1-nmol (1.0 mCi) injection of Lymphoseek (Subject 5). The sentinel node (arrow) was excised 27 h postadministration; it accumulated 1.9% of the dose. The gamma probe registered 1011 cps.
Fig. 3.
An image (anterior projection) acquired 22.3 h after a 1.2-mCi injection of unfiltered [Tc99m]sulfur colloid (Subject 8). Three sentinel lymph nodes (arrows) were detected. Four SLNs were excised 25.6 h postadministration. The primary SLN (lowest arrow) accumulated 7.52% of the dose and was positive for metastatic disease. The activity at the upper left corner is the imaging standard.
There were no adverse events that could be attributed to either radiopharmaceutical. One subject exhibited a Grade 3 hematologic adverse event, no Grade 4 hematologic adverse events occurred on this study and four nonhematologic adverse events occurred. The Grade 3 hematologic event consisted of an increase in glucose and GGT levels just prior to surgery. The subject (no. 11) was a diabetic; vital signs remained stable over the course of the observation and scanning. Four nonhematologic events occurred. One subject (no. 6) noted mild left shoulder pain 1.5 h after receiving a Lymphoseek injection in the left breast; this resolved spontaneously by the next morning. Another subject (no. 1) experienced a mild increased heart rate during the first 30 min after injection of unfiltered TcSC. Subject 11 developed a Grade 3 super ventricular tachycardia (SVT) on postoperative Day 3, which was successfully managed medically; this subject had a past history of SVT. She was later evaluated and treated with ablation therapy. Subject 2 received unfiltered TcSC the day prior to her scheduled surgery; her surgery was cancelled the next day due to a worsening upper respiratory infection.
None of the laboratory values were considered clinically relevant as they represent changes in nonfasting and fasting time periods, and sometimes after the induction of general anesthesia. There were changes in laboratory values between pre- and post-agent administration that reached statistical significance. Both Lymphoseek and unfiltered TcSC groups showed a statistically significant decrease in bicarbonate concentration and platelet count, and an increase in SGOT, LDH, total bilirubin and pH. The Lymphoseek group exhibited a decrease in total protein and MCHC, and an increase in albumin concentration, MCV and MPV. The unfiltered TcSC group exhibited a decrease in BUN, calcium concentration, triglyceride concentration, monocyte count and EOS count. All subjects received the same amount of Lymphoseek.
4. Discussion
This study was designed to assess the ability of Lymphoseek to detect sentinel lymph nodes after a prolonged interval between administration and mapping. We also compared the rates at which Lymphoseek and unfiltered TcSC clear the injection site after a single intradermal injection to the breast. The 2-day mapping procedure performed with Lymphoseek provided equivalent sentinel lymph node accumulation as unfiltered [99mTc]sulfur colloid, with an equivalent mean number of SLNs per study.
The clinically significant aspect of this work is the demonstration that Lymphoseek’s sentinel lymph node accumulation can persist up to 24 h after administration. A rapid egress of radioactivity from the sentinel lymph node could prevent this feature. This could occur by at least four processes. The first, and least likely, would be a loss of the technetium label within the SLN, with the resulting transport of a new labeled molecule out of the lymph node. Second, the reticuloendothelial receptor could bind Lymphoseek with an affinity that is significantly lower than what was measured in vitro [1], which would prevent internalization and result in a delayed egress from the SLN. Third, lysosomal catabolism of Lymphoseek could occur within the SLN, with the subsequent release of small Tc-99m-labeled metabolites back into the lymph channel. Fourth, the receptor could recycle back to the cell surface from the lysosome and release Lymphoseek into the lymph and eventually exit from the lymph node. Based on the documented biologic behavior of Tc-99m-labeled galactosyl-human serum albumin-diethylenetriamine-pentaacetic acid [13], which binds to its target cell via a similar mechanism and is technetium-99m labeled via DTPA chelation, we expected all of these processes to have a minimal effect on SLN retention.
The extended interval between administration and mapping provided by the 2-day protocol enabled both radiotracers to achieve higher primary sentinel lymph node accumulation. A protocol using a single intradermal injection of Lymphoseek [12] in five breast cancer patients produced a percent-of-injected dose of 1.1±0.5% when the time interval ranged from 3.4 to 6.1 h. This was lower, but not statistically significant (P=.76), than the 2-day protocol where the sentinel lymph node uptake was 1.5±1.7% and the time interval ranged from 19.4 to 27.1 h. Sentinel lymph node uptake of filtered TcSC [12] was 2.5±4.9% when the uptake time interval ranged from 3.5 to 7.3 h. This was lower than 3.5±3.1%, the uptake achieved by unfiltered TcSC when the uptake time interval ranged from 18.0 to 25.9 h. Although noteworthy, the increase in colloid SLN accumulation achieved by the 2-day protocol was not statistically significant. This is mainly due to the high biological variation in subject lymphatic flow and function.
The demonstration of rapid injection site clearance compared to TcSC, yet equivalent sentinel lymph node accumulation, is consistent with previous Lymphoseek trials [4,6] and the design of the radiopharmaceutical [1]. With the goal of producing a sentinel lymph node agent that rapidly clears the injection site, we designed Lymphoseek to enter both the blood capillaries and lymph channels. Consequently, although a high percentage of the dose clears the injection site over a given time interval, the same amount of Lymphoseek does not appear in the sentinel lymph node. A high fraction of the Lymphoseek dose leaves the injection site via the blood capillaries and localizes in the liver and urinary bladder.
This work is the first report of the injection site clearance rate of unfiltered TcSC. The mean of 0.012 h−1 includes four of the six studies with clearance rate constants of 0 h−1. This accounts for the high relative error of ±167%. The clearance rate constant of filtered TcSC [12] using the same injection protocol exhibited a mean and standard deviation of 0.029 ±0.021 h−1. The lowered relative error of ±72% occurred because only one of five studies was assigned a zero clearance rate due to a positive slope in the time–activity curve. Although the mean value of kc for unfiltered TcSC was half the value of filtered TcSC, the difference was not statistically significant (P=.255) due to the very high number of measurement errors resulting from the linear regression of time–activity data with very shallow slopes (Fig. 1).
The purpose of a Phase I clinical trial of a new radiopharmaceutical is to obtain a preliminary assessment of safety for a given injection route. Our implementation of a single 0.1-ml intradermal injection protocol was different from our initial Phase I study [4] which utilized four 1-ml peritumoral injections at 3-, 6-, 9- and 12-o’clock positions. Because we used a different injection route than the previous study, we considered this protocol a Phase I trial and duplicated the biodistribution and safety measurements.
The small scale of a Phase I clinical trial imposes certain limitations. These studies are not powered to detect small differences in biodistributions between groups or agents. This is especially true when the distributions have wide dispersions. The biologic variation in sentinel lymph node accumulation is extremely high [14]. Consequently, a larger Phase II or III trial will be required to demonstrate differences between sentinel lymph node accumulation of Lymphoseek and the current agents.
This study and a previous Phase I trial [12] employed entrance criteria designed to enroll subjects where there is likely to be more difficulty in identifying SLNs. These subjects are over 60 years old, have nonpalpable tumors or have upper-outer quadrant lesions. One Lymphoseek study [6] was terminated when at 16 h postinjection a sentinel lymph node was not visualized. A hot blue sentinel node was identified after peritumoral injections of unfiltered TcSC and Lymphazurin. This subject was 71 years of age, with a nonpalpable tumor in the upper outer quadrant, which is a situation that has the highest probability of poor lymphoscintigraphic visualization [15].
5. Conclusion
The sentinel lymph node accumulation of Lymphoseek persists for at least 24 h postadministration, which will permit 2-day SLN mapping of breast cancer.
Acknowledgments
We thank Dr. Ernest V Belezzuoli and the Neoprobe Corporation of Dublin, OH, for the use of a Model 2100 intraoperative radioisotope detector. Lymphoseek is a registered trademark of the Neoprobe Corporation.
Footnotes
This study was supported by the Quicktrials Program of the National Cancer Institute, grant number R21-CA097643.
References
- 1.Vera DR, Wallace AM, Hoh CK, Mattrey RF. A synthetic macromolecule for sentinel node detection: [99mTc]DTPA-mannosyl-dextran. J Nucl Med. 2001;42:951–959. [PubMed] [Google Scholar]
- 2.Steer CJ. In: Hepatology a textbook of liver disease. Zakim D, Boyer TD, editors. Philadelphia: W. B. Saunders; 1996. pp. 149–214. [Google Scholar]
- 3.Ellner SJ, Mendez J, Vera DR, Hoh CK, Ashburn WL, Wallace AM. Sentinel lymph node mapping of the colon and stomach using Lymphoseek in a pig model. Ann Surg Oncol. 2004;11:674–681. doi: 10.1245/ASO.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Wallace AM, Hoh CK, Vera DR, Darrah D, Schulteis G. Lymphoseek: a molecular radiopharmaceutical for sentinel node detection. Ann Surg Oncol. 2003;10:531–538. doi: 10.1245/aso.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Ellner SJ, Hoh CK, Vera DR, Darrah DD, Schulteis G, Wallace AM. Dose-dependent biodistribution of [99mTc]DTPA-mannosyl-dextran for breast cancer sentinel node mapping. Nucl Med Biol. 2003;30:805–810. doi: 10.1016/j.nucmedbio.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Wallace AM, Hoh CK, Ellner SJ, Darrah DD, Schulteis G, Vera DR. Lymphoseek: a molecular imaging agent for melanoma sentinel lymph node mapping. Ann Surg Oncol. 2007;14:913–921. doi: 10.1245/s10434-006-9099-4. [DOI] [PubMed] [Google Scholar]
- 7.Yeung H, Cody HS, III, Turlakow A, Riedel ER, Fey J, Gonen M, et al. Lymphoscintigraphy and sentinel node localization in breast cancer patients: a comparison between 1-day and 2-day protocols. J Nucl Med. 2001;42:420–423. [PubMed] [Google Scholar]
- 8.Hoh CK, Wallace AM, Vera DR. Preclinical studies of [99mTc]DPTA-mannosyl-dextran. Nucl Med Biol. 2003;30:457–464. doi: 10.1016/s0969-8051(03)00028-3. [DOI] [PubMed] [Google Scholar]
- 9.Linehan DC, Hill ADK, Akhurst T, Yeung H, Yeh S, Tran KN, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimize sentinel node identification in breast cancer patients. Ann Surg Oncol. 1999;6:450–454. doi: 10.1007/s10434-999-0450-4. [DOI] [PubMed] [Google Scholar]
- 10.Cody HS, Borgen PI. State-of-the-art approaches to sentinel node biopsy for breast cancer: study design, patient selection, technique, and quality control at Memorial Sloan-Kettering Cancer Center. Surg Oncol. 1999;8:85–91. doi: 10.1016/s0960-7404(99)00029-8. [DOI] [PubMed] [Google Scholar]
- 11.McMasters K, Wong S, Martin RI, Chao C, Tuttle T, Noyes R, et al. Dermal injection of radioactive colloid is superior to peritumoral injection for breast cancer sentinel lymph node biopsy: results of a multiinstitutional study. Ann Surg. 2001;233:676–687. doi: 10.1097/00000658-200105000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallace AM, Hoh CK, Darrah DD, Schulteis G, Vera DR. Sentinel lymph node mapping of breast cancer via intradermal administration of Lymphoseek. Nucl Med Biol. 2007;34:849–853. doi: 10.1016/j.nucmedbio.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokudo N, Vera DR, Makuuchi M. Clinical application of TcGSA. Nucl Med Biol. 2003;30:845–849. doi: 10.1016/s0969-8051(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 14.Gulec SA, Moffat FL, Carroll RG, Serafini AN, Sfakianakis GN, Allen L, et al. Sentinel lymph node localization in early breast cancer. J Nucl Med. 1998;39:1388–1393. [PubMed] [Google Scholar]
- 15.Chagpar AB, Martin RC, Scoggins CR, Carlson DJ, Laidley AL, El-Eid SE, et al. Factors predicting failure to identify a sentinel lymph node in breast cancer. Surgery. 2005;138:56–63. doi: 10.1016/j.surg.2005.03.003. [DOI] [PubMed] [Google Scholar]