Abstract
Objective
In a non-clinical trial setting, to determine the proportion of individuals with coronary artery disease (CAD) with optimal risk factor levels based on the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial.
Background
In COURAGE, the addition of percutaneous coronary intervention (PCI) to optimal medical therapy did not reduce the risk of death or myocardial infarction in stable CAD patients but resulted in more revascularization procedures.
Methods
REGARDS is a national prospective cohort study of 30,239 African American and White community-dwelling individuals aged >45 years enrolled in 2003-7. We calculated the proportion of 3,167 participants with self-reported CAD meeting 7 risk factor goals based on COURAGE: 1) aspirin use, 2) systolic blood pressure <130 mmHg and diastolic blood pressure <85 mmHg (<80 mmHg if diabetic), 3) low density lipoprotein cholesterol <85 mg/dL, high density lipoprotein cholesterol >40 mg/dL, and triglycerides <150 mg/dL, 4) fasting glucose <126 mg/dL, 5) nonsmoking status, 6) body mass index <25 kg/m,2 and 7) exercise ≥4 days per week.
Results
The mean age of participants was 69±9 years, 33% were African American, and 35% were female. Overall, the median number of goals met was 4. Less than a quarter met ≥5 of the 7 goals, and 16% met all 3 goals for aspirin, blood pressure, and LDL-C. Older age, white race, higher income, more education, and higher physical functioning were independently associated with meeting more goals.
Conclusions
There is substantial room for improvement in risk factor reduction among US individuals with CAD.
Keywords: coronary artery disease, prevention, risk factors
Introduction
Coronary artery disease (CAD) is highly prevalent in the United States [1]. The American Heart Association (AHA) estimates that 15,400,000 Americans have CAD and that CAD accounted for one in six deaths in the United States in 2009. The total estimated annual direct and indirect cost of CAD in the United States is $195.2 billion [1].
Current guideline recommendations for the management of patients with stable CAD involve intensive risk factor management and anti-ischemic therapies, with revascularization reserved for individuals whose symptoms persist or progress despite intensive medical therapy [2]. Despite these recommendations, many patients undergo revascularization, often because of emotional or psychological factors on the part of both patients and physicians [3, 4]. Over 1 million percutaneous coronary interventions (PCIs) are performed annually in the United States [1]. Although estimates vary, it appears that at least half of all PCIs in the United States are performed electively [5].
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial recently demonstrated that as compared to a strategy of PCI plus optimal medical therapy, an initial strategy of optimal medical therapy alone with PCI reserved for those with refractory angina had similar rates of death or non-fatal myocardial infarction in individuals with stable CAD who had undergone coronary angiography prior to randomization [6]. Also, over a median follow-up of 4.6 years, only 33% of individuals randomized to the optimal medical therapy group required revascularization [6], suggesting that two thirds of individuals with stable CAD could potentially avoid PCI during this time period if treated initially with optimal medical therapy alone [7].
Since clinical trial populations tend to be more adherent and health conscious, it is not clear to what extent individuals with stable CAD in the United States achieve the risk factor goals used in the COURAGE trial. Therefore, using data on participants who reported a history of CAD at baseline in the national REasons for Geographic and Racial Differences in Stroke (REGARDS) study, we sought to examine the proportion of individuals with risk factor levels similar to the goals used in the COURAGE trial. Additionally, we examined sociodemographic factors associated with being at these risk factor goals.
Methods
The REGARDS study has been described in detail previously [8]. Briefly, REGARDS is a cohort of 30,239 community dwelling individuals recruited between 2003 and 2007. Although the cohort is currently being followed longitudinally, this analysis utilizes data that were collected during the baseline examination. The cohort was designed to be balanced on race (white and African American) and sex; the final sample was 42% African Americans and 55% female. Because the primary goals of REGARDS are to elucidate regional and racial differences in stroke, residents of the Stroke Belt, located in the southeastern US, were over-sampled such that 20% of the overall cohort was selected from the “buckle” of the Stroke Belt (the coastal plain region of North Carolina, South Carolina, and Georgia); 30% from the rest of the Stroke Belt (the remaining parts of North Carolina, South Carolina, and Georgia plus Alabama, Mississippi, Louisiana, Arkansas, and Tennessee); and 50% from the remaining 40 contiguous states. Individuals were identified by commercially available lists and contacted by mail and telephone. Of those determined to be eligible, 49% agreed to participate. Upon enrollment, participants underwent a computer assisted telephone interview followed by an in-home examination. During the telephone interview, demographic and self-reported medical information was obtained. During the in-home examination, the participant's blood pressure, height, and weight were measured; and blood and urine samples were collected.
At enrollment, there were a total of 4,245 participants who reported a history of CAD, defined as a self-reported history of myocardial infarction, PCI, or coronary artery bypass graft surgery. Of these participants, 454 with missing data on blood pressure, glucose or lipid values, smoking status, height, weight, or physical activity were excluded. Additionally 624 individuals who had not fasted for their blood draw were excluded from the primary analysis. This resulted in a final sample size for our primary analyses of 3,167 participants with a self-reported history of prevalent CAD. Individuals excluded from our primary analyses were more likely to be African American (39% vs. 33%, p<0.001) and less likely to live in the Stroke Buckle (17% vs. 23%, p<0.001) than those included. Female sex did not differ significantly between those excluded (38%) and those included (35%, p=0.16). We conducted additional secondary analyses as described below without excluding the 624 individuals who had not fasted for their blood draw (n=3,791 for these analyses).
Risk Factor Treatment Goals
The risk factor goals used for this analysis were based on those used at the beginning of the COURAGE trial [9, 10] and are listed in Table 1. These goals were based on the American College of Cardiology (ACC) and AHA guidelines at the time that COURAGE was designed [9, 10] and do not vary significantly from current guideline recommendations [2, 11] or the AHA 2020 goals for ideal cardiovascular health [12], as shown in Table 1. Because our intent was to estimate the degree to which optimal risk factor levels are achieved in the general United States population in individuals with stable CAD, for our primary analyses, we defined the blood pressure goal as achieving both the systolic and diastolic blood pressure goals, the lipid goal as achieving all 3 lipid goals, and an optimal body mass index as <25 kg/m2. Exercise was defined as intense physical activity, enough to work up a sweat. Aspirin use was determined by participant self-report. Because hemoglobin A1C was not measured in the REGARDS study, we substituted a fasting glucose of <126 mg/dL in place of a hemoglobin A1C of <7% [13]. Because some of these goals have higher degrees of evidence to support their use and are easier to achieve than others, we also examined three goals in a secondary analysis: aspirin use, blood pressure control, and low density lipoprotein cholesterol (LDL-C) control (LDL-C <85 mg/dL)
Table 1. Comparison of Risk Factor Goals Evaluated in the 3,167 Participants with a Self-Reported History of Coronary Heart Disease at Baseline in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, 2003-2007.
| Our Analysis | 2012 Stable Ischemic Heart Disease Guideline2 | 2011 ACCF/AHA/AMA-PCPI Performance Measures11 | AHA 2020 Goals for Ideal Cardiovascular Health12 | |
|---|---|---|---|---|
| Aspirin use | Aspirin use recommended | Aspirin use recommended | Aspirin use | Not addressed |
| Blood Pressure | SBP <130 mmHg and DBP <85 mm Hg (<80 mmHg if diabetic) | SBP <140 mmHg and DBP <90 mmHg | SBP <140 mmHg and DBP <90 mmHg or ≥2 agents | SBP <120 mmHg and DBP <80 mmHg |
| Lipids | LDL-C <85 mg/dL, HDL-C >39 mg/dL, and triglycerides <150 mg/dL | Moderate or high dose statin therapy | LDL-C <100 mg/dL or statin therapy | Total cholesterol <200 mg/dL |
| Glucose | Fasting glucose <126 mg/dL | No Class I recommended target | Not addressed | Fasting glucose <100 mg/dL |
| Smoking status | Nonsmoking status | Nonsmoking status | Nonsmoking status or received cessation counseling | Nonsmoking status |
| Weight | Body mass index <25 kg/m2 | Body mass index <25 kg/m2 | Not addressed | Body mass index <25 kg/m2 |
| Physical Activity | Exercise ≥4 days per week | Exercise 30-60 minutes at a minimum of 5 days per week | Not addressed | ≥150 minutes per week of moderate intensity |
ACCF = American College of Cardiology Foundation, AHA = American Heart Association, AMA = American Medical Association, DBP = diastolic blood pressure, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, PCPI = Physician Consortium for Performance Improvement, SBP = systolic blood pressure
Sociodemographic Variables
Urban residence was defined based on census tract categories (urban, rural, or mixed). Functional status was assessed by the physical component summary score of the Short Form-12 [14]. Kidney function was estimated by the Modification of Diet in Renal Disease equation [15]. The presence of depressive symptoms was defined as a score of ≥ 4 on the four item version of the Centers for Epidemiologic Study Depressive Scale [16]. Finally, diabetes was defined as a self-reported history of diabetes, the use of diabetes medications, a fasting glucose ≥126 mg/dl, or a nonfasting glucose ≥200 mg/dl.
Statistical Analyses
We first calculated the proportion of participants in the study sample who met each risk factor goal listed in Table 1. Then, we calculated a composite score for each participant reflecting the total number of risk factor goals reached. To determine factors associated with the total number of goals achieved, we constructed a multivariable linear regression model, adjusting for age, sex, race, region of residence, income, education, urban residence, functional status, kidney function, and depressive symptoms. Similar methodology was utilized for the analyses examining meeting the aspirin, blood pressure, and LDL-C goals.
Additionally, because we required optimal levels of 3 lipid values to achieve the cholesterol goal, a body mass index <25 kg/m2, and a fasting glucose of <126 mg/dL in all participants regardless of their diabetes status in our definition of optimal risk factor levels, we conducted a series of secondary analyses in the 3,167 fasting participants. First, to examine control LDL-C alone, we calculated the proportion of individuals who had a LDL-C <100 mg/dL, <85 mg/dL, and <70 mg/dL, respectively. Second, we calculated the number of REGARDS participants with a body mass index <30 kg/m2. Third, to investigate glucose control in only those with diabetes, we calculated the proportion of individuals with diabetes at enrollment who had a fasting blood sugar <126 mg/dl. Also, because in our primary analyses, we excluded individuals who were not fasting at the time their blood was drawn, we calculated the number of total risk factor goals reached without excluding the 624 individuals who were non-fasting. For this portion of the analysis, we used a non-high density lipoprotein cholesterol (non-HDL-C) <100 mg/dL as the lipid criterion for all participants, and, for those who were non-fasting, we substituted a glucose level of <200 mg/dL for the glucose criterion in our definitions for risk factor goals (Table 1). All analyses were performed with SAS (version 9.2). All participants provided informed consent to participate, and this study was approved by the Institutional Review Board of the participating universities.
Results
The demographic and clinical characteristics of the study sample are shown in Table 2. Participants had a mean age of 69 ± 9 years. Approximately one-third was female and one-third was African American.
Table 2. Baseline Characteristics of the 3,167 Participants with a Self-Reported History of Coronary Heart Disease in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, 2003-2007.
| Age (years) | 69 ± 9 |
| Female | 35% |
| African American | 33% |
| Region | |
| Stroke Buckle | 23% |
| Stroke Belt | 34% |
| Non-Stroke Belt | 44% |
| Annual Income* | |
| <$20,000 | 24% |
| $20,000 - $35,000 | 31% |
| $35,000 - $75,000 | 32% |
| >$75,000 | 13% |
| Education | |
| <High School | 16% |
| High School | 29% |
| Some College | 27% |
| Completed College | 29% |
| Urban Group | |
| Rural | 21% |
| Mixed | 11% |
| Urban | 68% |
| Kidney Function | |
| eGFR ≥90 mL/min/1.73m2 | 29% |
| eGFR 60-89 mL/min/1.73m2 | 50% |
| eGFR 30-59 mL/min/1.73m2 | 18% |
| eGFR 15-29 mL/min/1.73m2 | 2% |
| eGFR <15 mL/min/1.73m2 | 1% |
| COURAGE Risk Factors | |
| Systolic Blood Pressure (mmHg) | 130 ± 18 |
| Diastolic Blood Pressure (mmHg) | 75 ± 10 |
| Total Cholesterol (mg/dL) | 174 ± 41 |
| LDL-C (mg/dL) | 100 ± 34 |
| HDL-C (mg/dL) | 46 ± 14 |
| Triglycerides (mg/dL) | 143 ± 94 |
| Fasting Glucose (mg/dL) | 109 ± 38 |
| Body Mass Index (kg/m2) | 29.4 ± 5.8 |
| Diabetes | 31% |
| PCS of SF-12 | 42 ± 11 |
| Depressive Symptoms | 14% |
eGFR = estimated glomerular filtration rate, HDL-C = high density lipoprotein cholesterol, LDL-C = low density lipoprotein cholesterol, PCS = physical component score, SF-12=Short Form 12
Date reported as mean ± standard deviation or %.
Income data are from the 2,812 participants who did not refuse to report their income.
The proportion of individuals meeting each risk factor goal is displayed in Figure 1. Nonsmoking status, fasting glucose <126 mg/dL, and the regular use of aspirin were the most frequently met risk factor goals. Overall, 50% of participants met the systolic and diastolic blood pressure goals and 25% or fewer participants met the physical activity, body mass index, or lipid goals. The total number of risk factor goals met is displayed in Figure 2. Overall, an average of 3.6 ±1.2 of the 7 total possible risk factor goals was met, while the median number of goals met was 4. Nearly all participants met at least 1 risk factor goal. However, fewer than a quarter met 5 or more goals, and only 17 of the 3,167 participants (0.5%) met all 7 goals. After multivariable adjustment, older age, white race, higher income, more education, and higher physical functioning were independently associated with meeting more treatment goals (Table 3). REGARDS participants enrolled in 2007 met slightly more risk factor goals (3.8+1.2) than those in previous years (3.5+1.2 in 2003 and 3.6+1.2 in each of years 2004-2006, p<0.01 for each comparison). When examining only the aspirin, blood pressure, and LDL-C goals, 91% of participants met at least 1 of these 3 goals and 16% met all 3. Male gender, white race, increased income, and more education were independently associated with meeting more treatment goals after multivariable adjustment (Table 4).
Figure 1. Proportion of the 3,167 Participants with a Self-Reported History of Coronary Heart Disease at Baseline in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study Meeting Each of the Risk Factor Goals,* 2003-2007.
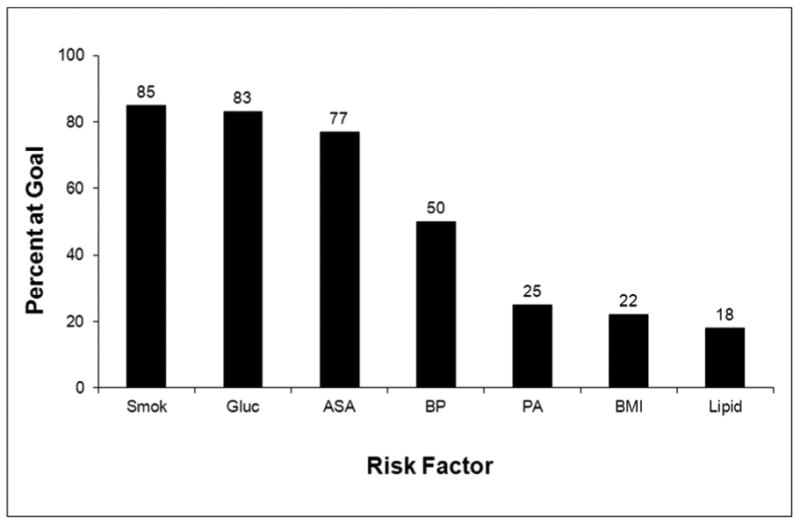
ASA = aspirin therapy, BMI = body mass index, BP = blood pressure, Gluc = glucose, PA = physical activity, Smok = smoking cessation
Numbers above the bars represent the percent achieved.
*See Table 1 for the definition of the risk factor goals.
Figure 2. Cumulative Number of Risk Factor Goals* Met in the 3,167 Participants with a Self-Reported History of Coronary Heart Disease at Baseline in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, 2003-2007.
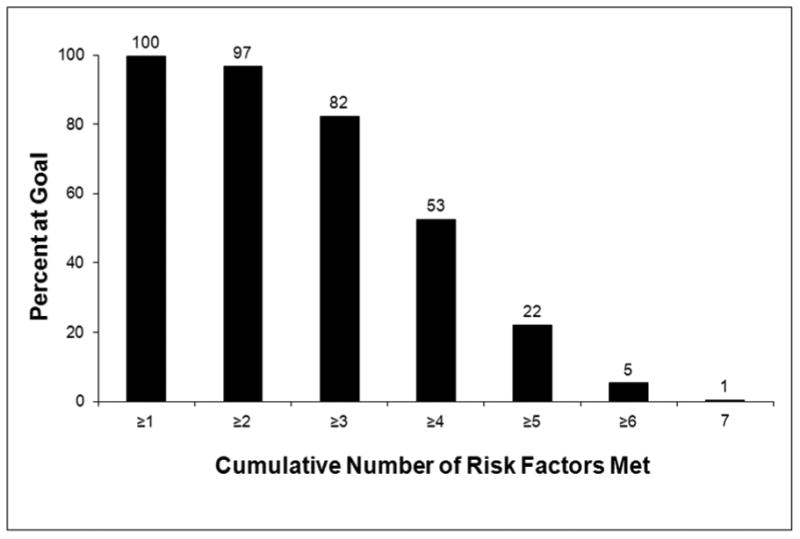
Numbers above the bars represent the percent achieved.
*See Table 1 for the definition of the risk factor goals.
Table 3. Factors Associated with Achievement of Risk Factor Goals* in the 3,167 Participants with a Self-Reported History of Coronary Heart Disease at Baseline in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, 2003-2007.
| Univariate Results | Multivariable Results | Standardized Coefficients | |
|---|---|---|---|
| Age (compared to ≥75 years) | |||
| 61-74 years | -0.26 (-0.35,-0.16) | -0.27 (-0.37, -0.18) | -0.11 |
| 45-60 years | -0.41 (-0.53, -0.29) | -0.37 (-0.50, -0.25) | -0.13 |
| Female (compared to male) | -0.31 (-0.40, -0.23) | -0.03 (-0.12, 0.07) | -0.01 |
| African American (compared to White) | -0.55 (-0.64, -0.46) | -0.36 (-0.45, -0.26) | -0.14 |
| Region (compared to non-Belt) | |||
| Stroke Belt | 0.09 (0.02, 0.16) | 0.04 (-0.05, 0.14) | 0.02 |
| Stroke Buckle | 0.11 (0.03, 0.19) | 0.08 (-0.03, 0.19) | 0.03 |
| Urban Group (compared to urban) | |||
| Mixed | 0.18 (0.05, 0.32) | 0.10 (-0.04, 0.24) | 0.03 |
| Rural | 0.18 (0.08, 0.29) | 0.08 (-0.02, 0.19) | 0.03 |
| Annual Income (compared to <$20,000) | |||
| $20,000 - $35,000 | 0.35 (0.23, 0.47) | 0.15 (0.02, 0.27) | 0.05 |
| $35,000 - $75,000 | 0.48 (0.37, 0.60) | 0.18 (0.05, 0.31) | 0.07 |
| >$75,000 | 0.70 (0.55, 0.85) | 0.29 (0.12, 0.46) | 0.08 |
| Education (compared to completed college) | |||
| Some College | -0.23 (-0.34, -0.12) | -0.10 (-0.21, 0.01) | -0.04 |
| High School | -0.27 (-0.38, -0.16) | -0.12 (-0.23, 0.00) | -0.04 |
| <High School | -0.53 (-0.66, -0.40) | -0.23 (-0.37, -0.08) | -0.07 |
| Depressive Symptoms | -0.13 (-0.22, -0.04) | -0.12 (-0.24, 0.01) | -0.03 |
| Renal Function (compared to eGFR ≥90 mL/min/ 1.73 m2) | |||
| eGFR 60-89 mL/min/ 1.73 m2 | 0.07 (0.00, 0.14) | 0.00 (-0.10, 0.09) | 0.00 |
| eGFR 30-59 mL/min/ 1.73 m2 | 0.07 (-0.02, 0.16) | -0.12 (-0.25, 0.01) | -0.04 |
| eGFR 15-29 mL/min/ 1.73 m2 | -0.23 (-0.45, 0.00) | -0.26 (-0.57, 0.05) | -0.03 |
| eGFR <15 mL/min/ 1.73 m2 | -0.14 (-0.45, 0.17) | -0.03 (-0.45, 0.39) | 0.00 |
| PCS (per 10 points higher score) | 0.24 (0.20, 0.27) | 0.17 (0.13, 0.21) | 0.16 |
eGFR = estimated glomerular filtration rate, PCS = physical component score of Short Form 12 Statistically significant findings are bolded and italicized. Multivariable model r-square is 0.11.
Number of additional treatment goals met after multivariable adjustment. For example, African Americans achieved 0.36 fewer treatment goals after multivariable adjustment compared to whites.
Table 4. Factors Associated with Achievement of Aspirin use, Blood Pressure Control, and Low Density Lipoprotein Cholesterol Control* in the 3,259 Participants with a Self-Reported History of Coronary Heart Disease at Baseline in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Study, 2003-2007.
| Univariate Results | Multivariable Results | Standardized Coefficients | |
|---|---|---|---|
| Age (compared to ≥75 years) | |||
| 61-74 years | 0.04 (-0.03, 0.11) | 0.06 (-0.01, 0.14) | 0.04 |
| 45-60 years | 0.06 (-0.03, 0.15) | 0.11 (0.02, 0.21) | 0.05 |
| Female (compared to male) | -0.22 (-0.28, -0.16) | -0.09 (-0.16, -0.02) | -0.05 |
| African American (compared to White) | -0.40 (-0.46, -0.34) | -0.34 (-0.41, -0.27) | -0.18 |
| Region (compared to non-Belt) | |||
| Stroke Belt | 0.07 (0.005, 0.14) | 0.03 (-0.04, 0.10) | 0.01 |
| Stroke Buckle | 0.11 (0.03, 0.18) | 0.06 (-0.02, 0.14) | 0.03 |
| Urban Group (compared to urban) | |||
| Mixed | 0.07 (-0.03, 0.16) | -0.04 (-0.14, 0.06) | -0.01 |
| Rural | 0.10 (0.03, 0.17) | 0.00 (-0.08, 0.08) | 0.00 |
| Annual Income (compared to <$20,000) | |||
| $20,000 - $35,000 | 0.17 (0.08, 0.25) | 0.05 (-0.03, 0.14) | 0.03 |
| $35,000 - $75,000 | 0.26 (0.17, 0.34) | 0.06 (-0.03, 0.16) | 0.03 |
| >$75,000 | 0.42 (0.31, 0.53) | 0.15 (0.02, 0.27) | 0.06 |
| Education (compared to completed college) 4 | |||
| Some College | -0.11 (-0.19, -0.03) | -0.06 (-0.14, 0.02) | -0.03 |
| High School | -0.16 (-0.24, -0.08) | -0.08 (-0.17, 0.00) | -0.04 |
| <High School | -0.33 (-0.43, -0.24) | -0.17 (-0.27, -0.06) | -0.07 |
| Depressive Symptoms | -0.13 (-0.22, -0.05) | -0.01 (-0.10, 0.08) | 0.00 |
| Renal Function (compared to eGFR ≥90 mL/min/ 1.73 m2) | |||
| eGFR 60-89 mL/min/ 1.73 m2 | 0.07 (0.00, 0.14) | 0.02 (-0.05, 0.09) | 0.01 |
| eGFR 30-59 mL/min/ 1.73 m2 | 0.07 (-0.01, 0.16) | 0.05 (-0.04, 0.14) | 0.02 |
| eGFR 15-29 mL/min/ 1.73 m2 | -0.23 (-0.45, 0.00) | -0.13 (-0.37, 0.09) | -0.02 |
| eGFR <15 mL/min/ 1.73 m2 | -0.15 (-0.46, 0.16) | 0.00 (-0.31, 0.30) | 0.00 |
| PCS (per 10 points higher score) | 0.06 (0.03, 0.08) | 0.02 (-0.01, 0.05) | 0.02 |
eGFR = estimated glomerular filtration rate, PCS = physical component score of Short Form 12
Statistically significant findings are bolded and italicized. Multivariable model r-square is 0.07.
Number of additional treatment goals met after multivariable adjustment. For example, African Americans achieved 0.33 fewer treatment goals after multivariable adjustment compared to whites.
In secondary analyses evaluating LDL-C level as a separate goal rather than simply 1 of 3 components of the lipid goal, 57% of individuals had an LDL-C <100 mg/dL, 38% had an LDL-C <85 mg/dL, and 18% had an LDL-C <70 mg/dL. Additionally, 61% of individuals had a body mass index <30 kg/m2 at enrollment, and 44% of individuals with diabetes had fasting glucose <126 mg/dL. After including individuals who had not fasted, the average number of risk factors goals met was still 3.6+1.2 with a median number of goals met of 4. When evaluating non-HDL-C as a separate measure, 23% of individuals had a non-HDL-C <100 mg/dL.
Discussion
In this analysis of community-dwelling individuals with self-reported CAD, we demonstrate that, on average, 4 out of a possible 7 risk factor goals studied in the COURAGE trial were met, and less than 1% of individuals achieved all 7 risk factor goals. Our results expand upon data recently published from the National Cardiovascular Data Registry that focused on the intensity of pharmacologic management in patients undergoing elective PCI [17]. Although risk factor levels were not reported, less than one-half of all patients undergoing elective PCI for stable angina were receiving pharmacologic therapy with anti-platelet agents, beta-blockers, and statins prior to undergoing PCI both before and after publication of the COURAGE trial [17].
The achievement of risk factor goals that we observed in this population-based sample was considerably lower than what participants in the COURAGE trial achieved [10]. At its conclusion, 96% of COURAGE trial participants were taking anti-platelet therapy compared with 77% of our study sample who reported regular aspirin use; 66% were exercising at least 150 minutes per week compared with 25% of our study sample who reported exercising ≥4 days per week; and approximately 60% reached the blood pressure goal compared with 50% of our study sample [10]. Smoking rates were similar with 19% of COURAGE participants smoking at the end of the study compared with 15% of our study sample [10]. This suggests that there is substantial opportunity to improve the risk factor profiles of individuals with CAD if optimal medical therapy, similar to that utilized in the COURAGE trial, were adopted on a wider scale. The COURAGE trial was not successful in reducing obesity as the mean body mass index in the COURAGE trial increased from 28.8 + 0.13 kg/m2 to 29.3 + 0.23 kg/m2 during the course of the study, which was similar to the mean body mass index (29.4 ± 5.8 kg/m2) present in REGARDS participants with CAD [10]. It is also noteworthy that in the COURAGE trial, not all participants achieved the study's goals either. A recent analysis of risk factor control among diabetic participants in three clinical trials, including COURAGE, demonstrated that risk factor goal attainment was poor even in a clinical trial setting [18]. These data suggest that while marked improvement can be made in risk factor control for United States adults with CAD, achieving all of these risk factor goals may be unrealistic for many people.
Similar to the findings in the COURAGE trial [9], multiple previous studies have demonstrated no reduction in death or myocardial infarction in stable CAD patients undergoing PCI compared to those medically managed [19-23]. However, these studies all suggest that the frequency and severity of anginal symptoms are reduced with PCI [19-23]. In the COURAGE trial, individuals randomized to the optimal medical therapy only arm received PCI for angina refractory to medical treatment or objective evidence of worsening ischemia on non-invasive testing. With this strategy, one-third of individuals required PCI over a median follow-up of 4.6 years. In COURAGE, the added cost of PCI was $10,000 without a significant increase in life years or quality adjusted life years gained [24]. Furthermore, PCI as an initial management strategy was associated with a cost of $206,229 per quality adjusted life year gained [24]. A recent publication from 10 institutions in the Northeastern United States suggests that at least in this consortium, the number of PCIs performed for stable angina has decreased since the publication of the COURAGE Trial [25]. These authors were unable to determine whether the observed decline in PCI was related to changes in risk factor modification. However, given the low number of risk factor targets achieved in the current study, efforts targeted towards improving risk factor control in CAD patients may result in substantial reductions in healthcare costs.
Previous studies have examined compliance with secondary prevention recommendations but were limited to specific populations or the examination of a limited number of risk factors [26-30]. Muntner, et al. demonstrated using 1999-2010 data from the National Health and Nutrition Examination Survey that many high-risk individuals had not achieved lipid goals [28]. Ho and colleagues examined achievement of lipid and blood pressure goals in Veterans Affairs facilities; only approximately one-half of patients were at goal LDL-C levels and fewer than half were at goal blood pressures [29]. In the Duke Databank for Cardiovascular Disease from 1995 to 2002, Newby, et al reported on changes in adherence to pharmacologic therapies but not actual risk factor goals [30]. Use of aspirin therapy in 2002 was 83% in their study [30], similar to the rate in REGARDS. Our study extends these aforementioned findings by examining a wide range of risk factor treatment goals in a large, nationwide, biracial community-based population.
Our study also provides insight into which types of patients are not achieving these risk factor goals. Similar to Newby, et al [30], African American race was associated with achievement of fewer risk factor goals. For age, we saw varying results based on the analysis performed. In the analysis looking at simply aspirin use, blood pressure control, and LDL-C control, younger age was associated with meeting more targets. However, in our analysis looking at all 7 targets, including behavioral targets, older age was associated with meeting more targets, suggesting that younger individuals with CAD may have better access to medications but are less likely to reach behavioral goals. In addition to age and race, we also observed that low income, less education, and poor functional status were associated with achievement of fewer risk factor goals. However, our model of sociodemographic factors explains only a small amount of the variance in risk factor control. While efforts should be concentrated on the vulnerable subgroups identified, achievement of risk factors goals was sufficiently low to justify a population-wide effort to identify and treat high risk individuals.
We studied the achievement of risk factor goals in order to draw conclusions about implications of the COURAGE trial [6,9]. Instead, had we chosen to study achievement of the ACC/AHA secondary prevention guideline goals [2] or clinical performance measures for the management of patients with stable CAD [11], we may have found different results, although the risk factor goals used in the COURAGE trial are, in most cases, very similar to those recommended by the ACC/AHA secondary prevention guidelines [2] as well as clinical performance measures for stable CAD [11] as shown in Table 1. However, our intent was not to examine quality of care but rather to estimate how close the management of more average CAD patients is to the treatment goals used in the COURAGE trial [6,9]. Additionally, unlike the goals used in the COURAGE trial, the secondary prevention guidelines and performance measures have never been prospectively studied. Based on the results of the COURAGE trial, a large percentage of individuals with stable CAD whose care is driven by these treatment goals can avoid PCI with good quality of life and no increased risk of myocardial infarction or death. As a result, given the significant number of PCIs performed annually in the United States, we were interested in studying the proportion of stable CAD patients who may not currently be achieving risk factor levels similar to those achieved in the COURAGE trial. The current results suggest that a large proportion of stable African American and white adults with CAD in the United States do not achieve risk factor levels similar to those used in the COURAGE trial, potentially contributing to more chronic angina, impaired quality of life, and potentially avoidable PCIs.
Our study has a number of limitations. First, the REGARDS participants were enrolled between 2003 and 2007, and the COURAGE trial was published in 2007. Therefore, we cannot evaluate whether increases in the proportion of CAD patients achieving these risk factor goals have occurred since the COURAGE trial was published. In addition, our mean age (69 years) is higher than the baseline age in COURAGE (61 years), and our sample included a higher proportion of African Americans (33% vs. 5%) and women (35% vs. 15%) than COURAGE. However, the baseline values for the risk factors studied were very similar in our participants to those of the COURAGE trial participants at baseline, suggesting that similar improvements in quality of life and reductions in the need for revascularization could be achieved with more intensive risk factor modification. Lastly, as is the case for most epidemiologic studies, we relied on self-report to identify individuals with CAD. Individuals with undiagnosed CAD or who failed to report a history of CAD were not included in this analysis.
In conclusion, on average, in the current study of United States black and white adults with a self-reported history of CAD, only half of the 7 possible modifiable risk factor goals were met. One strategy to enhance quality of life and reduce the number of PCIs performed may be to increase our focus on risk factor management for stable CAD patients.
Acknowledgments
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org. Dr. Brown's position is supported in part by grant 5KL2RR025776 from the UAB Center for Clinical and Translational Science with funding from the NIH National Center for Research Resources.
This project is supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, grant R01 HL80477 from the National Heart, Lung, and Blood Institute, and grant 5KL2RR025776 from the UAB Center for Clinical and Translational Science with funding from the NIH National Center for Research Resources. There was no relationship with industry for this project.
Abbreviations
- ACC
American College of Cardiology
- AHA
American Heart Association
- CAD
Coronary Artery Disease
- COURAGE
Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation Trial
- HDL-C
High Density Lipoprotein Cholesterol
- LDL-C
Low Density Lipoprotein Cholesterol
- REGARDS
REasons for Geographic and Racial Differences in Stroke study
- PCI
Percutaneous Coronary Intervention
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Lin GA, Dudley RA, Redberg RF. Cardiologists' use of percutaneous coronary interventions for stable coronary artery disease. Arch Intern Med. 2007;167:1604–1609. doi: 10.1001/archinte.167.15.1604. [DOI] [PubMed] [Google Scholar]
- 4.Rothberg MB, Sivalingam SK, Ashraf J, et al. Patients' and cardiologists' perceptions of the benefits of percutaneous coronary intervention for stable coronary disease. Ann Intern Med. 2010;153:307–313. doi: 10.7326/0003-4819-153-5-201009070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Feldman DN, Gade CL, Slotwiner AJ, Parikh M, Bergamn G, Wong SC, Minutello RM. Comparison of outcomes of percutaneous coronary interventions in patients of three age groups (< 60, 60 to 80, and > 80 years) (from the New York state Angioplasty Registry) Am J Cardiol. 2006;98:1334–1339. doi: 10.1016/j.amjcard.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 7.Brown TM, Bittner V. The management of stable patients with coronary heart disease: clinical implications of the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial. J Clin Lipidol. 2007;1:564–574. doi: 10.1016/j.jacl.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Howard VJ, Cushman M, Pulley LV, et al. The REasons for Geographic And Racial Differences in Stroke (REGARDS) study: objectives and design. Neuroepidemiology. 2005;25(3):135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 9.Boden WE, O'Rourke RA, Teo KK, et al. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial: Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151:1173–1179. doi: 10.1016/j.ahj.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Maron DJ, Boden WE, O'Rourke RA, et al. Intensive multifactorial intervention for stable coronary artery disease: optimal medical therapy in the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial. J Am Coll Cardiol. 2010;55:1348–1358. doi: 10.1016/j.jacc.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 11.Drozda JP, Messer JV, Spertus J. ACCF/AHA/AMA-PCPI 2011 performance measures for adults with coronary artery disease and hypertension. J Am Coll Cardiol. 2011;58:316–336. doi: 10.1016/j.jacc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Standards of medical care in diabetes – 2011. Diabetes Care. 2011;34(suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ware J, Kosinski M, Keller SD. A 12-Item Short-Form health survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. for the Modification in Renal Disease Study Group. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 16.Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educational and Psychological Measurement. 1993;53:1117–1125. [Google Scholar]
- 17.Borden WB, Redberg RF, Mushlin AI, Dai D, Kaltenbach LA, Spertus JA. Patterns and intensity of medical therapy in patients undergoing percutaneous coronary intervention. JAMA. 2011;305:1882–1889. doi: 10.1001/jama.2011.601. [DOI] [PubMed] [Google Scholar]
- 18.Farkouh ME, Boden WE, Bittner V, et al. Risk factor control for coronary artery disease secondary prevention in large randomized trials. J Am Coll Cardiol. 2013;61:1607–15. doi: 10.1016/j.jacc.2013.01.044. [DOI] [PubMed] [Google Scholar]
- 19.Parisi AF, Folland ED, Hartigan P, et al. A comparison of angioplasty with medical therapy in the treatment of single-vessel coronary artery disease. N Engl J Med. 1992;326:10–16. doi: 10.1056/NEJM199201023260102. [DOI] [PubMed] [Google Scholar]
- 20.RITA-2 trial participants. Coronary angioplasty versus medical therapy for angina: the second Randomized Intervention Treatment of Angina (RITA-2) trial. Lancet. 1997;350:461–468. [PubMed] [Google Scholar]
- 21.Pitt B, Waters D, Brown WV, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. N Engl J Med. 1999;341:70–76. doi: 10.1056/NEJM199907083410202. [DOI] [PubMed] [Google Scholar]
- 22.Hueb W, Soares PR, Gersh BJ, et al. The Medicine, Angioplasty, or Surgery Study (MASS-II): a randomized, controlled clinical trial of three therapeutic strategies for multivessel coronary artery disease: one-year results. J Am Coll Cardiol. 2004;43:1743–1751. doi: 10.1016/j.jacc.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 23.Henderson RA, Pocock SJ, Clayton TC, et al. Seven-year outcome in the RITA-2 trial: coronary angioplasty versus medical therapy. J Am Coll Cardiol. 2003;42:1161–1170. doi: 10.1016/s0735-1097(03)00951-3. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub WS, Boden WE, Zhang Z, et al. Cost-effectiveness of percutaneous coronary intervention in optimally treated stable coronary patients. Circ Cardiovasc Qual Outcomes. 2008;1:12–20. doi: 10.1161/CIRCOUTCOMES.108.798462. [DOI] [PubMed] [Google Scholar]
- 25.Ahmed B, Dauerman HL, Piper WD, et al. Recent changes in practice of elective percutaneous coronary intervention for stable angina. Circ Cardiovasc Qual Outcomes. 2011;4:300–405. doi: 10.1161/CIRCOUTCOMES.110.957175. [DOI] [PubMed] [Google Scholar]
- 26.Berger AK, Duval SJ, Armstrong C, Jacobs DR, Luepker RV. Contemporary diagnosis and management of hypercholesterolema in elderly acute myocardial infarction patients: a population-based study. Am J Geriatr Cardiol. 2007;16:15–23. doi: 10.1111/j.1076-7460.2007.04886.x. [DOI] [PubMed] [Google Scholar]
- 27.Bisognano JD, Townsend KA, Skyles AJ, Samuels KM. Prevalence of comorbidities and their influence on blood pressure goal attainment in geriatric patients. Am J Geriatr Cardiol. 2007;16:24–29. doi: 10.1111/j.1076-7460.2007.05543.x. [DOI] [PubMed] [Google Scholar]
- 28.Muntner P, Levitan EB, Brown TM, et al. Trends in prevalence, awareness, treatment, and control of high density lipoprotein-cholesterol among United States adults from 1999-2000 through 2009-2010. Am J Cardiol. 2013;112:664–70. doi: 10.1016/j.amjcard.2013.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho PM, Masoudi FA, Peterson ED, et al. Cardiology management improves secondary prevention measures among patients with coronary artery disease. J Am Coll Cardiol. 2004;43:1517–23. doi: 10.1016/j.jacc.2003.12.037. [DOI] [PubMed] [Google Scholar]
- 30.Newby LK, LaPointe NM, Chen AY, et al. Long-term adherence to evidence-based secondary prevention therapies in coronary artery disease. Circulation. 2006;113:203–12. doi: 10.1161/CIRCULATIONAHA.105.505636. [DOI] [PubMed] [Google Scholar]


