Abstract
Neurokinin B (NKB) is essential for human reproduction and has been shown to stimulate LH secretion in several species, including sheep. Ewes express the neurokinin-3 receptor (NK3R) in the retrochiasmatic area (RCh) and there is one report that placement of senktide, an NK3R agonist, therein stimulates LH secretion that resembles an LH surge in ewes. In this study, we first confirmed that local administration of senktide to the RCh produced a surge-like increase in LH secretion, and then tested the effects of this agonist in two other areas implicated in the control of LH secretion and where NK3R is found in high abundance: the preoptic area (POA) and arcuate nucleus (ARC). Bilateral microimplants containing senktide induced a dramatic surge-like increase in LH when given in the POA similar to that seen with RCh treatment. In contrast, senktide treatment in the ARC resulted in a much smaller, but significant, increase in LH concentrations suggestive of an effect on tonic secretion. The possible role of POA and RCh NK3R activation in the LH surge was next tested by treating ewes with SB222200, an NK3R antagonist, in each area during an E2-induced LH surge. SB222200 in the RCh, but not in the POA, reduced LH surge amplitude by about 40% compared to controls, indicating that NK3R activation in the former region is essential for full expression of the preovulatory LH surge. Based on these data, we propose that NKB actions in the RCh are an important component of the preovulatory LH surge in ewes.
Keywords: neurokinins, GnRH, oestrogen, NK3R, LH surge
INTRODUCTION
Although more than twenty years have passed since the initial study linking neurokinin B (NKB) to luteinising hormone (LH) secretion in women (1) and more recent evidence clearly demonstrated that NKB is critical for reproduction in humans (2), the details of how and where NKB acts to influence LH release remain largely unknown. Most work on NKB has focused on its possible roles in controlling tonic, episodic LH secretion. Low levels of tonic secretion of GnRH/LH are maintained during the luteal phase and early follicular phase by the negative feedback actions of oestradiol (E2) and progesterone (3, 4). However, these feedback actions of ovarian steroids on GnRH likely occur via interneurones since GnRH neurones do not express progesterone receptors (PR) (5, 6) or ERα (7), the ER isoform responsible for regulating GnRH secretion (8). NKB-containing neurones in the arcuate nucleus (ARC) are candidates for these steroid-responsive interneurones because they highly express ERα (9) and PR (10, 11).
Interest in NKB as a regulator of GnRH release began with the discovery that a subset of neurones coexpressing NKB and ERα in the infundibular nucleus undergo hypertrophy in postmenopausal women, suggesting that NKB is under E2-negative feedback control (1). These investigators further postulated that this hypertrophy was indicative of increased activity and thus NKB may contribute to the menopause-associated increase in LH release. Subsequent studies confirmed that E2 inhibits NKB as removal of steroid negative feedback via ovariectomy (OVX) increased NKB gene expression in the ARC of female monkeys (12, 13), sheep (14), rats (15), and mice (16, 17), while E2 treatment of OVX animals suppressed NKB gene expression in these same species (12, 15, 17-20). Furthermore, stimulation of LH secretion by NKB or senktide, a neurokinin-3 receptor (NK3R) agonist, has been described in non-rodent species including adult sheep (21, 22), prepubertal ewes (14) and prepubertal male monkeys (23). In rodents, the effects of NKB or senktide on GnRH/LH secretion appear to be dependent on steroid milieu (24). Thus in most reports, NK3R agonists stimulate LH secretion in gonadally-intact mice (25, 26) and rats (24, 27), but that they inhibit LH secretion in OVX mice (17) and rats (24, 27). In contrast, inconsistent effects of NK3R agonists have been observed in oestrogen-treated OVX rodents, with either inhibition or stimulation of LH release in rats (24, 27, 28) and no effect in mice (17).
While NKB is clearly an important regulator of LH secretion, it is unclear where NKB specifically acts to control GnRH release. The discovery that most ARC NKB neurones in ewes coexpress kisspeptin and dynorphin (thus named KNDy neurones) suggests that NKB closely interacts with kisspeptin (29), which is a potent stimulator of GnRH/LH secretion (30). More recently, NK3R was found to colocalize with a majority of NKB neurones in rats (31), mice (17), and sheep (32), while few or no GnRH cell bodies were found to express NK3R in rats (33) and sheep (32), respectively. In contrast, the vast majority of GnRH neurones express the kisspeptin receptor, Kiss1r, in both rodents (34, 35) and sheep (36), indicating that kisspeptin, but not NKB, directly affects GnRH neurones. These data, as well as others, have led to the hypothesis that NKB, acting via kisspeptin, is responsible for driving episodic GnRH secretion via a reciprocal KNDy neurone network while dynorphin acts as a brake to this system (17, 37, 38).
Although most NKB studies to date have focused on control of tonic GnRH secretion, this peptide may also be involved in the GnRH/LH surge. The initial report of NKB expression in sheep noted that females have twice as many NKB-positive ARC neurones than males and thus suggested a role in the preovulatory LH surge (9), but expression of mRNA for this peptide did not increase before or during the LH surge (9). Both injection of senktide in the third ventricle and placement of senktide-containing microimplants in the retrochiasmatic area (RCh) of the hypothalamus significantly stimulate LH secretion in ewes, with the high LH levels and pattern of secretion more closely resembling an LH surge than tonic LH secretion (21). However, it is unclear whether NK3R agonists have similar effects in other areas of the hypothalamus or if endogenous NKB plays a role in the LH surge. This work tested these important questions in three experiments. We first confirmed that local administration of senktide into the RCh induces a prolonged increase in LH secretion. We next tested effects of this agonist in two areas implicated in the control of GnRH that also contain neurones expressing NK3R: the preoptic area (POA) and ARC (32). Finally, based on the results of these experiments, we determined whether activation of NK3R in the RCh or POA is necessary for a normal LH surge.
MATERIALS AND METHODS
Animals
All experiments used adult ewes of predominantly Suffolk breeding that were housed indoors under a controlled photoperiod simulating natural outdoor day length. Ewes were fed a pelleted alfalfa diet and provided free access to water and supplemental minerals. The anoestrous experiment (Exp. 1) was performed in June in the middle of the anoestrous season (March through August), and breeding season experiments (Exps. 2 and 3) were done from October to mid-February. Blood samples (4 mL) were taken by jugular venipuncture, collected in heparinized tubes, and plasma stored at -20 C until assayed. All procedures were approved by the West Virginia University Animal Care and Use Committee and conducted in accordance with NIH guidelines on the care and use of animals in research.
Surgeries
All surgeries were performed under sterile conditions using isofluorane anaesthesia. Bilateral chronic 18-gauge guide cannulae were stereotaxically inserted into either the RCh, POA, or ARC as previously described (39). Ovariectomies were performed via midventral laparotomy (40). Ewes were treated with dexamethasone and penicillin pre- and post-operatively, and daily with analgesic (Banamine, Phoenix Pharmaceutical, St. Joseph, MO; 125 mg/sheep) starting at the time of anaesthesia to 5 days after surgery.
Experimental Approach
To determine whether the NK3R agonist senktide or the NK3R antagonist SB222200 (both obtained from Tocris Bioscience, Ellisville, MO) altered LH secretion, we administered the drugs by microimplantation into the RCh, POA or ARC as we have done with numerous receptor agonists and antagonists (21, 41). Microimplants were constructed from 22-gauge stainless steel tubing and cut to extend to the tip of the guide cannulae for ARC experiments or 1.5 mm beyond the tip for the RCh and POA. Our previous work showed that extending empty microimplants beyond the end of the guide tube within the ARC influenced LH release (41). Microimplants were filled by tamping at least 40 times in crystalline senktide or SB222200. As a control, empty microimplants were used.
Experiment 1: Effect of NKB or senktide in the RCh of anoestrous ewes
Because of the variability in response to senktide in rodents (24-28), we tested the repeatability of senktide actions in the RCh before embarking on extensive work exploring other possible sites of action of senktide in the ewe. This experiment was performed in anoestrous ewes to allow for data analysis to be completed before the start of Exp. 2 in the next breeding season; treatments of senktide in the RCh produced similar effects during both the breeding season and anestrus (21). Starting approximately two weeks after implantation of chronic guide tubes, blood samples were collected every 12 min for 24 min before and 4 h after insertion of senktide-filled or empty microimplants. Samples were then taken every 30 min for an additional 4 h, after which microimplants were removed and obturators replaced to occlude the guide cannulae. One week later, this protocol was repeated with ewes that had received senktide previously now receiving empty microimplants and vice versa. Animals were then killed and location of microimplants determined histologically (see below).
Experiment 2: Effect of senktide microimplants in the POA and ARC on LH release in follicular phase ewes
Based on the results of Exp. 1, we next proceeded to test the actions of senktide in the POA and ARC in the follicular phase of the cycle (18) during the subsequent breeding season. Bilateral guide tubes were implanted aimed at the ARC (n=6) or POA (n=6), and ovarian cycles synchronised as previously described (21). Briefly, two intramuscular (im) injections of prostaglandin F2α (5 mg/mL, Luteolyse, Pharmacia & Upjohn Co., NY, NY) were given 3 h apart, and this regimen was repeated seven days later. At this time two progesterone-containing controlled internal drug-releasing devices (CIDRs; Eazi-Breed, Pharmacia & Upjohn, New York, NY) were inserted intravaginally to produce luteal phase levels of progesterone. Seven days later PGF2α was again injected and CIDRs were removed. Starting 18 h after CIDR removal in the early follicular phase, blood samples were collected every 12 min from 24 min before to 4 h after insertion of senktide-filled or empty microimplants and less frequent sampling continued for another 4 hrs as in Exp. 1. Microimplants were then replaced with obturators and two CIDRs inserted intravaginally. Seven days later, CIDRs were removed, PGF2α was injected, and the treatment protocol repeated using a cross-over design so that all ewes received both treatments in a random order. Tissue was then collected to determine microimplantation sites (see below).
Experiment 3: Effect of SB222200 in the RCh or POA on the E2-induced LH surge
Based on the results of Exps 1 and 2, we next tested whether the actions of endogenous NKB in either the RCh or POA played a role in the oestrogen-induced LH surge. To do so, we used a protocol to produce an artificial follicular phase in OVX ewes in which exogenous E2 treatment simulates the normal preovulatory rise in circulating E2 (42, 43). Ewes were OVX and chronic guide cannulae were implanted aimed at the RCh (in September) or POA (in January). At the time of OVX, one short (1 cm) E2-containing Silastic implant was inserted subcutaneously (s.c.) and two CIDRs were inserted intravaginally. CIDRs were removed 10-14 days later. Twenty-four hours post-CIDR removal, four long (3 cm) E2-containing Silastic implants were inserted s.c., to establish an artificial follicular phase and produce an LH surge (43). This protocol has been used extensively by several different laboratory (42-45) and results in very consistent E2 concentrations in replicates and regardless of time of year (42, 45, 46). Either empty or SB222200-containing microimplants were inserted into the RCh or POA just before insertion of the long E2 implants. Starting 8 h later, jugular blood samples were taken every 2 h for 24 h then continued every 4 h for an additional 12 h. New SB222200-containing microimplants were inserted 24 h after original microimplants were inserted to maintain SB222200 delivery. At the conclusion of the sampling period, microimplants and the longer E2 implants were removed. SB222200 powder was still visible in the lumen of the microimplants at removal. CIDRs were reinserted 3 days later to reestablish an artificial luteal phase. Eight days after CIDR insertion, CIDRs were removed and the experiment was repeated with a crossover design so that each ewe received both control and SB222200 treatment. Thus the two replicates occurred two weeks apart, in November for RCh treatments and late January-early February for POA treatments.
Tissue collection
One or two days after conclusion of the final blood sampling period in all experiments, ewes were euthanised via an overdose of sodium pentobarbital (8-12 ml of Euthasol; 390 mg pentobarbital sodium/mL; Webster Veterinary, Devens MA). Two iv injections of heparin (25,000 U) were given 10 minutes prior to, and immediately before, pentobarbital. The head was quickly removed and perfused via the carotid arteries with 6 liters of 4% paraformaldehyde in 0.1 M phosphate buffer containing 0.1% NaNO3. After perfusion, a block of tissue containing the POA and hypothalamus was dissected from the brain and stored in paraformaldehyde overnight at 4 C. The tissue was then infiltrated with 30% sucrose, and coronal sections (50 μm thick) were cut on a freezing microtome. A series of every fifth section was mounted on slides and stained with cresyl violet to evaluate cannulae placement.
Assays
Using an RIA which has previously been validated in sheep (47), LH was measured in duplicate using 25-200 μl of plasma and expressed in terms of NIH-LH-S12. The minimal detectable concentration of LH in these assays averaged 0.10 ng/tube; intra- and interassay coefficients of variation were 15.0% and 18.2%, respectively. To confirm stage of the oestrous cycle in Exp. 2, circulating progesterone was measured in duplicate aliquots of 150 μl plasma with a commercially available solid-phase RIA kit (Coat-A-Count Kit, Diagnostics Products Corporation, Los Angeles, CA), which has been validated in sheep (39). All follicular-phase ewes had plasma progesterone concentrations less than 0.3 ng/ml; intra- and interassay coefficients of variation were 2.3% and 1.2%, respectively.
Statistical analysis
To distinguish between “surge-like” secretion and an increase in tonic (pulsatile) LH secretion we used two criteria: 1) once an LH increase began it had to be continuous until peak concentrations were reached, with no fall in LH concentrations between samples consistent with the metabolic half-life of LH and 2) peak concentrations had to be greater than ten-times basal LH concentrations. Differences in LH concentrations were analyzed by the Holm-Sidak one-way repeated measures ANOVA with post hoc analyses of pair-wise comparisons completed using paired t-tests. Data are reported as mean ± SEM. Differences were considered significant when P < 0.05.
RESULTS
Experiment 1: Effect of senktide treatment in the RCh on LH secretion in anoestrous ewes
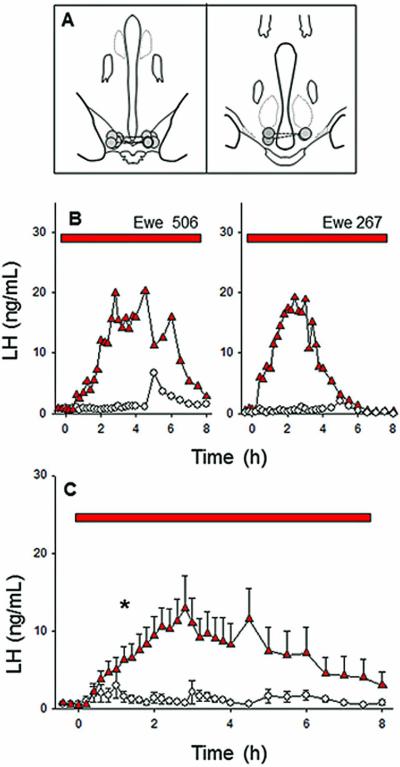
Five of the seven ewes had correctly-placed microimplant sites in the RCh (Fig. 1A). They were within 0.5-1.5 mm of the base of the brain and at the posterior end of the optic chiasm (Fig. 1A, left panel). Placement in two ewes was posterior to the RCh (Fig. 1A, right panel). The five ewes with appropriate placements responded to senktide microimplants with a significant and sustained increase in LH secretion (Fig. 1BC), a response similar to that which we previously reported (21). LH concentrations in response to senktide were first significantly higher than in control treatments at about 1.5 hrs after the start of treatment and remained significantly elevated for a total of 4.5 h. The average of peak LH concentrations in individual ewes was 16.9 ± 3.4 ng/mL and occurred on average 3 h after microimplant insertion. The observed elevation in LH secretion was continuous in all ewes, with peak concentrations ranging from 12.5 to 54 fold greater than basal levels. The two ewes with misplaced cannulae had a much lower LH peak (~3.5 ng/mL), which was not significantly different than peak control values. Occasional LH pulses were observed during treatment with empty microimplants (Fig. 1B) as expected for ovary-intact anoestrous ewes.
Figure 1.
Effect of microimplants of senktide in the RCh on LH secretion in anoestrous ewes. Top panel (A) depicts microimplant placement within the RCh, with bilateral placements in the same animal connected by a dashed line. The middle (B) and bottom (C) panels depict LH patterns in two representative ewes and the mean (± SEM) LH concentrations for the group (n=5), respectively, during treatment with senktide-containing (red triangles) or empty (open circles) microimplants. Red bars indicate the time period of microimplant treatment. Asterisks in panel C indicate the first point at which LH concentrations in senktide-treated ewes was significantly greater than in controls.
Experiment 2: Effect of senktide microimplants in the POA or ARC on LH secretion in follicular phase ewes
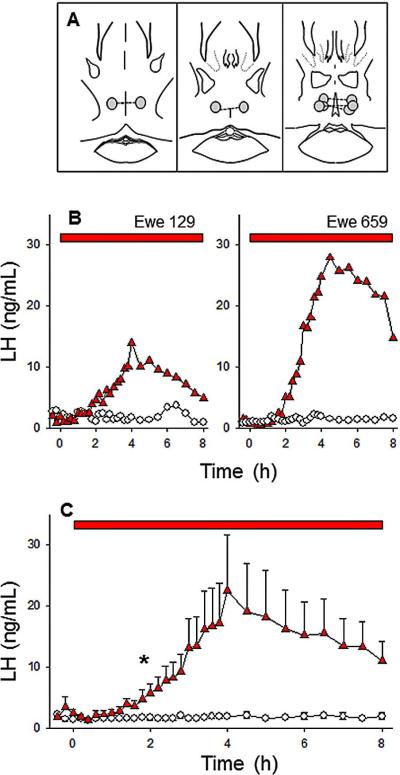
Five of six ewes had correct microimplant placements in the POA (Fig. 2A); placement in the other ewe was posterior to the POA (data not shown). Insertion of empty microimplants had no obvious effects on LH secretion. All five ewes with correct cannulae placement responded to senktide microimplants with a sustained increase in LH secretion (data from two ewes shown in Fig. 2B). The average peak LH concentration (22.4 ± 9.1 ng/mL) was similar to that seen with RCh treatments. The rise in LH was first significant 2 h after microimplant insertion and remained significantly elevated throughout the sampling period (Fig. 2C). The observed elevation in LH secretion was continuous (Fig. 2B) and peak LH concentrations were greater than ten-fold baseline levels in all five ewes (range: 10.3 to 31). The ewe with cannulae placement posterior to the POA had a lower maximum LH level of ~2.8 ng/mL, similar to that seen with control treatment.
Figure 2.
Effect of microimplants of senktide in the POA on LH secretion in follicular phase ewes. Top panel (A) depicts microimplant placement within the POA. The middle (B) and bottom (C) panels depict LH patterns in two representative ewes and the mean (± SEM) LH concentrations for the group, respectively, during treatment with senktide-containing (red triangles) or empty (open circles) microimplants. Red bars indicate the time period of microimplant treatment. Asterisks in panel C indicate the first point at which LH concentrations in senktide-treated ewes was significantly greater than in controls.
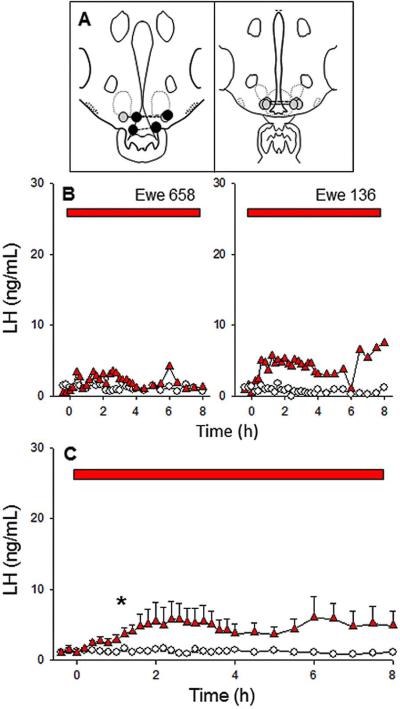
Four of six ewes had correctly placed microimplant sites in the ARC (Fig. 3A, grey symbols); in each of the other 2 ewes, one guide cannula was placed within the third ventricle (Fig. 3A, black symbols). All four ewes with correct cannulae placement responded to senktide microimplants with an increase in LH secretion as compared to empty implants (Fig. 3 B-C). However, the average peak LH secretion (5.8 ± 2.5 ng/mL) was much lower than that observed in the POA group and in no case was it 10 fold above basal concentrations (range: 3.7-6.1). In one ewe, the increase was continuous, but in the other three clear episodic secretion, indicative of tonic LH secretion, was evident. Each of the two ewes with one of the guide cannulae in the third ventricle had much higher peak LH values, averaging 52.2 ng/mL (20 and 80 fold greater than baseline), as expected based on previous work (21).
Figure 3.
Effect of microimplants of senktide in the ARC on LH secretion in follicular phase ewes. Top panel (A) depicts microimplant placement within the ARC. The middle (B) and bottom (C) panels depict LH patterns in two representative ewes and the mean (± SEM) LH concentrations for the group, respectively, during treatment with senktide-containing (red triangles) or empty (open circles) microimplants. Red bars indicate the time period of microimplant treatment. Asterisks in panel C indicate the first point at which LH concentration in senktide-treated ewes was significantly greater than in controls. Note that the scale of the y-axis is the same as Figs 1 and 2 to facilitate comparisons of the responses to senktide in all three areas.
Experiment 3: Effect of SB222200 in the RCh or POA on the E2-induced LH surge
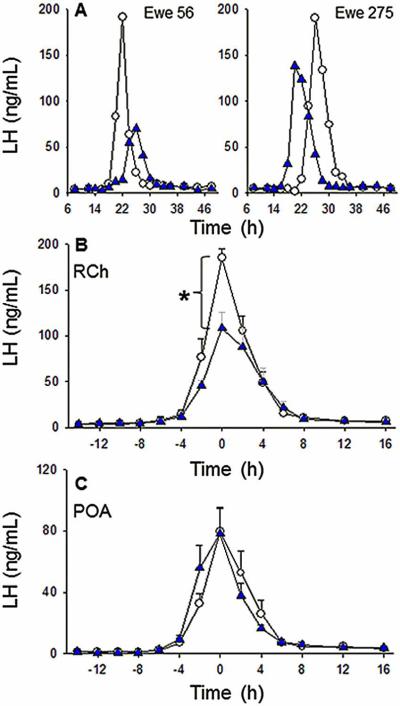
Four of the five ewes in the RCh group had correct placement of guide cannulae (data not shown), while the end of the guide tubes rested within the optic chiasm of the fifth ewe. LH surges are shown for two representative ewes with guide cannulae targeting the RCh in Fig. 4A. LH surges occurred in all five ewes, regardless of treatment, with peak LH release after insertion of the four E2 implants occurring at 24.5 ± 2.6 h in controls and 27.7 ± 2.8 h in SB222200-treated ewes (P > 0.05). All four ewes with correct placement of cannulae exhibited blunted LH surge amplitudes when treated with SB222200 in the RCh so that mean amplitude was significantly less compared to that during treatment with empty microimplants (108.4 ± 17.5 vs. 185.1 ± 10.2 ng/mL, respectively; Fig. 4B). In the ewe with missed cannulae placement, LH surge peak amplitude was higher during SB222200 treatment than during control treatment (232.2 ng/mL vs. 132.2 ng/mL, respectively).
Figure 4.
Effect of microimplants of SB222200 in the RCh and POA on the oestrogen-induced LH surge. The top panel (A) depicts LH profiles from two representative ewes from the RCh group with (blue triangles) or without (open symbols) SB222200 treatment during an E2-induced LH surge. The middle panel (B) shows the mean (± SEM) LH concentrations from all ewes (n=4) before and after the LH surge with SB222200-containing (blue symbols) or empty (open symbols) microimplants in the RCh. Asterisk: LH surge amplitude in the presence of SB222200 was significantly lower than the amplitude observed with empty microimplants. The bottom panel (C) presents the mean (± SEM) LH concentrations from all ewes (n=6) treated with SB222200-containing (blue symbols) or empty (open symbols) microimplants in the POA during an E2-induced surge. Note that time on the X-axis in panel A is from insertion of 4 E2 implants, while time in panels B and C is normalized to the peak of the LH surge. Time to LH peak did not differ by treatment in either the RCh or the POA groups.
All six ewes in the POA group had accurate guide cannulae placement and all exhibited E2-induced LH surges regardless of treatment, with peak LH values post-E2 treatment occurring at 23.6 ± 1.0 h in controls and at 25.0 ± 0.9 h in SB222200-treated ewes, which did not differ significantly. Average peak LH concentrations were not different between treatments (Fig. 4C).
DISCUSSION
Several lines of evidence, including the presence of NK3R and kisspeptin in KNDy neurones has led to the hypothesis that NKB elicits LH release by autocrine or paracrine actions on ARC KNDy neurones to cause kisspeptin release which in turn stimulates GnRH release (17, 22, 37, 48). However, NK3R expression is also relatively high in other areas of the hypothalamus (e.g., the POA and RCh). This raises the possibility that NKB may also act in these areas to influence GnRH/LH release. We found that senktide stimulated surge-like LH secretion when administered within the ovine RCh or POA but produced a more modest increase in the ARC. These results point to both the POA and RCh as possible sites for NKB action during the GnRH/LH surge, but only blockade of NK3R in the RCh reduced LH surge amplitude. This confirms and extends previous data from our laboratory that demonstrated stimulatory effects of senktide in the RCh (21), and demonstrates for the first time in any species that endogenous NKB signalling contributes to the oestrogen-induced LH surge.
The increase in LH release following administration of senktide in the RCh and POA resembled surge-like LH secretion, suggesting that NKB may be involved in the GnRH/LH surge, a hypothesis that until now has not been directly tested. Thus, although a number of studies have demonstrated stimulatory effects of NK3R agonists on LH secretion, this is the first to show, using a receptor antagonist, that NK3R activation is essential for the full LH surge. This effect seemed specific to the RCh as SB222200 placed in the POA, or in the one RCh ewe with misplaced guide cannulae, did not alter the E2-induced LH surge. In contrast, senktide treatment in both areas stimulated surge-like LH secretion. There are, however, several caveats that should be kept in mind in interpreting these data. First, amplitudes of LH surges during treatment with empty implants were noticeably higher in ewes of the RCh group compared to those in the POA group. This difference is likely due to the fact that the RCh work was carried out in the middle of the breeding season whereas the POA experiment was performed near the end of the breeding season. Previous work has shown that the amplitude of the LH surge using this surge-inducing paradigm is greater in November than in February (42). This seasonal difference also raises the possibility that the apparent effects of SB222200 in the RCh could reflect the order of treatment, since by chance three of these four ewes received SB222200 in the second replicate. However, both replicates were done in November and there is no evidence for changes in the amplitude of the estrogen-induced LH surge within the same month (43). Moreover, the percent decrease in LH surge amplitude for the ewe that received SB222200 first (32.5%) was within the range of those that received it second (27.4-63.3%). It is thus very unlikely that the order of treatment can explain the significant decrease in LH surge amplitude produced by the NK3R antagonist in the RCh.
While the lack of effect of SB222200 would appear to rule out a role for POA NK3R in the endogenous GnRH surge, it is important to note that neurones expressing NK3R in the POA are more widely scattered than NK3R-positive neurones in the RCh (32). This difference provides a possible alternative explanation for these data. Senktide, which is highly soluble in water, may have diffused far enough to reach sufficient receptors in both areas to stimulate surge-like LH release, while SB222200, which is relatively insoluble in water, may not have reached enough NK3R-positive cells in the POA to affect the LH surge, but did so when placed in the RCh. We also cannot rule out the possibility that the incomplete blockage of the LH surge is a result of the SB222200 treatment not fully blocking all NK3R in the RCh. Moreover, NK3R in multiple areas (other than just RCh) may need to be blocked in order to completely prevent the LH surge because several hypothalamic areas appear to participate in the LH surge in ewes. For example, in addition to the RCh, the POA and MBH are involved because Fos expression in kisspeptin (49) and GnRH (50) neurones in both the POA and MBH is increased at the time of surge.
The identity of neurones in the RCh that express NK3R is unknown, but they do not appear to be the A15 population of dopamine neurones (21) that are prominently involved in seasonal breeding (51, 52). Likewise, the pathway through which activation of RCh NK3R acts to stimulate LH secretion is yet to be identified, but may well involve kisspeptin neurones. The importance of kisspeptin to the LH surge in ewes has previously been demonstrated with intracerebroventricular administration of a Kiss1r antagonist suppressing the LH surge amplitude by ~50% in ewes (36). Our data showing a similar degree of suppression of the LH surge raise the possibility that the NK3R antagonist is blocking the same neural pathway as the Kiss1r antagonist in that study. Neither SB222200 treatment nor the Kiss1r antagonist (36) altered the timing of the surge, which may indicate that NKB and kisspeptin act only to ensure the full expression of the LH surge, but do not act to initiate the surge.
In contrast to the effects of senktide treatments in the RCh and POA, the significant but small LH response to ARC senktide treatment indicates that ARC NK3R are likely not involved in LH surge regulation. The relatively modest increase in mean LH concentrations (compared to senktide effects in the RCh and POA) and the presence of pulsatile LH patterns in most ewes point to a primary role for ARC NKB signalling in governing tonic LH secretion. This is consistent with several previous studies implicating NK3R in ARC neurones in the control of episodic GnRH secretion (22, 37, 38, 41). Thus there appear to be site-specific roles for NK3R in the regulation of tonic and surge secretion of LH.
While these data indicate that NKB contributes to the LH surge in sheep, their applicability to other species is unclear. There is currently no evidence that NKB plays a role in the LH surge in rodents, and this would be consistent with known differences in the mechanisms responsible for the LH surge in sheep and rodents. Oestrogen acts in the mediobasal hypothalamus (MBH) of sheep to induce the LH surge (53), but in the rostral periventricular area of the third ventricle (RP3V) in rodents (54-56). There are no reports of NKB-containing neurones in the RP3V and the population of kisspeptin neurones in this area is thought to mediate the positive feedback actions of oestrogen (57-59). Since the oestrogen-induced LH surge in sheep in independent of time of day (3), perhaps an NK3R-dependent signal from the RCh in sheep has replaced the afternoon signal from the SCN in rodents (60) as the trigger for kisspeptin release that drives the GnRH surge. In this regard, primates are similar to sheep in that the preovulatory LH surge is not tightly coupled to time of day and oestrogen positive feedback occurs in the MBH (61). These similarities raise the possibility that NK3R signalling may be important to the LH surge in primates, as it is in sheep. This possibility is consistent with the genetic data indicating that NKB/NK3R interaction is essential for reproduction in humans (2). In contrast, NK3R knockout mice remain fertile (62), albeit with some deficits in reproductive function (63).
In summary, we show herein that administration of the NK3R agonist senktide in either the POA or RCh stimulates surge-like LH secretion and that blockade of NK3R in the RCh attenuates the amplitude of the LH surge. In contrast, stimulation of NK3R in the ARC produced a much smaller increment in LH concentrations, which is more consistent with a role in regulation of tonic LH secretion. While it is clear that E2 acts in the MBH to induce a GnRH/LH surge in sheep, the neural mechanisms that transmit this signal to GnRH neurones are not well understood. Based on these data we hypothesize that activation of NK3R by NKB is an important part of this pathway.
Acknowledgments
We thank Heather Bungard and Cheri Felix at the West Virginia University Food Animal Research Facility and Dr. Margaret Minch for care of animals, and Paul Harton for his technical assistance in sectioning tissue. We also thank Dr. Al Parlow and the National Hormone and Peptide Program for reagents used to measure LH. This work was supported by grants from NIH (RO1-HD039916, RO1-HD017864).
References
- 1.Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–47. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 2.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nature genetics. 2009;41(3):354–8. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Neill J,D, editor. Knobil and Neill's Physiology of Reproduction. 3rd edition. Elsevier; Amsterdam: 2006. pp. 2389–447. [Google Scholar]
- 4.Zeleznik AJ, Pohl CR. Control of follicular development, corpus luteum function, the maternal recognition of pregnancy, and the neuroendocrine regulation of the menstrual cycle in higher primates. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. Third Edition. Elsevier; Amsterdam: 2006. pp. 2449–510. [Google Scholar]
- 5.Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–9. doi: 10.1210/endo.142.2.7956. [DOI] [PubMed] [Google Scholar]
- 6.Fox SR, Harlan RE, Shivers BD, Pfaff DW. Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology. 1990;51(3):276–83. doi: 10.1159/000125350. [DOI] [PubMed] [Google Scholar]
- 7.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887–95. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 8.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–80. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–25. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 10.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalisation of dynorphin a and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. Journal of neuroendocrinology. 2006;18(7):534–41. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 11.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–74. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 12.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. Journal of neuroendocrinology. 2004;16(2):146–53. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 13.Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17beta-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151(8):3783–94. doi: 10.1210/en.2010-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nestor CC, Briscoe AM, Davis SM, Valent M, Goodman RL, Hileman SM. Evidence of a role for kisspeptin and neurokinin B in puberty of female sheep. Endocrinology. 2012;153(6):2756–65. doi: 10.1210/en.2011-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology. 1994;60(4):337–45. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 16.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. American journal of physiology Endocrinology and metabolism. 2009;297(5):E1212–21. doi: 10.1152/ajpendo.00461.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(38):11859–66. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. The Journal of clinical endocrinology and metabolism. 1999;84(6):2111–8. doi: 10.1210/jcem.84.6.5689. [DOI] [PubMed] [Google Scholar]
- 19.Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor alpha. Endocrinology. 2004;145(2):736–42. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- 20.Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. Journal of neuroendocrinology. 2003;15(8):749–53. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 21.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–46. doi: 10.1210/en.2010-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakamoto K, Murata K, Wakabayashi Y, Yayou KI, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central Administration of Neurokinin B Activates Kisspeptin/NKB Neurons in the Arcuate Nucleus and Stimulates Luteinizing Hormone Secretion in Ewes During the Non-Breeding Season. The Journal of reproduction and development. 2012;58(6):700–6. doi: 10.1262/jrd.2011-038. [DOI] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–503. doi: 10.1210/en.2010-0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. American journal of physiology Endocrinology and metabolism. 2011;300(1):E202–10. doi: 10.1152/ajpendo.00517.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Galiano D, van Ingen Schenau D, Leon S, Krajnc-Franken MA, Manfredi-Lozano M, Romero-Ruiz A, Navarro VM, Gaytan F, van Noort PI, Pinilla L, Blomenrohr M, Tena-Sempere M. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–28. doi: 10.1210/en.2011-1260. [DOI] [PubMed] [Google Scholar]
- 26.Navarro VM, Gottsch ML, Wu M, Garcia-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–75. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinsey-Jones JS, Grachev P, Li XF, Lin YS, Milligan SR, Lightman SL, O'Byrne KT. The inhibitory effects of neurokinin B on GnRH pulse generator frequency in the female rat. Endocrinology. 2012;153(1):307–15. doi: 10.1210/en.2011-1641. [DOI] [PubMed] [Google Scholar]
- 28.Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain research. 2004;1026(2):307–12. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–60. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 30.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(5):1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. The Journal of comparative neurology. 2006;498(5):712–26. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 32.Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. Journal of neuroendocrinology. 2010;22(1):1–12. doi: 10.1111/j.1365-2826.2009.01930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. The Journal of comparative neurology. 2005;489(3):372–86. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 34.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–7. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 35.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–72. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–12. doi: 10.1210/en.2010-1225. [DOI] [PubMed] [Google Scholar]
- 37.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–89. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(8):3124–32. doi: 10.1523/JNEUROSCI.5848-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinizing hormone via dopamine neurones in anoestrous ewes. Journal of neuroendocrinology. 2001;13(12):1051–8. doi: 10.1046/j.1365-2826.2001.00726.x. [DOI] [PubMed] [Google Scholar]
- 40.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–42. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 41.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy S, Millar R, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–69. doi: 10.1210/en.2013-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. The Journal of endocrinology. 1981;89(2):229–40. doi: 10.1677/joe.0.0890229. [DOI] [PubMed] [Google Scholar]
- 43.Skinner DC, Harris TG, Evans NP. Duration and amplitude of the luteal phase progesterone increment times the estradiol-induced luteinizing hormone surge in ewes. Biology of reproduction. 2000;63(4):1135–42. doi: 10.1095/biolreprod63.4.1135. [DOI] [PubMed] [Google Scholar]
- 44.Harris TG, Robinson JE, Evans NP, Skinner DC, Herbison AE. Gonadotropin-releasing hormone messenger ribonucleic acid expression changes before the onset of the estradiol-induced luteinizing hormone surge in the ewe. Endocrinology. 1998;139(1):57–64. doi: 10.1210/endo.139.1.5662. [DOI] [PubMed] [Google Scholar]
- 45.Jackson LM, Mytinger A, Roberts EK, Lee TM, Foster DL, Padmanabhan V, Jansen HT. Developmental programming: postnatal steroids complete prenatal steroid actions to differentially organize the GnRH surge mechanism and reproductive behavior in female sheep. Endocrinology. 2013;154(4):1612–23. doi: 10.1210/en.2012-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ. Estradiol requirements for induction and maintenance of the gonadotropin-releasing hormone surge: implications for neuroendocrine processing of the estradiol signal. Endocrinology. 1997;138(12):5408–14. doi: 10.1210/endo.138.12.5558. [DOI] [PubMed] [Google Scholar]
- 47.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–67. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 48.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–45. doi: 10.1159/000329045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (Kisspeptin/Neurokinin B/Dynorphin) Neurons Are Activated during Both Pulsatile and Surge Secretion of LH in the Ewe. Endocrinology. 2012;153(11):5406–15. doi: 10.1210/en.2012-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moenter SM, Karsch FJ, Lehman MN. Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology. 1993;133(2):896–903. doi: 10.1210/endo.133.2.8344224. [DOI] [PubMed] [Google Scholar]
- 51.Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. Journal of neuroendocrinology. 2010;22(7):674–81. doi: 10.1111/j.1365-2826.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thiery JC, Gayrard V, Le Corre S, Viguie C, Martin GB, Chemineau P, Malpaux B. Dopaminergic control of LH secretion by the A15 nucleus in anoestrous ewes. Journal of reproduction and fertility Supplement. 1995;49(1):285–96. [PubMed] [Google Scholar]
- 53.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139(4):1752–60. doi: 10.1210/endo.139.4.5904. [DOI] [PubMed] [Google Scholar]
- 54.Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain research reviews. 2008;57(2):277–87. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wiegand SJ, Terasawa E, Bridson WE, Goy RW. Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology. 1980;31(2):147–57. doi: 10.1159/000123066. [DOI] [PubMed] [Google Scholar]
- 56.Petersen SL, Barraclough CA. Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain research. 1989;484(1-2):279–89. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 57.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431–6. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- 58.Pineda R, Garcia-Galiano D, Roseweir A, Romero M, Sanchez-Garrido MA, Ruiz-Pino F, Morgan K, Pinilla L, Millar RP, Tena-Sempere M. Critical roles of kisspeptins in female puberty and preovulatory gonadotropin surges as revealed by a novel antagonist. Endocrinology. 2010;151(2):722–30. doi: 10.1210/en.2009-0803. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: developmental roles and major regulatory actions. Journal of neuroendocrinology. 2012;24(1):22–33. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 60.Khan AR, Kauffman AS. The role of kisspeptin and RFamide-related peptide-3 neurones in the circadian-timed preovulatory luteinising hormone surge. Journal of neuroendocrinology. 2012;24(1):131–43. doi: 10.1111/j.1365-2826.2011.02162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plant TM. A comparison of the neuroendocrine mechanisms underlying the initiation of the preovulatory LH surge in the human, Old World monkey and rodent. Frontiers in neuroendocrinology. 2012;33(2):160–8. doi: 10.1016/j.yfrne.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 62.Kung TT, Crawley Y, Jones H, Luo B, Gilchrest H, Greenfeder S, Anthes JC, Lira S, Wiekowski M, Cook DN, Hey JA, Egan RW, Chapman RW. Tachykinin NK3-receptor deficiency does not inhibit pulmonary eosinophilia in allergic mice. Pharmacological research : the official journal of the Italian Pharmacological Society. 2004;50(6):611–5. doi: 10.1016/j.phrs.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]