Abstract
Background
Midlife Metabolic Syndrome (MetS) may impact cognitive health as a construct independently of hypertension, hyperlipidemia and other components.
Methods
10,866 participants aged 45 to 64 at baseline were assessed for MetS and completed cognitive testing at two later time points (3 and 9 years from baseline visit).
Results
MetS is associated with increased odds of low cognitive performance in the domains of executive function and word fluency, but not 6-year cognitive decline. Individual MetS components explained this association (hypertension, diabetes, low HDL, elevated triglycerides and increased waist circumference).
Conclusions
A focus on the individual risk factors as opposed to MetS during midlife is important to reduce the incidence of cognitive impairment in later life.
Keywords: metabolic syndrome, cognition, cognitive decline, obesity, hypertension and dementia
Introduction
Emerging evidence links the metabolic syndrome (MetS) with cognitive decline, but whether the syndrome contributes to worsening cognition beyond the components of the syndrome is less understood.1–3 Traditional cardiovascular risk factors such as diabetes,4–6 hypertension,7 obesity and smoking have also been associated with an increased risk of dementia,8, 9 and as a composite, MetS and vascular risk factors have been shown to be associated with short-term cognitive decline.10–12
MetS could influence cognition by creating a greater burden of small vessel disease and subclinical strokes. In addition, individuals with MetS may have a distinct biology that relates to cognitive function through signaling chemokines.13, 14 Prior studies demonstrate “residual risk” of cardiovascular outcomes or dementia in participants with MetS after adjusted for other risk factors.15, 16 This study hypothesized that MetS would be associated with 6-year cognitive decline and that there would be particularly “high risk” clusters of MetS components, such as elevated blood pressure, increased waist circumference (WC) and elevated fasting glucose.
Methods
Study Population
ARIC is a longitudinal, prospective, multi-site study of which the initial recruitment and study participation has been previously described.17 The study was conducted at four sites (Jackson, MS; Forsyth County, NC; Washington County, MD; suburban Minneapolis, MN), and includes a biracial population of adults, who upon initial recruitment at visit 1 were ages 45 to 64. Visit 1 occurred from 1987 to 1989 and visit 5 from 2011 to 2013.
MetS components were evaluated at visit 1 (1987 to 1989). Neurocognitive testing (see description below) was completed at visit 2 (1990 to 1992) and visit 4 (1996 to 1999). Only participants who attended visit 1, visit 2 and visit 4 were included. Of 11,656 participants who returned for visit 4, additional excluded participants included: missing cognitive test data (n=566) and adjudicated strokes (n=157). Because of small numbers, those who were neither white nor black (n= 30) and the black participants in the Minnesota (n= 12) and Washington County cohorts (n= 25) were excluded, leaving 10,866 in the analytic population. Participants missing data on any of the MetS parameters or other covariates were dropped from the analysis specific to the missing parameter. The Institutional Review Boards (IRB) at all institutions approved the study.
Measures
Cognitive testing
Three tests were used in the cognitive battery. The Delayed Word Recall (DWR)18 involves presenting the subject with a list of ten words, and after five minutes asking them to recall the list. This test has shown have a high predictive accuracy for Alzheimer’s dementia, and is primarily a test of verbal learning and recent memory.
The Digit-Symbol-Substitution test (DSST) is a test of cognitive processing speed and executive functioning, and is a part of the Weschler Adult Intelligence Scale-Revised (WAIS-R). In this test, a subject is provided with a list of number-symbol pairs, then presented with a list of numbers and asked to substitute the corresponding symbols. The score (0 to 93) is determined by translating the correct number of symbols into numbers in 90 seconds.
In the Word Fluency Test (WFT), participants are asked to produce words beginning with a particular letter and are given 60 seconds to complete the task. Three trials were completed with the letters “F”, “A” and “S, and proper nouns were excluded. This test assesses expressive language and executive function.
Metabolic Syndrome Definition
Five components were used in the definition of MetS assessed at visit 1: elevated blood pressure, increased waist circumference (WC), elevated triglycerides (TG), low high-density lipoprotein (HDL), and impaired fasting glucose.
Systolic and diastolic blood pressure were measured sitting in the right arm, and were recorded as the mean of the last two of three measurements using a random-zero sphygmomanometer. WC was measured in centimeters at the level of the umbilicus. Blood collection methods were described previously,19–21 and while the majority of participants were fasting for all tests, non-fasting participants were excluded from lipid measurements. TGs were measured by enzymatic methods, and HDL cholesterol was measured after dextran-magnesium precipitation. Serum glucose was measured with the hexokinase/glucose-6 phosphate dehydrogenase method.
MetS was categorized according to the American Heart Association (AHA)22 criteria. To be categorized as having MetS, participants had at least three of the following five criteria:
1) Elevated WD: WC >102 cm in men or > 88 cm in women; 2) low HDL: HDL < 40 mg/dL in men and <50 mg/dL in women; 3) elevated blood pressure ≥130 mmHg systolic or ≥85 mmHg diastolic or on medication for hypertension; 4) elevated TG ≥150 mg/dL and 5) Impaired fasting glucose or diabetes: elevated fasting glucose ≥ 100, elevated non-fasting glucose ≥ 200 or on medication for diabetes. Separate variables were created for MetS (yes/no) and the number of MetS components.
Covariates
A combined variable of race and field center (race-center) was created to account for the co-linearity, of which there five indicators. Diabetes was defined by: current use of diabetes medication, fasting glucose of ≥126 mg/dL, or a non-fasting glucose of ≥ 200. Covariates in adjusted models included: age, race-center, education, tobacco use, alcohol and coronary heart disease history. Participants also brought current medications to each visit, which were recorded by trained personnel. Participants categorized with coronary heart disease included those with a history of myocardial infarction, myocardial infarction determined by ECG adjudication, or history of coronary artery bypass graft (CABG) or angioplasty.
Statistical Methods
Baseline characteristics for participants were analyzed for each variable. Between-group comparisons were made stratifying for MetS and sex and race. Test scores at visit 2 and 4, and the difference between these two, each were main dependent variables in separate models, and were analyzed as both continuous and categorical variables. All analyses were performed using Stata version 12 (StataCorp, College Station, Texas). A two-sided p-value of <0.05 was considered significant for all analyses.
Cross-sectional Analysis
Logistic regression was used to examine performance in the lowest quintile at each visit compared with quintiles 2–5, with cumulative MetS components, presence of MetS, or individual MetS components as predictors in separate models. Interactions terms were evaluated between pairs of components, such as WC and elevated blood pressure, and were not included in final models.
A MetS cluster refers to groupings of 3 to 5 metabolic syndrome components. A separate linear regression model defined cognitive performance for each test (3 models total), with MetS components as predictors. A predicted test score for each cluster was created using a dummy variable for each MetS characteristic indicating its presence or absence, for a “typical subject” and the resulted score was computed. Predicted scores were compared across MetS clusters.
Longitudinal Association
The difference in test scores between visit 4 and visit 2 was calculated by subtracting the visit 4 score from the visit 2 score, with a positive number indicating a decline in test scores. The date at visit 2 was subtracted from the date at visit 4, and the score difference was divided by the date difference to obtain the mean decline per year. Because not all participants were tested exactly 6 years apart, to standardize years of cognitive decline this number was multiplied by 6. A linear regression model was created, with MetS components as predictors, adjusting for covariates described above. Interaction was examined as detailed for logistic regression models.
Results
Participants
Of 10,866 participants included in the study, 56.2% (n=6,109) were women, and 36.5% (n=3,830) had MetS at baseline (Table 1). Based on WC measurements, 50.3% (N=5,497) participants were classified as obese. Body mass index (BMI), WC and other parameters of MetS (elevated blood pressure, blood glucose, and TG) were considerably increased in participants that met criteria for MetS, by design. High-density lipoprotein (HDL), which is protective against vascular disease if elevated, was also significantly lower in MetS participants. The percentage of participants with each MetS component is shown in eTable1.
Table 1.
Baseline participant characteristics and cognitive test scores at visit 2 by sex and metabolic syndrome (MetS) status
| Sample Size (N) | Women | Men | |||
|---|---|---|---|---|---|
|
| |||||
| No MetS | MetS | No MetS | MetS | ||
| Age (y ± SD) | 10,500 | 52.9 ± 5.6 | 54.8 ± 5.5 | 54.1 ± 5.7 | 54.7 ± 5.6 |
| Education | 10,487 | ||||
| Less than high school | 508 (13.3) | 432 (25.7) | 491 (17.3) | 297 (16.9) | |
| High school to college | 1,787 (46.7) | 1,006 (48.6) | 1,023 (36.1) | 728 (41.5) | |
| Post-graduate training | 1,533 (40.0) | 532 (25.7) | 1,322 (46.6) | 728 (41.5) | |
| Race | 10,500 | ||||
| Black | 711 (18.6) | 579 (28.0) | 447 (15.8) | 226 (12.9) | |
| White | 3,121 (81.5) | 1,492 (72.0) | 2,391 (84.3) | 1,533 (87.2) | |
| Smoking | 10,493 | ||||
| Current or former | 1,735 (45.3) | 871 (42.1) | 1,887 (66.5) | 1,269 (72.2) | |
| Never | 2,092 (54.7) | 1,199 (57.9) | 951 (33.5) | 489 (27.8) | |
| Alcohol | 10,462 | ||||
| Current or former | 2,722 (71.3) | 1,250 (60.6) | 2,454 (86.8) | 1,544 (88.1) | |
| Never | 1,097 (28.7) | 813 (39.4) | 374 (13.2) | 208 (11.9) | |
| BMI (kg/m2) (± SD) | 10,495 | 25.4 ± 4.8 | 31.0 ± 5.8 | 26.1 ± 3.3 | 29.7 ± 4.0 |
| Symptomatic heart disease | 10,441 | 188 (5.0) | 172 (8.4) | 175 (6.2) | 176 (10.1) |
| MetS Characteristics | |||||
| Waist Circumference (cm) (± SD) | 10,496 | 88.5 ± 12.7 | 104.4 ± 13.6 | 95.2 ± 8.9 | 105.2 ± 9.8 |
| Systolic blood pressure (mmHg) (± SD) | 10,497 | 113.6± 15.7 | 126.7 ± 18.0 | 116.8 ±14.7 | 126.1 ± 16.3 |
| Diastolic blood pressure (mmHg) (± SD) | 10,496 | 69.6 ± 9.9 | 75.2 ± 10.2 | 72.9 ± 9.7 | 77.5 ± 10.5 |
| Triglycerides (mg/dL) (± SD) | 10,500 | 97.3 ± 44.2 | 168.1 ± 97.3 | 106.2 ± 53.1 | 194.7 ± 115.0 |
| HDL (mg/dL) (± SD) | 10,500 | 64.1 ± 16.1 | 48.0 ± 13.6 | 48.3 ± 12.7 | 36.9 ± 9.6 |
| Fasting glucose (mg/dL) (± SD) | 10,500 | 95.2 ± 14.9 | 116.0 ± 41.2 | 99.8 ± 15.9 | 115.6 ± 32.5 |
| Diabetes | 10,496 | 66 (1.7) | 395 (19.1) | 102 (3.6) | 289 (16.4) |
| On lipid medication | 10,426 | 15 (0.4) | 140 (6.8) | 32 (1.1) | 105 (6.0) |
| On antihypertensive medication | 10,447 | 401 (10.5) | 948 (46.0) | 298 (10.6) | 636 (36.3) |
| Cognitive Test Scores | |||||
| Delayed Word Recall Test (± SD) | 10,478 | 7.1 ± 1.4 | 6.8 ± 1.4 | 6.5 ± 1.5 | 6.3 ± 1.4 |
| Digit Symbol Substitution Test (± SD) | 10,478 | 50.4 ± 12.9 | 45.0 ± 14.1 | 44.5 ± 12.7 | 43.9 ± 11.9 |
| Word Fluency Test (± SD) | 10,476 | 36.1 ± 11.9 | 32.5 ± 11.5 | 33.8 ± 12.5 | 33.0 ± 12.0 |
Units are N (%) unless otherwise specified
Cross-sectional Association
The relationships of visit 1 MetS components and MetS to low cognitive performance at visit 2 and 4 are shown in Table 2. MetS was associated with increased odds of poor test performance for three tests across all visits (with the exception of DSST visit 2). Comparing visit 4 to visit 2, more MetS components were associated with poor test performance on DWR and DSST at visit 4, while a similar number of MetS components were associated with WFT at visits 2 and 4. Overall, the relationships observed were the strongest for the DSST, and were more consistent for visit 4 cognitive data than for visit 2 data.
Table 2.
Odds ratio (95 % confidence interval) for performance in the lowest quintile
| DWR | DSST | WFT | ||||
|---|---|---|---|---|---|---|
| Visit 2 | Visit 4 | Visit 2 | Visit 4 | Visit 2 | Visit 4 | |
| Increased waist circumference | 1.11 (1.00, 1.24) | 1.15 (1.04, 1.27) | 1.22 (1.07, 1.40) | 1.31 (1.15, 1.49) | 1.24 (1.11, 1.39) | 1.19 (1.07, 1.33) |
| Elevated blood pressure | 1.07 (0.96, 1.19) | 1.06 (0.96, 1.17) | 1.10 (0.96, 1.25) | 1.22 (1.08, 1.39) | 1.16 (1.04, 1.30) | 1.10 (0.99, 1.23) |
| Elevated triglycerides | 1.09 (0.98, 1.24) | 1.18 (1.06, 1.32) | 1.03 (0.90, 1.19) | 1.21 (1.05, 1.40) | 1.15 (1.02, 1.29) | 1.11 (0.98, 1.25) |
| Low HDL | 1.09 (0.98, 1.22) | 1.19 (1.08, 1.32) | 1.10 (0.96, 1.26) | 1.15 (1.00, 1.31) | 1.1 (0.99, 1.23) | 1.11 (1.00, 1.24) |
| Elevated fasting glucose or diabetes | 1.02 (0.92, 1.14) | 1.00 (0.91, 1.11) | 1.14 (1.00, 1.29) | 1.17 (1.03, 1.33) | 1.06 (0.95, 1.18) | 1.08 (0.97, 1.20) |
| MetS | 1.12 (1.01, 1.15) | 1.13 (1.02, 1.25) | 1.11 (0.98, 1.26) | 1.24 (1.09, 1.41) | 1.25 (1.12, 1.40) | 1.18 (1.06, 1.32) |
Each component of the Metabolic Syndrome (MetS) is shown in a separate model for visit 2 and 4; all are adjusted for age, combined race and test center, education, sex, coronary artery disease, history of smoking and history of drinking. Results for composite MetS are shown in a separate model with the same covariates.
All MetS components were significantly associated with increased odds of performing in the lowest quintile on the DSST for visit 4, but only increased WC and elevated glucose achieved statistical significance at visit 2. For DSST at visit 4, increased WC and elevated blood pressure had the strongest associations, with a low HDL reaching marginal statistical significance. WC was the only variable that maintained significance across all three tests.
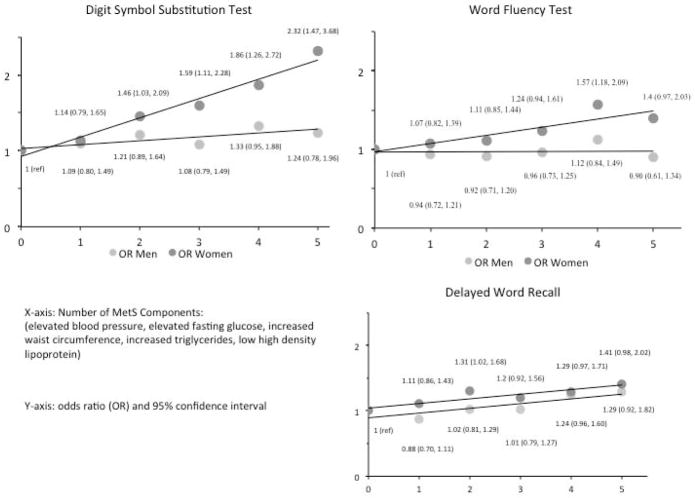
Increasing number of MetS components were associated with increased odds of performance in the lowest quintile for women only. Again, these results were stronger for the DSST, where having five components versus no components was associated with increased odds of performance in the lowest test quintile (Figure 1). Interaction terms for MetS components and gender were significant in DSST and WFT, but not DWR (adjusted OR MetS*gender (95% CI)): DWR 1.00 (0.93, 1.07); DSST 1.13 (1.03, 1.23); WFT 1.1 (1.02, 1.18).
Figure 1.
Number of metabolic syndrome (MetS) components at baseline and adjusted odds of lowest test quintile performance at visit 4 with 95% confidence intervals. Covariates include age, education, race-center number, sex, smoking and drinking status; reference (ref) category was zero MetS components.
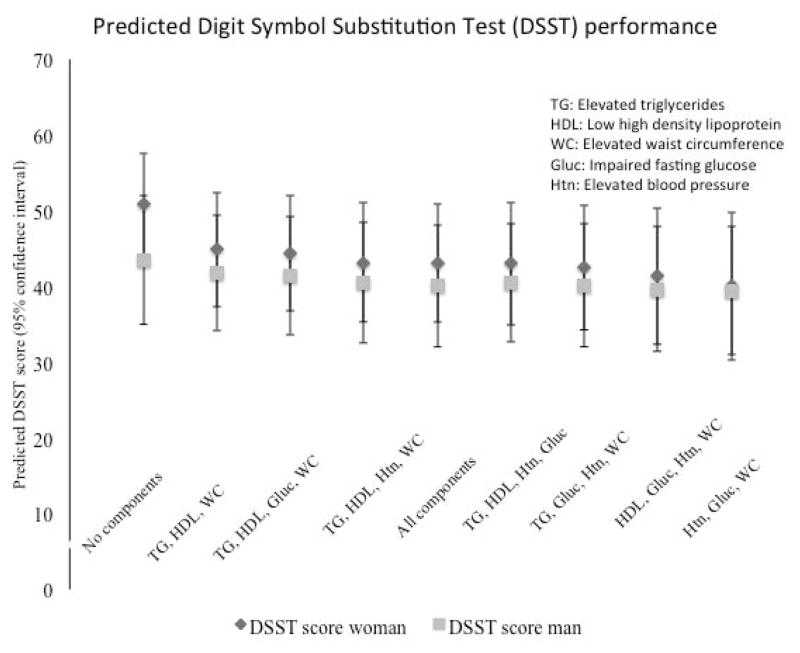
MetS clusters, in the multivariable model of MetS components as predictors of performance on the DSST, were not significantly different from each other (Figure 2). Having no MetS components trended toward higher test scores at visit 4 than having all five components. When MetS was included in the model with all of the individual components, it did not reach statistical significance for worse cognitive performance, indicating that MetS did not contribute to worse cognitive function above and beyond the individual factors (MetS adjusted β (95% confidence interval) DWR:−0.04(−0.15, 0.07); DSST −0.10(−0.81, 0.62); WFT −0.21(−1.03, 0.61)).
Figure 2.
Predicted mean digit symbol substitution test (DSST) score for the “average” participant with each metabolic syndrome (MetS) cluster. Scores shown for men and women with error bars for standard deviation. Model is adjusted for age, education, race-center number, smoking and drinking status.
Longitudinal Association
MetS was not a significant predictor of 6-year change in test score (Table 3). Diabetes was the only component associated with decline on all three tests; however in participants, impaired fasting glucose (greater than 100 mg/dL) or diabetes was not associated with cognitive decline. In men, elevated blood pressure (DSST), elevated TG (DWR) and low HDL (WFT) were associated with cognitive decline in the respective cognitive tests, but WC carried no association with more decline. In women, elevated TG (DWR) and diabetes (DWR, DSST, and WFT) were associated with cognitive decline. When stratified by race, it diabetes was associated with test score decline in white participants only, however interaction terms for diabetes and race were not significant (eTable 2).
Table 3.
6-year change in cognitive test scores (95% confidence interval) by sex, between visit 2 and visit 4
| DWR | DSST | WFT | ||||
|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | |
| Increased waist circumference | 0.03 (−0.06, 0.12) | 0.05 (−0.03, 0.15) | −0.02 (−0.39, 0.35) | 0.28 (−0.11, 0.67) | 0.34 (−0.13, 0.82) | −0.28 (−0.71, 0.14) |
| Elevated blood pressure | −0.05 (−0.14, 0.48) | −0.01 (−0.09, 0.08) | 0.39 (0.01, 0.76) | 0.05 (−0.36, 0.45) | 0.11 (−0.37, 0.60) | 0.41 (−0.03, 0.85) |
| Elevated triglycerides | 0.01 (−0.09, 0.11) | 0.10 (0.01, 0.20) | 0.01 (−0.38, 0.39) | 0.58 (0.13, 1.03) | 0.23 (−0.26, 0.73) | −0.15 (−0.64, 0.34) |
| Low HDL | −0.02 (−0.11, 0.07) | 0.05 (−0.04, 0.13) | 0.14 (−0.23, 0.51) | 0.24 (−0.16, 0.63) | 0.77 (0.29, 1.24) | 0.30 (−0.73, 0.13) |
| Elevated fasting glucose or diabetes | 0.03 (−0.06, 0.12) | 0.01 (−0.07, 0.09) | 0.22 (−0.15, 0.58) | −0.13 (−0.51, 0.26) | 0.21 (−0.26, 0.68) | −0.15 (−0.57, 0.27) |
| Diabetes | 0.14 (−0.01, 0.30) | 0.16 (0.01, 0.30) | 1.04 (0.42, 1.67) | 0.71 (0.04, 1.39) | 0.81 (0.001, 1.61) | 1.21 (0.47, 1.95) |
| MetS | 0.01 (−0.08, 0.11) | 0.05 (−0.04, 0.14) | 0.20 (−0.17, 0.58) | 0.10 (−0.31, 0.50) | 0.44 (−0.04, 0.92) | −0.28 (−0.72, 0.16) |
Positive values indicate a decline in cognitive test score. Results for composite Metabolic Syndrome (MetS) are shown in a separate model. Delayed Word Recall (DWR) has a range of 0 to 10, Digit Symbol Substitution Test (DSST) from 0 to 92 and Word Fluency Test (WFT) from 0 to 88.
Discussion
This prospective study found that the presence of MetS at baseline was associated with worse cognitive performance on three tests at individual visits in later life. The contribution of MetS to poor performance was not above and beyond the contribution made by individual vascular risk factors. This result was robust for the DSST, which is a test of processing speed and executive function, as well as for WFT, which measures language and executive function.
The decrement in function in the DSST and the WFT was more concordant with changes in function related to subcortical white matter disease or vascular cognitive impairment as opposed to Alzheimer’s disease pathology. Lacunar infarcts, which are a marker of cerebral small vessel disease, have been linked to decreased performance on the DSST,23 most likely because subcortical disease increases task-processing time. Risk factors such as obesity and hypertension are associated with psychomotor slowing and executive function deficits.24 The relationship between low performance on the DWR and MetS was the weakest, as this test is preferentially affected in Alzheimer’s disease.18
These findings are consistent with other large studies, which have shown that traditional vascular risk factors are associated with dementia.8, 15, 25 One study25 suggested that a composite score of four cardiovascular risk factors (hypertension, hyperlipidemia, smoking and diabetes) at midlife was associated with a greater of dementia in later life in a dose-dependent fashion. Similarly, the clustering of midlife obesity, hypertension and elevated cholesterol was shown to be an additive in risk for dementia.8
Our results emphasize that MetS is no more than a risk profile of individual risk factors with respect to its impact on cognition. The MetS definition does not include risk factors that are known to impact cognition, such as smoking. WC seemed to be a particularly robust predictor of the worst cognitive performance at visit 4. One possible explanation is that WC was the most prevalent MetS characteristic (see eTable 1), and may be an early indicator of poor cardiovascular health, even before other risk factors develop.
Change in cognitive performance, compared with performance on tests administered at one point in time, has less potential for confounding, particularly for factors that are stable within individuals, such as their education or early experiences. However, the cumulative MetS or the number of components was not associated with 6-year cognitive decline in this age group; with the exception of diabetes (Table 3). Of note the effect sizes observed are small, and may not be clinically significant at the time studied. As this study only measures six-year decline, those with some decline may be more likely to progress to dementia. ARIC investigators have previously shown that both diabetes and elevated blood pressure are associated with cognitive decline.11 The risk of cardiovascular disease contributed by MetS in ARIC was not in excess of the level explained by the individual components,26 and the present study suggests that the same pattern holds for cognitive decline.
An important study of the contribution of midlife vascular risk factors to cognitive health at older ages was The Honolulu-Asia Aging Study, which included middle-age Japanese men in longitudinal follow-up over 40 years. This cohort followed participants to a diagnosis of dementia in their elderly years, and found that higher MetS z -scores were associated with a higher likelihood of vascular dementia.15 The present analysis adds to this literature by examining midlife risk factors to describe cognitive function even before the onset of clinical dementia or mild cognitive impairment.
The American Heart Association has termed cardiovascular health as including 7 potentially modifiable risk factors that contribute to cardiovascular morbidity,27 and has set goal levels for middle-aged adults. A recent study suggested that a greater number of these ideal cardiovascular metrics were associated with better cognitive function.28 As opposed to MetS, the 7 risk factors may be a better way to think about health, so that the emphasis for public health messages can be that changing each factor individually reduces morbidity.
Another important result of this study is that women with more MetS components have increased odds of performing in the lowest quintile for the DSST and the WFT, as compared with men. MetS and insulin resistance have been associated with poorer executive cognitive function in middle-age women but not men29 and other studies demonstrated that MetS increases risk of cardiovascular disease in women more than men.26, 30 Taken together, this literature suggests that a sex difference exists in vascular risk factors influence on cerebrovascular burden of disease and brain health. This is in contradiction to other findings that more MetS components are associated with worse cognitive outcomes and obesity was a risk factor for lower cognitive functioning in men only31–33. More work is needed to explore sex-specific outcomes in cognitive decline.
The strengths of the analysis are the size and larger percentage of blacks in the population. Change in cognition was used as an outcome measure, which is more robust than cognitive measures obtained at one visit to potential confounding. Our failure to find associations with cognitive change in participants may reflect that participants are relatively young at study onset, and less likely to have developed mild-cognitive impairment or dementia. The cognitive measures used are more robust than chart reviews or the mini-mental state examination, which do not delineate cognitive domains of impairment.
The study limitations include the possibility of unmeasured confounders to this relationship, such as the presence of obesity in childhood or young adulthood, and early-life cognitive measures, such as intelligence quotient (IQ). This study by design looked at the contribution of mid-life metabolic syndrome on later cognitive change, and did not include those that developed metabolic syndrome components after visit 1. This may underestimate the effect size seen with cognitive change, and reflects the most conservative estimate of the results. In addition, the included participants do not include those lost to attrition in ARIC, and therefore may reflect a selection bias. It may be that the “unhealthiest” are the participants who did not return to follow up visits, and therefore our population may not include those who are most likely to have cognitive decline, again underestimating the effect size.
In this cohort of young-elderly participants, the number of MetS components was associated with increased odds of poor cognitive performance on tests of executive and language function. MetS was not associated with 6-year cognitive change. The individual components, such as WC, elevated blood pressure and diabetes, explained the poor cognitive function observed. Our results support efforts by AHA and other groups, to target markers of cardiovascular health, rather that grouping heterogeneous risk factors (such as in MetS), which may not add information above and beyond the individual components. Future directions should focus on defining accurate biomarkers of cardiovascular health in relationship to cognition so that health behaviors can be linked with pathophysiology. This will further clarify midlife vulnerabilities to dementia so that efforts can be focused on treatment and intervention before disease onset.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative Study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
The authors thank the staff and participants of the ARIC study for their important contributions.
References
- 1.McEvoy LK, Laughlin GA, Barrett-Connor E, et al. Metabolic syndrome and 16-year cognitive decline in community-dwelling older adults. Ann Epidemiol. 2012;22:310–317. doi: 10.1016/j.annepidem.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A. Impact of metabolic syndrome on cognition and brain: a selected review of the literature. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2060–2067. doi: 10.1161/ATVBAHA.112.252759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller M, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic Syndrome and Dementia Risk in a Multiethnic Elderly Cohort. Dementia and Geriatric Cognitive Disorders. 2007;24:185–192. doi: 10.1159/000105927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christman AL, Matsushita K, Gottesman RF, et al. Glycated haemoglobin and cognitive decline: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. 2011;54:1645–1652. doi: 10.1007/s00125-011-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes--systematic overview of prospective observational studies. Diabetologia. 2005;48:2460–2469. doi: 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]
- 6.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet neurology. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 7.Knopman DS, Mosley TH, Catellier DJ, Coker LH Atherosclerosis Risk in Communities Study Brain MRIS. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K, Ebly E, Hachinski V, Hogan D. Presence and treatment of vascular risk factors in patients with vascular cognitive impairment. Archives of neurology. 1997;54:33–39. doi: 10.1001/archneur.1997.00550130019010. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 11.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 12.Raffaitin C, Feart C, Le Goff M, et al. Metabolic syndrome and cognitive decline in French elders: the Three-City Study. Neurology. 2011;76:518–525. doi: 10.1212/WNL.0b013e31820b7656. [DOI] [PubMed] [Google Scholar]
- 13.Hutley L, Prins JB. Fat as an endocrine organ: relationship to the metabolic syndrome. Am J Med Sci. 2005;330:280–289. doi: 10.1097/00000441-200512000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Lieb W, Beiser AS, Vasan RS, et al. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalmijn S, Foley D, White L, et al. Metabolic cardiovascular syndrome and risk of dementia in Japanese-American elderly men. The Honolulu-Asia aging study. Arteriosclerosis, thrombosis, and vascular biology. 2000;20:2255–2260. doi: 10.1161/01.atv.20.10.2255. [DOI] [PubMed] [Google Scholar]
- 16.Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. American journal of epidemiology. 1989;129:687–702. [PubMed] [Google Scholar]
- 18.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Archives of neurology. 1989;46:141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 19.Warnick GR, Benderson J, Albers JJ. Dextran sulfate-Mg2+ precipitation procedure for quantitation of high-density-lipoprotein cholesterol. Clin Chem. 1982;28:1379–1388. [PubMed] [Google Scholar]
- 20.Nagele U, Hagele EO, Sauer G, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22:165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study--I. Development of a blood collection and processing system suitable for multicenter hemostatic studies. Thrombosis and haemostasis. 1989;61:15–19. [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 23.van de Pol LA, Korf ES, van der Flier WM, et al. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Archives of neurology. 2007;64:1023–1028. doi: 10.1001/archneur.64.7.1023. [DOI] [PubMed] [Google Scholar]
- 24.Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111–116. doi: 10.2174/156720507780362263. [DOI] [PubMed] [Google Scholar]
- 25.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 26.McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28:385–390. doi: 10.2337/diacare.28.2.385. [DOI] [PubMed] [Google Scholar]
- 27.Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reis JP, Loria CM, Launer LJ, et al. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuur M, Henneman P, van Swieten JC, et al. Insulin-resistance and metabolic syndrome are related to executive function in women in a large family-based study. Eur J Epidemiol. 2010;25:561–568. doi: 10.1007/s10654-010-9476-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pischon T, Hu FB, Rexrode KM, Girman CJ, Manson JE, Rimm EB. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2008;197:392–399. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Lower cognitive function in the presence of obesity and hypertension: the Framingham heart study. Int J Obes Relat Metab Disord. 2003;27:260–268. doi: 10.1038/sj.ijo.802225. [DOI] [PubMed] [Google Scholar]
- 32.Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol Aging. 2005;26 (Suppl 1):11–16. doi: 10.1016/j.neurobiolaging.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 33.Cavalieri M, Ropele S, Petrovic K, et al. Metabolic syndrome, brain magnetic resonance imaging, and cognition. Diabetes Care. 2010;33:2489–2495. doi: 10.2337/dc10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]