Abstract
Background
Previously, we have demonstrated that genetically disrupting retinoblastoma protein (Rb) expression in enterocytes results in taller villi, mimicking resection-induced adaption responses. Rb deficiency also results in elevated IGF-2 expression in villus enterocytes. We propose that postoperative disruption of Rb results in enhanced adaptation which is driven by IGF-2.
Methods
Inducible, intestine-specific Rb-null mice (iRbIKO) and wild-type littermates (WT) underwent a 50% proximal small bowel resection (SBR) at 7–9 weeks of age. They were then were given tamoxifen on POD 4–6, and harvested on POD 28. The experiment was then repeated on double knockouts of both IGF-2 and Rb (IGF-2 null/iRbIKO).
Results
iRbIKO mice demonstrated enhanced resection-induced adaptive villus growth after SBR and increased IGF-2 mRNA in ileal villus enterocytes compared to their WT littermates. In the IGF-2 null/iRbIKO double knockout mice, there was no additional villus growth beyond what was expected of normal resection-induced adaptation.
Conclusions
Adult mice in which Rb is inducibly deleted from the intestinal epithelium following SBR have augmented adaptive growth. IGF-2 expression is necessary for enhanced adaptation associated with acute intestinal Rb deficiency.
Keywords: small bowel adaptation, Retinoblastoma protein, Insulin-like Growth Factor-2
Introduction
Following small bowel resection (SBR), the remaining bowel undergoes a compensatory response known as adaptation, in which enhanced rates of enterocyte proliferation lead to increases in villus height and crypt depth. The mechanism(s) for adaptation remains an important area of research as efforts to enhance this process would benefit the short gut population by attenuating the need for parenteral nutrition and its associated morbidities.
Retinoblastoma protein (Rb) is a prototype tumor suppressor and cell cycle regulator.[1–3] Rb is expressed in virtually all tissues and controls cell cycle progression via interactions with the E2F family of transcription factors.[4,5] The contribution of Rb to normal intestinal homeostasis has been revealed using mutant mice in which Rb expression has been genetically disrupted within the intestinal epithelium in the fetal period. In these mice, the intestinal phenotype mirrors what is observed during the adaptive to SBR and consists of enhanced rates of enterocyte proliferation, taller villi, and deeper crypts. [6,7] These findings therefore offer the possibility that Rb expression may be involved in resection-induced adaptation responses.
In order to test whether Rb or other Rb family member pocket proteins p107 and p130 are involved in adaptation, we performed SBR procedures in mice in whom Rb, p107, or p130 expression was disrupted in the intestinal epithelium from birth. [7,8] We found that adaptation after SBR occurred normally in the p107 or p130-mutant mice, but not in the Rb-mutant mice in which rates of enterocyte proliferation and morphology of villi and crypts were magnified at baseline. In other words, although these constitutively Rb intestine specific knockouts have an augmented phenotype at baseline, they do not have any further villus growth after small bowel resection. It could therefore be argued that Rb is not involved in the process of resection-induced adaptation. On the other hand, lack of resection-associated villus and crypt growth in the Rb mutant mice could also be a direct consequence of disrupted Rb expression.
The purpose of the present study was to resolve the above conflicting interpretations. To this end, we crossed Rb (flox/flox) mice line to Villin Cre-ER mouse line in order to manipulate Rb expression in adult mice subsequent to the stimulus of intestinal resection. Specifically, we sought to determine whether resection-induced adaptation could be further enhanced by disrupting Rb expression in the postoperative period.
In addition, we have previously found that intestinal disruption of Rb results in increased expression of insulin-like growth factor-2 (IGF-2) within villus enterocytes.[9] IGF-2 is an intestinotrophic growth factor, and so we theorized that it may be a driving force behind Rb mediated mucosal growth. Mice who have both an Rb and IGF-2 deficiency no longer demonstrate a hyperplastic intestinal mucosa at baseline.[9] IGF-2 itself is also not required for normal adaptation after SBR (Sun RC et al, in press). Thus, we generated a double knockout mouse strain in which IGF-2 expression is absent at baseline, along with an inducible intestinal epithelial Rb deficiency in order to determine if IGF-2 is a driving force behind Rb-mediated adaptive growth.
Materials and Methods
Animals
The protocol for this study was approved by the Washington University Institutional Animal Care and Use Committee (Protocol 20100103 and 20130308). Inducible, intestine-specific Rb-null mice were generated by crossing mice with a tamoxifen-inducible Cre-fusion protein under control of the villin promoter (obtained via generous donation from Sylvie Robine, Curie Institute, Paris, France) with mice in which the Rb gene had been floxed for recombination (Jackson Laboratories, Bar Harbor, ME, USA). Hereafter, these mice are designated vil-Cre-ERT2(+) Rb(flox/flox), or iRbIKO. Littermates lacking Cre (vil-Cre-ERT2(−);Rb(flox/flox)) were used as wild type (WT) controls. These mice were then bred with IGF2 knockout mice (a generous gift from Dr. Carla Kim, Department of Genetics; Harvard Medical School) to generate IGF-2 null/vil-Cre-ERT2(+) Rb(flox/flox) (IGF-2 null/iRbIKO) and IGF-2 null/vil-Cre-ERT2(−) Rb(flox/flox) (IGF-2 null/WT mice). Mice were operated on at aged 7–9 weeks and kept on a 12-h light-dark schedule.
Experimental Design
In the first series of experiments, we tested whether the acute disruption of intestinal Rb expression in normal adult mice after resection would affect normal adaptation responses to massive SBR. We therefore performed SBR procedures on iRbIKO (n = 9) and WT (n = 9) mice. These mice were then administered intraperitoneal TAM for 3 days on postoperative days (POD) 4–6. Mice were then harvested on POD 28.
In the next series of experiments, we tested whether the deletion of IGF-2 would abolish adaptation responses to massive SBR when Rb is deleted post resection. We therefore performed SBR procedures on IGF-2 null/iRbIKO (n=5) and IGF-2 null/WT mice (n=8) mice. These mice were also then administered intraperitoneal TAM for 3 days on POD 4–6 and harvested on POD 28.
Small Bowel Resection Procedure
Mice underwent 50% proximal SBR as we have previously illustrated.[10] Briefly, mice that underwent SBR had transection of the bowel at a point 12-cm proximal to the ileal-cecal junction and also at a point 1 to 2-cm distal to the ligament of Treitz. The mesentery was ligated, and the intervening bowel was removed. Intestinal continuity was restored with an end-to-end single-layer, interrupted anastomosis using 9-0 monofilament suture. After the operation, mice were provided free access to water for the first 24 hours after surgery.
All mice were placed on a liquid rodent diet (Micro-stabilized Rodent Liquid Diet LD101, Purina Mills, St Louis, MO, USA) 1 day before operation. Postoperatively, the animals received water only for the first 24 h, followed by the same liquid diet until sacrifice. On POD 4–6, each mouse received a daily 100ul (1mg) intraperitoneal injection of tamoxifen.
Tamoxifen Preparation
Tamoxifen (TAM) was prepared by measuring 50mg of stock (Sigma LifeSciences T5648-5G) and adding 500ul of 100% Ethanol followed by 2250ul of Sunflower Oil. The mixture was then sonicated until clear, and another 2250ul of Sunflower Oil were added.[11]
Small Bowel Harvest and Enterocyte Isolation
At the time of harvest, mice were first anesthetized with an intraperitoneal injection of ketamine, xylazine, and acepromazine (4:1:1 proportion). The abdominal cavity was then opened, the intestinal anastomosis identified, and the remaining distal bowel excised from the mesentery and cecum. The mice were then sacrificed via cervical dislocation following removal of the intestine. The intestine was then immediately flushed with ice-cold phosphate buffered saline.
The first centimeter distal to the anastomosis was discarded, and the next 2 cm were fixed for histology in 10% neutral-buffered formalin. Crypts and villi were separated from the resected intestine using our previously described technique of enterocyte isolation involving calcium chelation and mechanical dissociation.[8]
Histology
A 2-cm segment of tissue was removed from the distal portion of the resected bowel and fixed for histology in formalin during the time of operation. During harvest, a 2-cm segment was removed 12-cm proximal to the terminal ileum, adjacent to the anastomosis, and fixed in formalin.
All histologic measurements were performed by one investigator who was blinded with regard to mouse strain. Five-micrometer-thick longitudinal sections of paraffin-embedded tissue sections were mounted on glass slides. Hematoxylin-and-eosin-stained sections were used to measure villus height and crypt death with a video-assisted computer program (Metamorph, UIC; Dowington, PA). At least 20 well-oriented crypts and villi were counted per slide. Crypts were counted only if the crypt-villus junctions on both sides of the crypt were intact and if Paneth cells were present at the base of the crypt. Villi were counted only if the central lymphatic channel extended from the villus base to the tip and if the mucosal surface was in continuity with an intact crypt. The increase in villus height was calculated as a percentage by taking the difference between the villus height of postoperative and intraoperative samples and dividing it by the intraoperative measurement.
Rates of Enterocyte Proliferation and Immunostaining
Formalin-fixed tissue sections were embedded in paraffin, sectioned, deparaffinized, and blocked with 3% hydrogen peroxide in methanol. Antigen retrieval was performed using Diva Decloaking solution (Biocare Medical, Concord, CA) (120°C for 5 minutes followed by 100°C for 10 minutes). Slides were blocked with avidin-pink and biotin-blue (Biocare Medical), labeled with anti-p-Histone H3 (Ser 10) antibody (Cell Signaling Technology; Danvers, MA) in DaVinci Green (Biocare Medical), stored overnight at 4°C, and visualized with biotinylated goat anti-rat IgG (Accurate Chemical; Westbury, NY), followed by streptavidin-horseradish peroxidase (HRP; Invitrogen, Camarillo, CA) and diaminobenzidine (DAB; Sigma-Aldrich, St Louis, MO), and hematoxylin counterstaining. The number of positively-staining p-Histone-H3 (Ser 10) crypt enterocytes and the total number of cells per crypt were counted from at least 20 well-oriented crypts by blinded scoring. A proliferative index was calculated from the ratio of these measurements.
Western Blot
Frozen isolated villus samples were thawed, reconstituted with Tris buffer, and sonicated for 10 seconds. The samples were lysed with sodium dodecyl sulfate sample buffer. The lysate was then heated for 5 minutes at 100°C and the protein concentration was determined by using the RC DC kit (Bio-Rad; Hercules, CA). Proteins were loaded in equal amounts for Western blotting. Total Rb (5541436, BD Pharmingen; San Diego CA) and actin (Cell Signaling Technology; Danvers MA) antibodies were used.
RT-PCR
RNA was prepared from harvested ileal crypts and villi as previously described and were homogenized in lysis buffer (RNAqueous kit, Ambion, Austin, TX).[8] The RNA was extracted according to kit instructions and stored at −80°C. Total RNA concentration was determined using a NanoDrop Spectrophotometer (ND-1000, NanoDrop Technologies, Wilmington, DE) and RNA quality evaluated using an Experion System with an RNA StdSens Chip and reagents (Bio-Rad Laboratories, Richmond, CA). β-Actin was used as the endogenous control, and whole bowel was used as a calibrator. IGF-2 gene expression was determined using primers and reagents from Applied Biosystems (Foster City, CA) and using an Applied Biosystems 7500 Fast Real-Time PCR system (Foster City, CA).
Apoptosis
Each postoperative H&E stained sections were analyzed for apoptotic bodies (pyknotic nuclei, condensed chromatin).[12] An apoptosis index was measured by counting the number of apoptotic bodies found per 50 crypts.
Statistics
For most experiments, means were calculated and compared using a Student’s t-test. Linear regression was calculated using SigmaPlot 12.5 software (San Jose, CA). Values in the text are means ± SEM. Differences were considered significant at p≤0.05.
Results
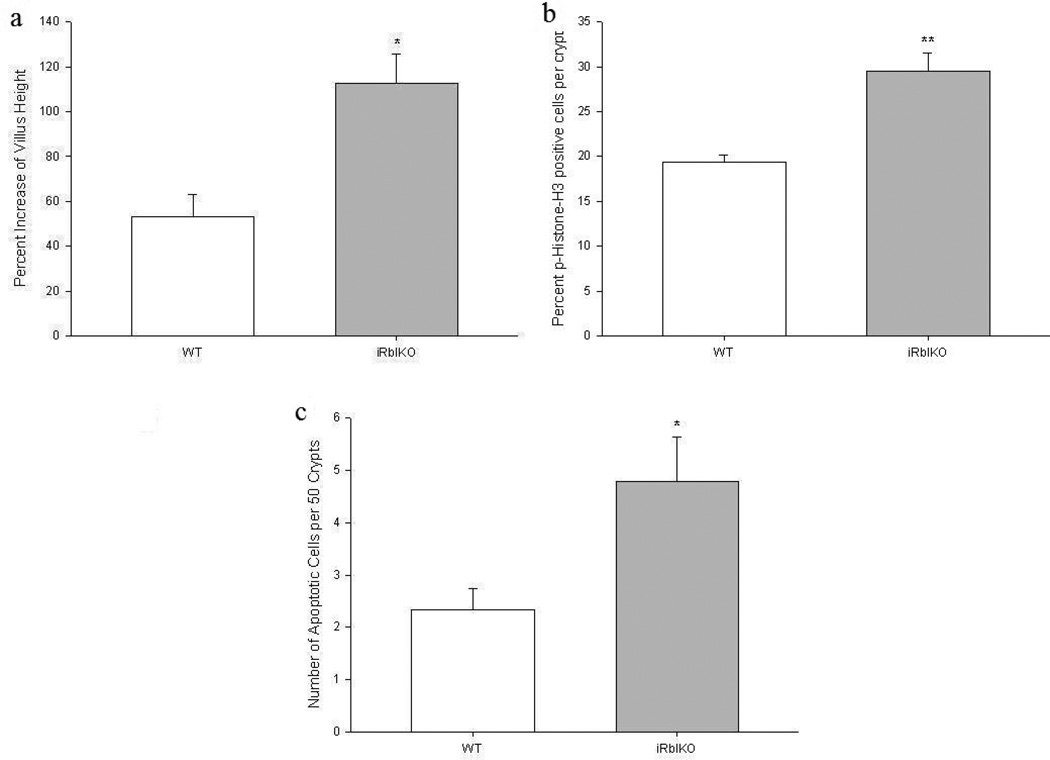
In the first series of experiments, we sought to determine if resection-induced adaptation could be enhanced by genetic ablation of intestinal epithelial cell Rb expression in adult mice following SBR. As anticipated, the WT mice demonstrated normal adaptation responses as gauged by an increase in villus height from baseline (Figure 1a). However, iRbIKO mice experienced a statistically significant (2-fold) increase in villus growth compared to WT. This enhanced resection-induced adaptive villus growth seen in iRbIKO mice was also mirrored as enhanced rates of proliferation and apoptosis compared to WT (Figures 1b and 1c). Deletion of Rb in villus enterocytes was confirmed with Western Blot (Figure 2).
Figure 1.
Effects of 50% proximal small bowel resection on (a) Percent change in villus height, (b) rates of enterocyte proliferation as measured by percent of p-Histone-H3 positive cells per total number of crypt cells, and (c) apoptosis as measured as total number of apoptotic cells per 50 crypts. WT = Wild Type mice, iRbIKO= Inducible, intestinal Rb Knockout mice. * p < 0.05
Figure 2.
Rb protein expression is successfully deleted in enterocytes of adult iRbIKO mice. Western blot of protein from ileal villus enterocytes probed for Rb. Actin was used as protein binding control. WT = Wild Type mice, iRbIKO= Inducible intestinal Rb Knockout mice.
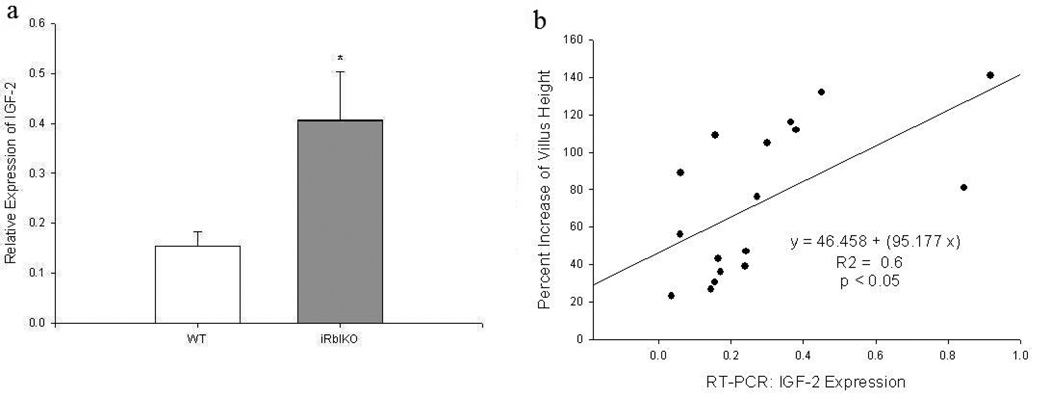
mRNA was isolated from villus enterocytes for real-time PCR. The iRbIKO mice had greater than a 2-fold increase in IGF-2 mRNA expression compared to WT mice (Figure 3a). Additionally, there existed a significant positive linear relationship between IGF-2 mRNA expression and villus growth (Figure 3b).
Figure 3.
(a) mRNA expression of Insulin-like Growth Factor-2 (IGF-2) as measured by RT-PCR in villus enterocytes. B-actin was used as the endogenous control, and whole bowel was used as a calibrator. (b) Linear regression of IGF-2 expression and percent villus growth. WT = Wild Type mice, iRbIKO= Inducible, intestinal Rb Knockout mice. * p < 0.05
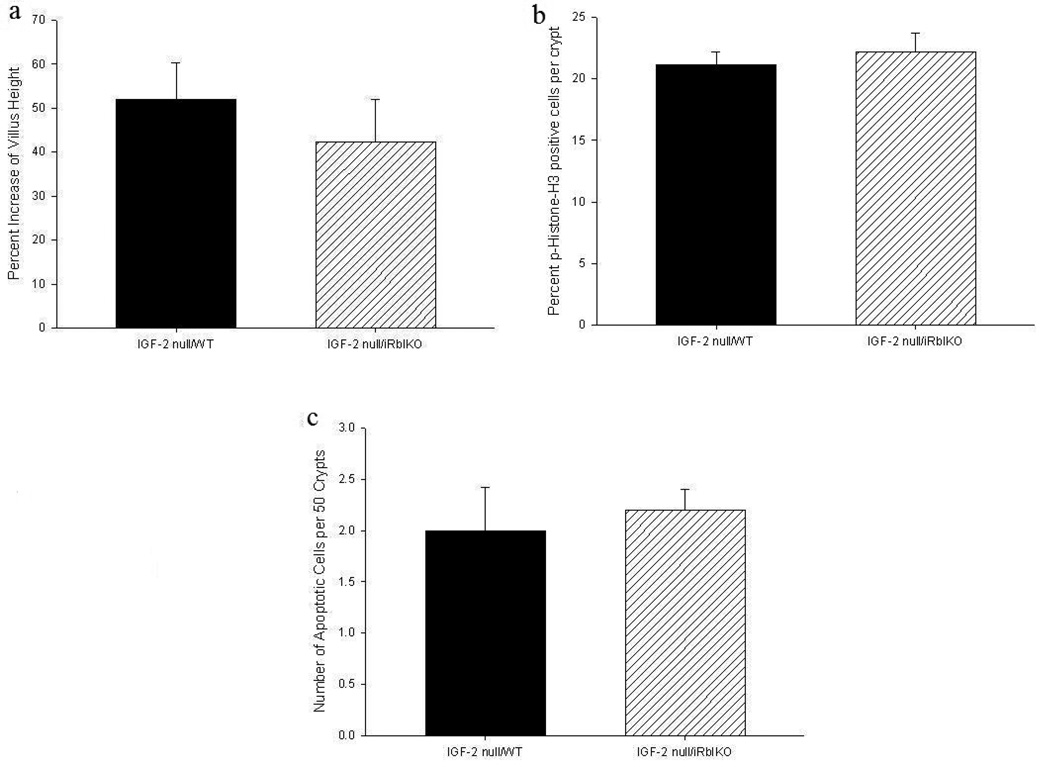
The next series of experiments tested whether IGF-2 played a role in the regulation of Rb-mediated enhanced adaptation. IGF-2 deletion was confirmed by RT-PCR of villus enterocytes while absent Rb expression was verified by Western Blot (data not shown). We found that mice in whom both Rb and IGF-2 were deleted (IGF-2 null/iRbIKO) did not have further increase in villus growth than WT controls (IGF-2 null/WT) (Figure 4a). There were also no differences in proliferative rate or apoptosis (Figures 4b and 4c).
Figure 4.
Effects of 50% proximal small bowel resection on (a) percent increase of villus height, (b) rates of enterocyte proliferation as measured by percent of p-Histone-3 positive cells per total number of crypt cells, and (c) apoptosis as measured as total number of apoptotic cells per 50 crypts. WT = Wild Type mice, iRbIKO= Inducible, intestinal Rb Knockout mice.
Discussion
In the present study, we have demonstrated that acute disruption of Rb protein expression within intestinal epithelial cells of adult mice following SBR augments adaptation. These mice also have increased IGF-2 mRNA expression in villus enterocytes. Disrupting IGF-2 expression prevented enhanced adaptation in the iRbIKO mice after SBR. Taken together, these data suggest that perturbation of intestinal Rb expression and/or activity leads to enhanced IGF-2 expression that drives enhanced resection-induced intestinal adaptation.
Rb controls the G1/S transition by its interactions with the E2F family of transcription factors by suppressing E2F activity and arresting cells in G1, effectively functioning as a growth inhibitor. [13,14] Phosphorylation of Rb results in dissociation of the Rb-E2F complex with subsequent transcription of genes necessary for cells to enter S phase. [15,16] Despite the fact that the intestinal epithelium comprises some of the most actively proliferating cells in the body, the role of Rb and E2F in the small intestine has only recently been explored. Polyomaviruses that encode the large T-antigen protein have provided an outlet of further study of Rb. Binding of the T-antigen to Rb results in Rb inactivation and promotes activation of the E2F transcription factor. [17] It has been previously demonstrated that this interaction between large T-antigen and Rb results in increased enterocyte proliferation as well as intestinal hyperplasia. [18,19]
Since germline deletion of Rb is embryonic lethal, studies of the effects of Rb deficiency has therefore been conditional within specific tissues. Prior reports in which Rb protein expression has been disrupted in the fetal intestine have had disparate findings. In one study, an intestinal fatty acid binding promoter (I-FABP) was used to drive Cre expression in Rb (flox/flox) mice.[6] The generated Rb intestine conditional nulls still expressed Rb and these mice did not demonstrate an abnormal small intestinal phenotype. Another group crossed collagen 1A1 Cre mice with Rb (flox/flox) mice to delete Rb expression in the small intestine.[20] Despite significant increases in proliferating cells within crypts and villi, the mice died shortly after birth. Since the collagen 1A1 promoter is not enterocyte-specific, it is possible that the mice died as a consequence of Rb deficiency in other tissues. Finally, we and others have employed villin Cre transgenic mice crossed with Rb (flox/flox) mice to delete Rb in the intestine as the villin promoter appears to be the most efficient and accurate for targeting intestinal epithelial cells.[8,21]
Since villin expression occurs in the embryonic period, it is possible that the observed hyperplastic intestine may have developed as a consequence of compensatory alterations in other developmental genes. To control for this possibility, we have characterized a model for induced expression of villin Cre to ablate Rb expression in the intestinal epithelium of adult Rb(flox/flox) mice. Through this unique model, we have established that Rb is critical for maintenance of the crypt/villus architecture in adult mice – beyond the phase of intestinal development.
We have previously shown that SBR did not stimulate additional mucosal growth in mice in which Rb expression was already deleted within the intestinal epithelium.[7] In the present study, we now show that the induction of intestinal Rb deficiency subsequent to SBR is additive in terms of mucosal growth. The exact timing of interventions designed to enhance intestinal adaptation responses after SBR appears to be crucial. Consistent with this notion is our prior finding that administration of epidermal growth factor (EGF) was effective in enhancing adaptation responses only when administered during the immediate postoperative interval.[22] EGF was ineffective when the same dose was given several weeks after adaptation had fully developed. The immediate postoperative period therefore seems to be the most opportune time for success with interventions designed to enhance adaptation. We opted to wait until POD4 in order to minimize stress to the animals immediately after surgery. Future studies will be needed to determine the optimal timing for induction of Rb deficiency in the intestinal epithelium of resected mice to enhance adaptation responses.
Although IGF-2 is a known intestinotrophic factors[23], it had never been previously linked to Rb, and our previous discovery that targeted enterocyte deficiency of Rb enhanced expression of epithelial IGF-2 was novel.[9]. When IGF-2 is eliminated in context of Rb disruption, the trophic effects of Rb were negated, strongly suggesting that IGF-2 is required to Rb mediated intestinal hyperplasia.[9] Similarly, our hypothesis that IGF-2 is required for Rb-mediated enhanced adaptation was confirmed when we found that levels of IGF-2 were elevated in iRbIKO mice with increased adaptation and that the elimination of IGF-2 subsequently reversed the hyperplastic phenotype and only normal adaptation was observed. As we know that IGF-2 is dispensable to normal adaptation responses and lacks significant phenotype in the unoperated intestine (Sun RC et al, in press), we are confident that our own present observations are the result of increased IGF-2 in the context of Rb-deficiency and not increased IGF-2 alone.
Increases in IGF-2 expression through loss of imprinting (LOI) has been associated with increased cellular proliferation and many common cancers.[24] The allelic dosage of IGF-2 has been found to correlate with intestinal mucosal surface area and crypt cell number.[25] Additionally, ectopic overexpression of IGF-2 in smooth muscle cells results in a significantly longer intestine[26] while administration of an IGF receptor inhibitor[27] and transgenic expression of a decoy IGF receptor[24] reverses these enterotrophic effects. Taken together, these findings suggest an important role for IGF-2 in normal homeostasis of the intestinal mucosa.
IGF-2 ligands bind to IGF-1R, which is found in both the intestinal epithelium and mesenchyme and mediates all known physiologic activities of IGF-2.[23,28,29] Just as the hyperplastic effects of Rb deficiency were reversed with concomitant IGF-2 deletion, similar results were also observed when Rb and IGF-1R were absent from epithelial enterocytes.[9] Additionally, epithelial IGF-1R is also not required for normal adaptation (Sun RC et al, in press). Thus, we hypothesize that deletion of epithelial IGF-1R may also negate the Rb mediated enhanced adaptation, in which case we may determine that IGF-2 has localized effect on the epithelium and not the mesenchyme. Our lab is presently generating an inducible, intestine specific double knockout strain in order to test this theory.
Our findings of stimulated mucosal growth in unperturbed mice and enhanced adaptation responses in mice undergoing SBR through the induction of intestinal epithelial cell Rb deficiency underscores important regulatory roles for Rb under normal and pathologic conditions. The mechanism for how Rb deficiency stimulates IGF-2 expression as well as the downstream effects of IGF-2 in intestinal adaptation remains unknown. Further mechanistic characterization of Rb and IGF-2 in the context of resection-induced intestinal adaptation may offer the potential of new therapeutic interventions intended to enhance resection-induced adaptation in patients suffering from short gut syndrome.
Acknowledgments
This work was supported by National Institutes of Health Grants T32 GM008795 (Choi), P30DK52574 - Morphology and Murine Models Cores of the Digestive Diseases Research Core Center of the Washington University School of Medicine, and the Children’s Surgical Sciences Institute of the St. Louis Children’s Hospital Foundation. Dr. Choi is also supported by The Marion and Van Black Endowed Pediatric Surgical Research Fellowship.
Footnotes
This paper was presented as a Poster Presentation at the 55th Annual Meeting of the Society for Surgery of the Alimentary Tract on May 5, 2014.
References
- 1.Dimova DK, Dyson NJ. The E2F transcriptional network: old acquaintances with new faces. Oncogene. 2005;24:2810–2826. doi: 10.1038/sj.onc.1208612. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, McCormick F. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–112. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 3.Zheng L, Lee WH. The retinoblastoma gene: a prototypic and multifunctional tumor suppressor. Exp Cell Res. 2001;264:2–18. doi: 10.1006/excr.2000.5129. [DOI] [PubMed] [Google Scholar]
- 4.Attwooll C, Lazzerini Denchi E, Helin K. The E2F family: specific functions and overlapping interests. EMBO J. 2004;23:4709–4716. doi: 10.1038/sj.emboj.7600481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cobrinik D. Pocket proteins and cell cycle control. Oncogene. 2005;24:2796–2809. doi: 10.1038/sj.onc.1208619. [DOI] [PubMed] [Google Scholar]
- 6.Haigis K, Sage J, Glickman J, Shafer S, Jacks T. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J Biol Chem. 2006;281:638–647. doi: 10.1074/jbc.M509053200. [DOI] [PubMed] [Google Scholar]
- 7.Leinicke JA, Longshore S, Wakeman D, Guo J, Warner BW. Regulation of retinoblastoma protein (Rb) by p21 is critical for adaptation to massive small bowel resection. J Gastrointest Surg. 2012;16:148–155. doi: 10.1007/s11605-011-1747-8. discussion 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo J, Longshore S, Nair R, Warner BW. Retinoblastoma protein (pRb), but not p107 or p130, is required for maintenance of enterocyte quiescence and differentiation in small intestine. J Biol Chem. 2009;284:134–140. doi: 10.1074/jbc.M806133200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi P, Guo J, Erwin CR, Warner BW. IGF-2 mediates intestinal mucosal hyperplasia in retinoblastoma protein (Rb)-deficient mice. J Pediatr Surg. 2013;48:1340–1347. doi: 10.1016/j.jpedsurg.2013.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmrath MA, VanderKolk WE, Can G, Erwin CR, Warner BW. Intestinal adaptation following massive small bowel resection in the mouse. J Am Coll Surg. 1996;183:441–449. [PubMed] [Google Scholar]
- 11.Metzger D, Chambon P. Site- and time-specific gene targeting in the mouse. Methods. 2001;24:71–80. doi: 10.1006/meth.2001.1159. [DOI] [PubMed] [Google Scholar]
- 12.Wakeman D, Schneider JE, Liu J, Wandu WS, Erwin CR, Guo J, Stappenbeck TS, Warner BW. Deletion of p38-alpha mitogen-activated protein kinase within the intestinal epithelium promotes colon tumorigenesis. Surgery. 2012;152:286–293. doi: 10.1016/j.surg.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson DG, Schwarz JK, Cress WD, Nevins JR. Expression of transcription factor E2F1 induces quiescent cells to enter S phase. Nature. 1993;365:349–352. doi: 10.1038/365349a0. [DOI] [PubMed] [Google Scholar]
- 14.Dyson N. The regulation of E2F by pRB-family proteins. Genes Dev. 1998;12:2245–2262. doi: 10.1101/gad.12.15.2245. [DOI] [PubMed] [Google Scholar]
- 15.Burke JR, Deshong AJ, Pelton JG, Rubin SM. Phosphorylation-induced conformational changes in the retinoblastoma protein inhibit E2F transactivation domain binding. J Biol Chem. 2010;285:16286–16293. doi: 10.1074/jbc.M110.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L, Timmers C, Maiti B, Saavedra HI, Sang L, Chong GT, Nuckolls F, Giangrande P, Wright FA, Field SJ, et al. The E2F1-3 transcription factors are essential for cellular proliferation. Nature. 2001;414:457–462. doi: 10.1038/35106593. [DOI] [PubMed] [Google Scholar]
- 17.White MK, Khalili K. Interaction of retinoblastoma protein family members with large T-antigen of primate polyomaviruses. Oncogene. 2006;25:5286–5293. doi: 10.1038/sj.onc.1209618. [DOI] [PubMed] [Google Scholar]
- 18.Saenz-Robles MT, Markovics JA, Chong JL, Opavsky R, Whitehead RH, Leone G, Pipas JM. Intestinal hyperplasia induced by simian virus 40 large tumor antigen requires E2F2. J Virol. 2007;81:13191–13199. doi: 10.1128/JVI.01658-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathi AV, Saenz Robles MT, Cantalupo PG, Whitehead RH, Pipas JM. Simian virus 40 T-antigen-mediated gene regulation in enterocytes is controlled primarily by the Rb-E2F pathway. J Virol. 2009;83:9521–9531. doi: 10.1128/JVI.00583-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang HS, Hinds PW. pRb-mediated control of epithelial cell proliferation and Indian hedgehog expression in mouse intestinal development. BMC Dev Biol. 2007;7:6. doi: 10.1186/1471-213X-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kucherlapati MH, Nguyen AA, Bronson RT, Kucherlapati RS. Inactivation of conditional Rb by Villin-Cre leads to aggressive tumors outside the gastrointestinal tract. Cancer Res. 2006;66:3576–3583. doi: 10.1158/0008-5472.CAN-05-2699. [DOI] [PubMed] [Google Scholar]
- 22.Shin CE, Helmrath MA, Falcone RA, Jr, Fox JW, Duane KR, Erwin CR, Warner BW. Epidermal growth factor augments adaptation following small bowel resection: optimal dosage, route, and timing of administration. J Surg Res. 1998;77:11–16. doi: 10.1006/jsre.1998.5336. [DOI] [PubMed] [Google Scholar]
- 23.Lund PK. Molecular basis of intestinal adaptation: the role of the insulin-like growth factor system. Ann N Y Acad Sci. 1998;859:18–36. doi: 10.1111/j.1749-6632.1998.tb11108.x. [DOI] [PubMed] [Google Scholar]
- 24.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 25.Harper J, Burns JL, Foulstone EJ, Pignatelli M, Zaina S, Hassan AB. Soluble IGF2 receptor rescues Apc(Min/+) intestinal adenoma progression induced by Igf2 loss of imprinting. Cancer Res. 2006;66:1940–1948. doi: 10.1158/0008-5472.CAN-05-2036. [DOI] [PubMed] [Google Scholar]
- 26.Zaina S, Pettersson L, Thomsen AB, Chai CM, Qi Z, Thyberg J, Nilsson J. Shortened life span, bradycardia, and hypotension in mice with targeted expression of an Igf2 transgene in smooth muscle cells. Endocrinology. 2003;144:2695–2703. doi: 10.1210/en.2002-220944. [DOI] [PubMed] [Google Scholar]
- 27.Kaneda A, Feinberg AP. Loss of imprinting of IGF2: a common epigenetic modifier of intestinal tumor risk. Cancer Res. 2005;65:11236–11240. doi: 10.1158/0008-5472.CAN-05-2959. [DOI] [PubMed] [Google Scholar]
- 28.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidegger I, Pircher A, Klocker H, Massoner P. Targeting the insulin-like growth factor network in cancer therapy. Cancer Biol Ther. 2011;11:701–707. doi: 10.4161/cbt.11.8.14689. [DOI] [PubMed] [Google Scholar]