Abstract
Although post-traumatic stress disorder (PTSD) and addiction are very different disorders, both are characterized by hyperreactivity to trauma- or drug-related cues, respectively. We investigated whether an appetitive conditioning task, Pavlovian conditioned approach, which predicts vulnerability to reinstatement of cocaine-seeking, also predicts fear incubation, which may be a marker for vulnerability to PTSD. We classified rats based on whether they learned to approach and interact with a food predictive cue (sign-trackers), or, whether upon cue presentation they went to the location of impending food delivery (goal-trackers). Rats were then exposed to extensive Pavlovian tone-shock pairings, which causes the fear response to increase or “incubate” over time. We found that the fear incubation effect was only present in sign-trackers. The behavior of goal-trackers was more consistent with a normal fear response – it was most robust immediately after training and decayed slowly over time. Sign-trackers also had lower levels of brain-derived neurotrophic factor (BDNF) protein in the prefrontal cortex than goal-trackers. These results indicate that, while many factors likely contribute to the disproportionate co-occurrence of PTSD and substance abuse, one such factor may be a core psychological trait that biases some individuals to attribute excessive motivational significance to predictive cues, regardless of the emotional valence of those cues. High levels of BDNF in the prefrontal cortex may be protective against developing excessive emotional and motivational responses to salient cues.
Keywords: addiction, post-traumatic stress disorder, autoshaping, vulnerability, individual differences, brain-derived neurotrophic factor
1. Introduction
Addiction is highly comorbid with post-traumatic stress disorder (PTSD). The overall prevalence of addiction in the United States is about 15% [1], while the prevalence of addiction among people with PTSD is up to 52% in the community and as high as 75% in treatment-seeking populations [2, 3]. Similarly, overall prevalence of PTSD is about 7–10% [4], whereas prevalence among patients with addiction has been reported as high as 42% [5]. Some possible explanations that have been proposed for the relationship between PTSD and addiction include self-medication of anxiety with drugs or alcohol [6, 7], increased exposure to traumatic events due to activities involved in acquiring illegal substances [5, 8, 9], or addictive substances altering the brain’s sensitivity to stress to make users more vulnerable to PTSD [10]. These possibilities are not mutually exclusive, and empirical support exists for each of them. However, another possibility is that common factors intrinsic to the individual can increase vulnerability to both disorders. For example, a number of twin studies have indicated significant overlap in genetic predisposition to PTSD and addiction [11–13].
There are many obvious phenomenological differences between addiction and PTSD, but there are also some striking similarities in the core psychological processes underlying both disorders. In particular, both disorders involve excessive motivational responses to cues associated with emotionally salient events. Excessive reactivity to trauma-related cues is especially well-documented in the case of PTSD and is described in no less than three of the diagnostic criteria for PTSD [14–16]. Similarly, addiction is characterized in part by excessive emotional and motivational responses to drug-related cues, i.e. cue-induced craving [17]. The extent to which drug-related cues induce such motivational responses in an individual is positively correlated with a number of clinically relevant variables, such as addiction severity, risk of relapse, and poor treatment outcomes [18, 19]. Thus, a general tendency to attribute excessive motivational salience to conditioned cues, regardless of the emotional valence of those cues, would likely predispose an individual to developing both addiction and PTSD.
Pavlovian conditioned approach (PCA) behavior has been used to assess the propensity of individual animals to attribute motivational salience to a reward cue [20, 21]. In this procedure, a discrete cue, i.e. a conditioned stimulus (CS), predictive of a food reward, is separated spatially from the location of reward delivery. All animals learn the predictive value of the CS, but a subset of animals (sign-trackers; STs) are especially prone to attribute motivational value to the CS, as evidenced by approach and physical interaction with it. Other animals (goal-trackers; GTs) learn to approach the location of reward delivery upon CS presentation but rarely approach the CS. STs also attribute more motivational salience to drug-paired cues and are more susceptible to drug- and cue-induced reinstatement of drug-seeking behavior than GTs [22–25]. In addition to differences in conditioned appetitive behaviors, STs show more fear toward a CS paired with footshock than GTs [26]. This may indicate that STs would be more likely to develop abnormal fear responses in procedures that model PTSD-like behavior.
In typical fear-conditioning paradigms, in which a tone (CS) is paired with footshock, a fear response to the CS develops quickly, after as little as one tone-shock pairing, and then either remains stable or slowly decays over time [27–30]. However, if the tone-shock pairing is repeated extensively, the fear response increases or “incubates” over time and becomes maximal ~30 days after conditioning [31], similar to the delayed-onset pattern of symptom development often seen in PTSD patients [32]. Interestingly, fear incubation shows considerable individual variability, with some animals showing a large incubation effect while others show no incubation at all [33]. Here, we sought to test whether individual variation in the attribution of motivational value to reward cues, as measured by PCA behavior, would predict incubation of conditioned fear.
To address possible neurobiological differences that could account for individual differences in behavior, we also measured expression of brain-derived neurotrophic factor (BDNF). This molecule was chosen because the BDNF Val66Met polymorphism has been implicated in the development of both addiction [34–39] and PTSD [40, 41]. Heterozygote BDNF knockout mice self-administer more alcohol than wild-type mice [42, 43], and exhibit impaired extinction of conditioned fear [44]. In addition to these effects of global differences in BDNF expression, several pre-clinical studies have shown effects of BDNF manipulation on both drug-seeking behavior and conditioned fear, with BDNF either increasing or decreasing conditioned motivational behavior in a highly region- and circuit-specific manner [45, 46]. We therefore tested STs and GTs for differences in BDNF expression in multiple brain regions within the emotional circuitry relevant to both PTSD and addiction [47].
2. Material and Methods
All procedures were approved by the University Committee on the Use and Care of Animals.
2.1 Subjects
Ninety-four male Sprague-Dawley rats weighing 275–300 g were obtained from Harlan and Charles River for use in these studies. Subjects were counterbalanced for supplier throughout all phases of the experiment. The rats were housed individually with ad libitum access to water throughout the study. As detailed below, food was also provided ad libitum until training on the PCA task was complete. Subsequent to PCA training, rats were food-restricted to maintain 85% of free-feeding weight, and food restriction continued until brains were harvested at the end of the experiment. The vivarium was kept on a 12:12-h light:dark schedule with temperature maintained at 70–73 °F and humidity at 65–70%. All experimental procedures were performed during the light portion of the cycle.
2.2 Pavlovian conditioned approach
Training on the PCA task took place in Med Associates behavioral testing chambers (24.1×20.5 cm floor area, 29.2 cm high; Med Associates, St. Albans, VT). Each chamber had its own sound-attenuating enclosure and a ventilating fan that provided masking noise. Red room lights were used throughout each session, and a red house light in each chamber was illuminated during testing, as well. A recessed food cup was located in the center of one wall of each testing chamber, into which 45-mg banana-flavored pellets could be delivered from a pellet dispenser using a programmable schedule. To the left or right of the food cup, according to a counterbalanced design, was a retractable stainless-steel lever. Whenever the lever was extended into the chamber, an LED mounted inside the lever mechanism illuminated the slot through which the lever protruded. A tray with corn-cob bedding was placed beneath the stainless-steel grid floor.
For two days prior to training, rats received ~15 banana pellets in their home cages to familiarize them with this food. On each of the next two days, rats underwent a pretraining session consisting of 25 pellets delivered non-contingently into the food cup on a variable interval (VI) 30-s schedule, i.e. one food pellet was delivered on average every 30 seconds, but the actual interval between pellets varied randomly between 1 and 60 seconds. The lever remained retracted throughout the pretraining sessions. Two rats did not consume all 25 pellets by the end of a second pretraining session and were eliminated from the study. PCA training sessions then commenced for the next 5 days. Each session consisted of 25 trials in which the lever CS was extended for eight seconds, and a food pellet-US was delivered immediately upon retraction of the lever. Trials were initiated on a 90-s VI schedule, resulting in a total session length of ~35–40 min. Head-entries into the food cup were detected by an infrared photobeam ~1.5 cm above the base of the food cup, and lever deflections were recorded as “lever presses,” but neither response had any influence on delivery of the food-US.
2.3 Operant conditioning
After PCA training was complete, rats were food-restricted to maintain 85% of free-feeding weight for the remainder of the experiment. For appetitive operant conditioning, rats were placed in a different set of standard behavioral test chambers (Med Associates) of the same dimensions and in the same room as those used in PCA training. The chambers were configured with nose-poke holes to the left and right of a food receptacle, 3 cm above the floor. According to a counter-balanced design, one nose-poke hole was designated as inactive such that nose pokes were recorded but had no programmed consequences. Nose pokes in the active hole were reinforced with a chocolate-flavored food pellet under a fixed-ratio (FR) 1 schedule (i.e., only 1 response was required for reinforcement) for the first 5 pellets, then an FR 7 schedule for the next 5 pellets, then a variable-ratio (VR) 20 schedule (i.e., an average of 20 responses are required before reinforcement). Rats received daily 50-min sessions for 5 days until responding stabilized at a consistent rate of ~1 response/sec.
2.4.1 Fear conditioning: apparatus
A separate set of eight behavioral test chambers (Med Associates) housed in a different room were used for Pavlovian fear conditioning. Each chamber rested on top of a load cell platform, and displacement of the chamber was used to assess freezing behavior [48]. The floor of each chamber consisted of stainless steel rods connected to a constant-current electrical stimulation source and solid-state grid scrambler for delivery of aversive foot shocks. Each chamber also contained a speaker for delivery of auditory stimuli. The chambers were cleaned with a 1% acetic acid solution to create a distinctive odor, and a white house light inside each chamber was illuminated. Background noise (~70 dB) was supplied by fans in the cabinets in which each chamber was housed, and the room was illuminated by overhead fluorescent lighting.
2.4.2 Fear conditioning: procedure
Starting the day after completion of operant conditioning, rats received 10 auditory fear conditioning sessions (one session per day for 10 days). The rats were transported to the conditioning chambers 3 min after being placed in the chamber. Each conditioning session consisted of 10 trials in which a tone CS (30 s, 4 KHz, 80 dB SPL) co-terminated with a footshock US (2 s, 1 mA) on a pseudo-random variable-interval (VI) schedule with an average intertrial interval (ITI) of 4 min. Rats remained in the chamber for 3 min after the last tone-shock pairing. This procedure was repeated for a total of 10 consecutive days.
2.5 Conditioned suppression
Separate groups of rats were returned to the operant conditioning chambers and re-trained on the operant conditioning task for 3 days to ensure stable responding, beginning either one or thirty days after fear conditioning. For the conditioned suppression test, operant conditioning was conducted as before, but beginning 30 min into the session, six 30-s presentations of the tone CS were superimposed on the ongoing operant program using a pseudo-random VI 420-s schedule. The extent to which operant responding slowed during presentation of the tone CS relative to the preceding baseline was used as a measure of conditioned fear.
2.6 Enzyme-linked immunosorbent assay (ELISA)
One day after the final behavioral test, rats were euthanized by overdose of pentobarbital (100 mg/kg, i. p.) and rapid decapitation. Four major brain areas, i.e. the prefrontal cortex, striatum, hippocampus, and amygdala, were collected using standard gross dissection methods [49] and homogenized in extraction buffer containing 100mM Tris±HCl, pH 7.2, 400mM NaCl, 4mM EDTA, 0.2mM PMSF, 0.2mM benzethonium chloride, 2mM benzamidine, 40 U/ml aprotinin, 0.05% sodium azide, 2% BSA, 0.5% gelatin, and 0.2% Triton X-100 (Sigma Chemical, USA). Detection and quantification of BDNF protein was performed by the University of Michigan Immunology Core using ELISA kits from R&D Systems according to the manufacturer’s protocol. Samples were all run in duplicate, and data were normalized to the total amount of protein for each sample.
2.7 Data analysis
Rats were categorized as STs or GTs based on an “Approach Index” score derived from the number, latency and probability of lever contacts and magazine entries upon CS presentation during PCA training, as described previously[50]. Briefly, we used the following formula: [Response bias (lever contacts − magazine entries)/(lever contacts + magazine entries) + Probability (lever contact probability − magazine entry probability) + Contact Latency (lever contact latency − magazine entry latency)/(8 s)]/3. Average scores from sessions 4 and 5 were used as the final “Approach Index”. Rats with an Approach Index of less than − 0.5 were designated goal-trackers (GTs; twice as likely to direct behavior towards the magazine), those above +0.5 as sign-trackers (STs; twice as likely to direct behavior towards the CS Ȓ lever), and those between −0.5 and +0.5 as intermediate responders (IRs).
Freezing behavior was the dependent variable used to assess conditioned fear during the fear conditioning procedure. Load-cell activity generated by displacement of the chamber was digitized at 5 Hz (Threshold Activity, Med Associates), and freezing behavior was scored if the rat was immobile for at least one second. The load cell gain was set such that small head movements involved in grooming, head turning, sniffing, etc., which would not be scored as freezing by a human observer, would also exceed the freezing threshold and be scored as activity by the detection software [48]. For each session, freezing behavior was expressed as a percentage of total observations. For the conditioned suppression tests, nose pokes were recorded in 2-s bins throughout the session. Fear to the tone was measured by calculating the conditioned suppression ratio (nose-pokes during the 30-s tone / [nose-pokes during the 30-s tone + nose-pokes during the 30 s immediately preceding the tone]). On this scale, a ratio of 0.5 = no suppression, and 0.0 = complete suppression.
Between-group comparisons of repeated measures data from the PCA training were performed using linear mixed-effects models. Average freezing during fear conditioning was compared using a one-way ANOVA. One-sample t-tests were used to test whether conditioned suppression ratios were different from 0.5. Overall effects of PCA phenotype and incubation time on fear expression were tested with 2-way ANOVAs. Between-group suppression ratios were also compared using planned unpaired t-tests. BDNF expression levels were compared using one-way ANOVAs and planned unpaired t-tests.
3. Results
3.1 Pavlovian conditioned approach
Over 5 days of PCA training, two different conditioned responses (CRs) developed to lever-CS presentation: a sign-tracking response directed toward the lever, and a goal-tracking response directed toward the food cup. As illustrated in Figure 1, rats classified as STs developed a strong bias toward lever-directed responses during CS presentation (average GT lever presses per day = 15 ± 2; IR = 22 ± 2; ST = 32 ± 2; F2, 153 = 17.8, p < 0.001), while GTs developed a comparably strong bias toward food cup-directed responses during CS presentation (average GT magazine entries per day = 51 ± 3; IR = 33 ± 3; ST = 9 ± 3; F2, 74 = 60.2, p < 0.001), and IRs performed both responses with relatively little bias. Similar results were obtained by an analysis of latency to lever press (data not shown; average GT latency = 6.9 ± 0.1 sec; IR = 6.5 ± 0.1 sec; ST = 5.8 ± 0.1 sec; F2, 142 = 21.0, p < 0.001), and latency to magazine entry (data not shown; average GT latency = 3.8 ± 0.2 sec; IR = 5.2 ± 0.2 sec; ST = 7.4 ± 0.2 sec; F2, 102 = 145, p < 0.001).
Figure 1.
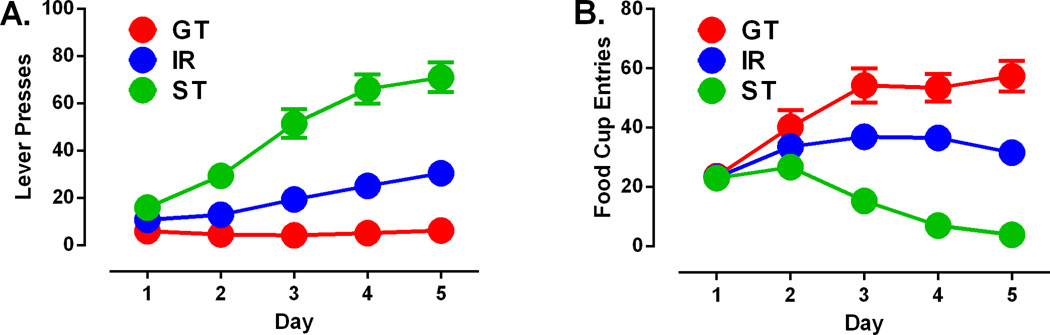
Pavlovian conditioned approach. Development of different conditioned responses in sign-trackers (ST, n = 28), intermediate responders (IR, n = 37), and goal-trackers (GT, n = 29) during Pavlovian pairing of a lever CS and a food US, as a function of day of training. Data points are mean ± SEM for (A) number of lever contacts, and (B) number of food cup entries during the lever presentation.
3.2 Fear conditioning
There were no significant differences in the acquisition of conditioned freezing among PCA phenotypes (Figure 2A). All groups acquired a robust conditioned freezing response to the tone CS within one session, and this response was maintained throughout all 10 days of the training period. For purposes of data analysis, rats were classified into “HI” and “LO” groups based on a median split of their average freezing during fear conditioning (Figure 2B). The distribution of average freezing responses was similar across PCA phenotypes (ANOVA F2, 91 = 0.014, p = 0.986).
Figure 2.
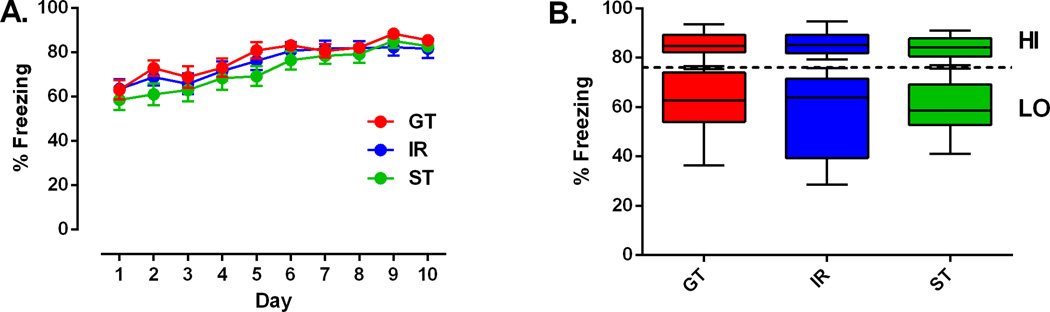
Acquisition of a conditioned freezing response. (A) Percentage of freezing (mean ± SEM) for sign-trackers (ST, n = 28), intermediate responders (IR, n = 37), and goal-trackers (GT, n = 29) over 10 days. For each day, freezing during the 30-sec tone presentations was averaged across the entire session. (B) Rats were designated based on a median split of average freezing as high-conditioning (ST, n = 14; IR, n = 20; GT, n = 13) and low-conditioning (ST, n = 14; IR, n =17; GT, n = 16). Data are depicted as box plots with maximum and minimum values (whiskers) for each group. The dotted line is the median for the overall sample. Rats received 10 trials per day for 10 days, each trial consisting of a 30-sec tone presentation immediately followed by a foot shock.
3.3 Fear incubation
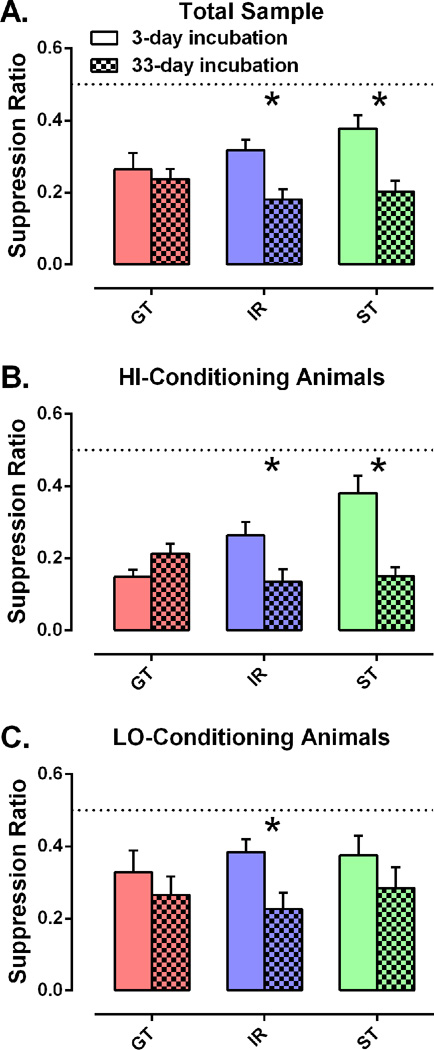
Baseline rates of operant nose-pokes for food pellets were ~1 response per second, and a 2-way ANOVA showed no significant effect of phenotype (F2, 88 = 0.18, p = 0.83), incubation time (F1, 88 = 2.65, p = 0.11), or phenotype×incubation (F2, 88 = 0.22, p = 0.81) on baseline operant responding. For reference, raw averages are presented in Table 1 for nose-poke response rates both at baseline and during tone presentations. When tone CSs were presented in the operant conditioning chambers after an incubation period of 3 or 33 days, a fear incubation effect was evident in the overall sample of 94 animals, such that the fear-induced suppression of operant responding was more pronounced after 33 days. A 2-way ANOVA revealed a significant main effect of incubation time (suppression ratio = 0.32 ± 0.02 after 3 days vs. 0.21 ± 0.02 after 33 days; F1, 88 = 16.8, p < 0.0001), but no main effect of PCA phenotype. The phenotype×incubation interaction approached, but did not reach statistical significance when all 3 phenotypes were included (F2, 88 = 2.50, p = 0.0884); however, planned a priori comparisons of STs to GTs revealed a significant phenotype×incubation interaction (F1, 53 = 4.36, p = 0.0416). When each PCA phenotype was analyzed separately (Figure 3A), the fear incubation effect remained significant among STs (t26 = 3.60, p = 0.0013) and IRs (t35 = 3.24, p = 0.0027), but not GTs (t27 = 0.511, p = 0.613). With the analysis limited to just the “HI” conditioning animals (Figure 3B), again the fear incubation effect was seen in both STs (t12 = 4.61, p = 0.0006) and IRs (t18 = 2.25, p = 0.0375) but not GTs (t11 = −1.68, p = 0.120). However among the “LO” conditioning animals (Figure 3C), the fear incubation effect was significant only in IRs (t15 = 2.74, p = 0.0151), but not in GTs (t14 = 0.769, p = 0.455) or STs (t12 = 1.09, p = 0.296).
Table 1.
Nose-poke response rates. Data are presented as average nose-poke responses per second ± SEM both during baseline and during tone presentations, split by PCA phenotype and incubation time.
| 3-day incubation responses / sec at baseline | 33-day incubation responses / sec at baseline | 3-day incubation responses / sec during tone | 33-day incubation responses / sec during tone | |
|---|---|---|---|---|
| GT | 1.08 ± 0.15 | 1.34 ± 0.14 | 0.40 ± 0.12 | 0.39 ± 0.09 |
| IR | 1.20 ± 0.19 | 1.34 ± 0.25 | 0.64 ± 0.08 | 0.32 ± 0.08 |
| ST | 1.13 ± 0.13 | 1.54 ± 0.26 | 0.62 ± 0.10 | 0.32 ± 0.07 |
Figure 3.
Conditioned fear expression after 3 days or 33 days of incubation. Plots are shown for (A) the total sample at 3 days (GT, n = 14; IR, n = 18; ST, n = 15) and 33 days (GT, n = 15; IR, n = 19; ST, n = 13); (B) HI-conditioning animals at 3 days (GT, n = 5; IR, n = 10; ST, n = 6) and 33 days (GT, n = 8; IR, n = 10; ST, n = 8); and (C) LO-conditioning animals at 3 days (GT, n = 9; IR, n = 8; ST, n = 9) and 33 days (GT, n = 7; IR, n = 9; ST, n = 5). Six tones were presented without shock in a new context while the rats were continuously nose-poking for food at a rate of ~1 nose-poke per second. Fear to the tone is indicated by a reduction in the conditioned suppression ratio (tone responses/[tone responses + pre-tone responses]). Data are presented as mean ratios ± SEM. On this scale, a ratio of 0.5 = no suppression, and 0.0 = complete suppression. *Significant difference 3-day vs 33-day incubation p < 0.05.
3.4 Brain-derived neurotrophic factor expression
BDNF protein levels were measured by ELISA in prefrontal cortex, amygdala, striatum, and hippocampus at the end of the experiment. Because there were no significant differences in BDNF levels between short and long incubation animals in any brain region, data from these two conditions were combined. ANOVAs involving all 3 PCA phenotypes did not reveal a significant effect of phenotype for BDNF levels in any brain region. Planned a priori comparisons showed that STs had significantly less BDNF in the prefrontal cortex than GTs (Figure 4A; t55 = 2.270, p = 0.0271), but no differences in BDNF levels in amygdala (t55 = 0.863, p = 0.392), striatum (t55 = 1.37, p = 0.176), or hippocampus (t55 = 0.682, p = 0.498). In addition, “HI” conditioning animals had lower levels of BDNF than “LO” conditioning animals in hippocampus (Figure 4B; t92 = −2.63, p = 0.0104), but not in prefrontal cortex (t92 = 0.357, p = 0.722), amygdala (t92 = −1.212, p = 0.229), or striatum (t92 = −0.277, p = 0.783). There was no significant interaction between the effects of PCA phenotype and fear conditioning on BDNF levels in any brain region.
Figure 4.
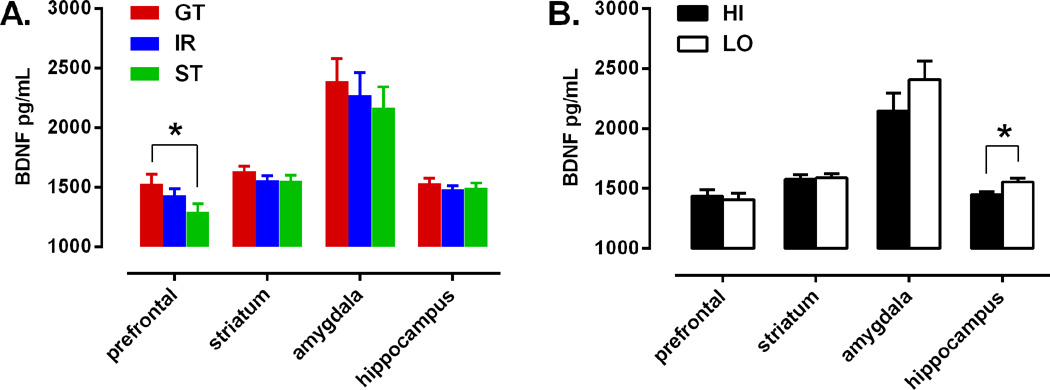
Regional BDNF expression. Data are divided by (A) PCA phenotype (GT, n = 29; IR, n = 37; ST, n = 28) or (B) fear-conditioning phenotype (HI, n = 47; LO, n = 47). Data are presented as mean BDNF protein concentration + SEM. *Significant difference p < 0.05.
4. Discussion
We replicated the results of Pickens et al. 2009 [33], who showed that protracted exposure of rats to tone-shock pairings (100 pairings delivered over 10 days), produces fear that increases over time rather than slowly decaying or remaining stable. In addition, we found that this fear incubation effect occurred predominantly in STs, i.e. animals that are prone to attribute motivational value to discrete reward cues, especially in those animals that had exhibited the highest level of freezing behavior during the fear conditioning procedure. The fear incubation procedure did not seem to affect expression of BDNF in the PFC, striatum, hippocampus, or amygdala. However, the sign-tracking trait was associated with decreased BDNF expression in the PFC, and increased freezing during fear acquisition was associated with lower BDNF expression in the hippocampus.
The fear responses measured 3 days after fear conditioning did not replicate our previous findings of enhanced fear conditioning to discrete cues among STs relative to GTs [26]. In fact, in the present study STs showed considerably less fear to the tone CS than GTs when tested 3 days after conditioning. The apparent discrepancy between the two studies is likely attributable to the intensity of the initial fear conditioning procedure. The fear conditioning procedure in our previous study consisted of just 5 tone-shock pairings delivered in a single session, as opposed to 100 pairings delivered over 10 days in this study. The fear incubation effect only occurs after extensive repetition of tone-shock pairings, which presumably is a more intensely aversive experience. The fact that STs and GTs showed comparable levels of fear when tested 33 days after conditioning in the present study indicates that STs did not have a weaker fear response than GTs; rather, the expression of that response was just delayed. The lack of a fear incubation effect in GTs may actually indicate that they developed less fear to the tone than STs and therefore were less likely to show fear incubation. In support of this interpretation is the fact that STs that did not have a strong freezing response to the tone during initial fear conditioning also did not show a significant fear incubation effect. It is possible that an even more intense or extended fear conditioning procedure would produce fear incubation among GTs as well as STs, particularly considering that the intensity and chronicity of a traumatic experience is known to have a strong influence on the probability of an individual developing pathological behavior like PTSD in response to that experience [51]. It is important to note that the shift in contexts between training and testing may have contributed to the relatively high levels of conditioned suppression seen in this study, as previous studies have found that a change in context can augment conditioned suppression[52, 53]. There is some evidence that GTs are more sensitive to contextual cues than STs [26, 54], however contextual changes are not likely to have influenced the observed incubation effects because the context was shifted for both the 3-day and the 33-day incubation groups.
An interesting aspect of the fear incubation effect is that it appears not to be a result of fear gradually increasing above the level normally produced by limited tone-shock pairings, but rather, it is an initial inhibition of fear behavior that gradually dissipates over time [33, 55, 56]. This is not unlike the clinical phenomenon of dissociation, in which the emotional response to a salient event is often suppressed or dis-integrated from the explicit memory of the event, a symptom also known as “emotional numbing” [14, 57]. Dissociation is a common reaction to trauma, particularly if the trauma is chronic or prolonged [58–61]. Several theorists have proposed an important role for dissociation in the etiology of PTSD [15, 62–65], and clinical studies have found peri-traumatic dissociation to be the most predictive risk factor for development of PTSD after exposure to trauma [66, 67]. The current findings may suggest that an emotional style involving the attribution of excessive motivational salience to predictive cues can also lead to pathological dissociation in response to traumatic events.
Our findings that STs had lower levels of BDNF expression in the PFC than GTs is in agreement with other studies of the role of BDNF in conditioned fear and appetitive responding. BDNF injection into the PFC enhances extinction and reduces reinstatement of cocaine self-administration in rats [68, 69], while shRNA knockdown of BDNF in the same region of the PFC increases the cocaine self-administration breakpoint [70]. Similarly, Peters et al. 2010 [71] found that BDNF injection into the infralimbic region of the PFC enhances extinction and reduces expression of conditioned fear. Our finding of reduced BDNF levels in the hippocampus of rats with high levels of freezing during fear conditioning is also supported by Peters et al. 2010 [71], who showed that BDNF infusion into the hippocampus reduces expression of conditioned fear. However, selective viral knockdown of hippocampal BDNF expression in mice does not appear to affect fear acquisition or expression [72]. The effects of hippocampal BDNF manipulation on drug-seeking behaviors have not been reported. Because BDNF enhances glutamatergic activity in PFC and hippocampal neurons, promotes synaptogenesis, and enhances long-term potentiation [73, 74], our results imply that enhanced activity within the PFC and hippocampus may be protective against excessive fear responses and cue-motivated behavior in general. However, several caveats must be considered before making such an interpretation. First, our tissue samples consisted of relatively large regions, whereas neurotrophic effects on motivated behaviors may be more finely site-specific, as demonstrated in reported differences between the effects of BDNF within the infralimbic versus prelimbic PFC on conditioned fear [71, 75]. Second, though we found no evidence of effects on BDNF expression by fear incubation, BDNF is induced by several behavioral conditioning procedures, including fear conditioning and selfadministration [76–84]. Therefore, since all our measurements of BDNF were taken after behavioral testing, it is not clear whether our observed differences in BDNF protein represent individual differences in baseline expression, or individual differences in evoked BDNF activity in response to the training procedures we employed. Finally, our observations are correlational and cannot be taken alone as evidence of a causal relationship between BDNF expression and the behavioral traits we measured.
Our results are consistent with the interpretation that pathological fear reactions likely result from the interplay of multiple phenotypic domains, including both the initial emotional reactivity to traumatic stimuli, and the ability of conditioned cues to acquire motivational value. It is reasonable to ask why such traits exist at all. Traits that tend to increase pathological responses to such ubiquitous human experiences as trauma and exposure to addictive substances would seem to be maladaptive and therefore unlikely to be maintained by natural selection. However, maladaptive combinations of traits can be maintained at relatively high frequencies if the individual component traits are each adaptive in themselves [85–87]. Furthermore, it may often be the case that two extremes of a behavioral trait are each adaptive in different situations, ensuring the persistence of variability in that trait within the population [88]. For example, the sign-tracking trait may be advantageous in times of scarcity and danger, when quick reactions to salient stimuli are necessary for survival, while the goal-tracking trait might be preferable in circumstances of relative security, when a more subtle cognitive approach can be used to contextualize events and guide behavior rather than compelling an immediate response.
The findings presented here highlight the importance of assessing the interplay of phenotypic domains in animal models of psychiatric disorders [89, 90]. Neither the sign-tracking trait nor initial conditionability to aversive stimuli appear sufficient to predispose toward fear incubation, but in combination these two traits may produce a new, “higher order” trait that predisposes to pathological behavior [91]. A focus on either of the component traits alone could lead to the inaccurate conclusion that neither is associated with fear incubation, especially if the sample population happens to be enriched for one or the other low-risk trait, such that the high-risk combination becomes too rare to detect. In addition, a focus on fear incubation itself without consideration of underlying component behavioral traits can greatly impede efforts at understanding the pathophysiology of abnormal fear reactions, especially if non-susceptible animals are consistently included in the analysis. Important interactions with other behaviors and comorbidities can also be overlooked if the interplay of component traits is neglected. For example, involvement of the sign-tracking trait with susceptibility to abnormal fear reactions may help to explain the high rates of comorbidity between PTSD and addiction [26, 92].
5. Conclusions
The relationship we detected between PCA behavior and fear incubation indicates that certain personality traits may confer vulnerability to multiple psychiatric disorders, including both disorders of approach, like addiction, and disorders of avoidance, like PTSD. We have identified one such trait as the tendency to assign excessive motivational value to predictive cues, regardless of emotional valence. This trait may interact with other traits, such as “conditionability” or “emotional reactivity” to produce different forms of behavioral pathology in response to various environmental challenges. Though we have some evidence of broad areas of overlap in the neurobiology of frequently comorbid psychiatric disorders, e.g. decreased regulation of affective responses by the PFC and hippocampus, further delineation of relevant psychological and behavioral endophenotypes will be necessary to more precisely determine the sources of individual variation in vulnerability or resilience to psychiatric disturbance.
Highlights.
Fear to a tone extensively paired with shock “incubated”, i.e. increased over time.
Fear incubation only occurred in rats that preferentially approached reward cues.
Rats that approached reward cues also had less prefrontal cortical BDNF.
Prefrontal BDNF may protect against both addiction and pathological fear responses.
Acknowledgements
We wish to thank Philip Presnell, Christopher Fitzpatrick, Vedran Lovic, and Elizabeth LaRose for their technical assistance with these experiments. This work was supported by grants from the NIH to SM (R01MH065961) and TER (R37DA04294).
Abbreviations
- BDNF
brain-derived neurotrophic factor
- CS
conditioned stimulus
- ELISA
enzyme-linked immunosorbent assay
- FR
fixed-ratio
- GT
goal-tracker
- IR
intermediate responder
- ITI
intertrial interval
- PCA
Pavlovian conditioned approach
- PTSD
post-traumatic stress disorder
- ST
sign-tracker
- US
unconditioned stimulus
- VI
variable interval
- VR
variable-ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jonathan D. Morrow, Email: jonmorro@umich.edu.
Benjamin T. Saunders, Email: Benjamin.Saunders@ucsf.edu.
Stephen Maren, Email: maren@tamu.edu.
Terry E. Robinson, Email: maren@tamu.edu.
References
- 1.O'Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: Human and non-human animal models. Pharmacology & Therapeutics. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 3.Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, et al. Trauma and the Vietnam War generation: Report of findings from the National Vietnam Veterans Readjustment Study. New York: Brunner/Mazel; 1990. [Google Scholar]
- 4.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of general psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 5.Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. The American journal of psychiatry. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- 6.Ouimette P, Read JP, Wade M, Tirone V. Modeling associations between posttraumatic stress symptoms and substance use. Addictive behaviors. 2010;35:64–67. doi: 10.1016/j.addbeh.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. The American journal of psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- 8.Brady KT, Dansky BS, Sonne SC, Saladin ME. Posttraumatic stress disorder and cocaine dependence. Order of onset. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 1998;7:128–135. [PubMed] [Google Scholar]
- 9.Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addictive behaviors. 1998;23:827–840. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 10.Stewart SH, Conrod PJ. Psychosocial models of functional associations between posttraumatic stress disorder and substance use disorder. In: Ouimette P, Brown PJ, editors. Trauma and substance abuse: causes, consequences, and treatment of comorbid disorders. Washington, DC: American Psychological Association; 2003. pp. 29–55. [Google Scholar]
- 11.Xian H, Chantarujikapong SI, Scherrer JF, Eisen SA, Lyons MJ, Goldberg J, et al. Genetic and environmental influences on posttraumatic stress disorder, alcohol and drug dependence in twin pairs. Drug and alcohol dependence. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 12.McLeod DS, Koenen KC, Meyer JM, Lyons MJ, Eisen S, True W, et al. Genetic and environmental influences on the relationship among combat exposure, posttraumatic stress disorder symptoms, and alcohol use. Journal of traumatic stress. 2001;14:259–275. doi: 10.1023/A:1011157800050. [DOI] [PubMed] [Google Scholar]
- 13.Sartor CE, McCutcheon VV, Pommer NE, Nelson EC, Grant JD, Duncan AE, et al. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychological medicine. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Psychiatric Association. American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. DSM-5 Task Force. [Google Scholar]
- 15.Ehlers A, Clark DM. A cognitive model of posttraumatic stress disorder. Behaviour research and therapy. 2000;38:319–345. doi: 10.1016/s0005-7967(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 16.Casada JH, Amdur R, Larsen R, Liberzon I. Psychophysiologic responsivity in posttraumatic stress disorder: generalized hyperresponsiveness versus trauma specificity. Biol Psychiatry. 1998;44:1037–1044. doi: 10.1016/s0006-3223(98)00182-6. [DOI] [PubMed] [Google Scholar]
- 17.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 18.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and alcohol dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. doi: 10.1016/j.biopsych.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flagel SB, Akil H, Robinson TE. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology. 2009;56(Suppl 1):139–148. doi: 10.1016/j.neuropharm.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yager LM, Robinson TE. A classically conditioned cocaine cue acquires greater control over motivated behavior in rats prone to attribute incentive salience to a food cue. Psychopharmacology. 2013;226:217–228. doi: 10.1007/s00213-012-2890-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders BT, Robinson TE. Individual variation in the motivational properties of cocaine. Neuropsychopharmacology. 2011;36:1668–1676. doi: 10.1038/npp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders BT, Robinson TE. A cocaine cue acts as an incentive stimulus in some but not others: implications for addiction. Biol Psychiatry. 2010;67:730–736. doi: 10.1016/j.biopsych.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders BT, Yager LM, Robinson TE. Cue-evoked cocaine "craving": role of dopamine in the accumbens core. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrow JD, Maren S, Robinson TE. Individual variation in the propensity to attribute incentive salience to an appetitive cue predicts the propensity to attribute motivational salience to an aversive cue. Behav Brain Res. 2011;220:238–243. doi: 10.1016/j.bbr.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell BA, Campbell EH. Retention and extinction of learned fear in infant and adult rats. Journal of comparative and physiological psychology. 1962;55:1–8. doi: 10.1037/h0049182. [DOI] [PubMed] [Google Scholar]
- 28.Wendt G. Two and one-half year retention of a conditioned response. The Journal of General Psychology. 1937;17:178–80. [Google Scholar]
- 29.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning & memory. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear NE, Miller JS, Jagielo JA. Animal memory and learning. Annual review of psychology. 1990;41:169–211. doi: 10.1146/annurev.ps.41.020190.001125. [DOI] [PubMed] [Google Scholar]
- 31.Pickens CL, Golden SA, Nair SG. Incubation of fear. Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] 2013;Chapter 6(Unit 6):27. doi: 10.1002/0471142301.ns0627s64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andrews B, Brewin CR, Philpott R, Stewart L. Delayed-onset posttraumatic stress disorder: a systematic review of the evidence. The American journal of psychiatry. 2007;164:1319–1326. doi: 10.1176/appi.ajp.2007.06091491. [DOI] [PubMed] [Google Scholar]
- 33.Pickens CL, Golden SA, Adams-Deutsch T, Nair SG, Shaham Y. Long-lasting incubation of conditioned fear in rats. Biol Psychiatry. 2009;65:881–886. doi: 10.1016/j.biopsych.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lang UE, Sander T, Lohoff FW, Hellweg R, Bajbouj M, Winterer G, et al. Association of the met66 allele of brain-derived neurotrophic factor (BDNF) with smoking. Psychopharmacology. 2007;190:433–439. doi: 10.1007/s00213-006-0647-1. [DOI] [PubMed] [Google Scholar]
- 35.Cheng CY, Hong CJ, Yu YW, Chen TJ, Wu HC, Tsai SJ. Brain-derived neurotrophic factor (Val66Met) genetic polymorphism is associated with substance abuse in males. Brain research Molecular brain research. 2005;140:86–90. doi: 10.1016/j.molbrainres.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Nedic G, Perkovic MN, Sviglin KN, Muck-Seler D, Borovecki F, Pivac N. Brain-derived neurotrophic factor Val66Met polymorphism and alcohol-related phenotypes. Progress in neuropsychopharmacology & biological psychiatry. 2013;40:193–198. doi: 10.1016/j.pnpbp.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Zhang XY, Chen da C, Xiu MH, Luo X, Zuo L, Haile CN, et al. BDNF Val66Met variant and smoking in a Chinese population. PLoS One. 2012;7:e53295. doi: 10.1371/journal.pone.0053295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia W, Shi JG, Wu B, Ao L, Zhang R, Zhu YS. Polymorphisms of brain-derived neurotrophic factor associated with heroin dependence. Neuroscience letters. 2011;495:221–224. doi: 10.1016/j.neulet.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 39.Matsushita S, Kimura M, Miyakawa T, Yoshino A, Murayama M, Masaki T, et al. Association study of brain-derived neurotrophic factor gene polymorphism and alcoholism. Alcoholism, clinical and experimental research. 2004;28:1609–1612. doi: 10.1097/01.alc.0000145697.81741.d2. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Benedek DM, Fullerton CS, Forsten RD, Naifeh JA, Li XX, et al. PTSD risk is associated with BDNF Val66Met and BDNF overexpression. Molecular psychiatry. 2014;19:8–10. doi: 10.1038/mp.2012.180. [DOI] [PubMed] [Google Scholar]
- 41.Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. doi: 10.1111/j.1749-6632.2010.05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hensler JG, Ladenheim EE, Lyons WE. Ethanol consumption and serotonin-1A (5-HT1A) receptor function in heterozygous BDNF (+/−) mice. Journal of neurochemistry. 2003;85:1139–1147. doi: 10.1046/j.1471-4159.2003.01748.x. [DOI] [PubMed] [Google Scholar]
- 43.McGough NN, He DY, Logrip ML, Jeanblanc J, Phamluong K, Luong K, et al. RACK1 and brain-derived neurotrophic factor: a homeostatic pathway that regulates alcohol addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:10542–10552. doi: 10.1523/JNEUROSCI.3714-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Psotta L, Lessmann V, Endres T. Impaired fear extinction learning in adult heterozygous BDNF knock-out mice. Neurobiology of learning and memory. 2013;103:34–38. doi: 10.1016/j.nlm.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 45.McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain research. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Musumeci G, Minichiello L. BDNF-TrkB signalling in fear learning: from genetics to neural networks. Reviews in the neurosciences. 2011;22:303–315. doi: 10.1515/RNS.2011.031. [DOI] [PubMed] [Google Scholar]
- 47.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maren S. Overtraining does not mitigate contextual fear conditioning deficits produced by neurotoxic lesions of the basolateral amygdala. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:3088–3097. doi: 10.1523/JNEUROSCI.18-08-03088.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu K, Lau WM, Lau HT, So KF, Chang RC. Micro-dissection of rat brain for RNA or protein extraction from specific brain region. Journal of visualized experiments : JoVE. 2007:269. doi: 10.3791/269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Meyer PJ, Lovic V, Saunders BT, Yager LM, Flagel SB, Morrow JD, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. doi: 10.1371/journal.pone.0038987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breslau N. The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma Violence Abuse. 2009;10:198–210. doi: 10.1177/1524838009334448. [DOI] [PubMed] [Google Scholar]
- 52.Bouton ME, Frohardt RJ, Sunsay C, Waddell J, Morris RW. Contextual control of inhibition with reinforcement: adaptation and timing mechanisms. Journal of experimental psychology Animal behavior processes. 2008;34:223–236. doi: 10.1037/0097-7403.34.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaye H, Mackintosh NJ. A change of context can enhance performance of an aversive but not of an appetitive conditioned response. The Quarterly journal of experimental psychology B, Comparative and physiological psychology. 1990;42:113–134. [PubMed] [Google Scholar]
- 54.Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012;36:2521–2532. doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mednick MT. Mediated generalization and the incubation effect as a function of manifest anxiety. Journal of abnormal psychology. 1957;55:315–321. doi: 10.1037/h0044440. [DOI] [PubMed] [Google Scholar]
- 56.Golin S, Golin AK. Incubation and inhibition. Journal of experimental psychology. 1966;71:208–211. doi: 10.1037/h0022827. [DOI] [PubMed] [Google Scholar]
- 57.Panzer A, Viljoen M. Dissociation: a developmental psychoneurobiological perspective. 2004 [Google Scholar]
- 58.Wolf EJ, Lunney CA, Miller MW, Resick PA, Friedman MJ, Schnurr PP. The dissociative subtype of PTSD: a replication and extension. Depression and anxiety. 2012;29:679–688. doi: 10.1002/da.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stovall-McClough KC, Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. Journal of consulting and clinical psychology. 2006;74:219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- 60.Bremner JD, Southwick S, Brett E, Fontana A, Rosenheck R, Charney DS. Dissociation and posttraumatic stress disorder in Vietnam combat veterans. The American journal of psychiatry. 1992;149:328–332. doi: 10.1176/ajp.149.3.328. [DOI] [PubMed] [Google Scholar]
- 61.van der Kolk BA, Pelcovitz D, Roth S, Mandel FS, McFarlane A, Herman JL. Dissociation, somatization, and affect dysregulation: the complexity of adaptation of trauma. The American journal of psychiatry. 1996;153:83–93. doi: 10.1176/ajp.153.7.83. [DOI] [PubMed] [Google Scholar]
- 62.Janet P. The major symptoms of hysteria. 1907 [Google Scholar]
- 63.Van der Kolk BA, Van der Hart O. Pierre Janet and the breakdown of adaptation in psychological trauma. The American journal of psychiatry. 1989 doi: 10.1176/ajp.146.12.1530. [DOI] [PubMed] [Google Scholar]
- 64.Spiegel D, Cardena E. Disintegrated experience: The dissociative disorders revisited. Journal of abnormal psychology. 1991;100:366. doi: 10.1037//0021-843x.100.3.366. [DOI] [PubMed] [Google Scholar]
- 65.Brewin CR. A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behaviour research and therapy. 2001;39:373–393. doi: 10.1016/s0005-7967(00)00087-5. [DOI] [PubMed] [Google Scholar]
- 66.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 67.Breh DC, Seidler GH. Is peritraumatic dissociation a risk factor for PTSD? Journal of trauma & dissociation : the official journal of the International Society for the Study of Dissociation. 2007;8:53–69. doi: 10.1300/J229v08n01_04. [DOI] [PubMed] [Google Scholar]
- 68.Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, et al. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- 69.Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, et al. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing efficacy of cocaine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Molecular psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nature reviews Neuroscience. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 74.Vicario-Abejon C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nature reviews Neuroscience. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- 75.Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Translational psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takei S, Morinobu S, Yamamoto S, Fuchikami M, Matsumoto T, Yamawaki S. Enhanced hippocampal BDNF/TrkB signaling in response to fear conditioning in an animal model of posttraumatic stress disorder. Journal of psychiatric research. 2011;45:460–468. doi: 10.1016/j.jpsychires.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 77.Rattiner LM, Davis M, Ressler KJ. Differential regulation of brain-derived neurotrophic factor transcripts during the consolidation of fear learning. Learning & memory. 2004;11:727–731. doi: 10.1101/lm.83304. [DOI] [PubMed] [Google Scholar]
- 78.Gabriele A, Setlow B, Packard MG. Cocaine self-administration alters the relative effectiveness of multiple memory systems during extinction. Learning & memory. 2009;16:296–299. doi: 10.1101/lm.1253409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mizuno K, Dempster E, Mill J, Giese KP. Long-lasting regulation of hippocampal Bdnf gene transcription after contextual fear conditioning. Genes, brain, and behavior. 2012;11:651–659. doi: 10.1111/j.1601-183X.2012.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chapman TR, Barrientos RM, Ahrendsen JT, Hoover JM, Maier SF, Patterson SL. Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiology of aging. 2012;33(832):e1–e14. doi: 10.1016/j.neurobiolaging.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jones SV, Stanek-Rattiner L, Davis M, Ressler KJ. Differential regional expression of brainderived neurotrophic factor following olfactory fear learning. Learning & memory. 2007;14:816–820. doi: 10.1101/lm.781507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albeck DS, Beck KD, Kung LH, Sano K, Brennan FX. Leverpress escape/avoidance training increases neurotrophin levels in rat brain. Integrative physiological and behavioral science : the official journal of the Pavlovian Society. 2005;40:28–34. doi: 10.1007/BF02734186. [DOI] [PubMed] [Google Scholar]
- 83.Kadar E, Aldavert-Vera L, Huguet G, Costa-Miserachs D, Morgado-Bernal I, Segura-Torres P. Intracranial self-stimulation induces expression of learning and memory-related genes in rat amygdala. Genes, brain, and behavior. 2011;10:69–77. doi: 10.1111/j.1601-183X.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- 84.Kadar E, Huguet G, Aldavert-Vera L, Morgado-Bernal I, Segura-Torres P. Intracranial self stimulation upregulates the expression of synaptic plasticity related genes and Arc protein expression in rat hippocampus. Genes, brain, and behavior. 2013;12:771–779. doi: 10.1111/gbb.12065. [DOI] [PubMed] [Google Scholar]
- 85.Eaves LJ, Martin NG, Heath AC, Hewitt JK, Neale MC. Personality and reproductive fitness. Behavior genetics. 1990;20:563–568. doi: 10.1007/BF01065872. [DOI] [PubMed] [Google Scholar]
- 86.Wakefield JC. High mental disorder rates are based on invalid measures: Questions about the claimed ubiquity of mutation-induced dysfunction. Behavioral and Brain Sciences. 2006;29:424–426. [Google Scholar]
- 87.Wakefield JC. Evolutionary versus prototype analyses of the concept of disorder. Journal of abnormal psychology. 1999;108:374–399. doi: 10.1037//0021-843x.108.3.374. [DOI] [PubMed] [Google Scholar]
- 88.Wolf M, van Doorn GS, Leimar O, Weissing FJ. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447:581–584. doi: 10.1038/nature05835. [DOI] [PubMed] [Google Scholar]
- 89.Kalueff AV, Ren-Patterson RF, LaPorte JL, Murphy DL. Domain interplay concept in animal models of neuropsychiatric disorders: a new strategy for high-throughput neurophenotyping research. Behav Brain Res. 2008;188:243–249. doi: 10.1016/j.bbr.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 90.LaPorte JL, Egan RJ, Hart PC, Bergner CL, Cachat JM, Canavello PR, et al. Qui non proficit, deficit: experimental models for 'integrative' research of affective disorders. Journal of affective disorders. 2010;121:1–9. doi: 10.1016/j.jad.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 91.Stewart AM, Kalueff AV. Developing better and more valid animal models of brain disorders. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 92.Flagel SB, Robinson TE, Clark JJ, Clinton SM, Watson SJ, Seeman P, et al. An animal model of genetic vulnerability to behavioral disinhibition and responsiveness to reward-related cues: implications for addiction. Neuropsychopharmacology. 2010;35:388–400. doi: 10.1038/npp.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]