Abstract
The presentation of two 19 year old males with stage I non-Hodgkin lymphoma in the proximal tibia prompted an extensive review of institutional and national databases to assess if there is any statistical evidence that these reflected a previously overlooked syndromic pattern of presentation. The institutional records of a single institution were reviewed for presentation of non-Hodgkin lymphoma in bone. The records of two additional institutions were reviewed for all reports of non-Hodgkin lymphoma in the tibia. Analysis was performed on data from SEER (Surveillance, Epidemiology, and End Results) dichotomized to bone presentation in the lower extremity versus other bones. Institutional databases included 20 patients with tibial presentation of lymphoma with a median age of 22.5 years (versus 42 for all bone lymphomas; p<0.001). 18/20 were diffuse large B cell lymphoma, and all patients ≤ 40 achieved remission and apparent cure. Distinctive and unusual features were a tendency for bilateral involvement of the tibia and sclerotic changes on X-ray. SEER data included 808 cases of bone lymphoma; the fraction of cases presenting in the lower extremity vs other bone sites is higher at ages ≤ 40 years (38% vs 19%; p < 0.0001). Presentation in the lower extremity, as compared to other bone sites, confers 97% overall survival in patients ≤ 40 (vs. 82%; p = 0.01). This survival effect was independent of stage. In contrast, no significant difference in overall survival was identified for lower extremity versus non-lower extremity site for age > 40. These data show a previously undescribed syndromic pattern of disease presentation: bone lymphoma in young patients is likely to present in the lower extremity, specifically the proximal tibia, has atypical sclerotic features on x-ray, is often bilateral, and has an excellent prognosis compared to bone lymphomas at other sites matched for stage and age.
Keywords: non-Hodgkin lymphoma, bone lymphoma, tibia, epidemiology
Introduction
Lymphoma of the bone is a rare malignancy, first suggested by Oberling in 1928 and further described by Parker and Jackson in a series on “reticulum cell sarcoma of bone.” 1, 2 Primary bone lymphoma, as originally defined by Ostrowski, is a lymphoma of bone without disease present elsewhere for at least 6 months after initial diagnosis, account for less than 1% of all malignant lymphomas and occur at a median age of 46 years.2 The characteristics of bone lymphoma have been further defined by a number of case series: the most common locations of bone lymphoma are variously reported as the spine, the ischium, and long bones, particularly the femur; the most common presenting symptom is pain associated with swelling; the most common histology is diffuse large B-cell lymphoma, and the phenotype may be Germinal Center- or Activated B Cell-like. 1-10 The current treatment of primary bone lymphoma appears to typically be a combination of chemotherapy and radiotherapy. 9
We encountered two male patients, both age 19, whose chief complaint was knee pain and each had a biopsy of the proximal tibia that was diagnostic of a B-cell lymphoma. In both cases, the proximal tibia was preferentially involved. One patient had bilateral proximal tibia involvement by diffuse large B cell lymphoma, not otherwise specified. The other had unilateral tibia involvement by a low grade B cell lymphoma (not further classifiable) that was treated with local radiation and recurred 17 months later in the contralateral tibia. Both patients’ bone x-rays showed a sclerotic pattern. These cases prompted us to review all lymphomas with an initial diagnosis of lymphoma in bone at one institution, to identify all patients with tibial lymphoma at two additional institutions, and to examine the association of age and relative incidence of lower limb bone lymphoma within a national cancer database.
Materials and Methods
Correlation of the clinical and pathology records of the University of Rochester Medical Center from 2003-2011 identified all lymphomas in patient’s whose initial diagnosis was based on a bone biopsy (excluding iliac crest) directed at a radiographic lesion. The demographics, symptoms at presentation, radiologic findings, clinical stage, treatment, and follow-up were identified for the subset of bone lymphoma specimens for which the site was tibia in the pathology report (table1). The demographics and clinical stage were also obtained for the subset of bone lymphoma specimens for which the site was femur. Immunohistochemical stains were reviewed. Radiologic images of the cases at the index institution were reviewed. Reproducibility of findings was assessed by searching the pathology records of two other institutions for lymphomas diagnosed in the tibia; the latter searches were preformed without age restriction and without the stipulation that the specimen represent the patient’s initial diagnosis.
Table 1.
Subjects’ clinical and pathologic characteristics. For specimens 1-11, definitive positive and negative results for the following studies are listed: CD10, BCL6, MUM1. If the specimen was inadequate to perform these studies or the result was inconclusive, the phenotype is not listed. For specimens 12-20, the phenotypes are as described in the original surgical pathology reports.
| Sex | Age | Diagnosis | Phenotype | XRAY | Stage | Sites | Therapy | Follow-up | |
|---|---|---|---|---|---|---|---|---|---|
|
1
(index) |
M | 19, 21 | Low grade B- cell; centrocyte- like cytology, diffuse |
+CD20/79a; Clonal IGH PCR --BCL6 |
Sclerosis | IE | Bilateral proximal tibia |
XRT | 59 months NED (post recurrence in opposite tibia) |
| 2 | M | 32 | DLBCL Centroblastic |
+CD20/79a --CD10 |
Sclerosis | IVB | Proximal tibia, iliac, sacrum, LNs |
R-CHOP | 77 months, NED |
| 3 | M | 17 | Pre-B lymphoblastic lymphoma |
+CD79a +TdT |
Sclerosis | III – IV | Tibia, femur, elbow, mediastinum, LNs |
Pediatric ALL protocol |
63 months NED |
| 4 | F | 24 | DLBCL centroblastic |
+CD20/79a/10 +BCL6/kappa --BCL2 --MUM1 |
Sclerotic & Lytic |
IV | Bilateral proximal tibia, LN |
R-CHOP + XRT |
43 months NED |
| 5 | M | 38 | DLBCL necrotic |
+CD20 | Sclerosis | IV | Tibia and LN | R-CHOP + XRT |
30 months NED |
|
6
(index) |
M | 19 | DLBCL centroblastic |
+CD20 +lambda --CD10 |
Sclerotic & Lytic |
IVA | Bilateral proximal tibia |
R-CHOP | 30 months NED |
| 7 | M | 19 | DLBCL necrotic |
+CD20/79a/10 | Sclerosis | IE | Proximal tibia | R-CHOP + XRT |
129 months NED |
| 8 | M | 34 | DLBCL centroblastic |
+CD20/79a --CD45ro |
Lytic cyst |
IE | Proximal Tibia | CHOP + XRT |
78 months NED |
| 9 | F | 13 | DLBCL | NA | IE | Tibia | NA | NA | |
| 10 | M | 56 | DLBCL necrosis |
+CD20/79a +BCL2/6 --TdT/CD34 |
Lytic | IV | Bilateral tibia, clavicle |
R-CHOP + auto SCT |
20 months died of disease at |
| 11 | F | 85 | DLBCL with fibrosis and necrosis |
+CD20/10 +BCL6 --BCL2− CD3 |
NA | IE | Tibia | R-CHOP + XRT |
72 months NED |
| 12 | M | 15 | DLBCL with fibrosis and necrosis |
+CD20 --CD30/15 --CD43/45ra --MPO/Lys |
Sclerosis | NA | Tibia and femur | NA | NA |
| 13 | M | 28 | DLBCL | +CD20/45 --CD30/99 |
NA | IE | Tibia | CHOP + XRT |
74 months NED |
| 14 | F | 21 | DLBCL | +CD20 | Sclerosis | IE | Tibia | CHOP + XRT |
14 months NED |
| 15 | F | 21 | DLBCL with fibrosis |
+CD20 −CD3 −CD45RO |
NA | NA | Tibia and femur | NA | NA |
| 16 | M | 19 | DLBCL with dense fibrosis |
+CD20/79a --TdT |
NA | 1E | Proximal tibia | RCHOP + XRT |
86 months NED |
| 17 | M | 48 | DLBCL, with fibrosis |
+CD20 --BCL2 --cyclin D1 |
NA | 1E | Proximal tibia | RCHOP + XRT |
19 months NED |
| 18 | M | 31 | DLBCL | +CD20 | NA | 1E | Tibia predominantly distal with proximal extension |
RCHOP | 70 months NED |
| 19 | M | 20 | DLBCL, immunoblastic |
+CD20/10 +PAX5 +BCL6 |
NA | 1E | Proximal tibia | RCHOP | 60 months NED |
| 20 | M | 29 | DLBCL | +CD20/45 −CD45ro |
NA | NA | Proximal tibia | NA | NA |
Abbreviations: S = sclerosis, L = Lytic, XRT = external beam radiation therapy, NED= No evidence of disease, DLBCL = Diffuse Large B cell Lymphoma, NA = not available, R-CHOP = Rituxin- Cyclophosphamide, Hydroxydaunorubicin, Oncovin (vincristine), Prednisone.
The Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute is a population-based national cancer database comprised of data on incident cancer cases contributed from registries across the United States. SEER began collecting data from 9 registries in 1973; currently SEER includes data captured on incident cases from 17 participating registries, a catchment area representing approximately 28% of the US population. 11, 12
In order to limit the influence of variability in referral and diagnosis patterns, data from the 2000-2009 SEER dataset was used for this analysis; this dataset was comprised of incident cancer cases captured in 17 SEER site registries. Inclusion in the analysis dataset was limited to patients with a lymphoma diagnosis (ICDO3 codes 9590, 9591, 9650, 9663, 9670, 9671, 9673, 9675, 9680, 9684, 9687, 9690, 9691, 9695, 9698, 9699, 9702, 9714, 9727, 9728, 9729, 9920, and 9930; n=129,097), and bone as their primary site of disease (only included if site was C40.0, C40.1,C40.2, C40.3, C40.8, C40.9, C41.0, C41.1, C41.2, C41.3, C41.4, C41.8, C41.9;n=1,315)12. Patients diagnosed with plasma cell tumors (ICDO3 9730-9739) were excluded from this analysis (n=923). Our final analysis dataset included 808 lymphomas with bone involvement diagnosed from 2000-2009. Histology was grouped by ICDO3 codes into malignant lymphomas not otherwise specified or diffuse, Hodgkin lymphomas, non-Hodgkin lymphoma - mature T- and NK-cell lymphomas, non-Hodgkin lymphoma -precursor cell lymphoblastic lymphoma, myeloid leukemias, and non-Hodgkin lymphoma -mature B-cell lymphomas, which was further categorized into diffuse large B cell lymphoma, not otherwise specified and other. Stage was captured according to the SEER collaborative staging algorithm.
Involvement of the tibia, specifically, is not captured with the ICDO3 site codes; therefore, patients with primary involvement of the long bones of the lower limb and associated joints (C40.2) were designated as the lower extremity cases and were compared to other bone lymphoma patients with involvement of all other sites. From this point forward, patients with lymphoma involvement of any long bones of the lower limb and associated joints will be referred to as ‘lower extremity’ cases.
Descriptive analyses included presentation of case characteristics by frequency and percents, stratified by age, site of bone involvement (lower extremity versus other bone sites) and gender. Differences in age and stage of lymphoma at diagnosis by site of bone involvement were analyzed using chi-squared tests. Overall Survival was defined as time from diagnosis to date of death from any cause; patients who were still alive at the end of follow-up were censored as of December 31, 2009. Overall survival was estimated by standard Kaplan-Meier survival techniques. We compared overall survival between cases with lower extremity involvement and cases with lymphoma involvement of other bone sites using the log-rank test. A Cox proportional hazards model was used for multivariable analyses. Analyses were implemented using SAS software system (SAS Institute, Cary, NC; Version 9.3).
Results
An institutional search identified all bone lymphoma specimens obtained from 2003-11. 49 cases of non-Hodgkin lymphoma with biopsy-proven initial presentation in bone were identified at the index institution; these had a median age at presentation of 42 years (data not shown). Of these, 10 patients (~20%) had tibial lymphoma. These patients were significantly younger (median age of 21.5 years; p<0.001). In all of these 10 cases, review of the clinical data indicated that knee pain or swelling was the primary symptom. None of these specimens included any other involved sites although subsequent imaging suggested the involvement of other bone sites and lymph nodes in several patients (table 1); 6 fulfilled criteria for “primary bone lymphoma” 2. The remaining 4 cases showed predominant involvement of the tibia and clinically were thought most likely to represent primary tibial lymphoma. Eleven patients had initial presentation of lymphoma in the femur and these patients were strikingly older than those who presented with tibial disease (median age of 74; p<0.001). Similar searches of bone lymphomas (without restrictions with regard to patient age or initial site of presention) at 2 other institutions identified 10 additional tibial non-Hodgkin lymphoma cases; the median age was 24.5 years. Together, the 20 cases of tibial lymphoma had an age range of 13 – 85, median age of 22.5 years and a male:female ratio of 3:1 (Table 1). With the exception of a 56 year old patient, the available radiographs for all patients at the index institution showed sclerotic changes (figure 1). No radiographic features or case histories were particularly suggestive of antecedent Osgood-Schlatter disease.
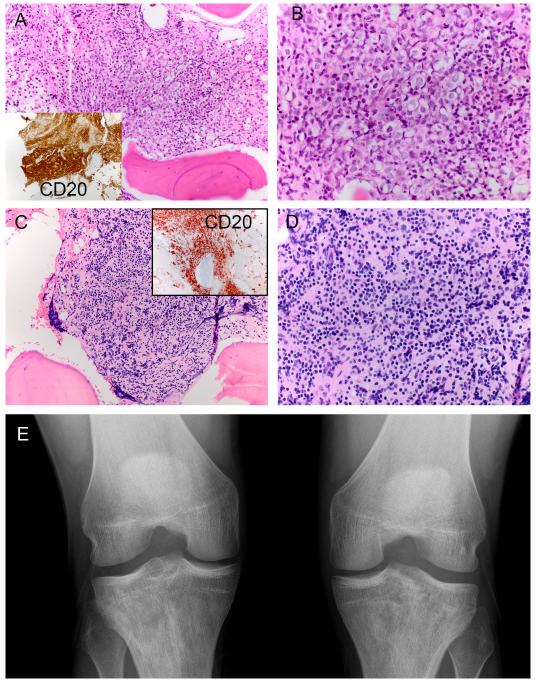
Figure 1.
H&E and CD20 stain of for index patient 6 (original magnification: A 200×, B 400×). H&E and CD20 stain of for index patient 1 (original magnification: C 200×, D 400×). X-ray of patient 6 at presentation shows bilateral sclerosis of the proximal tibias (E). The decreased and variable radiolucency of the tibial epiphyses and metaphyses is best appreciated by comparison to homogenous lucency of the femurs and fibulas.
All tumors were B-cell lymphomas; all but two had diffuse large B-cell lymphoma histology and were positive for CD20 and/or CD79a. One patient (table 1, patient 1) showed a low-grade histology composed of monomorphic small lymphocytes; this material was the subject of extensive extramural consultation which corroborated the diagnosis. This latter lymphoma recurred after 17 months in the contralateral tibia. One patient with atypically widespread disease had precursor B cell lymphoblastic leukemia/lymphoma (3 in table 1). The cases often had a low stage and an overall good prognosis. Of the 17 cases where stage was available, 10 cases were limited to the tibia (stage IE). Bilateral tibia involvement was identified in 4 of the 10 index institution cases. For cases where therapy was known, most were treated with combined modalities of chemotherapy and radiation. Of the 9 cases at the index institution with follow-up (average 56.4 months), one recurred in the contralateral tibia, one died of his lymphoma (56 years old at presentation), and 7 had no recurrence of disease.
The SEER database (2000 to 2009) includes 808 cases of lymphoma with bone presentation. Because the SEER database does not specify bone site beyond lower extremity, we used this subgroup as a surrogate for the proximal tibia. The most common histology for the lymphomas with bone involvement was diffuse large B cell lymphoma, not otherwise specified, regardless of site of involvement. No difference was apparent in the median ages for presentation in the lower extremity (187 cases, median age 57) versus all other bone sites (621 cases, median age 63).
In younger patients, a significantly larger fraction of bone lymphoma present in the lower extremity bones compared to other bones (38% for ≤40 vs 19% for > 40 years, p<0.0001) (table 2). This difference is most striking for males aged 10-30 years (Figures 2a and 2b). Over all, half (53.3%) of bone lymphomas, regardless of age were limited stage disease (Stage I/IE). A significantly greater fraction of lower extremity bone lymphomas were low stage compared to other sites regardless of age (table 3).
Table 2.
Comparing age of lymphoma diagnosis by site of bone involvement; SEER 17 registry data, 2000-2009
| Age at Diagnosis count (% of column) |
|||
|---|---|---|---|
| ≤ 40 | >40 | ||
| Lower Extremity | 66 (38%) | 121 (19%) | p<0.0001 |
| Other Bone | 107 (62%) | 514 (81%) | χ 2 |
| Total | 173 | 635 | |
Figure 2.
Percent of lymphoma cases with bone involvement by sex (male/female, A/B), age group, and stratified by site of bone involvement. Filled bars: lower extremity. Open bars: all other bone sites. (SEER 17 registry data, 2000-2009).
Table 3.
Comparing stage of lymphoma diagnosis by site of bone involvement; SEER 17 registry data, 2000-2009
| Age ≤ 40 | Age >40 | |||
|---|---|---|---|---|
| n=167 | n=598 | |||
| Other Bone | L. Ext. | Other Bone | L. Ext. | |
| Stage I/IE | 45 (44%) | 49 (75%) | 262 (54%) | 75 (65%) |
| Stage II, III, IV | 57 (56%) | 16 (25%) | 221 (46%) | 40 (35%) |
| χ2 p-value | 0.0001 | 0.033 | ||
Young patients with lower extremity bone presentation of non-Hodgkin lymphoma had a superior overall survival compared to young patients with other bone sites at presentation (figure 3). As expected, survival was worse in patients over 40 years (figure 3B) in comparison to patients ≤40 (figure 3a). More striking is the differential effect of site of bone involvement when stratified by age. Five-year overall survival in patients ≤40 with lower extremity presentation was 97% versus 82% in non-lower extremity presentation (figure 3A). The difference in overall survival by site of bone involvement among patients ≤40 is highly significant (p=0.0086). After controlling for stage, the effect of lower extremity presentation on overall survival remains statistically significant (p=0.0485). Among patients >40 (figure 3B), two-year overall survival among those with lower extremity involvement was 73% versus 68% in non-lower extremity bone involvement and five-year overall survival was 68% versus 57%, (log-rank p=0.15). Because the curves repeatedly cross and do not separate until 12 months, site may have no real effect on survival in those >40.
Figure 3.
Kaplan-Meier curves for bone lymphoma, stratified by site of involvement. A) Overall survival in patients 40 and younger; B) Overall survival in patients older than 40. Circles: lower extremity. Cross: all other bone sites. (SEER, 2000-2009; median follow-up of 48 months).
Discussion
Our data reveal a syndromic pattern of presentation of lymphoma that occurs specifically in the proximal tibia of young people (≤ 40 years old) presenting with knee pain and/or swelling, is frequently bilateral, and shows sclerotic changes on imaging. Epidemiologic data indicate that lymphoma of lower extremity bones has excellent prognosis compared to bone non-Hodgkin lymphoma presenting in young patients at other sites. Furthermore, in our patients the lymphoma was often highly restricted to the proximal tibia and showed a profound tropism for this site: of 10 patients at the index institution, 6 patients had lymphoma solely in the proximal tibia as the only bone site and 4 patients demonstrated bilateral proximal tibial involvement with relative sparing of other bone sites. Our institutional data show a striking bias toward tibial rather than femoral presentation in young patients (25 versus 74 years median age of presentation). Strikingly, the youngest patient (18 years old) with femoral presentation of lymphoma at this institution had disease characterized by the surgeon as “knee and distal femur”, suggesting a similarity to the presentations seen in the proximal tibia.
Although the SEER database does not include localization specifically to the proximal tibia, the data on the lower extremity corroborates and extend our institutional findings. Most importantly, cases of lower extremity lymphoma in young patients had significantly better overall survival in comparison to lymphomas of non-lower extremity bone sites in young patients. Although lower extremity presentation tended to be associated with a lower stage, low stage did not fully account for the improved overall survival; site of bone involvement remained statistically significant after adjustment for stage in the multivariate Cox proportional hazards model.
A sclerotic radiographic pattern was identified in all 7 cases at the index institution in patients ≤ 40 for whom imaging was available. Overall, 9/11 patients with available radiologic information had either purely sclerotic or mixed sclerotic lesion, and only 2 had a purely lytic lesion. This finding is in contrast with the typical lytic appearance of Non-Hodgkin lymphomas of the bone which is either permeative or moth-eaten, with bone destruction as a significant feature 10, 13, 14. A predominately sclerotic image, while more often seen in classical Hodgkin lymphoma of the bone, is described in just 2% of bone non-Hodgkin lymphoma 15. Therefore, radiographic sclerosis may be a diagnostic clue for non-Hodgkin lymphoma presenting in the proximal tibia.
While a range of common studies was attempted (e.g. immunohistochemical studies for CD10, MUM1, BCL-2, BCL-6, EBV-LMP, and Ki-67, as well as in-situ hybridization for EBER), no shared histologic, immunophenotypic, or genetic features that further characterized these B cell tumors were identified. The specimens were in general small and crushed, and the decalcification performed on most hindered any PCR-based analyses. Bone lymphomas are often difficult to diagnose and classify and the osteoblastic/sclerotic nature of these tumors makes them particularly prone to crush artifact and limited sample size. In fact, several of the patients required repeat procedures to obtain sufficient material for the final diagnosis.
This syndromic pattern of presentation of lymphoma may have escaped notice in prior studies of “primary bone lymphoma” because many of our patients (for example patients 2-5) would have been excluded from those series since there was lymph node involvement at the time of presentation. Furthermore, the patient ages straddled the cut-off between adult and pediatric series; our two index cases were both seen in adult clinics and were not enrolled on pediatric trials. Still, the SEER data show that the highest incidence of lower extremity bone lymphomas for males is in the second decade (see figure 2). Our review of the literature identified 9 publications describing 7 case series of pediatric bone lymphomas16-24 (the two largest series are each reported in two parts, with a publication describing a follow-up time point21, 22 or the clinical and histologic data described separately18, 24). These series document that pediatric primary bone lymphoma commonly presents in the lower limb. While the femur may be a more common site of lymphoma than the tibia, it is unclear if the femoral presentation is associated with the same syndromic pattern of presentation involving a complaint of knee pain with sclerosis on bone x-ray. Regardless, one of these series of pediatric primary bone lymphomas showed a striking similarity to the series we describe here; Zhao et al describe a series of 10 pediatric patients with “primary bone lymphoma”, all male, four with tibial presentation, one of which was bilateral24.
The bilateral presentation in the proximal tibias with sparing of other sites has also been described by others. For example, two recent case reports both show striking images of bilateral proximal tibial lymphoma, although both in older males 25,26.
Several non-Hodgkin lymphoma subtypes with site-specific biology, prognostic and treatment characteristics, and even distinctive molecular features have been described and incorporated into the World Health Organization’s classification of lymphoid tumors27. The site-specific pathogenesis of a subset of these is thought to be dependent on chronic, local inflammation, the most common of which are the various forms of mucosal associated lymphoid tissue (MALT) lymphoma. The WHO also recognizes “diffuse large B cell lymphoma associated with chronic inflammation”, the prototypical form of which occurs in males in the seventh decade and specifically in the pleural cavity; it has also been described associated with inflammation around metal implants in the femur 28. A small case series of bone lymphomas putatively associated with trauma has been reported 29. Our reviews of the patients’ medical records did not suggest any obvious etiologies for the cases of proximal tibial non-Hodgkin lymphoma, but one possible explanation relates to the increased athletic activities of this age group that might result in repetitive, microtrauma and subsequent inflammation of the proximal tibia. While evidence of EBV infection is typically found in cases of “diffuse large B cell lymphoma associated with chronic inflammation”, we were unable to detect EBV in any of the tumors from patients < 40 years old in this series.
In sum, we describe a strong association between proximal tibial lymphoma (often bilateral), young age, sclerotic lesions on X-ray and excellent prognosis, associations that are reinforced by analyses of the SEER database. Due to the uncommon nature of these tumors, an increased awareness of the possibility of this unusual lymphoma in young patients is essential, since ample diagnostic material is required for diagnostic studies, including immunohistochemical and molecular studies, and prognosis may vary significantly by site of involvement. In addition, this syndromic pattern of presentation suggests intriguing hypotheses regarding the relationship between microtrauma and the development of lymphoma.
Acknowledgments
This publication was supported by a grant from the Upstate New York Translational Research Network to WRB, administered through the University of Rochester CTSA under award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure/Conflict of Interest
There are no conflicts of interest to declare.
References
- 1.K. Krishnan Unni CYI, Bridge Julia A., Kindblom Lars-Gunnar, Wold Lester E. Malignant Lymphoma. AFIP Atlas of Tumor Pathology: Tumors of the Bones and Joints. :231–248. [Google Scholar]
- 2.Ostrowski ML, Unni KK, Banks PM, et al. Malignant lymphoma of bone. Cancer. 1986;58:2646–2655. doi: 10.1002/1097-0142(19861215)58:12<2646::aid-cncr2820581217>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Alencar A, Pitcher D, Byrne G, et al. Primary bone lymphoma--the University of Miami experience. Leuk Lymphoma. 51:39–49. doi: 10.3109/10428190903308007. [DOI] [PubMed] [Google Scholar]
- 4.Beal K, Allen L, Yahalom J. Primary bone lymphoma: treatment results and prognostic factors with long-term follow-up of 82 patients. Cancer. 2006;106:2652–2656. doi: 10.1002/cncr.21930. [DOI] [PubMed] [Google Scholar]
- 5.Bhagavathi S, Micale MA, Les K, et al. Primary bone diffuse large B-cell lymphoma: clinicopathologic study of 21 cases and review of literature. Am J Surg Pathol. 2009;33:1463–1469. doi: 10.1097/PAS.0b013e3181b314ce. [DOI] [PubMed] [Google Scholar]
- 6.de Leval L, Braaten KM, Ancukiewicz M, et al. Diffuse large B-cell lymphoma of bone: an analysis of differentiation-associated antigens with clinical correlation. Am J Surg Pathol. 2003;27:1269–1277. doi: 10.1097/00000478-200309000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Gianelli U, Patriarca C, Moro A, et al. Lymphomas of the bone: a pathological and clinical study of 54 cases. Int J Surg Pathol. 2002;10:257–266. doi: 10.1177/106689690201000403. [DOI] [PubMed] [Google Scholar]
- 8.Lima FP, Bousquet M, Gomez-Brouchet A, et al. Primary diffuse large B-cell lymphoma of bone displays preferential rearrangements of the c-MYC or BCL2 gene. Am J Clin Pathol. 2008;129:723–726. doi: 10.1309/CMPGPYTMFRM6RVQR. [DOI] [PubMed] [Google Scholar]
- 9.Ramadan KM, Shenkier T, Sehn LH, et al. A clinicopathological retrospective study of 131 patients with primary bone lymphoma: a population-based study of successively treated cohorts from the British Columbia Cancer Agency. Ann Oncol. 2007;18:129–135. doi: 10.1093/annonc/mdl329. [DOI] [PubMed] [Google Scholar]
- 10.Unni KK, Dahlin DC. Dahlin’s bone tumors: general aspects and data on 11,087 cases. Lippincott-Raven; Philadelphia: 1996. [Google Scholar]
- 11.Harlan LC, Hankey BF. The surveillance, epidemiology, and end-results program database as a resource for conducting descriptive epidemiologic and clinical studies. J Clin Oncol. 2003;21:2232–2233. doi: 10.1200/JCO.2003.94.023. [DOI] [PubMed] [Google Scholar]
- 12.Surveillance, Epidemiology and End Results (SEER) Program National Cancer Institute ( www.seer.cancer.gov) Research Data. 1973-2008 www.seer.cancer.gov Available Released April 2011, based on the November 2010 submission.
- 13.Barbieri E, Cammelli S, Mauro F, et al. Primary non-Hodgkin’s lymphoma of the bone: treatment and analysis of prognostic factors for Stage I and Stage II. Int J Radiat Oncol Biol Phys. 2004;59:760–764. doi: 10.1016/j.ijrobp.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Tong MYT, Wu WC, Lam JJ, Lee AWM. Primary Non-Hodgkin’s Lymphoma of Bone: a Rare Cause of Lytic Bone Lesion. J HK Coll Radiol. 2004;7:24–30. [Google Scholar]
- 15.O’Neill J, Finlay K, Jurriaans E, et al. Radiological manifestations of skeletal lymphoma. Curr Probl Diagn Radiol. 2009;38:228–236. doi: 10.1067/j.cpradiol.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Coppes MJ, Patte C, Couanet D, et al. Childhood malignant lymphoma of bone. Med Pediatr Oncol. 1991;19:22–27. doi: 10.1002/mpo.2950190105. [DOI] [PubMed] [Google Scholar]
- 17.Furman WL, Fitch S, Hustu HO, et al. Primary lymphoma of bone in children. J Clin Oncol. 1989;7:1275–1280. doi: 10.1200/JCO.1989.7.9.1275. [DOI] [PubMed] [Google Scholar]
- 18.Glotzbecker MP, Kersun LS, Choi JK, et al. Primary non-Hodgkin’s lymphoma of bone in children. J Bone Joint Surg Am. 2006;88:583–594. doi: 10.2106/JBJS.D.01967. [DOI] [PubMed] [Google Scholar]
- 19.Howat AJ, Thomas H, Waters KD, et al. Malignant lymphoma of bone in children. Cancer. 1987;59:335–339. doi: 10.1002/1097-0142(19870115)59:2<335::aid-cncr2820590228>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler JS, Tarbell NJ, Kozakewich H, et al. Primary lymphoma of bone in children: analysis of treatment results with adriamycin, prednisone, Oncovin (APO), and local radiation therapy. J Clin Oncol. 1986;4:496–501. doi: 10.1200/JCO.1986.4.4.496. [DOI] [PubMed] [Google Scholar]
- 21.Lones MA, Perkins SL, Sposto R, et al. Non-Hodgkin’s lymphoma arising in bone in children and adolescents is associated with an excellent outcome: a Children’s Cancer Group report. J Clin Oncol. 2002;20:2293–2301. doi: 10.1200/JCO.2002.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Suryanarayan K, Shuster JJ, Donaldson SS, et al. Treatment of localized primary non-Hodgkin’s lymphoma of bone in children: a Pediatric Oncology Group study. J Clin Oncol. 1999;17:456–459. doi: 10.1200/JCO.1999.17.2.456. [DOI] [PubMed] [Google Scholar]
- 23.Wollner N, Lane JM, Marcove RC, et al. Primary skeletal non-Hodgkin’s lymphoma in the pediatric age group. Med Pediatr Oncol. 1992;20:506–513. doi: 10.1002/mpo.2950200604. [DOI] [PubMed] [Google Scholar]
- 24.Zhao XF, Young KH, Frank D, et al. Pediatric primary bone lymphoma-diffuse large B-cell lymphoma: morphologic and immunohistochemical characteristics of 10 cases. Am J Clin Pathol. 2007;127:47–54. doi: 10.1309/LRQVTE5NM8B9ANLY. [DOI] [PubMed] [Google Scholar]
- 25.Giardino AA, Shinagare AB, Shinagare SA, et al. Primary bone lymphoma involving bilateral tibia. Am J Hematol. 2012;87:924–925. doi: 10.1002/ajh.23245. [DOI] [PubMed] [Google Scholar]
- 26.Wong CL, Mansberg R, Yosufzai M. Symmetric bony involvement of the lower extremities with lymphoma demonstrated on gallium scintigraphy. Clin Nucl Med. 2005;30:353–355. doi: 10.1097/01.rlu.0000159907.63432.6a. [DOI] [PubMed] [Google Scholar]
- 27.Jaffe ES, Harris NL, Stein H, Campo E, Pileri SA, Swerdlow SH. Introduction and overview of the classification of the lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC; Lyon: 2008. pp. 158–199. [Google Scholar]
- 28.Cheuk W, Chan AC, Chan JK, et al. Metallic implant-associated lymphoma: a distinct subgroup of large B-cell lymphoma related to pyothorax-associated lymphoma? Am J Surg Pathol. 2005;29:832–836. doi: 10.1097/01.pas.0000157747.10967.f4. [DOI] [PubMed] [Google Scholar]
- 29.Stein ME, Lewis DC, Gershuny AR, et al. Trauma as an etiologic factor of primary bone lymphoma: a report of 4 cases. J BUON. 2003;8:163–166. [PubMed] [Google Scholar]