Abstract
Invariant natural killer T (iNKT) cells can provide help for B cell activation and antibody production. Since B cells are also capable of cytokine production, antigen presentation and T cell activation, we hypothesized that iNKT cells will also influence these activities. Furthermore, subsets of iNKT cells based on CD4 and CD8 expression that have distinct functional activities may differentially affect B cell functions. We investigated the effects of co-culturing expanded human CD4+, CD8α+ and CD4−CD8α− double negative (DN) iNKT cells with autologous peripheral B cells in vitro. All iNKT cell subsets induced IgM, IgA and IgG release by B cells without needing the iNKT cell agonist ligand α-galactosylceramide (α-GC). Additionally, CD4+ iNKT cells induced expansions of cells with phenotypes of regulatory B cells. When co-cultured with α-GC-pulsed B cells, CD4+ and DN iNKT cells secreted Th1 and Th2 cytokines but at 10–1,000-fold lower levels than when cultured with dendritic cells. CD4+ iNKT cells reciprocally induced IL-4 and IL-10 production by B cells. DN iNKT cells expressed the cytotoxic degranulation marker CD107a upon exposure to B cells. Remarkably, while iNKT cell subsets could induce CD40 and CD86 expression by B cells, iNKT cell-matured B cells were unable to drive proliferation of autologous and alloreactive conventional T cells, as seen with B cells cultured in the absence of iNKT cells. Therefore, human CD4+, CD8α+ and DN iNKT cells can differentially promote and regulate the induction of antibody and T cell responses by B cells.
Introduction
T cell-dependent B cell responses require engagement of antigen by the BCR resulting in antigen internalization, processing and cell-surface presentation on MHC molecules (1–3). Upon recognition of cognate peptide-MHC class II complexes, the T cell upregulates CD40L which engages CD40 on the B cell, inducing proliferation and differentiation. The T cell also secretes cytokines that are required for immunoglobulin isotype switching. Cognate T cell-B cell interactions can result either in extrafollicular proliferation of B cells into short-lived plasmablasts that do not undergo affinity maturation and mediate transient innate-like responses (2), or germinal centre proliferation of B cells which undergo somatic hypermutation and affinity maturation resulting in the generation of long-lived plasma cells or memory B cells (3).
Invariant natural killer T (iNKT)5 cells can provide help for B cell maturation and antibody production. iNKT cells are cytotoxic T cells that express a TCR composed of an invariant α-chain (Vα14Jα18 in mice and Vα24Jα18 in humans) that pairs with a limited number of β-chains and recognizes glycolipid antigens presented by the MHC class I-like molecule CD1d (4, 5). iNKT cells are thought to play central roles in innate and adaptive immunity through their ability to activate or induce differentiation of NK cells (6), dendritic cells (DC)5 (7, 8) and T cells (9) and to release multiple helper T (Th) cell-polarising cytokines (10–13). iNKT cells can also regulate, enhance and sustain humoral immune responses. In murine models, CD1d and iNKT cells are required for the generation of protective antibody responses against pathogens, including Plasmodium falciparum (14), Streptococcus pneumoniae (15) and Borrelia species (16). CD1d and iNKT cells are also required for the production of allergen-specific IgE in an experimental asthma mouse model (17). The adjuvant effect of iNKT cells on humoral immune responses is antigen-specific. Co-administration of the iNKT cell agonist ligand α-galactosylceramide (α-GC)5 with immunizing antigen to mice results in enhanced production of antibodies specific for the antigen (18–20). This help provided by iNKT cells results in the induction of long-lived antibody-secreting plasma cells, affinity maturation and the generation of memory B cells (20–22). iNKT cells can also provide help for B cells specific for lipid-containing antigens internalized through the BCR (23, 24). Such B cell help results in the formation of extrafollicular plasmablasts and germinal centres, affinity maturation and robust IgG antibody responses but not long-lived memory cells (25).
Although iNKT cells express semi-invariant TCRs, they can be divided into distinct populations based on CD4 and CD8 expression. Humans have varying ratios of CD4+CD8− (CD4+), CD4−CD8α−β− (double-negative or DN)5 and CD4−CD8α+β− (CD8α+) iNKT cells (11, 13, 26). CD4+ iNKT cells release the most Th2 cytokines and CD8α+ and DN iNKT cells predominantly exhibit Th1 phenotypes and cytotoxic activity (11, 13, 27). To date, 2 studies (28, 29) have examined the relative contributions of human iNKT cells subsets to B cell help and found that both CD4+ and CD4− iNKT cells similarly induced B cell proliferation, but CD4+ iNKT cells induced higher levels of antibody production.
In addition to their roles in antibody production, B cells are potent APCs that can prime CD4+ T cells without the participation of DCs or macrophages (30). Similar to DC, B cells can produce both Th1- and Th2-type cytokines and can be polarized towards one or the other subset subsequent to interaction with CD4+ Th1 or Th2 cells (31). The unique abilities of iNKT cells to selectively secrete Th1, Th2, Th17 or regulatory T cell cytokines (10–13) and to induce DC maturation (7, 8, 32) led us to hypothesise that iNKT cells may exert stimulatory and/or regulatory control over antigen presentation and T cell activation by B cells. Here we have examined the outcomes of culturing human peripheral B cells with expanded autologous iNKT cells or sorted CD4+, CD8α+ and DN iNKT cell subsets in vitro, in the absence or presence of α-GC. We show that the iNKT cell subsets differentially induce phenotypic differentiation, antibody secretion and T cell stimulation by B cells. We also show that CD4+ iNKT cells promote the development of cells with phenotypes of regulatory B (Breg)5 cells and the production of IL-10 by some B cells. Thus, CD4+, CD8α+ and DN iNKT cells can differentially promote T cell or antibody responses via their interactions with B cells. This observation has implications for iNKT cell-based therapies for cancer and autoimmune disease, which may require the selective targeting of functionally-distinct subsets of iNKT cells.
Materials and Methods
Antibodies and flow cytometry
Fluorochrome-conjugated mAbs specific for human CD1d, CD3, CD4, CD5, CD8α, CD19, CD20, CD22, CD24, CD27, CD38, CD40, CD58, CD69, CD80, CD83, CD86, CD95, CD107a, HLA-DR, IFN-γ, IL-4, IL-10, IL-13, IL-21, the TCR Vα24 and Vβ11 chains, the CDR3 of the iNKT cell TCR (6B11), CXCR5, PD-1 and isotype control mAbs were purchased from Immunotools (Friesoythe, Germany), eBioscience (Hatfield, UK) and BD Biosciences (Oxford, UK). 105 cells were labelled with mAbs and analysed using a CyAN ADP flow cytometer (Beckman Coulter, High Wycombe, UK). Data were analysed with the Summit v4.3 software (Dako, Colorado, USA) and FlowJo v7.6 (Treestar Inc, New Jersey, USA). In co-cultures of expanded iNKT cells and B cells, iNKT cells were analysed by gating on the CD3+6B11+ lymphocytes and on their CD4+, CD8+ and DN subsets. Follicular helper T (TFH) cells were defined as CXCR5+PD-1+ or IL-21+. B cells were analysed by gating on CD19+ lymphocytes and B cell subsets were defined as naïve (CD27−IgD+), unswitched memory (CD27+IgD+), switched memory (CD27+IgD−), CD27− memory (CD27−IgD−) B cells and two putative Breg cell subsets (CD1dhiCD5+ and CD24hiCD38hi). Single stained controls were used to set compensation parameters and fluorescence-minus-one (FMO)5 and isotype-matched antibody controls were used to set analysis gates.
Isolation of human B cells
PBMC were isolated from blood samples obtained from healthy donors or from buffy coat packs (kindly provided by the Irish Blood Transfusion Service) by density gradient centrifugation over Lymphoprep (Nycomed Pharma AS, Oslo, Norway). B cells were purified by magnetic bead sorting using CD19 Microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany), and purity of B cells was determined to be >99% by flow cytometric analysis of CD20 expression. Enriched B cells were cryopreserved or maintained in iNKT cell medium, which consisted of RPMI 1640 containing 0.05 mM L-glutamine, 10% v/v HyClone FBS (Thermo-Scientific, Logan, UT), 0.02M HEPES buffer, 100 U/ml penicillin and 100 μg/ml streptomycin, 2.5 μg/ml amphotericin B Fungizone, 1X MEM non-essential amino acids and 0.05 mM β-mercaptoethanol (Gibco BRL, Paisley, UK).
Generation of monocyte-derived DC
Monocytes were enriched to >90% purity from PBMC by positive selection using CD14 Microbeads (Miltenyi Biotec). The monocytes were allowed to differentiate into immature DC (iDC)5 by culturing them for 6 days in the presence of GM-CSF and IL-4 as described previously (32). Flow cytometry was used to verify that differentiation into iDC had taken place and cells expressed HLA-DR and CD11c but not CD14.
Generation of iNKT cell lines
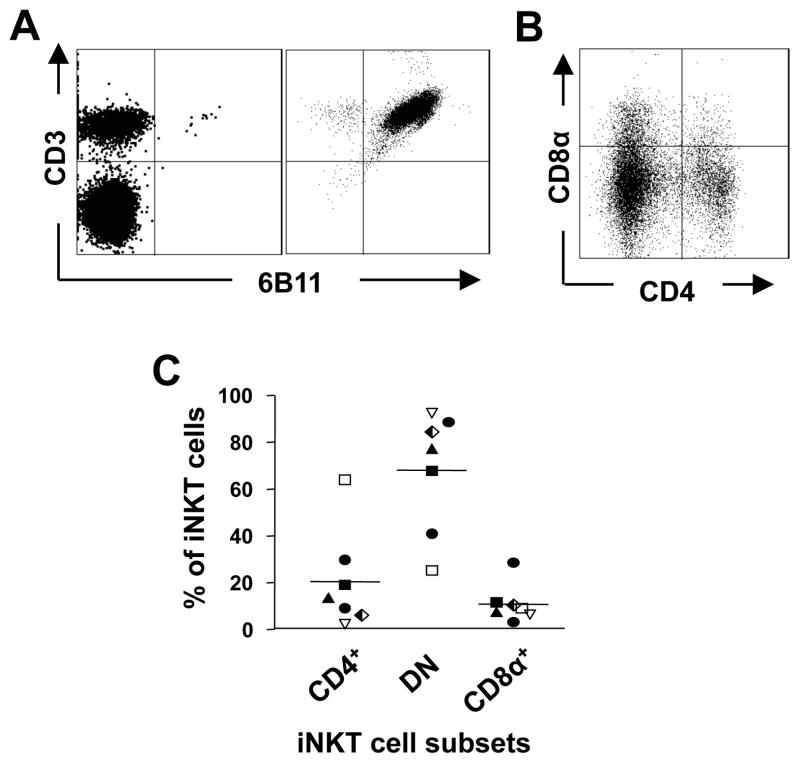
iNKT cells were enriched from PBMC by staining with a PE-conjugated anti-iNKT cell mAb (clone 6B11) followed by positive selection of the PE-positive cells by magnetic bead separation (Miltenyi Biotec). In later experiments anti-iNKT cell Microbeads were used. Enriched iNKT cells were then purified to >99% purity by flow cytometric sorting of CD3+Vα24+Vβ11+ cells using a MoFlo™ XDP Cell Sorter (Beckman Coulter). Sorted iNKT cells were cultured in iNKT cell medium and expanded by one of 2 methods, described previously (13, 32). In the first method, iNKT cells were subjected to a single stimulation with plate-bound anti-CD3 (HIT3A) mAb (BD Biosciences) in the absence of irradiated feeder cells and cultured in the presence of recombinant IL-2. In the second method, iNKT cells were stimulated with PHA in the presence of irradiated allogeneic PBMC and IL-2. Purity and phenotype of iNKT cell lines were assessed by flow cytometry after staining the cells with mAbs specific for CD3, 6B11, CD4 and CD8. Both methods resulted in 750–1,000-fold enrichment of iNKT cells and yielded cell lines of which >98% displayed 6B11+CD3+ phenotypes (Fig. 1A). iNKT cell lines were phenotyped for expression of CD4 and CD8α (Fig. 1B) and means of 20±21%, 68 ± 26% and11 ± 8% of 6B11+ cells were CD4+, DN and CD8α+, respectively (Fig. 1C). Expanded iNKT cell lines were further sorted into CD4+, CD8+ and DN cells by MoFlo™ cell sorting.
Figure 1. Expansion of iNKT cell subsets.
A, Flow cytometric analysis of CD3 and the Vα24Jα18 TCR (6B11) expression by freshly-isolated PBMC and a 6-week-old iNKT cell line. Plots are representative of iNKT lines generated from 10 healthy donors. B, Flow cytometric analysis of CD4 and CD8 expression by expanded iNKT cells, after electronically gating on CD3+6B11+ cells. C, Mean proportions of CD4+, double negative (DN) and CD8+ iNKT cell subsets found in 7 iNKT cell lines.
Co-culture of B cells with iNKT cells
B cells were co-cultured with iNKT or as controls non-iNKT cells (total PBMC expanded with anti-CD3 mAb or PHA and IL-2 as done with iNKT cells) for 3 or 10 days at 1:1 ratios in 96-well round-bottom plates (Corning Life Sciences, MA, USA) at cell densities of 106 cells/ml. The following stimulators or blockers were added: 100 ng/ml of α-GC (Funakoshi, Tokyo, Japan), 10 ng/ml PMA and 1 μg/ml of ionomycin (both from Sigma); 10 μg/ml each of anti-CD1d, anti-CD40, anti-CD154, anti-IL-4 and anti-IL-13 mAb. Before adding to cultures, α-GC was subjected to heating, sonication and vortexing as described previously (13). Supernatants were harvested and frozen at -20°C until they were analysed for cytokine and Ig production. Cells were recovered for phenotypic analysis by flow cytometry or for use as stimulators of T cells.
Analysis of cytokine and antibody secretion
Cytometric bead array (CBA)5 kits were used to quantify the levels of cytokines and Igs in supernatants from the B-iNKT cell co-cultures, according to the manufacturer’s instructions (BD Biosciences, UK). The cytokines assayed for were IFN-γ, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70 and IL-13. The immunoglobulins assayed for were IgA, IgM, total IgG, IgG1, IgG2 and IgE. Flow cytometric data was generated using a CyAN ADP flow cytometer and geometric means of the individual bead populations were analysed using Summit v4.3 software. GraphPad Prism v5.0 (GraphPad Software Inc, La Jolla, CA, USA) was used to draw standard curves and obtain sample concentration values.
Analysis of intracellular cytokine production by iNKT cell and B cell subsets
Total B cells were co-cultured for 3 days in medium alone or with equal numbers of sorted CD4+ iNKT cells in the absence or presence of α-GC as described above. Monensin (0.05 mM, Sigma-Aldrich) was added for the last 4 hours to promote intracellular accumulation of cytokines. Cells were then washed and stained for cell surface expression of 6B11, CD19, CD1d, CD5, CD24 and CD38 and intracellular expression of IFN-γ, IL-10, IL-4 and IL-21 using fluorochrome-conjugated mAb obtained from BD Biosciences or eBioscience and analysed by flow cytometry (13).
Cytotoxicity assays
Cytolytic degranulation by iNKT cells in response to B cells in the absence and presence of α-GC was examined by analysis of cell-surface CD107a expression. iNKT cells and B cells were co-cultured for 4 hours at 1:1 ratios in the presence of anti-CD107a PECy7 mAb. Monensin (2 μM) was added after 1 hour to prevent proteolysis of the mAb conjugate upon reinternalization of CD107a. Frequencies of CD107a expression by iNKT subsets were determined by flow cytometry after electronically gating on CD4+, DN and CD8α+ subsets.
Analysis of the capacity of B cells to drive T cell proliferation
B cells were co-cultured with equal numbers of autologous iNKT cells at densities of 106 cells of each type per ml in the presence or absence of 100 ng/ml of α-GC for 3 days. B cells and iNKT cells were also cultured separately in medium alone as negative controls. The co-cultured cells were harvested, washed and examined for expression of CD40, CD69, CD80, CD83, CD86 and HLA-DR by flow cytometry or used as stimulators for autologous or allogeneic conventional T cells that were enriched by magnetic selection using CD3 Microbeads (Miltenyi Biotec). The T cells were labelled using the CellTrace™ Violet Cell Proliferation Kit following manufacturer’s instructions (Invitrogen, Paisley, UK) and cultured with the B/iNKT cells at ratios of 3:1 with the following stimuli: medium only (negative control), 10 μg/ml purified protein derivative of tuberculin (PPD; Statens Serum Institut, Copenhagen, Denmark)5, 1 μg/ml Staphylococcal enterotoxin B (SEB; Sigma-Aldrich)5 or 5 μg/ml PHA (Sigma-Aldrich). Proliferation of the T cells was assayed by flow cytometric examination of dilution of the CellTrace dye after 6 days in culture. Acquired data was analysed using FlowJo v7.6.
Statistical analyses
Statistical analysis was performed using GraphPad Prism v5.0. For comparison between 2 groups, the Mann-Whitney U test was used to compare unpaired data and the Wilcoxon matched pairs test was used to compare paired data. For comparison between 3 or more groups, the Kruskal-Wallis test was used to compare unpaired data and the Friedman’s test was used to compare paired data. Dunn’s multiple comparison tests were performed post-hoc to compare individual groups within an experiment. Two-way ANOVA with post-hoc Bonferroni’s test was used to compare the effect of treatments.
Results
CD1d is uniformly expressed across all B cell subsets
PBMC from 7 healthy donors were stained with mAbs specific for CD19 and CD1d and either CD5, CD22, CD38 or CD27, which detect B-1, mature, plasma and memory B cells, respectively. CD1d expression by each B cell subset was determined by comparing the intensity of staining with anti-CD1d mAb with that of the corresponding FMO control, in which the anti-CD1d mAb was omitted (Fig. 2A). Up to 30% of circulating B cells expressed phenotypes associated with plasma cells, while 19–44% were B-1 cells and 12–31% were memory cells (Fig. 2B). CD1d was expressed at the cell surface of similar proportions (48–92%) of each B cell subset. Memory B cells displayed the highest proportion of CD1d expression (Fig. 2C), while the mean fluorescence intensity (MFI)5 of CD1d expression was also highest on this B cell subset. (Fig. 2D). One-way ANOVA showed no significant difference between the mean proportions of or MFI of CD1d expression on each B cell subset. Whereas CD1d expression by B cells is reported to be downregulated by activation (33), we found that co-culturing with iNKT cells did not affect the level of CD1d expression (Fig. 2E).
Figure 2. CD1d is uniformly expressed across all human B cell subsets.
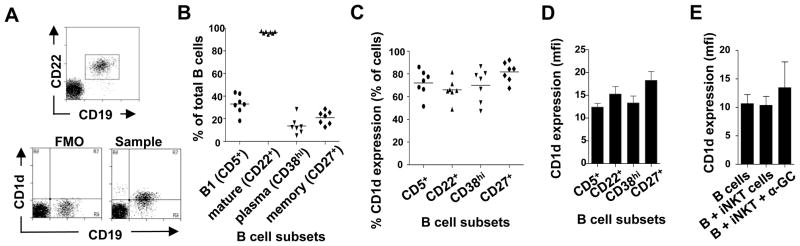
A, Representative flow cyometry dot plot showing the identification of B cell subsets and the gating strategy for measuring CD1d expression by B cell subsets (top). The bottom left plot shows the FMO control lacking the anti-CD1d mAb, while the bottom right plot shows CD1d expression. B, Proportions of B cell subsets in peripheral blood of 7 donors. C, Proportions of B cell subsets that express cell surface CD1d. Horizontal lines represent means from seven donors. D, Average (± SEM) mean fluorescence intensities (MFI) of CD1d expression by B cell subsets. One way ANOVA showed that there were no significant differences in CD1d expression between the subsets. E, Average (± SEM) MFI of CD1d expression by total B cells cultured for 3 days in the absence and presence of iNKT cells and α-GC.
CD4+, DN and CD8α+ iNKT cells can induce secretion of IgG, IgA and IgM, but not IgE, by B cells
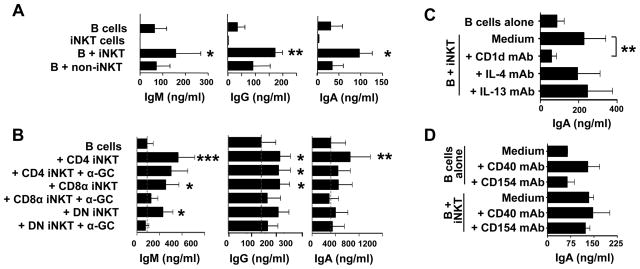
iNKT cells can provide help to B cells for the production and secretion of antibodies in vivo (18–24). We investigated whether sorted subsets of CD4+, DN and CD8α+ iNKT cells differed in their capacity to induce antibody production. Initially, B cells were cultured with total iNKT cells or non-iNKT cells in the absence of added antigen and cell supernatants were removed after 3 days (data not shown) or 10 days (Fig. 3A) and assayed for antibody production by multiplex CBA analysis. Relative to B cells cultured alone, there was increased production of IgA and IgM (p<0.05) after 3 days of culture with iNKT cells and of total IgG (p<0.01), IgM and IgA (p<0.05) after 10 days of B cell co-culture with iNKT (Fig. 3A). In contrast, non-iNKT cells did not induce the release of these antibodies by the same B cells. No IgE was detected in any of the stimulations or co-cultures (data not shown). When sorted subsets of CD4+, DN and CD8+ iNKT cells were cultured for 10 days with B cells, all three subsets induced IgM, IgA and IgG production (Fig. 3B). Surprisingly, the addition of α-GC to the co-cultures did not result in enhanced antibody production. The activation of B cells in the absence of α-GC may thus be due to the presence of a self-glycolipid presented by CD1d on the B cell.
Figure 3. CD4+, CD8+ and DN iNKT cells induce secretion of IgG, IgA and IgM, but not IgE, by B cells.
A, Levels of IgM, IgG and IgA in supernatants from 10-day cultures of B cells, iNKT cells and co-cultures of B cells with iNKT cells or non-iNKT cells (PBMC expanded by anti-CD3 mAb and IL-2 stimulation). IgE was also assayed for and not detected in any cultures. Bars represent means ± SEM from 3 independent experiments. B, Levels of IgM, IgG and IgA released by B cells cultured for 10 days in medium alone or with CD4+, CD8+ or DN iNKT cells, in the absence or presence of 100 ng/ml α-GC. Results are means ± SEM from 7 experiments. C and D, Levels of IgA in supernatants of B cells cultured for 10 days in medium alone or with iNKT cells in the absence or presence of blocking mAbs specific for CD1d, IL-4 and IL-13 (C) and CD40 and CD154 (D). Results are means ± SEM from 3 and 6 donors for C and D, respectively. *p<0.05; **p<0.01 and ***p<0.001 compared to the levels released by B cells cultured in medium alone except where indicated by bars.
To investigate the requirements for cell-cell contact and for CD1d and cytokines in iNKT cell-mediated B cell help for antibody production, B cells were cultured alone or with equal numbers of total iNKT cells for 10 days together or separated using transwell plates and in the absence or presence of blocking antibodies against CD1d, IL-4, IL-13, CD40 or CD154. Supernatants from the co-cultures were removed and assayed for IgG, IgM and IgA release. When B cells and iNKT cells were separated in transwell plates or when blocking antibodies against CD1d were added to the iNKT–B cell co-cultures, antibody secretion was inhibited (see Fig. 3C for IgA). When anti-IL-4, anti-IL-13 (Fig. 3C), anti-CD40 or anti-CD154 mAbs (Fig. 3D) were added to the cultures there was no inhibition of IgG, IgM or IgA release and the anti-CD40 mAb may have a weak agonistic effect on B cell activation. Therefore, all 3 subsets of human iNKT cells can provide B cell help for antibody production by a mechanism that requires cell contact and CD1d but not α-GC, and does not appear to require CD40-CD154 interactions or Th2 cytokine secretion.
CD4+ iNKT cells induce the expansion of unswitched memory and CD1dhiCD5+ B cells
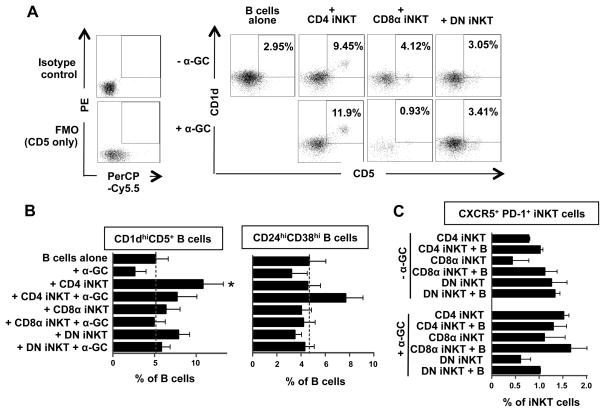
Total B cells were cultured for 3 or 10 days in medium alone or with equal numbers of expanded total, CD4+, CD8+ or DN iNKT cells or non-iNKT cells (PBMC expanded by anti-CD3 mAb and IL-2). Changes in the percentages of naïve (CD27−IgD+), unswitched memory (CD27+IgD+), switched memory (CD27+IgD−) and CD27− memory (CD27−IgD−) B cells and two putative Breg cell subsets (CD1dhiCD5+ and CD24hiCD38hi) (34, 35) were analysed by flow cytometry. The expression of the TFH (CXCR5+PD-1+) phenotype (36, 37) by the iNKT cells was also examined. Total and CD4+ iNKT cells, but not CD8+ nor DN iNKT cells, in the presence of α-GC induced modest expansions of unswitched memory B cells (28% to 46%) after 10 days with concomitant reductions in naive B cells (35% to 22%) by a mechanism that required cell-cell contact (data not shown). However, the numbers of switched and CD27− memory B cells were unaffected. Sorted CD4+ iNKT cells also induced significant expansions of CD1dhiCD5+ B cells (Fig. 4A and B). In contrast, CD8+ and DN iNKT cells induced upregulation of CD5 but not CD1d on B cells. Induction of CD1dhiCD5+ B cells by CD4+ iNKT cells required cell-cell contact but not prior activation of the iNKT cells with α-GC. CD4+ iNKT cells in the presence of α-GC also induced a moderate expansion of CD24hiCD38hi B cells (Fig. 4B). These results provide evidence that resting CD4+ iNKT cells may induce the differentiation of Breg cells in vitro.
Figure 4. CD4+ iNKT cells induce the expansion of CD1dhiCD5+ and CD24hiCD38hi B cells.
B cells from 7 donors were cultured for 3 days in medium alone or with equal numbers of expanded CD4+, CD8+ or DN iNKT cells in the absence or presence of α-GC. A, Representative flow cytometry dot plots showing isotype control mAb staining and FMO for CD1d mAb staining (left) and CD1d and CD5 expression (right) by gated CD19+ cells. B, Mean (± SEM) percentages of B cells that expressed CD1dhiCD5+ (left) or CD24hiCD38hi (right) phenotypes. C, Mean (± SEM) percentages of iNKT cells that expressed CXCR5+PD-1+ phenotypes.
Although iNKT cells provided B cell help for antibody secretion (Fig. 3), <2% of CD4+, CD8α+ or DN expressed the the CXCR5+PD-1+ phenotype found on TFH cells and murine iNKT cells (36, 37). The presence of B cells or α-GC did not significantly alter the frequencies of iNKT cells that express TFH phenotypes (Fig. 4C).
B cells present α-GC to CD4+ and DN iNKT cells resulting in the release of low levels of IFN-γ, TNF-α, IL-4, IL-5, IL-10 and IL-13
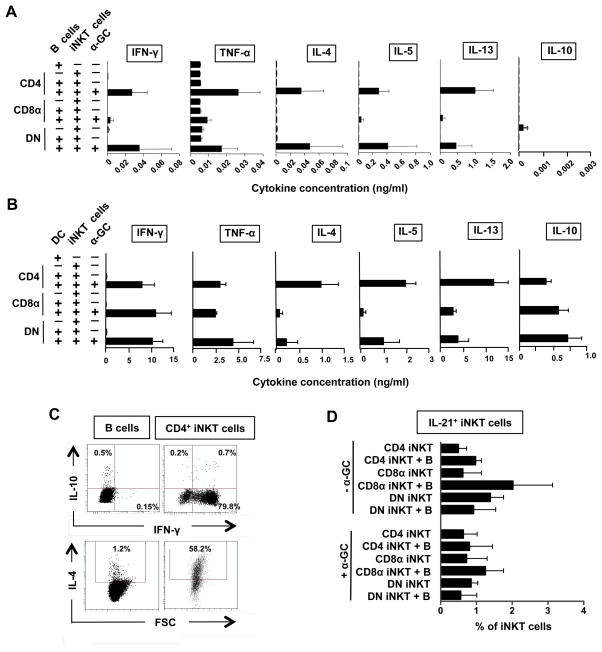
The ability of B cells to present α-GC to iNKT cells resulting in cytokine release was investigated by co-culturing B cells alone or with autologous sorted CD4+, DN or CD8+ iNKT cells in the absence or presence of α-GC. Cell supernatants were removed after 3 days and assayed for cytokine levels by multiplex CBA analysis. We found that co-culturing B cells with any of the iNKT cell subsets in the absence of α-GC did not lead to significant increases in IFN-γ, TNF-α, IL-2, IL-4, IL-5, IL-10 or IL-13 release compared to B cells, iNKT cells or non-iNKT cells cultured alone. When α-GC was present, low levels of IFN-γ, TNF-α, IL-4, IL-5 and IL-13, but not IL-2 nor IL-10, were released by the co-cultures of B cells with CD4+ or DN iNKT cells (Fig. 5A). Although CD4+ iNKT cells induced the expansion of cells with phenotypes associated with Breg cells (Fig. 4), no IL-10 was detected in the supernatents of these co-cultures. When monocyte-derived DC were used as APCs for α-GC, all subsets of iNKT cells released the above cytokines, including IL-10, with 100–1,000-fold more IFN-γ and TNF-α and 10–100-fold more IL-4, IL-5 and IL-13 released compared to when B cells were used as APCs (Fig. 5B). It is unlikely that the low amounts of cytokines produced by co-cultures of B cells and iNKT cells are due to contaminating monocytes or DC, because the B cells were enriched to purities of >99.5%, as shown by flow cytometric analysis of CD19 and CD20 expression. These results suggest that B cells can present α-GC to iNKT cells but are 10–1,000-times less efficient than DC at stimulating cytokine production by the cells.
Figure 5. Co-cultures of α-GC-pulsed B cells and CD4+ or DN iNKT cells produce low levels of IFN-γ, TNF-α, IL-4, IL-5, IL-10 and IL-13.
A, Cytokine levels released by B cells, CD4+, CD8+ and DN iNKT cells and B cells co-cultured for 3 days with autologous CD4+, CD8+ and DN iNKT cells in the absence or presence of α-GC. B, Cytokine levels released by dendritic cells (DC), iNKT cell subsets and 3-day co-cultures of DC and iNKT cells. C, Flow cytometry dot plots showing intracellular expression of IFN-γ and IL-10 (top) and IL-4 (bottom) by gated B cells (left) and CD4+ iNKT cells (right) co-cultured for 3 days in the presence of α-GC. D, Mean (± SEM) percentages of CD4+, CD8+ and DN iNKT cells that produced IL-21 after 3 days of culture with B cells in the absence and presence of α-GC. Bars represent means (± SEM) from 3 (A and B) or 4 (D) independent experiments.
To determine the cellular sources of these cytokines and to further investigate if the CD1dhiCD5+ B cells that were induced by CD4+ iNKT cells have cytokine profiles typical of Breg cells, total B cells were cultured for 3 days in medium alone or with equal numbers of expanded CD4+ iNKT cells in the absence or presence of α-GC. Monensin was added to the cultures for the final 4 hours and the expression of IFN-γ, IL-10 and IL-4 by gated CD19+ (B) cells and 6B11+ (iNKT) cells was examined by flow cytometry. Upon stimulation with B cells pulsed with α-GC, most iNKT cells produced IFN-γ and IL-4 but these cytokines were produced by <2% of B cells (Fig. 5C). Interestingly, when B cells were co-cultured with CD4+ iNKT cells, up to 1% of the B cells and up to 2% of the iNKT cells expressed IL-10. However, due to the small numbers of CD1dhiCD5+ B cells, we were unable to determine with certainty if this putative Breg population was the source of B cell-derived IL-10. Thus, while B cells present α-GC to CD4+ iNKT cells resulting in Th1 and Th2 cytokine production, CD4+ iNKT cells induce the expansion of B cells with phenotypes of Breg cells and can induce IL-10 production by some B cells.
The ability of iNKT cell subsets to produce the TFH cell cytokine IL-21 was also tested by intracellular staining for IL-21 in iNKT cell subsets after exposure for 3 days to B cells in the presence and absence of α-GC. Figure 5D shows that <2% of untreated CD4+, CD8α+ and DN iNKT cells produced IL-21 and these frequencies were not changed by the addition of B cells or α-GC. Thus, although iNKT cells can provide B cell help for antibody production, <2% express classical TFH cell phenotypes (Fig 4C) or cytokine profiles (Fig. 5D).
DN iNKT cells degranulate in the presence of B cells
To determine if subsets of iNKT cells can potentially kill autologous B cells, total iNKT cells were cultured with equal numbers of sorted B cells and cytolytic degranulation by iNKT cell subsets was measured by CD107a externalisation. Figure 6 shows that CD4+ and CD8+ iNKT cells did not degranulate in response to B cells whether or not α-GC was present, but these cells expressed CD107a at the cell surface when stimulated with PMA and ionomycin. Surprisingly, DN iNKT cells displayed significant CD107a expression in response to B cells, whether or not α-GC was present. Thus, it is likely that DN iNKT cells can kill autologous B cells.
Figure 6. DN iNKT cells can kill autologous B cells.
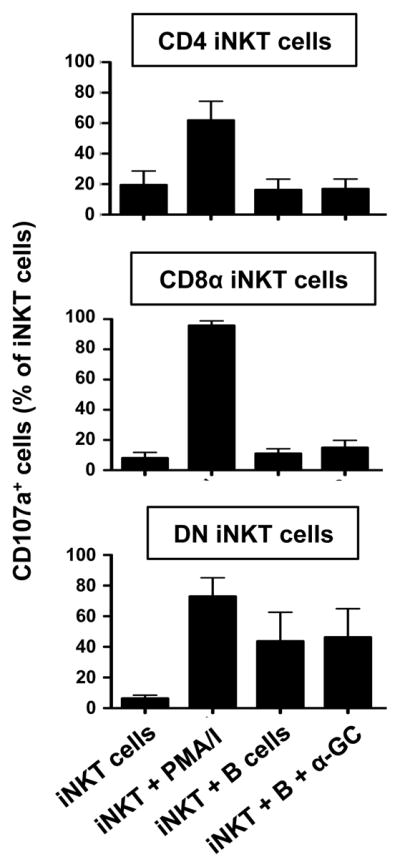
A, Mean (± SEM) percentages of CD4+ (top), CD8α+ (middle) and DN (bottom) iNKT cells from 4 donors that express cell surface CD107a after culture in medium alone or with autologous B cells in the absence and presence PMA and ionomycin (PMA/I) and α-GC.
iNKT cells induce expression of activation and costimulatory markers by B cells
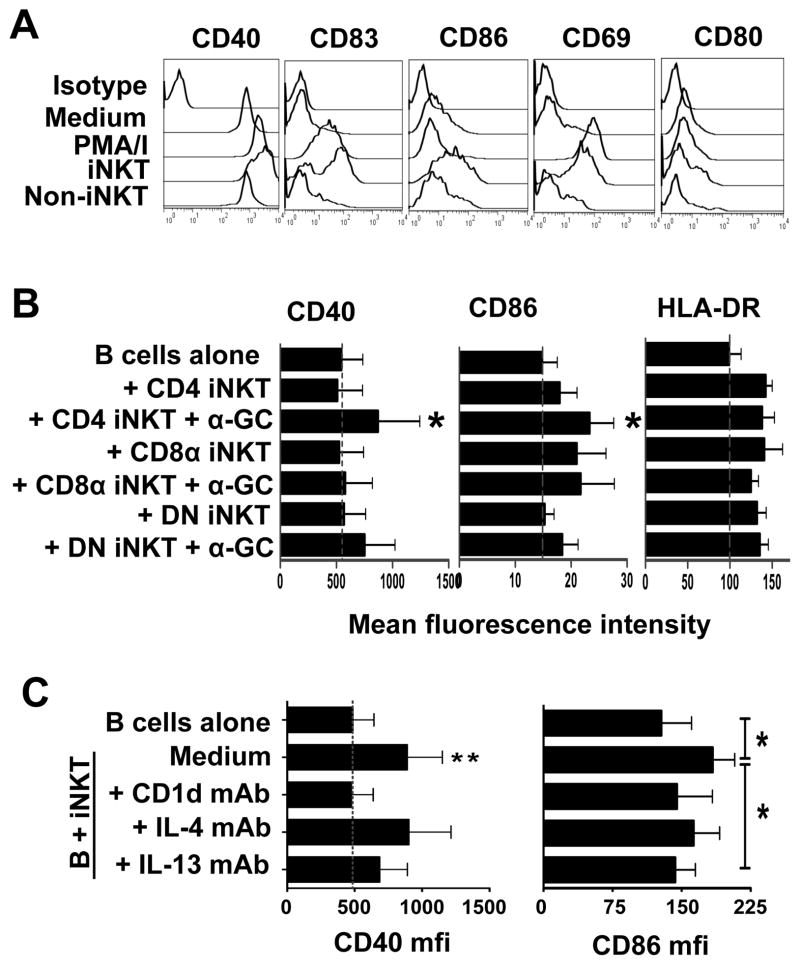
To determine if iNKT cells can induce maturation of B cells into cells with APC phenotypes, total B cells were cultured for 3 or 10 days in medium alone, with PMA and ionomycin or with equal numbers of expanded iNKT cells or non-iNKT cells. Changes in cell-surface expression of CD40, CD83, CD86, CD69, CD80 and HLA-DR by total B cells and naïve, unswitched memory, switched memory, CD27− memory B cells, and CD1dhiCD5+ and CD24hiCD38hi B cells were analysed by flow cytometry. We observed increased expression of CD40 (p<0.05), CD95 (p<0.01), CD86 (p<0.05) and CD83 (not significant), but not CD80 nor HLA-DR on total B cells after 3 days of co-incubation with total iNKT cells (Fig. 7A). After 10 days of co-incubation with iNKT cells, these markers were not expressed at significantly higher levels than on B cells cultured alone. When non-iNKT cells were substituted for iNKT cells, the levels of the above-mentioned markers were similar to those on B cells cultured alone (Fig. 7A). CD40 and CD86 were upregulated on naïve, unswitched memory, switched memory, CD27− memory B cells and the two putative Breg cell subsets (CD1dhiCD5+ and CD24hiCD38hi) (data not shown).
Figure 7. NKT cells induce upregulation of activation and costimulatory molecules by B cells.
B cells were cultured in medium alone, with PMA and ionomycin or with equal numbers of expanded iNKT cells or non-iNKT cells for 3 to 10 days. Changes in cell-surface expression of CD40, CD83, CD86, CD69 and CD80 by gated B cells (CD19+) were analysed by flow cytometry. A, Offset histogram overlays showing mean fluorescence intensity (MFI) of staining of gated B cells with mAbs specific for the indicated markers or isotype control mAb (top histograms) after culturing cultured (from 2nd from top to bottom) in medium alone, or with PMA and ionomycin, iNKT cells or non-iNKT cells. B, Bar charts showing average (± SEM) MFI of staining for CD40 and CD86 expression by B cells from 7 donors after incubation for 3 days with medium alone or with autologous CD4+, CD8+ or DN iNKT cells, in the absence or presence of 100 ng/ml α-GC. *p<0.05 compared to MFI on B cells cultured in medium alone. C, MFI of CD40 (left) and CD86 (right) expression by B cells from 6 donors cultured for 3 days in medium alone or with iNKT cell in the absence or presence of blocking mAbs specific for CD1d, IL-4 and IL-13.
When sorted CD4+, DN and CD8+ iNKT cells were cultured with the B cells, only the CD4+ subset was found to significantly induce CD40 and CD86 expression and this occurred only when α-GC was present. CD8+ and DN iNKT cell subsets also weakly induced CD86 expression, but while CD4+ and DN iNKT cells required the presence of α-GC to do so, CD8+ iNKT cells did not (Fig. 7B). Therefore, iNKT cells induce the expression of activation and costimulatory molecules by B cells, but the CD4+, DN and CD8+ iNKT cell subsets differ in their abilities to do so and in their requirements for ligand activation.
The requirements for cell-cell contact, CD1d and cytokines in iNKT cell-mediated upregulation of CD40, CD83 and CD86 expression by B cells were tested using transwell plates and in the absence or presence of blocking mAbs against CD1d, IL-4 or IL-13. When B cells and iNKT cells were separated in transwell plates or when anti-CD1d blocking antibodies were added to the iNKT–B cell co-cultures, the upregulation of all 3 markers was reduced (Fig. 7C). Blocking IL-13 resulted in moderate inhibition of CD86 expression, only, but blocking IL-4 had no effect on the expression of any of these markers.
iNKT cells prevent the induction of T cell proliferation by B cells
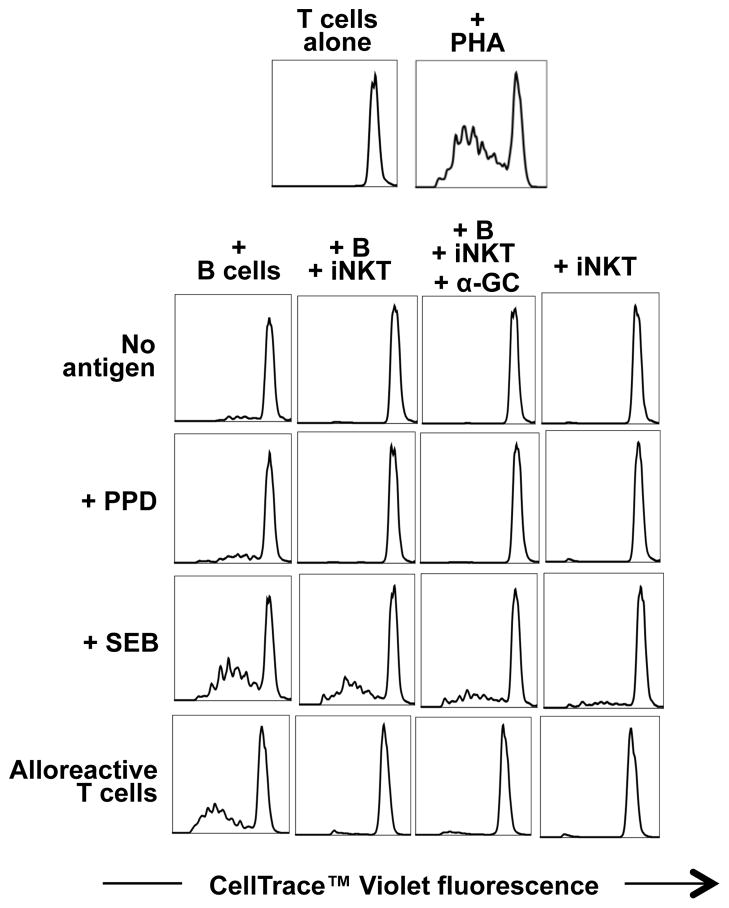
The effect of total iNKT cells on the ability of B cells to promote proliferation of autologous and allogeneic T cells was investigated using the CellTrace™ Violet Cell Proliferation assay. B cells that were cultured in medium alone were able to induce proliferation of autologous T cells in the absence of antigen, and to a greater degree in the presence of PPD, SEB or PHA (Fig. 8). However, B cells that were pre-cultured with iNKT cells were greatly reduced in their ability to induce T cell proliferation. Non-specific and PPD-stimulated T cell proliferation was abrogated by the presence of iNKT cells, whereas SEB-specific T cell proliferation was not. Allogeneic T cell proliferation was also abrogated by iNKT cells. This inhibition of B cell-stimulated T cell proliferation occurred whether or not α-GC was present in the iNKT-B cell co-cultures.
Figure 8. iNKT cells inhibit the induction of T cell proliferation by B cells.
B cells were co-cultured with equal numbers of autologous iNKT cells in the absence or presence of 100 ng/ml of α-GC for 3 days. B cells and iNKT cells were also cultured separately in medium alone as negative controls. The co-cultured cells were harvested, washed and used as stimulators of sorted autologous or allogeneic conventional CD3+ T cells, that were labelled using the CellTrace™ Violet. The T cells were cultured with the B/iNKT cells at a ratio of 3:1 with medium only, 10 μg/ml PPD, 1 μg/ml SEB or 5 μg/ml PHA. Proliferation of the T cells was assayed by flow cytometric examination of dilution of the CellTrace dye after 6 days in culture. Plots are representative of 3 independent experiments.
Discussion
iNKT cells provide cognate and non-cognate help for lipid-reactive and protein-reactive B cells. They are required for the generation of protective antibody responses against some murine pathogens (14–16) and they dramatically augment antibody responses to co-administered antigens in vivo (18–22). These findings have led to interest in iNKT cells and their ligands as adjuvants for the development of vaccines and immunotherapies (38, 39). However, despite their name, iNKT cells are heterogeneous and multifunctional in nature. Human CD4+, CD8α + and DN iNKT cells can release Th1 (IFN-γ and TNF-α) and Th2 (IL-4, IL-5 and IL-13) cytokines when activated with α-GC presented by CD1d+ cells. The relative amounts of the cytokines follow a striking CD8α>DN>CD4 pattern for Th1 and a CD4>DN>CD8α pattern for Th2, whereas CD4+ iNKT cells, only, produce IL-9 and IL-10 (11, 13, 27). These variable helper T cell cytokine profiles prompted us to compare the relative abilities of CD4+, CD8α + and DN iNKT cell lines to provide help for B cell differentiation and antibody production in vitro. We also have investigated the influence iNKT cell subsets have on cytokine production, antigen presentation and T cell activation by B cells.
Two previous in vitro co-culture studies of B cells with fresh (29) and expanded (28) human iNKT cells and one on murine iNKT cells (40) have shown that iNKT cells can induce antibody production by human B cells in vitro. We found that all subsets of expanded iNKT cell lines induced the secretion of IgG (both IgG1 and IgG2), IgA and IgM by autologous B cells. Also observed by Galli and co-workers (28), we found that IgE was not induced by any of the 3 iNKT cell subsets. This may reflect a need for conventional CD4+ T cells, which are required for iNKT cell-mediated IgE production by murine B cells (41). iNKT cell-mediated help for B cells required cell-cell contact and CD1d, but did not appear to involve CD40-CD154 interaction and it was not inhibited by neutralising antibodies against IL-4 or IL-13. These findings compare and contrast with those from in vivo studies in mice immunised with α-GC and protein antigen, which showed that iNKT cell-mediated B cell help required B cell expression of CD1d (21, 24, 42) and CD40 (24, 37) but did not require IL-4 (24). iNKT cell-mediated enhancement of antibody responses in vivo also required DC but did not require the expression of CD1d by DC (43). Another difference between these in vivo models of α-GC-mediated B cell help and our system, is that α-GC was not required for iNKT cell-mediated B cell help in vitro. Galli and coworkers (28) also found that human CD4+ iNKT cells, in the absence or presence of antigen, induced IgM production by B cells in vitro. Our findings confirm that the direct interaction between iNKT cells and B cells is sufficient to stimulate antibody production in vitro and that exogenous antigen is not required.
The ability of iNKT cells to provide B cell help for antibody production prompted us to examine whether these iNKT cells express CXCR5+PD-1+ phenotypes of TFH cells or produce IL-21, attributes that are associated with murine iNKT cells stimulated in vivo with α-GC (25, 36, 37). We found that up to 2% of expanded iNKT cells expressed TFH phenotypes or released IL-21 and this frequency was not increased by co-culturing them with B cells in the absence or presence of α-GC. Similar proportions of iNKT cells displaying TFH phenotypes were found in mice immunised with antigen and α-GC (36, 37).
The ability of iNKT cells to induce antibody secretion by B cells highlights them as potential targets for therapeutic boosting of antibody responses in vaccines and infections or inhibition of pathogenic antibody production in autoimmune and allergic disease. Devera and coworkers (42) exploited iNKT help to B cells to enhance and sustain neutralising antibody responses towards the Bacillus anthracis lethal toxin, which led to sustained survival and good health in mice challenged with the toxin. However, because of the multiple effector and immunoregulatory activities of iNKT cells, a complete understanding of the mechanisms underlying the interaction between iNKT cells and B cells is essential to predict or programme the outcomes in vivo.
Activation of human iNKT cells requires the presence of immature but not mature B cells (44). However, we found that CD1d is expressed at comparable levels by mature, plasma, memory and B-1 B cells, suggesting that all B cell subsets can present glycolipids to iNKT cells. CD4+ iNKT cells were found to promote expansions of unswitched (IgD+) memory B cells in a contact-dependent manner, resulting in decreased frequencies of naïve B cells. Despite inducing antibody release by B cells, none of the iNKT cell subsets induced significant expansions of switched (IgD−) memory B cells or the expression of IgM or IgG by any memory B cell subset. This suggests that iNKT cells promote antibody release by class-switched memory B cells rather than inducing class switching. Thus, it appears that iNKT cells may interact differently with naive and memory B cells, promoting maturation of naïve B cells into unswitched memory cells that are not required for iNKT cell activation while promoting maturation of switched memory B cells into antibody-secreting plasma cells. Future experiments involving stimulation of B cells with iNKT cell subsets followed by detection of specific antibody-secreting memory B cells are required to confirm that iNKT cells can restimulate memory B cells.
We investigated if iNKT cells could induce the expansion of putative regulatory B (Breg) cells. The CD1dhiCD5+ B cell phenotype defines a subset of murine B cells that downregulate immune responses via secretion of IL-10 and inhibits the development of autoimmune disease (34, 45, 46). In humans, an IL-10-producing B cell population that inhibits Th1 cell differentiation resides in the CD24hiCD38hi B cell compartment and this subset is impaired in patients with systemic lupus erythematosus (35, 47). The majority of human CD1dhiCD5+ B cells are reported to be contained in the CD24hiCD38hi B cell subset (35), therefore, we investigated both subsets as putative Breg cells. We found that co-culturing B cells with total iNKT cells did not significantly affect CD1d expression. However, CD4+ iNKT cells induced the expansion of a population of CD1dhiCD5+ B cells B cells by a mechanism that required cell-cell contact but not activation of the iNKT cells with α-GC. CD4+ iNKT cells in the presence of α-GC also induced a moderate expansion of CD24hiCD38hi B cells. Although, we did not detect IL-10 in the supernatants of co-cultures of CD4+ iNKT cells with B cells using multiplex CBA analysis, up to 1% of the B cells in these cultures expressed intracellular IL-10. IL-10 production is a hallmark feature of Breg cells (34, 35, 48–50). We were unable to show convincingly that this IL-10 was produced by the CD1dhiCD5+ subset of B cells, however, our data indicate that CD4+ iNKT cells induced IL-10 production by some B cells, suggesting that they promote Breg cell differentiation. We also found that up to 1 ng/ml of IL-10 was released by co-cultures of DC with all subsets of iNKT cells, suggesting that iNKT cells can induce regulatory DC as well as B cells.
DC can present α-GC to iNKT cells resulting in the rapid secretion of Th1 and Th2 cytokines in vivo and in vitro (7, 8, 13, 32, Fig. 5B). However, experiments aimed at demonstrating that B cells can similarly present α-GC and induce cytokine production by iNKT cells have been conflicting. Bialecki and co-workers (51) reported no IFN-γ or IL-4 production by co-cultures of murine iNKT cells and marginal zone B (MZB)4 cells presenting α-GC in vitro, but both cytokines were produced when DC were added. Two other studies demonstrated weak Th2 (IL-4 and IL-13) production by murine iNKT cells after stimulation with α-GC-pulsed total B cells (52) or MZB cells (53) in vitro and in vivo and these cytokine profiles were skewed towards Th1 when DC were present. We found that when α-GC-pulsed human B cells were cultured with CD4+ or DN iNKT cells, but not CD8+ iNKT cells, IFN-γ, TNF-α, IL-4, IL-5 and IL-13 were secreted into the supernatants. Using intracellular cytokine staining and flow cytometry, we showed that iNKT cells are the main source of these cytokines. However, the amounts of cytokines released were 10–1,000-fold lower than those when DC were used as APC for α-GC. Glycolipid presentation by B cells appears to be required for optimal activation of iNKT cells by DC, since removal of MZB cells reduced iNKT cell activation by murine spleen cells pulsed with α-GC in vitro (51) and human PBMC depleted of B cells failed to support iNKT cell expansion and cytokine release in response to α-GC (44). In contrast, Besbradica and co-workers (52) reported that B cells suppressed murine DC-mediated iNKT cell activation in vivo. Collectively, these findings support the view that B cells can present glycolipid antigens to iNKT cells, but rather than being potent stimulators of cytokine secretion, B cells modulate cytokine production by iNKT cells activated by DC. Reciprocally, iNKT cells can induce cytokine production by DC (7, 8, 32) and by small proportions of B cells.
Since CD8+ and DN iNKT cells, and to a lesser degree CD4+ iNKT cells, are potent cytotoxic T cells capable of killing CD1d+ cells presenting α-GC (13), we investigated if these iNKT cell subsets could kill autologous B cells presenting this glycolipid. Interestingly, only the DN subset of iNKT cells degranulated in response to B cells and this occurred whether or not α-GC was present. Thus, while iNKT cells can promote antibody production by B cells, DN iNKT cells may regulate this activity by killing the B cells.
iNKT cells can induce maturation of DC into APC that express costimulatory and adhesion molecules and can prime naïve conventional T cells (7, 8, 32). B cells are also professional APCs (30) and they express CD1d, therefore we investigated the influence that iNKT cells and their subsets have on the APC function of B cells. Extending the findings of Kitamura and co-workers (54), we found that iNKT cells induced the expression of CD40, CD69, CD83 and CD86, but not CD80 nor HLA-DR, on B cells by a mechanism that was dependent on cell-cell contact and CD1d. Of the 3 iNKT cell subsets, CD4+ iNKT cells were the most potent inducers of costimulatory molecule expression by B cells. Blocking IL-13 resulted in moderate inhibition of CD86 expression but blocking IL-4 had no effect on the expression of these markers by B cells. These phenotypic changes suggest that iNKT cells induce maturation of B cells into APCs. We found that B cells presented antigens, superantigens and mitogens to T cells resulting in their proliferation. However, B cells that were pre-cultured with iNKT cells were greatly reduced in their ability to induce T cell proliferation. Non-specific and PPD-stimulated T cell proliferation was abrogated by iNKT cells, whereas SEB-specific T cell proliferation was not. As SEB does not require intracellular processing to be presented on MHC, our results suggest that iNKT cells may inhibit intracellular antigen processing by B cells. Allogeneic T cell proliferation was also abrogated by iNKT cells. This inhibition of B cell-stimulated T cell proliferation occurred whether or not α-GC was present in the iNKT-B cell co-cultures. These data are consistent with a model whereby iNKT induce the maturation of B cells into tolerogenic APC that inhibit conventional T cell activation. Subsets of B cells that are tolerogenic APCs have been described (55, 56) and although not shown here, it is possible that the induction of T cell proliferation by B cells may be inhibited by CD1dhiCD5+ Breg cells or by IL-10 released by other B cells.
CD4+ T cell help for B cells is a key requirement for the generation of antibody-secreting plasma cells and memory B cells. The interaction between the two cells generally takes place in secondary lymphoid organs, involves presentation of processed antigen by the B cell to the T cell, signalling through costimulatory molecules such as CD40, and the production of cytokines (1–3). Our study demonstrates that human B cells can present glycolipid antigen to iNKT cells resulting in low-level Th1 and Th2 cytokine secretion in vitro. Reciprocally, iNKT cells can have diverse effects on B cells, depending on the iNKT cell subset, on whether or not glycolipid antigen is added, and possibly on the differentiation status of the B cell. Firstly, CD4+, CD8+ and DN iNKT cells can all stimulate antibody production by B cells by a mechanism that requires CD1d but not exogenous glycolipid antigen nor CD40-CD154 interaction. Secondly, CD4+ iNKT cells in the presence of glycolipid antigen promote maturation of naïve B cells into memory cells, but they do not appear to promote Ig isotype switching by these cells. Thirdly, CD4+ iNKT cells in the absence of exogenous glycolipid antigen induce the expansions of B cells that exhibit phenotypes of Breg cells and B cells that produce IL-10, whereas DN iNKT cells in the absence of added glycolipid may kill autologous B cells. Finally, iNKT cells (and in particular the CD4+ subset) induce maturation of all B cell subsets into cells with APC phenotypes but these APCs fail to stimulate proliferation of conventional T cells, as seen when untreated B cells are used. Collectively, these results indicate that the presence of exogenous α-GC is a major determinant of the outcome of B-iNKT cell interactions: in the presence of α-GC, B cells promote cytokine secretion by iNKT cells which will boost T cell-mediated immunity, whereas in the absence of α-GC iNKT cell activation by B cells results in antibody-mediated immune responses and suppression of T cell-mediated immunity. The dependence of these functional outcomes on the absence or presence of α-GC raises the question of how other glycolipid antigens will modify B cell responses to iNKT cells and in this regard, several chemical analogues of α-GC that can skew cytokine responses of iNKT cells are being tested as potential immunomodulators for the treatment of disease (32, 57, 58). The diverse outcomes of iNKT-B cell interactions have important implications for therapy for autoimmune disease, where induction of Breg cells or prevention of pathogenic antibody responses may be beneficial (34, 35, 45, 46, 48–50) and for cancer and infectious disease where T cell responses are required (4, 5). The presence of iNKT cell subsets with opposing roles in immune responses might explain why clinical trials involving these cells to date have been unsuccessful (38, 39). Therapeutic manipulation of iNKT cells may necessarily require the sorting of iNKT cells into functionally-distinct subsets and/or selective activation of particular effector functions using customized glycolipid antigens.
Footnotes
This work was supported by grants from the Irish Health Research Board (SGZ and VO’R), Science Foundation Ireland (AEH), the Irish Research Council (YGG) and NIH grant CA 143748 (MAE).
Abbreviations used in this paper: Breg, regulatory B cell; CBA, cytometric bead array; DC, dendritic cell; DN, double negative CD4−CD8−; FMO, fluorescence minus one (flow cytometry control); α-GC, α-galactosylceramide; iDC, immature dendritic cell; iNKT cells, invariant natural killer T cells; MFI, mean fluorescence intensity; MZB, marginal zone B cells; PPD, purified protein derivative of tuberculin; SEB, Staphylococcal enterotoxin B; TFH cell, follicular helper T cell.
References
- 1.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan IC, Toellner KM, Cunningham AF, Serre K, Sze DM, Zúñiga E, Cook MC, Vinuesa CG. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 3.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 4.Brigl M, Brenner M. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 5.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 6.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: Cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–4650. [PubMed] [Google Scholar]
- 7.Kitamura H, Iwakabe K, Yahata T, Nishimura S, Ohta A, Ohmi Y, Sato M, Takeda K, Okumura K, Van Kaer L, Kawano T, Taniguchi M, Nishimura T. The natural killer T (NKT) cell ligand α-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells. J Exp Med. 1999;189:1121–1128. doi: 10.1084/jem.189.7.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- 9.Singh N, Hong S, Scherer DC, Serizawa I, Burdin N, Kronenberg M, Koezuka Y, Van Kaer L. Cutting edge: activation of NK T cells by CD1d and α-galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 10.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–636. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORγt and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Reilly V, Zeng SG, Bricard G, Atzberger A, Hogan AE, Jackson J, Feighery C, Porcelli SA, Doherty DG. Distinct and overlapping effector functions of expanded human CD4+, CD8α+ and CD4-CD8α− invariant natural killer T cells. PLoS One. 2011;6:e28648. doi: 10.1371/journal.pone.0028648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schofield L, McConville M, Hansen D, Campbell A, Fraser-Reid B, Grusby M, Tachado S. CD1d-restricted immunoglobulin G formation to GPI-anchored antigens mediated by NKT cells. Science. 1999;283:225–229. doi: 10.1126/science.283.5399.225. [DOI] [PubMed] [Google Scholar]
- 15.Kobrynski LJ, Sousa AO, Nahmias AJ, Lee FK. Cutting edge: antibody production to pneumococcal polysaccharides requires CD1 molecules and CD8+ T cells. J Immunol. 2005;174:1787–1790. doi: 10.4049/jimmunol.174.4.1787. [DOI] [PubMed] [Google Scholar]
- 16.Belperron A, Dailey C, Bockenstedt L. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174:5681–5686. doi: 10.4049/jimmunol.174.9.5681. [DOI] [PubMed] [Google Scholar]
- 17.Lisbonne M, Diem S, Keller A, Lefort J, Araujo L, Hachem P, Fourneau J, Sidobre S, Kronenberg M, Taniguchi M, Van Endert P, Dy M, Askenase P, Russo M, Vargaftig B, Herbelin A, Leite-de-Moraes M. Cutting Edge: Invariant Va14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2005;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 18.Ko S, Ko H, Chang W, Park S, Kweon M, Kang C. α-galactosylceramide can act as a nasal vaccine adjuvant inducing protective immune responses against viral infection and tumor. J Immunol. 2005;175:3309–3317. doi: 10.4049/jimmunol.175.5.3309. [DOI] [PubMed] [Google Scholar]
- 19.Lang G, Exley M, Lang M. The CD1d-binding glycolipid α-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner. Immunology. 2006;119:116–125. doi: 10.1111/j.1365-2567.2006.02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galli G, Pittoni P, Tonti E, Malzone C, Uematsu Y, Tortoli M, Maione D, Volpini G, Finco O, Nuti S, Tavarini S, Dellabona P, Rappuoli R, Casorati G, Abrignani S. Invariant NKT cells sustain specific B cell responses and memory. Proc Natl Acad Sci USA. 2007;104:3984–3989. doi: 10.1073/pnas.0700191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang G, Devera T, Lang M. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devera T, Shah H, Lang G, Lang M. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38:1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci USA. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leadbetter E, Brigl M, Illarionov P, Cohen N, Luteran M, Pillai S, Besra G, Brenner M. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci USA. 2008;105:8339–8344. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King IL, Fortier A, Tighe M, Dibble J, Watts GF, Veerapen N, Haberman AM, Besra GS, Mohrs M, Brenner MB, Leadbetter EA. Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat Immunol. 2011;13:44–50. doi: 10.1038/ni.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin H, Nieda M, Rozenkov V, Nicol AJ. Analysis of the effect of different NKT cell subpopulations on the activation of CD4 and CD8 T cells, NK cells, and B cells. Exp Hematol. 2006;34:289–295. doi: 10.1016/j.exphem.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human Vα24 natural killer T cells. J Exp Med. 2002;195:637–641. doi: 10.1084/jem.20011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossignol A, Barra A, Herbelin A, Preud’homme JL, Gombert JM. Freshly isolated Vα24+ CD4+ invariant natural killer T cells activated by α-galactosylceramide-pulsed B cells promote both IgG and IgE production. Clin Exp Immunol. 2007;148:555–563. doi: 10.1111/j.1365-2249.2007.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Harris D, Haynes L, Sayles P, Duso D, Eaton S, Lepak N, Johnson L, Swain S, Lund F. Reciprocal regulation of polarised cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 32.Hogan AE, O’Reilly V, Dunne MR, Dere RT, Zeng SG, O’Brien C, Amu S, Fallon PG, Exley MA, O’Farrelly C, Zhu X, Doherty DG. Activation of human invariant natural killer T cells with a thioglycoside analogue of α-galactosylceramide. Clin Immunol. 2011;140:196–207. doi: 10.1016/j.clim.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 33.Allan LL, Stax AM, Zheng DJ, Chung BK, Kozak FK, Tan R, van den Elzen P. CD1d and CD1c expression in human B cells is regulated by activation and retinoic acid receptor signaling. J Immunol. 2011;186:5261–5272. doi: 10.4049/jimmunol.1003615. [DOI] [PubMed] [Google Scholar]
- 34.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Blair PA, Noreña LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Chang PP, Barral P, Fitch J, Pratama A, Ma CS, Kallies A, Hogan JJ, Cerundolo V, Tangye SG, Bittman R, Nutt SL, Brink R, Godfrey DI, Batista FD, Vinuesa CG. Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat Immunol. 2011;13:35–43. doi: 10.1038/ni.2166. [DOI] [PubMed] [Google Scholar]
- 37.Tonti E, Fedeli M, Napolitano A, Iannacone M, von Andrian UH, Guidotti LG, Abrignani S, Casorati G, Dellabona P. Follicular helper NKT cells induce limited B cell responses and germinal center formation in the absence of CD4+ T cell help. J Immunol. 2012;188:3217–3222. doi: 10.4049/jimmunol.1103501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cerundolo V, Silk JD, Masri SH, Salio M. Harnessing invariant NKT cells in vaccination strategies. Nat Rev Immunol. 2009;9:28–38. doi: 10.1038/nri2451. [DOI] [PubMed] [Google Scholar]
- 39.Exley MA, Nakayama T. NKT-cell-based immunotherapies in clinical trials. Clin Immunol. 2011;140:117–118. doi: 10.1016/j.clim.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–165. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto T, Min B, Sugimoto T, Hayashi N, Ishikawa Y, Sasaki Y, Hata H, Takeda K, OkumuraL K, Van Kaer L, Paul WE, Nakanishi K. Nonredundant roles for CD1d-restricted natural killer T cells and conventional CD4+ T cells in the induction of immunoglobulin E antibodies in response to interleukin 18 treatment of mice. J Exp Med. 2003;197:997–1005. doi: 10.1084/jem.20021701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devera T, Aye L, Lang G, Joshi S, Ballard J, Lang M. CD1d-dependent B cell help by NK-like T cells leads to enhanced and sustained production of Bacillus anthracis lethal toxin-neutralising antibodies. Infect Immun. 2010;78:1610–1617. doi: 10.1128/IAI.00002-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joshi SK, Lang GA, Devera TS, Johnson AM, Kovats S, Lang ML. Differential contribution of dendritic cell CD1d to NKT cell-enhanced humoral immunity and CD8+ T cell activation. J Leukoc Biol. 2012;91:783–790. doi: 10.1189/jlb.1111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosma A, Abdel-Gadir A, Isenberg DA, Jury EC, Mauri C. Lipid-antigen presentation by CD1d+ B Cells is essential for the maintenance of invariant natural killer T cells. Immunity. 2012;36:477–490. doi: 10.1016/j.immuni.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang M, Sun L, Wang S, Ko KH, Xu H, Zheng BJ, Cao X, Lu L. Novel function of B cell-activating factor in the induction of IL-10-producing regulatory B cells. J Immunol. 2010;184:3321–3325. doi: 10.4049/jimmunol.0902551. [DOI] [PubMed] [Google Scholar]
- 47.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 48.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 49.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 51.Bialecki E, Paget C, Fontaine J, Capron M, Trottein F, Faveeuw C. Role of marginal zone B lymphocytes in invariant NKT cell activation. J Immunol. 2009;182:6105–6113. doi: 10.4049/jimmunol.0802273. [DOI] [PubMed] [Google Scholar]
- 52.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA, Thomas JW, Unutmaz D, Van Kaer L, Joyce S. Distinct roles of dendritic cells and B cells in Vα14Jα18 natural T cell activation in vivo. J Immunol. 2005;174:4696–4705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 53.Zietara N, Łyszkiewicz M, Krueger A, Weiss S. ICOS-dependent stimulation of NKT cells by marginal zone B cells. Eur J Immunol. 2011;41:3125–3134. doi: 10.1002/eji.201041092. [DOI] [PubMed] [Google Scholar]
- 54.Kitamura H, Ohta A, Sekimoto M, Sato M, Iwakabe K, Nakui M, Yahata T, Meng TH, Koda T, Nishimura S, Kawano T, Taniguchi M, Nishimura T. α-galactosylceramide induces early B cell activation through IL-4 production by NKT cells. Cell Immunol. 2000;199:37–42. doi: 10.1006/cimm.1999.1602. [DOI] [PubMed] [Google Scholar]
- 55.Eynon EE, Parker DC. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuchs EJ, Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 57.Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–534. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- 58.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, Wong CH, Yu AL. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci USA. 2007;104:10299–10304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]