Abstract
Mesenchymal stem cells (MSCs) are useful in tissue repair, but also possess immunomodulatory properties. Murine and uncontrolled human trials suggest efficacy of MSCs in treating lupus. Autologous cells are preferable, however, recent studies suggest that lupus derived MSCs lack efficacy in treating disease. Thus, the optimum derivation of MSCs for use in lupus is unknown. It is also unknown which in vitro assays of MSC function predict in vivo efficacy. The objectives for this study were to provide insight into the optimum source of MSCs and to identify in vitro assays that predict in vivo efficacy. We derived MSCs from four umbilical cords (UC), four healthy bone marrows (HBM) and four lupus bone marrows (LBM). In diseased MRL/lpr mice, MSCs from HBM and UC significantly decreased renal disease, while LBM-MSCs only delayed disease. Current in vitro assays did not differentiate efficacy of the different MSCs. Inhibition of B cell proliferation did differentiate based on efficacy. Our results suggest that autologous MSCs from lupus patients are not effective in treating disease. Furthermore, standard in vitro assays for MSC licensing are not predictive of in vivo efficacy, while inhibiting B cell proliferation appears to differentiate effective from ineffective MSCs.
Keywords: Stem Cells, Autoimmunity, Systemic Lupus Erythematosus, Inflammation
Introduction
Mesenchymal stem cells (MSCs) are multi-potent progenitor cells readily isolated from a variety of tissues including bone marrow, umbilical cords, adipose tissue, and dental pulp. Upon stimulation, MSCs can undergo differentiation into many cell types including, but not limited to, chondrocytes, osteoblasts, and adipocytes. Their differentiation capacity contributes to their well-studied biological niche in wound healing, as they home to sites of injury and initiate tissue repair and regeneration. Recent recognition of the immunoregulatory functions of MSCs led to exploration of new therapeutic functions for MSCs.
MSCs modulate the immune system by exerting regulatory effects on various immune cells such as T and B lymphocytes, natural killer cells, and dendritic cells (1–4). Many factors contribute to MSCs capacity to impact a wide array of immune cells. The abilities of MSCs to secrete anti-inflammatory cytokines, expand regulatory T cells and down-regulate co-stimulatory molecules on antigen presenting cells are well-established (4–7). The expansive immunomodulatory properties of MSCs make them an attractive candidate for the cellular therapy of autoimmune diseases. To date, MSC therapy has shown positive results and relative safety in uncontrolled trials of human immune diseases, including systemic lupus erythematosus (SLE) (8).
SLE is an autoimmune disease characterized by autoantibody production and subsequent immune complex formation leading to chronic inflammation and end organ damage. Although the etiology of SLE remains unclear, numerous studies suggest that aberrant immune activation, due to ineffective immune regulation, contributes to the development of disease (9, 10). Current treatment options for SLE have broad immune impacts and often interfere with critical immune functions. As such, many patients experience significant side effects, primarily infections, that may rival the manifestations of disease itself in severity (11, 12). Thus, new treatment options, such as MSC therapy, may provide a novel treatment option where benefits surpass risks.
Although results have varied depending on the source of origin, preclinical and clinical studies alike show promise in the use of MSC for the treatment of SLE. We previously showed allogeneic transplant of bone marrow derived MSCs from C57BL/6 and syngeneic transplant of MSCs from young lupus prone mice improved established disease in MRL/lpr and NZB/NZW(F1) lupus prone mice however, no impact on disease was seen with MSC transplants derived from older, diseased lupus prone mice (13). These results are consistent with prior work where bone marrow derived MSCs from B6 mice transplanted into lupus prone MRL/lpr mice ameliorated disease (14–16). Conversely, other groups found syngeneic transplant of bone marrow derived MSCs in (NZB/NZW)F1 mice did not impact disease outcome (17, 18). Positive effects on disease were also seen when MSCs derived from healthy human bone marrow, umbilical cord and adipose tissue were infused into MRL/lpr mice (16, 19, 20). Moreover, uncontrolled human lupus trials suggest improvement in organ dysfunction in standard of care resistant SLE patients treated with allogeneic MSC derived from bone marrow or umbilical cords. However, more limited use of autologous bone marrow derived MSCs did not elicit such improvement (21–23). In addition to these limited in vivo studies, in vitro examination of lupus patient derived MSCs suggest defective MSC differentiation and early signs of cellular senescence (14, 22). Together, these results show variance in the disease modulating capacity of MSCs based on their source of origin, with questions remaining regarding the most beneficial source to be used for treatment of SLE.
In any cellular therapy in humans, use of autologous cells is preferred to allogeneic cells, matched or unmatched for immune markers, to prevent allogeneic responses. The aim in this study was to compare the in vivo and in vitro immunoregulatory capacity of lupus patient derived MSCs to MSCs from healthy control bone marrow or umbilical cords to determine the differential efficacy of human MSCs based on their source of origin. We also determined if current in vitro assays used for “licensing” MSC lots for human use are predictive of efficacy in vivo. Finally, we used in vitro assays, which were more lupus specific, to predict in vivo disease modulating capacity.
Materials and Methods
Mice
MRL/lpr mice were purchased from Jackson Laboratory (Bar Harbor, ME). Mice were maintained in the specific-pathogen-free animal facility of the Ralph H. Johnson Veterans Affairs Medical Center, and the Institutional Animal Care and Use Committee approved all animal procedures. When the mice developed 30mg/dl proteinuria (Chemstrip 7), the 15–17-week-old MRL/lpr mice were randomized into groups as follows: (i) UC-MSC transplantation group (n=8), (ii) HBM-MSC transplantation group (n=8), (iii) LBM-MSC transplantation group (n=16), or (iv) phosphate-buffered saline (PBS) injection group (n=8). 3 UC-MSC lines were injected into 2–3 mice, 3 HBM-MSC lines were injected into 2–3 mice, and 3 LBM-MSC lines were injected into 5–6 mice. A total of 1×106 cells/mouse were administered intravenously at a single time and all mice were sacrificed at 8 weeks after treatment. Mice were euthanized if they demonstrated signs of distress such as lack of grooming, eating or drinking, weight loss of >15%, and proteinuria >500mg/dl. At any of these endpoints we euthanized the mice and collected organs for processing.
Isolation and culture of human MSC
The use of human UC-MSC and BM-MSC was approved by the IRB of the Medical University of South Carolina (MUSC) and all donors were given informed consent. The MSC were isolated and cultured as previously described with slight modification (3, 24). Briefly, human UC-MSC harvested from the Wharton’s Jelly in umbilical cords of healthy babies born by C section at the MUSC hospital were expanded in Alpha-MEM (Gibco) supplemented with 10% human platelet lysate (Emory University), 2 mM L-glutamine (Lonza BioWhittaker), and antibiotics (100 U/ml penicillin and 100 µg/ml streptomycin; Lonza BioWhittaker), and placed in a humidified cell culture incubator containing 5% CO2 at 37°. Non-adherent cells were removed by changing medium every 3 days after the initial plating. After 7 days of incubation, when UC-MSC colonies took up at least 60–80% of the total surface area, the cells were subcultured.
Both healthy and lupus patient bone marrow mononuclear cells were collected from bone marrow aspirates, separated by gradient centrifugation and seeded at a density of 1×106 cells/cm2 in the same media used for UC-MSC. After 3 days of culture, non-adherent cells were removed and the medium was changed twice weekly thereafter. Once 60–80% confluence was reached, adherent cells were re-plated at a density of 104 cells/cm2 in medium for expansion. Human BM-MSCs were derived from fresh whole bone marrow of healthy donors (ALLCELLS) and 4 lupus patients. Lupus patients consisted of 3 female and 1 male African Americans between the ages of 28 to 41. Two patients had active disease with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) scores >6. 2 patients had inactive disease with a SLEDAI score <2. All patients were on prednisone with a dose ≤5mg and either on azathioprine or mycophenylate mofetil.
After three passages, the cells were harvested. MSC were identified according to the criteria of International Society for Cellular Therapy (25). Briefly, flow cytometric analysis confirmed the cells expressed CD44, CD90, CD105, but not CD31, CD45, HLA-DR. The capacity of MSC to differentiate along adipogenic and osteogenic lineages was evaluated as described previously (26). Cells of passage 3–6 were used in the experiments. Initial MSC characterization confirmed the purity of each cell line. However, MSC purity in cultures post cryopreservation and prior to use, was determined by an additional flow confirmation of MSC specific markers and absence of non-MSC markers. In all experiments, analyses indicated >95% purity.
Urine albumin excretion
All mice were placed in metabolic cages for 24-h urine collection before treatment and every 2 weeks after treatment. Urinary albumin excretion was determined by enzyme-linked immunosorbent assay (ELISA) using a standard curve of known concentrations of mouse albumin (Bethyl Laboratories) as previously described [26]. Results are expressed as milligrams of albumin per mouse per day.
Measurement of serum anti-double-stranded DNA (anti-dsDNA) antibody levels
Serum was collected from each mouse before treatment and every two weeks after treatment. Anti-dsDNA antibody levels were measured by ELISA as previously described [27, 28]. Briefly, 96-well ELISA plates were coated with 5µg/ml double-stranded calf thymus DNA (Sigma-Aldrich) in sodium salt citrate buffer at 37°C overnight. After washing, sera were added in serial dilutions starting at 1/100. Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (γ-chain specific; Sigma-Aldrich) was added, followed by 3,3’, 5,5’-tetramethylbenzidine (Sigma-Aldrich) for color development. Optical density (OD) at A450 was measured by a microtiter plate reader (Multiskan Ascent, Thermo Electron Corporation).
Evaluation of IgG and C3 deposition in glomeruli
At the time of sacrifice, one kidney was snap frozen in liquid nitrogen and placed in OCT medium (Tissue-Tek). 4-mm thick frozen sections were stained with fluorescein-conjugated anti-mouse IgG (MP Biomedical) or anti-mouse C3 (MP Biomedical). The average intensities of deposits in five independent fields of one kidney section per animal were quantitated by NIS-Elements BR 3.0.
Histology and pathology assessment of kidney
Kidneys were removed when the mice were sacrificed at 8 weeks after treatment. One kidney was fixed with 10% buffered formalin, embedded in paraffin, and then sectioned. The sections were stained with hematoxylin and eosin (H&E). Photos were taken at 40X using a Nikon Eclipse 80i microscope equipped with a Nikon digital sight color camera and NIS-Elements BR 3.0. For pathology assessment, kidney slides were examined in a blinded fashion and graded for hypercellularity, mesangial expansion, necrosis, crescents, and membrane thickening. Scores from 0 to 3+ (0, none; 1+, mild; 2+, moderate; and 3+, severe) were assigned for each of these features and then added together to yield a final renal pathology score. The scores for crescent formation and necrosis were doubled to reflect the severity of those lesions.
Flow cytometry
Bone marrow cells were incubated with unlabeled mouse anti-CD16/CD32 for blocking Fc receptors, and stained with mouse PE-labeled anti-CD138, PerCP-labeled anti-B220, APC-labeled anti-TCR-β and unlabeled anti-mouse Igκ. Spleen cells were incubated with unlabeled mouse anti-CD16/CD32 for blocking Fc receptors. The following anti-mouse antibodies were incubated with the spleen cells to characterize spleen cell subsets: APC-labeled anti-CD3, PerCP-labeled anti-CD4, FITC-labeled anti-CD8 and PE-labeled anti-Foxp3. Flow samples were analyzed on a flow cytometer (Becton Dickinson FACSCalibur) and analyzed using BD CellQuest Pro 5.2.1. All antibodies were purchased from BD Biosciences or eBioscience.
Urine cytokine analysis
IL17A, CSF1, IFNγ and MCP1 cytokine ELISAs were performed on urine from MSC injected mice. ELISA kits were obtained from eBioscience (CSF1) and BioLegend (IL17A, IFNγ and MCP1), the recommended procedure was followed to the manufacturer’s specifications. Plates were read using a Multiskan Ascent at 450 nm.
PBMC proliferation assay
The proliferation assay was performed in 24-well plates (Costar) in a total volume of 0.5ml alpha-MEM (Cellgro) supplemented with 5% human platelet lysate, 2 mM L-glutamine, 55 µM 2-mercaptoethanol (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin. PBMCs were isolated from healthy human blood using lymphocyte separation medium (Cellgro) and were labeled with 2µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen). 5×105 PBMCs were stimulated with 1µg/ml anti-human CD3/CD28 antibodies in the presence or absence of varying concentrations of MSC. 3 UC-MSC lines, 2 HBM-MSC lines, and 3 LBM-MSC lines were tested 1–2 times. Plates were incubated for 72 hours at 37°C in a humidified atmosphere with 5% CO2. PBMCs were then collected, stained with anti- human APC-labeled CD3, PerCP-labeled CD4 and PE-labeled CD8, and the proliferation status was analyzed by flow cytometry. All antibodies were purchased from BioLegend.
Cytokine secretion analysis
Human IFNγ and IL17A ELISAs were performed on supernatants from PBMC proliferation assays. ELISA kits were obtained from BioLegend and the procedure was followed to the kit’s specifications. Plates were read using a Multiskan Ascent at 450 nm.
B cell proliferation assay
Peripheral blood mononuclear cells from the blood of healthy donors (n=3) were isolated using lymphocyte separation medium (Cellgro). CD19+ B cells were isolated by CD19 microbeads (Miltenyi) according to the manufacturer’s instructions. B cells were labeled with 2µM carboxyfluorescein diacetate succinimidyl ester (CFSE) (Invitrogen). 5×104 CD19+ cells were co cultured with 5×104 MSCs from various sources in a 96-well flat bottom plate (Costar) with a total volume of 200ul RPMI 1640 (Cellgro) supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 55 µM 2-mercaptoethanol (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin. B cells received the following stimuli: 2.5µg/ml CpG oligonucleotide 2006 (Miltenyi), 1µg/ml soluble CD154 (CD40L) (BioLegend), 2.5µg/ml F(ab’)2 anti-human IgM/IgA/IgG (Jackson ImmunoResearch) and 1000U/ml interleukin 2 (IL2) (BioLegend). 3 UC-MSC lines, 2 HBM-MSC lines, and 3 LBM-MSC lines were tested 1–2 times. Plates were incubated for 4 days at 37°C in a humidified atmosphere with 5% CO2. CD19+ cells were then collected, stained with anti-human PE-labeled CD19 (Miltenyi), APC-labeled IgG (BioLegend) and APC-labeled IgA (Miltenyi). Cell proliferation status was analyzed by flow cytometry.
IFNγ licensing assay
The IFNγ licensing assay was performed in 24-well plates (Costar) in a total volume of 0.5ml alpha-MEM (Cellgro) supplemented with 5% human platelet lysate, 2 mM L-glutamine, 55 µM 2-mercaptoethanol (Gibco), 100 U/ml penicillin and 100 µg/ml streptomycin. 5×104 MSCs were stimulated with 0, 5, 10, and 50 ng/ml of recombinant human IFNγ. Plates were incubated for 24 hours at 37°C in a humidified atmosphere with 5% CO2. MSCs were then collected, stained with anti- human APC-labeled CD274 (B7-H1) and PerCP-labeled HLA-ABC then analyzed by flow cytometry. All antibodies were purchased from BioLegend.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
One µg of total RNA was isolated from cultured MSC and mouse kidney tissue. RNA was used to generate cDNA (SA Biosciences) for reverse transcriptase RT-PCR. iQ SYBR Green Supermix (BioRad, Hercules, CA, USA) and gene specific primers (SAbioscience) at 200nM were used to amplify relative amounts of cDNA on a CFX Connect Real-Time System (BioRad, Hercules, CA, USA). The amplification was performed by one 5 minute cycle at 95°C which was required for enzyme activation; followed by 39 cycles of denaturation (95°C, 15s), annealing (55°C, 30s), and extension (72°C, 30s). Melting Curve analysis was performed to confirm amplicon specificity. The relative expression was calculated using the double ΔCT method (i.e. using the equation 2−ΔΔCT) using BioRad software.
Statistical analysis
All data were analyzed using Prism version 5.0 software (GraphPad, San Diego, CA). Survival significance was determined via Log-Rank (Mantel-Cox) analysis of the survival curve. Two-way ANOVA was used for proteinuria curve and in vitro assay analysis. One-way ANOVA (Tukey posttest) and Kruskal-Wallis (Dunns post test) were utilized to test for significance between groups in single group comparisons. A 95% confidence limit, defined by p values ≤ 0.05, was considered to be statistically significant and indicated within the figures.
Results
Improved survival of lupus prone mice receiving human MSCs
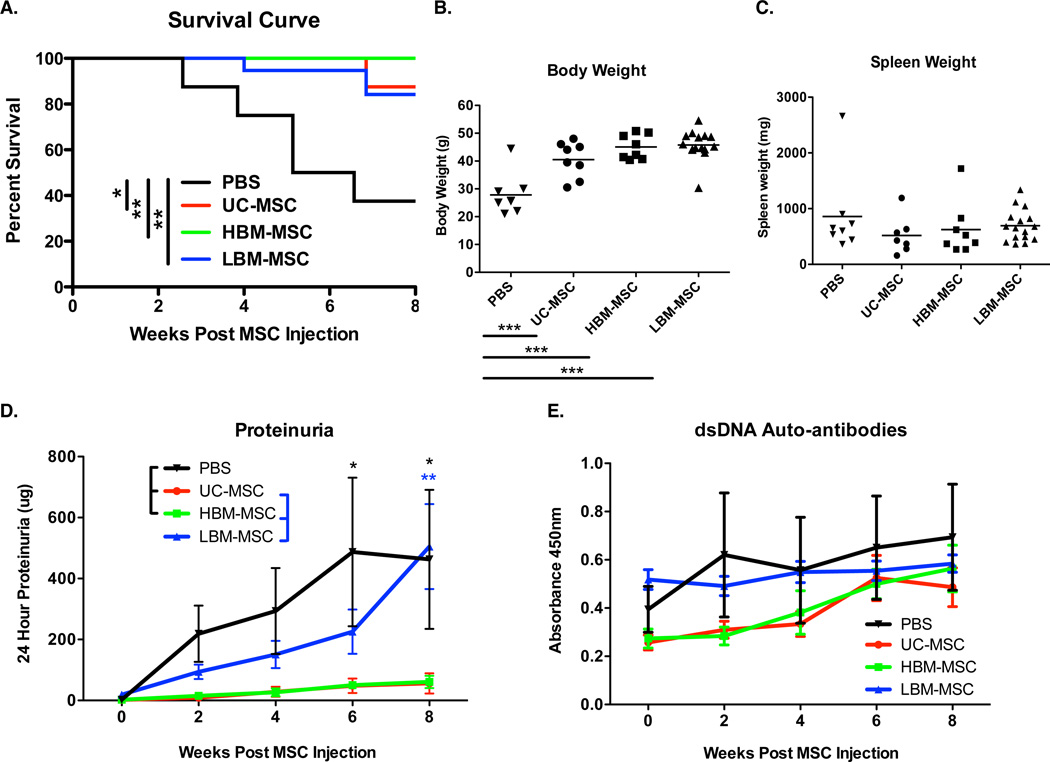
Human MSC (hMSC) transplantation, regardless of source, significantly enhanced the survival of MRL/lpr mice when given at disease onset versus the PBS control group. By 60 weeks of age, 62% mortality was observed in the PBS group compared to 0–15% in the hMSC treated groups (Figure 1A). Furthermore, there was a significant increase in the body weight of hMSC mouse groups in comparison to the PBS control mice (Figure 1B). Trends toward lower spleen weights in hMSC treated mice corresponded with increased body weight. However, this correlation was not reach significance (Figure 1C).
Figure 1. Survival curve, body and spleen weight, urinary albumin excretion, and serum dsDNA auto-antibody levels of MRL/lpr mice.
A. Kaplan Meier survival curve of MRL/lpr mice receiving hMSCs. Mortality was recorded until the time of sacrifice (8 weeks post transplant) B. Body weight of MRL/lpr mice at time of sacrifice. C. Spleen weights of MRL/lpr mice at time of sacrifice. Results are expressed as the mean ±SEM. D. Urinary albumin excretion over time post MSC transpant. Results are expressed as the mean 24 hour albumin excretion (ug/mouse/day) ±SEM. E. Serum dsDNA auto-antibodies overtime post MSC transplant. Results are expressed as the mean ±SEM. PBS (n=8), UC-MSC (n=8), HBM-MSC (n=8), LBM-MSC (n=16). Statistical comparisons were performed using Log-rank test, 1way ANOVA with Tukey posttest, and 2way ANOVA with Bonferroni posttest. *p≤ 0.05; **p≤.01; ***p≤0.001.
Next, we sought to determine whether improved survival of hMSC treated mice was due to effects on renal disease. Urine was collected before and every two weeks post MSC transplantation to assess 24-hour urine albumin excretion. As expected, the PBS mouse group experienced increasing proteinuria over time. In contrast, mice transplanted with healthy donor MSCs from umbilical cord (UC-MSC) or bone marrow (HBM-MSC) had significantly less proteinuria. Lupus patient MSC (LBM-MSC) treated mice, however, had delayed onset of proteinuria (Figure 1D). Mice that died prior to sacrifice had elevated proteinuria implicating renal disease as causative in their mortality. Due to the role of anti-dsDNA antibodies in lupus nephritis, we then assessed serum anti-dsDNA antibody levels before and every two weeks post MSC transplantation. Anti-dsDNA antibody levels were not impacted by hMSC treatment (Figure 1E), suggesting the effect of MSCs on lupus nephritis is post autoantibody production/immune complex deposition.
Effect of hMSC on kidney pathology varies based on source of origin
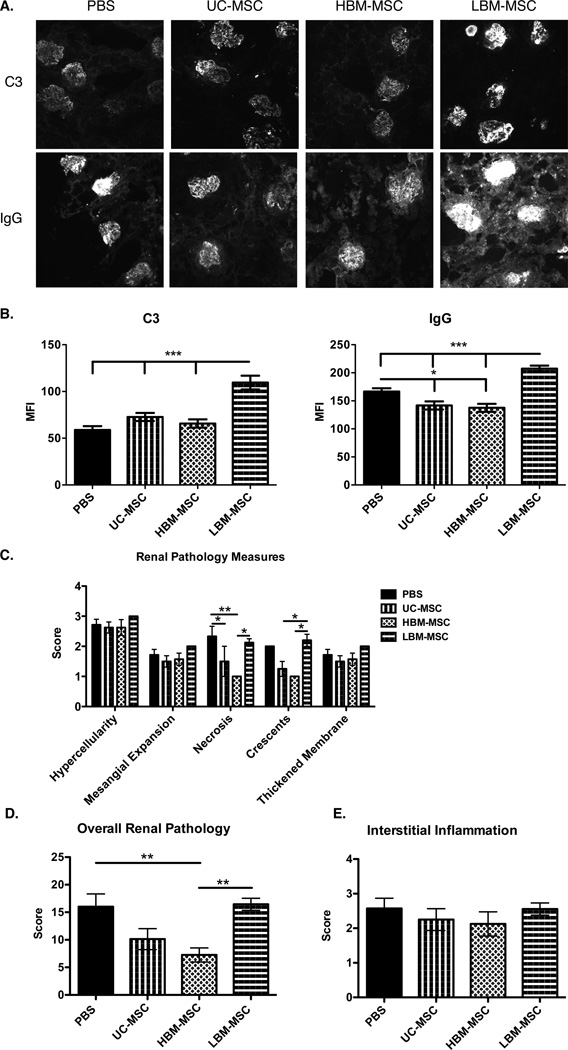
We then assessed whether the different sources of hMSC had a differential effect on histopathological changes in the kidneys of MRL/lpr mice. We found that IgG, but not C3, deposition in the glomeruli was decreased in UC-MSC and BM-MSC treated mice when compared to PBS controls (Figure 2A and 2B). In contrast, LBM-MSC treatment significantly increased C3 and IgG deposition in the glomeruli when compared to all other treatment groups, including PBS controls (Figure 2A and 2B). H&E stained kidney slides were scored in a blinded fashion. The following renal pathology measures make up the over all renal pathology score: hypercellularity, mesangial expansion, necrosis, crescents, and membrane thickening (Figure 2C). We observed lower necrosis and crescents in UC-MSC and HBM-MSC when compared to LBM-MSC, which were not different than PBS treated mice (Figure 2C). Significantly lower total pathology scores were seen in HBM-MSC treated mice with UC-MSC treated mice approaching significance (p=0.0706), but not in LBM-MSC treated mice, when compared to PBS controls (Figure 2D). Together these results suggest that UC-MSC and HBM-MSC, but not LBM-MSC, are effective in reducing kidney pathology in MRL/lpr mice. In fact, these data suggest potential exacerbation of kidney disease by LBM-MSCs.
Figure 2. Glomerular C3 intensity, IgG intensity, and overall glomerular pathology score in MRL/lpr mice.
At time of sacrifice kidneys were embedded in OCT and paraffin for sectioning. OCT embedded kidney labeled with fluorescein-conjugated anti-mouse C3 or IgG. A. Representative images of C3 and IgG deposits (20x) in glomeruli. B. Graphical assessment of MFI for C3 and IgG deposition. C. H&E staining of paraffin embedded kidney were examined and graded for hypercellularity, mesangial expansion, necrosis, cresents, and membrane thickening. D. Scores are added together to yield overall renal pathology score. E. Interstitial inflammation scores. Results are expressed as the mean ±SEM. PBS (n=8), UC-MSC (n=8), HBM-MSC (n=8), LBM-MSC (n=9). Statistical comparisons were performed using 1way ANOVA with Tukey posttest. *p≤ 0.05; **p≤.01; ***p≤0.001.
LBM-MSC not effective in reducing inflammatory markers
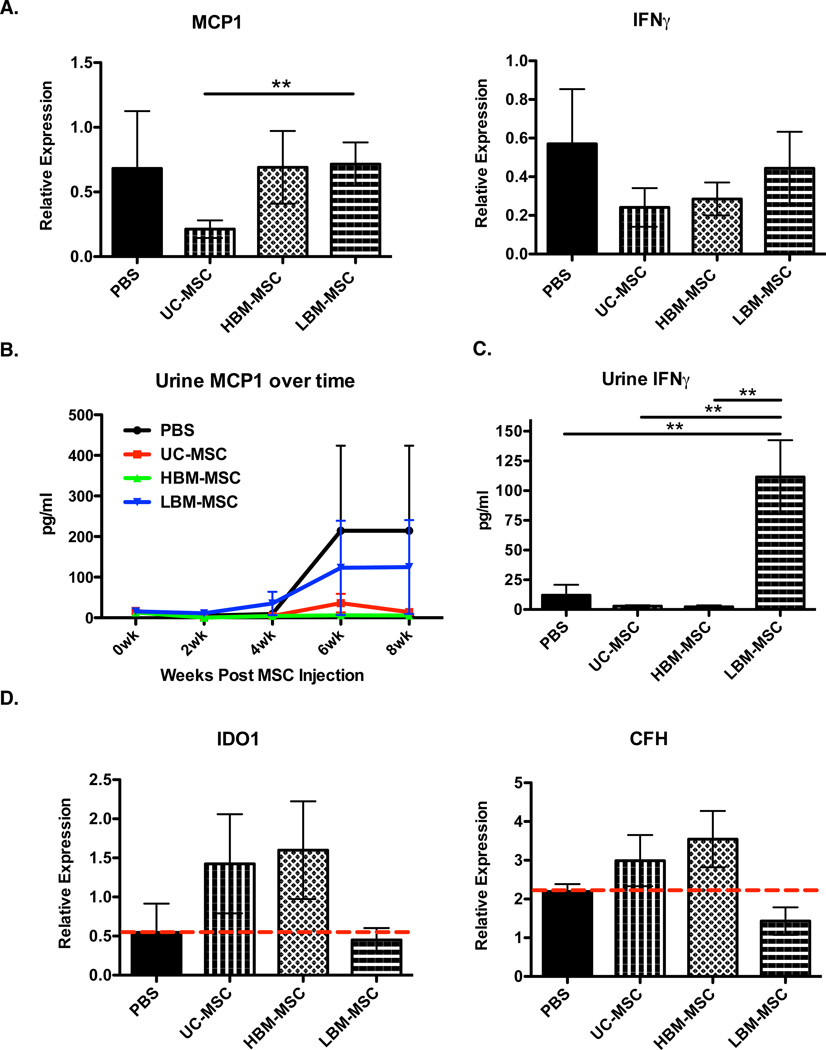
Upon finding the differences in the ability of hMSC to modulate kidney pathology, we examined inflammatory cytokines in the kidney and urine of treated mice. CSF1, MCP1, IL17 and IFNγ are all cytokines indicative of immune activation in active nephritis (27) (28–30). As shown in Figure 3A, UC-MSC treated mice experienced reduced MCP1 expression when compared to LBM-MSC treated mice. Both UC-MSC (p=0.4351) and HBM-MSC (p=0.6216) treated mice appeared to have lower IFNγ expression in the kidney compared to PBS controls, albeit not significant. (Figure 3A). CSF1 and IL17A gene expressions were undetectable in the kidneys regardless of treatment group (data not shown). We then looked at the presence of these cytokines in the urine of mice. Over the course of treatment, there was less urinary MCP1 in UC-MSC and HBM-MSC treated mice when compared to LBM-MSC and PBS treated mice, although this finding did not reach significance (Figure 3B). However, at 6 weeks post MSC transplantation, we observed significantly higher levels of urinary IFNγ in LBM-MSC treated mice over all other treatment groups including controls, consistent with the kidney pathology results (Figure 2 and Figure 3C). Urinary IL17A was not detected and no differences in urinary CSF1 were found at any time point post MSC transplant (Data Not Shown and Supplemental Figure 1). The inability of LBM-MSCs to inhibit inflammatory cytokines in the kidney and urine of MRL/lpr mice further support that lupus patient MSCs, when compared to healthy donor MSCs, are not as effective as disease modulators as MSCs from other sources.
Figure 3. Inflammatory markers and human gene expression in MRL/lpr mice.
At time of sacrifice kidneys were isolated for qPCR analysis. A. Expression of mouse MCP1 and IFNγ expression in the kidney of MRL/lpr mice relative to GAPDH. B. Urinary MCP1 measured over time post MSC transplant by ELISA. C. Urinary IFNγ at 6 weeks post MSC transplant measured by ELISA. D. Human IDO1 and CFH expression relative to GAPDH from the kidney of MRL/lpr mice at time of sacrifice (8 weeks post transplant). Results are expressed as the mean ±SEM. PBS (n=4), UC-MSC (n=8), HBM-MSC (n=8), LBM-MSC (n=12). Statistical comparisons were performed using 1way ANOVA with Tukey posttest and 2way ANOVA with Bonferroni posttest. *p≤ 0.05; **p≤.01.
Reduced human gene expression in the kidney of LBM-MSC mice
Due to the inability of LBM-MSC to inhibit kidney pathology and inflammatory cytokine production, we hypothesized that LBM-MSCs were not homing to the site of inflammation. To assess the presence of hMSC in the kidneys of MRL/lpr mice, we looked for the expression of human IDO1 and CFH, which are expressed by hMSC. MSC presence in the kidney of hMSC treated mice is associated with gene expression higher than that of PBS control mice. Our results show positive gene expression for human IDO1 and CFH only in UC-MSC and HBM-MSC groups (Figure 3D). Together, these results suggest that LBM-MSC either have lower expression of IDO1/CFH or that there are fewer hMSCs in the kidney of mice treated with LBM-MSCs, either due to decreased migration capacity or decreased survival.
Increased T and B cells in the bone marrow of mice treated with lupus patient MSC
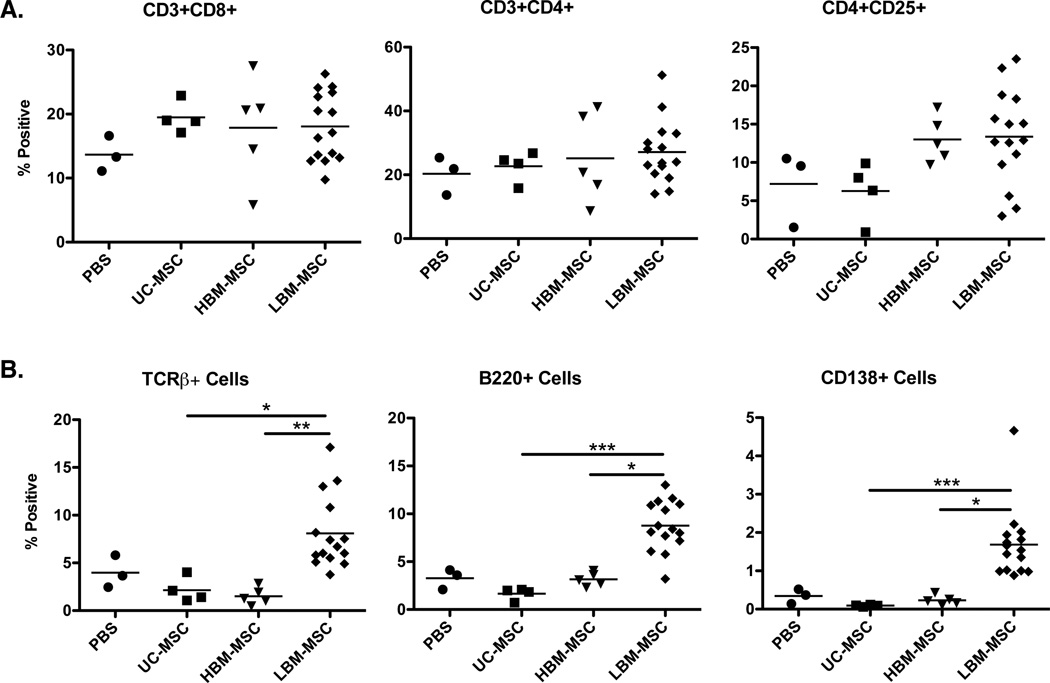
Our results support an exacerbation of kidney disease in LBM-MSC treated mice, although these mice still experienced improved survival, versus PBS controls. To assess potential mechanisms by which MSCs from the various sources impact lupus disease activity, we characterized various immune cell populations in the spleen and bone marrow of mice from each group via flow cytometry. When examining the spleen, no differences were seen in the percentages of CD8+ T cells, CD4+ T cells, activated CD4+CD25+ T cells, or Foxp3+ T cells between mouse groups (Figure 4A and data not shown). In the bone marrow, no significant changes in TCRβ+ cells, B220+ cells, or plasma (CD138+) cells were observed in the mice treated with UC-MSC (p=0.2286, 0.0571, 0.0571) and HBM-MSC (p=0.0714, 1, 0.5714), though percentages trended lower in the hMSC treatment groups compared to PBS controls. However, a significant percentage increase in all three cell subsets was seen in LBM-MSC treated mice when compared to healthy hMSC treated mice (Figure 4B).
Figure 4. Percentages of inflammatory cells in the spleen and bone marrow of MRL/lpr mice.
Flow cytometry analysis was performed on spleen and bone marrow isolated from mice injected with MSC at time of sacrifice. A. Percentages of CD3+CD8+, CD3+CD4+, and CD3+CD4+CD25+ T cells in the spleen. B. Percentages of TCRβ+, B220+, and CD138+ cells in the bone marrow. Results are expressed as the mean ±SEM. PBS (n=3), UC-MSC (n=4), HBM-MSC (n=5), LBM-MSC (n=15). Statistical comparisons were performed using Kruskal-Wallis test with Dunn’s multiple comparison posttest. *p≤ 0.05; **p≤.01; ***p≤0.001.
In vitro differences in MSC are dependent on tissue origin rather than donor
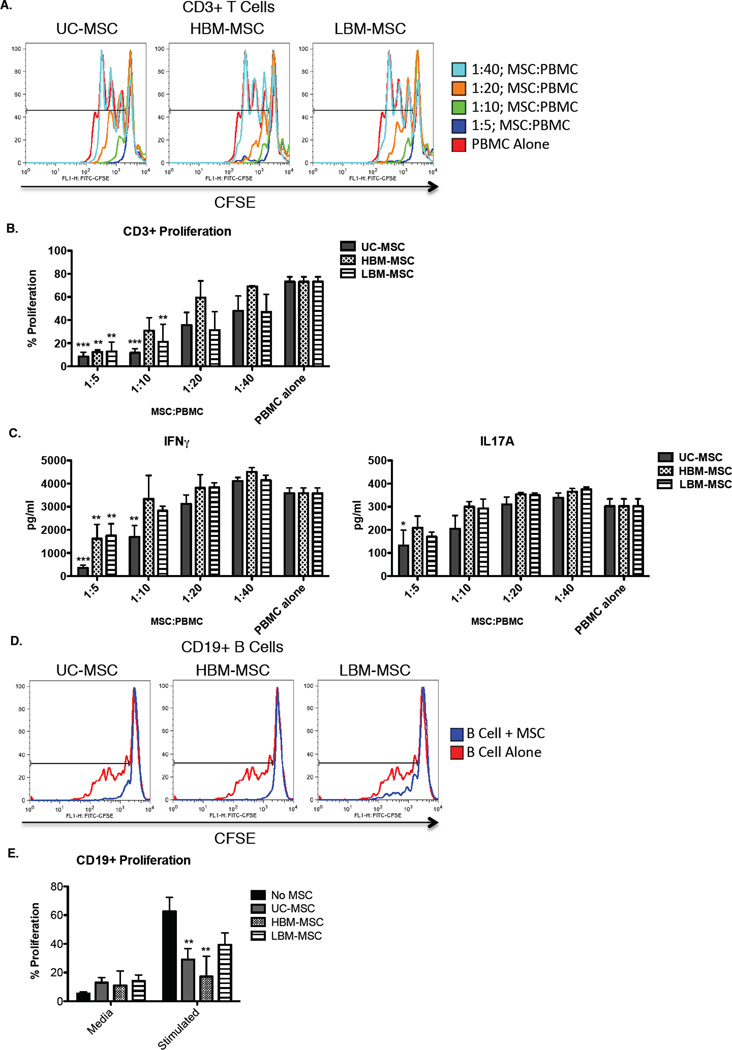
Inhibition of T cell and B cell proliferation in vitro is a known effect of MSCs and is proposed as a measure of in vivo effectiveness. Due to our results showing the inability of LBM-MSC to inhibit progression of disease in MRL/lpr mice, we next tested the in vitro efficacy assays. We were only able to detect suppression of mouse CD3+ T cells when co-cultured at a 1:20 dilution with UC-MSCs, but not HBM-MSCs or LBM-MSCs, although this effect was not enhanced at higher concentrations (Supplemental Figure 2). Due to our inability to potently suppress mouse splenocytes, we examined the effects of hMSCs on healthy human PBMCs. As shown in Figure 5A and 5B, human CD3+ T cell proliferation is reduced in a dose dependent fashion by all MSC groups. However, when examining IFNγ cytokine production, we observed that MSCs derived from the bone marrow were not able to inhibit IFNγ production to the same capacity as umbilical cord MSCs (Figure 5C). Moreover, we examined IL17A production as it was previously shown that co-culture with MSCs can increase IL17 production by PBMCs (31). Our findings did not show increased IL17 production, but rather, presence of MSCs slightly reduced IL17 production, although only UC-MSC at the highest concentration provided a significant decrease (Figure 5C). There are varied results in the literature regarding B cell suppression by MSCs. One study examining the effects of BM-MSC on CD19+ B cells found suppression of B cell proliferation, while another reported expansion(2, 32). In order to explain these results, and determine if variances in suppression could be detected in our various MSC lines, we conducted similar co-culture assays using the same cell stimulants and cell ratios. Our study did show expansion of CD19+ B cells, however, we found that UC-MSCs and HBM-MSCs, but not LBM-MSC (p=0.1119), were able to significantly reduce B cell proliferation (Figure 5D and 5E).
Figure 5. T cell and B cell proliferation in CFSE dilution assays co-cultured with different sources of MSC and IFNγ production of T cell proliferation assay.
A. Representative plots of CD3+ T cell proliferation in CFSE dilution assay co-cultured with each source of MSC. B. Proliferation of CD3+ T cells incubated alone (n=5) or with varying ratios of UC-MSC (n=5), HBM-MSC (n=4), and LBM-MSC (n=5). Cultures were stimulated with 1µg/ml of anti-human CD3/CD28 antibodies. C. Supernatants were collected from PBMC co-culture assay, IFNγ and IL17A cytokine levels were analyzed by ELISA. D. Representative plots of CD19+ B cell proliferation in CFSE dilution assay co-cultured with each source of MSC. E. Proliferation of CD19+ B cells incubated alone (n=4) or 1:1 ratio with UC-MSC (n=5), HBM-MSC (n=4), and LBM-MSC (n=5). Cultures were stimulated with 2.5µg/ml CpG, 1µg/ml), 2.5µg/ml F(ab’)2 anti-human IgM/IgA/IgG, and 1000U/ml IL2. Results are expressed as the mean percentage of proliferating cells or pg/ml IFNγ ±SEM. Statistical comparisons were performed 2way ANOVA with Bonferroni posttest. **p≤.01; ***p≤0.001.
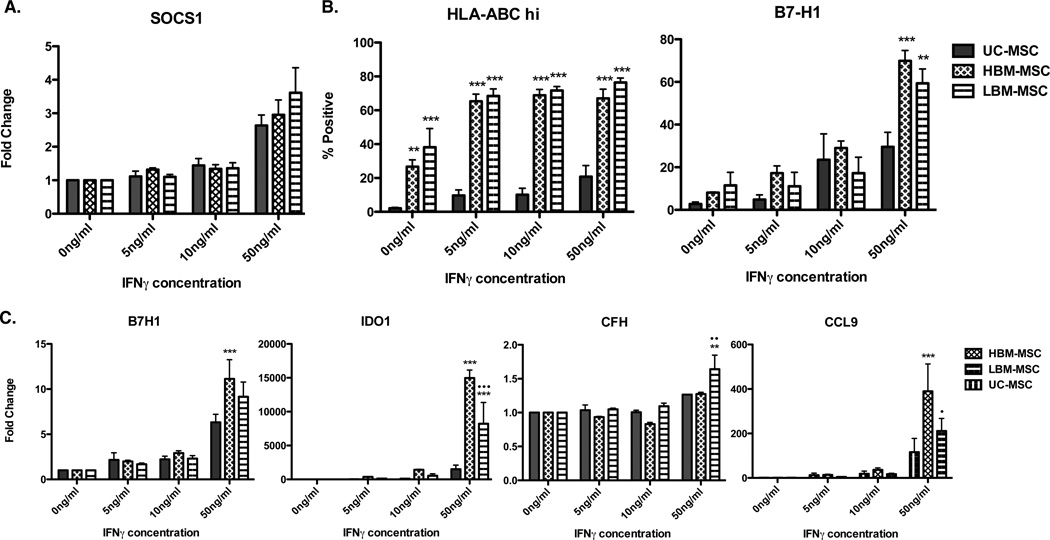
We next turned to the examination of MSC surface markers and gene expression to explain the inconsistency of suppressive trends in vitro. IFNγ licensing was previously shown to enhance and be indicative of suppressive function and is an inflammatory cytokine associated with lupus. Thus, we examined the effects of IFNγ stimulation on MSCs from all three sources (33–35). While no difference was observed in surface expression of HLA-DR (data not shown), we saw a dose dependent increase in HLA-ABC to IFNγ with significantly higher surface expression on MSCs derived from bone marrow (Figure 6B). Heightened basil expression of HLA-ABC on bone marrow derived MSC could suggest higher immunogenicity of these cells when compared to MSC derived from umbilical cords. Significantly higher surface expression of B7-H1 was seen in bone marrow derived MSCs over UC-MSC at the highest dose of IFNγ (Figure 6B). B7-H1, IDO1 and CFH are highly expressed in early passage MSCs with suppressive function, and this expression increases with IFNγ licensing (36–38). Our findings show IFNγ licensing significantly increased expression of B7-H1 in HBM-MSC compared to UC-MSC. Furthermore, IDO1 and CCL9 expression was increased in HBM-MSC and LBM-MSC when compared to UC-MSC. However, only LBM-MSC experienced a significant increase in CFH expression when compared to both UC-MSC and HBM-MSC (Figure 6C). Our findings show heightened IFNγ responsiveness in MSC from bone marrow, regardless of the disease status of the donor, when compared to UC-MSC. Together, our in vitro results suggest that IFNγ licensing is not a sufficient measure for predicting suppressive capacity in vivo.
Figure 6. Flow cytometry and qPCR analysis of MSC markers after in vitro IFNγ licensing.
UC-MSC (n=), HBM-MSC (n=), and LBM-MSC (n=) were cultured with 0, 5 10, or 50 ng/ml of IFNγ. A. SOCS1 expression relative to GAPDH in IFNγ licensed MSCs B. Percentage of HLA-ABC+ and B7-H1+ MSCs cultured with varying concentrations of IFNγ. C. IDO1, B7H1, CFH, and CCL9 expression relative to GAPDH in IFNγ licensed MSCs. n=3 for each MSC source. Results are expressed as the mean ±SEM. Statistical comparisons were performed using 2way ANOVA with Bonferroni posttest. *denotes comparison to UC-MSC; •denotes comparison to HBM-MSC. *p≤.05 **p≤.01; ***p≤0.001.
Discussion
Although many uncontrolled studies investigating the use of MSC cell therapy in various diseases were effective and well-tolerated, studies in SLE patients are missing. Our present study directly compares the immunoregulatory capacity, both in vivo and in vitro, of MSCs from lupus patients and healthy controls. Additionally, we investigated the capacity of in vitro MSC assays to predict in vivo efficacy. To examine whether lupus patient derived bone marrow MSCs are effective as disease modulators we compared them directly to healthy donor bone marrow and umbilical cord MSCs. We observed improved survival and increased body weight in the groups of mice that received human MSC regardless of their source of origin or whether the cells were derived from a lupus patient or healthy control. Supporting previous studies examining healthy human or mouse MSC in murine lupus, HBM and UC-MSC were significantly more effective than LBM-MSC in improving lupus disease manifestations including proteinuria, renal pathology, and urinary inflammatory markers (13–16, 19, 20). While our study strongly supports the efficacy of healthy human MSCs to modulate disease in murine lupus, we uncovered a negative impact on renal disease manifestations in mice treated with lupus patient derived MSCs.
Consistent with our previous murine MSC study, lupus patient MSCs were unable to prevent renal disease progression including their inability to reduce proteinuria and inhibit renal pathology, both of which were positively impacted by UC and HBM-MSC (13). LBM-MSC treated mice experienced increased proteinuria when compared to HBM-MSC and UC-MSC mice at 8 weeks post treatment. Although no differences were detected in serum anti-dsDNA antibody levels in the various mouse groups, we observed significantly higher C3 and IgG glomerular deposition in LBM-MSC treated mice compared to all other treatment groups, including the control. Moreover, overall renal pathology was not impacted in LBM-MSC mice. The discrepancy in auto-antibody levels in the serum compared to IgG deposition in the kidney may be explained by the active renal disease activity in the LBM-MSC mice at the time of sacrifice. Serum anti-dsDNA antibodies may decrease in the serum of patients with renal flares due to their deposition into the tissue (39). Thus, serum levels may remain constant or decrease as antibodies are deposited in the kidney.
Despite the active kidney disease at the time of sacrifice, we observed improved survival of mice in the LBM-MSC treatment groups when compared to PBS treated controls. As in all similar murine studies, we anticipate that the mice that died had severe renal disease falsely lowering the renal disease scores of the control mice. Improved survival in the LBM-MSC treated mice, despite active renal disease, reflects a time delay in onset of disease in the LBM-MSC mice, but no effect on overall severity of disease. Between 4 and 6 weeks post MSC transplant, PBS control mice developed increased proteinuria followed shortly by death. While UC-MSC and HBM-MSC treated mice did not have an increase in proteinuria, LBM-MSC treated mice developed increased proteinuria at 8 weeks, 2–4 weeks after the PBS treated group. If allowed to proceed an additional 2–4 weeks, we would likely observe increased mortality in the LBM-MSC treated mice. Moreover, LBM-MSC treated mice sacrificed at 8 weeks were at peak disease activity, explaining the heightened kidney pathology and increased inflammatory cell populations in the bone marrow. It is notable that not all aspects of lupus nephritis pathology were impacted by the MSC treatments. Mesangial proliferation, among other features of lupus nephritis, was not impacted by MSC treatment. The significant impact of MSC treatment was in preventing the more severe manifestations of renal disease, crescents and necrosis. This is in contrast to the effect of using control murine MSCs to treat disease where all aspects of lupus nephritis were impacted. These differences may reflect known differences in mechanisms of effect of mouse MSCs (nitric oxide mediated) versus human MSCs (IDO mediated) (35, 40).
Several issues with LBM-MSC may factor into the relative short term benefit of the LBM-MSCs. Previous studies show early senescence and defective differentiation of LBM-MSCs, which may explain a short term, but not long term benefit (14, 22). Defects in LBM-MSC migration, premature death, and increased cytokine production may also play a role in the lack of a full protective effect. Our inability to detect human IDO1 and CFH gene expression in the kidney of LBM-MSC treated mice suggests these abnormalities may impact in vivo efficacy. These findings suggest either that the cells never migrated to the diseased kidney, that they migrated to the kidney, but died prematurely or that they in vivo are not activated to express IDO and CFH. These findings are consistent with the lack of efficacy of MSCs derived from diseased lupus mice in treating murine lupus and the limited reports of a lack of efficacy of autologous MSC treatment in human lupus (13, 23).
One of the more unexpected findings in this study was that there was consistency in effect of the MSCs depending on their source of origin. Both in vitro and in vivo there was very little variation in the effect of the MSCs. We used four different MSC cell lines from each source (healthy human bone marrow, lupus bone marrow and healthy umbilical cord). All of the healthy BM-MSC lines and all the UC-MSC lines had similar in vivo efficacy and in vitro effects. Perhaps most surprising was that of the four lupus MSC bone marrow derived lines, all 4 had similar effects on murine disease and similar effects in in vitro assays, despite marked differences in disease activity and medications.
Given the differential efficacy of the MSCs, we next sought to determine if there is an in vitro assay that could predict in vivo efficacy. The obvious goal of this research is to treat human lupus patients with MSCs in a controlled trial. It is important that we select for this trial the most effective MSC origin, which we could hopefully identify using in vitro assays prior to infusion. Thus, we performed a number of immune assays, including standard assays used now for assessment of MSCs for human treatment as well as additional measures to differentiate effective (UC-MSC/HBM-MSC) from ineffective (LBM-MSC) derivations. The most widely used assay to determine immune competence of MSCs is suppression of T cell proliferation. We found that all the MSC lines suppressed T cell proliferation in a dose dependent fashion, although UC-MSCs were more effective in suppressing IFNγ production. Thus, the standard assay does not allow identification of in vivo inefficacy. Our results also showed only healthy UC-MSCs and HBM-MSCs were able to significantly suppress B cell proliferation, although LBM-MSC did reduce B cell proliferation albeit not significantly. We observed more significant differences between BM derived MSCs versus UC derived MSCs than differences between lupus versus controls MSCs
Lastly, we used IFNγ licensing to examine markers indicative of MSC function. Prior studies suggest that treating MSCs with IFNγ may enhance in vivo efficacy and that these assays are predictive of in vivo efficacy (34, 35, 41). Therefore we were interested in whether IFNγ licensing differentiated lupus versus control MSCs and if treatment with IFNγ could enhance the immunosuppressive abilities of lupus MSCs. A previous study examined the effects of CD8+ T cell produced IFNγ on UC-MSC and LBM-MSC and found defective IDO production by LBM-MSCs (35). Conversely, we observed increases in B7-H1 and IDO1 suppressive gene expression in both healthy and lupus bone marrow derived MSCs similar to findings by another group only examining bone marrow derived MSC (34). We also saw increased CFH expression in LBM-MSC. Our in vitro results show that MSCs from various sources, including lupus patients, are able to suppress T and B cells to some capacity. Furthermore, IFNγ licensing of LBM-MSC revealed their ability to upregulate markers indicative of suppression, suggesting that standard assays of MSC function are not likely to identify or explain the inability of LBM-MSCs to maintain inhibition of disease in lupus prone mice. These in vitro findings suggest that the failure of LBM-MSCs to impact lupus disease to the same capacity as healthy MSCs is likely due to defects in migration, premature death, or inability to maintain suppressive functions in an excessive inflammatory environment. Whether the defective immunosuppressive function in lupus derived MSCs is intrinsic or a result of disease remains unanswered. Our prior reports suggest that it is an effect of disease as MSCs derived from predisease lupus prone mice are as effective as control MSCs, while MSCs from mice after disease onset are not effective (13). The follow-up question of whether lupus derived MSCs can be “rescued” remains to be answered.
In conclusion, our study demonstrated that, like murine LBM-MSCs, human LBM-MSCs are not as effective in ameliorating disease in lupus prone mice as UC-MSC and HBM-MSC. Moreover, in vitro assessments of immunomodulatory functions detected a reduced capacity of LBM-MSC to inhibit IFNγ production and CD19+ B cell proliferation, although inhibition of CD3+ proliferation and IFNγ licensing results were indicative of immune activity by LBM-MSC. Together, the overall effectiveness of LBM-MSC in vitro, other than B cell inhibition, suggest that they are effective in cell-to-cell inhibition indicating their ineffectiveness in vivo is due to other functional defects. These defects of LBM-MSCs may lie in their migration to inflamed tissues or inability to persist and function during systemic inflammation. Studies regarding chemotactic and migratory properties of LBM-MSC in vivo are yet to be completed, but may provide insight into the defects uncovered in this study. Additionally, future studies regarding LBM-MSCs responsiveness to IFNγ stimulation may prove to be useful in enhancing the properties of these cells both in vitro and in vivo. Although these studies have shown that LBM-MSC are not yet a suitable source of MSC for cell therapy in lupus, it is important to continue to define differences in MSCs as it appears that donors and source of origin impact their function. Finally, these studies were infusing human MSCs into mice. It is possible that the LBM-MSCs are effective when given to patients, however, our studies in mice and humans and the limited in vivo studies in patients at this time suggest that allogeneic MSCs are more suitable for human trials.
Supplementary Material
Acknowledgements
The authors greatly appreciate Dr. Osama Naga, Medical University of South Carolina, for his technical assistance with these experiments.
This work was funded by a grant from the Lupus Foundation and NIH grant number UL1 RR029882 (NCRR) and UL1 TR000062 (NCATS) as well as the Medical Research Service, Ralph H. Johnson VAMC.
Abbreviations used in this paper were
- MSC
Mesenchymal stem cell
- hMSC
Human mesenchymal stem cells
- SLE
Systemic Lupus Erythematosus
- SLEDAI
Systemic Lupus Erythematosus Disease Activity Index
- UC
Umbilical cord
- HBM
Healthy bone marrow
- LBM
Lupus bone marrow
Footnotes
The authors declare that they have no competing interests.
References
- 1.Klyushnenkova E, Mosca JD, Zernetkina V, Majumdar MK, Beggs KJ, Simonetti DW, Deans RJ, McIntosh KR. T cell responses to allogeneic human mesenchymal stem cells: immunogenicity, tolerance, and suppression. J Biomed Sci. 2005;12:47–57. doi: 10.1007/s11373-004-8183-7. [DOI] [PubMed] [Google Scholar]
- 2.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi G, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 3.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions Between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells. 2006;24:74–85. doi: 10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- 4.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, Galun E, Rachmilewitz J. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105:2214–2219. doi: 10.1182/blood-2004-07-2921. [DOI] [PubMed] [Google Scholar]
- 5.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell. 2008;2:141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Prevosto C, Zancolli M, Canevali P, Zocchi MR, Poggi A. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888. doi: 10.3324/haematol.11240. [DOI] [PubMed] [Google Scholar]
- 7.English K, Barry FP, Mahon BP. Murine mesenchymal stem cells suppress dendritic cell migration, maturation and antigen presentation. Immunology Letters. 2008;115:50–58. doi: 10.1016/j.imlet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin. Biol. Ther. 2011;11:893–909. doi: 10.1517/14712598.2011.574119. [DOI] [PubMed] [Google Scholar]
- 9.Tsokos GC. Systemic lupus erythematosus. N. Engl. J. Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 10.Nowling TK, Gilkeson GS. Mechanisms of tissue injury in lupus nephritis. Arthritis Res. Ther. 2011;13:250–259. doi: 10.1186/ar3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lo MS, Tsokos GC. Treatment of systemic lupus erythematosus: new advances in targeted therapy. Ann. N. Y. Acad. Sci. 2012;1247:138–152. doi: 10.1111/j.1749-6632.2011.06263.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtman EI, Helfgott SM, Kriegel MA. Emerging therapies for systemic lupus erythematosus-focus on targeting interferon-alpha. Clin. Immunol. 2012;143:210–221. doi: 10.1016/j.clim.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu F, Molano I, Ruiz P, Sun L, Gilkeson GS. Differential effect of allogeneic versus syngeneic mesenchymal stem cell transplantation in MRL/lpr and (NZB/NZW)F1 mice. Clin. Immunol. 2012;145:142–152. doi: 10.1016/j.clim.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 14.Sun L, Akiyama K, Zhang H, Yamaza T, Hou Y, Zhao S, Xu T, Le A, Shi S. Mesenchymal stem cell transplantation reverses multiorgan dysfunction in systemic lupus erythematosus mice and humans. Stem Cells. 2009;27:1421–1432. doi: 10.1002/stem.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji S, Guo Q, Han Y, Tan G, Luo Y, Zeng F. Mesenchymal stem cell transplantation inhibits abnormal activation of Akt/GSK3β signaling pathway in T cells from systemic lupus erythematosus mice. Cell. Physiol. Biochem. 2012;29:705–712. doi: 10.1159/000178590. [DOI] [PubMed] [Google Scholar]
- 16.Feng X, Yao G, Hou Y, Lu L, Gilkeson G. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010 doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- 17.Youd M, Blickarz C, Woodworth L, Touzjian T, Edling A, Tedstone J, Ruzek M, Tubo R, Kaplan J, Lodie T. Allogeneic mesenchymal stem cells do not protect NZBxNZW F1 mice from developing lupus disease. Clinical & Experimental Immunology. 2010;161:176–186. doi: 10.1111/j.1365-2249.2010.04158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schena F, Gambini C, Gregorio A, Mosconi M, Reverberi D, Gattorno M, Casazza S, Uccelli A, Moretta L, Martini A, Traggiai E. Interferon-γ-dependent inhibition of B cell activation by bone marrow-derived mesenchymal stem cells in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2010;62:2776–2786. doi: 10.1002/art.27560. [DOI] [PubMed] [Google Scholar]
- 19.Chang J-W, Hung S-P, Wu H-H, Wu W-M, Yang A-H, Tsai H-L, Yang L-Y, Lee OK. Therapeutic effects of umbilical cord blood-derived mesenchymal stem cell transplantation in experimental lupus nephritis. Cell Transplant. 2011;20:245–257. doi: 10.3727/096368910X520056. [DOI] [PubMed] [Google Scholar]
- 20.Choi EW, Shin IS, Park SY, Park JH, Kim JS, Yoon EJ, Kang SK, Ra JC, Hong SH. Reversal of serologic, immunologic, and histologic dysfunction in mice with systemic lupus erythematosus by long-term serial adipose tissue-derived mesenchymal stem cell transplantation. Arthritis Rheum. 2012;64:243–253. doi: 10.1002/art.33313. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, Zhang H, Liang J, Li X, Feng X, Wang H, Hua B, Liu B, Lu L, Gilkeson GS, Silver RM, Chen W, Shi S, Sun L. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years experience. Cell Transplant. 2012 doi: 10.3727/096368911X582769c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Zhang H, Feng X, Hou Y, Lu L, Fan L. Abnormality of bone marrow-derived mesenchymal stem cells in patients with systemic lupus erythematosus. Lupus. 2007;16:121–128. doi: 10.1177/0961203306075793. [DOI] [PubMed] [Google Scholar]
- 23.Carrion F, Nova E, Ruiz C, Diaz F, Inostroza C, Rojo D, Monckeberg G, Figueroa F. Autologous mesenchymal stem cell treatment increased T regulatory cells with no effect on disease activity in two systemic lupus erythematosus patients. Lupus. 2010;19:317–322. doi: 10.1177/0961203309348983. [DOI] [PubMed] [Google Scholar]
- 24.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 25.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 26.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Chu Y, Yang X, Gao D, Zhu L, Yang X, Wan L, Li M. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 28.Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR. Circulating CSF-1 Promotes Monocyte and Macrophage Phenotypes that Enhance Lupus Nephritis. Journal of the American Society of Nephrology. 2009;20:2581–2592. doi: 10.1681/ASN.2009050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh R, Usha, Rathore S, Behura S, Singh N. Urinary MCP-1 as diagnostic and prognostic marker in patients with lupus nephritis flare. Lupus. 2012;21:1214–1218. doi: 10.1177/0961203312452622. [DOI] [PubMed] [Google Scholar]
- 30.Chan RW-Y, Tam L-S, Li EK-M, Lai FM-M, Chow K-M, Lai K-B, Li PK-T, Szeto C-C. Inflammatory cytokine gene expression in the urinary sediment of patients with lupus nephritis. Arthritis Rheum. 2003;48:1326–1331. doi: 10.1002/art.11062. [DOI] [PubMed] [Google Scholar]
- 31.Guo Z, Zheng C, Chen Z, Gu D, Du W, Ge J, Han Z, Yang R. Fetal BM-derived mesenchymal stem cells promote the expansion of human Th17 cells, but inhibit the production of Th1 cells. Eur. J. Immunol. 2009;39:2840–2849. doi: 10.1002/eji.200839070. [DOI] [PubMed] [Google Scholar]
- 32.Traggiai E, Volpi S, Schena F, Gattorno M, Ferlito F, Moretta L, Martini A. Bone marrow-derived mesenchymal stem cells induce both polyclonal expansion and differentiation of B cells isolated from healthy donors and systemic lupus erythematosus patients. Stem Cells. 2013;26:562–569. doi: 10.1634/stemcells.2007-0528. [DOI] [PubMed] [Google Scholar]
- 33.Ren G, Su J, Zhang L, Zhao X, Ling W, L'huillie A, Zhang J, Lu Y, Roberts AI, Ji W, Zhang H, Rabson AB, Shi Y. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 34.Chinnadurai R, Copland IB, Patel SR, Galipeau J. IDO-Independent Suppression of T Cell Effector Function by IFN--Licensed Human Mesenchymal Stromal Cells. J. Immunol. 2014;192:1491–1501. doi: 10.4049/jimmunol.1301828. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Feng X, Lu L, Konkel JE, Zhang H, Chen Z, Li X, Gao X, Lu L, Shi S, Chen W, Sun L. A CD8 T cell-IDO axis is required for mesenchymal stem cell suppression of human SLE. Arthritis Rheumatol. 2014 doi: 10.1002/art.38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.X N, YQ J, WT M, L Z, Y Z. Expression of B7-H1 molecule on human bone marrow mesenchymal stem cells and its effects on T lymphocyte proliferation. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:990–993. [PubMed] [Google Scholar]
- 37.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–4621. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 38.Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-γ does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clinical & Experimental Immunology. 2007;149:353–363. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ho A, Magder LS, Barr SG, Petri M. Decreases in anti-double-stranded DNA levels are associated with concurrent flares in patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:2342–2349. doi: 10.1002/1529-0131(200110)44:10<2342::aid-art397>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, Muroi K, Ozawa K. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–234. doi: 10.1182/blood-2006-02-002246. [DOI] [PubMed] [Google Scholar]
- 41.Polchert D, Sobinsky J, Douglas GW. IFN-γ activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.