Abstract
Pancreatic ductal adenocarcinoma (PDA) arises at the convergence of genetic alterations in KRAS with a fostering microenvironment shaped by immune cell influx and fibrotic changes; identification of the earliest tumorigenic molecular mediators evokes the proverbial chicken and egg problem. Matrix metalloproteinases (MMPs) are key drivers of tumor progression that originate primarily from stromal cells activated by the developing tumor. Here matrix metalloproteinase-3 (MMP3), known to be expressed in PDA, was found to be associated with expression of Rac1b, a tumorigenic splice isoform of Rac1, in all stages of pancreatic cancer. Using a large cohort of human PDA tissue biopsies specimens, both MMP3 and Rac1b are expressed in PDA cells, that the expression levels of the two markers are highly correlated, and that the subcellular distribution of Rac1b in PDA is significantly associated with patient outcome. Using transgenic mouse models, co-expression of MMP3 with activated KRAS in pancreatic acinar cells stimulates metaplasia and immune cell infiltration, priming the stromal microenvironment for early tumor development. Finally, exposure of cultured pancreatic cancer cells to recombinant MMP3 stimulates expression of Rac1b, increases cellular invasiveness, and activation of tumorigenic transcriptional profiles.
Implications
MMP3 acts as a co-conspirator of oncogenic KRAS in pancreatic cancer tumorigenesis and progression, both through Rac1b-mediated phenotypic control of pancreatic cancer cells themselves, and by giving rise to the tumorigenic microenvironment; these findings also point to inhibition of this pathway as a potential therapeutic strategy for pancreatic cancer.
Keywords: Pancreatic cancer, Pancreatitis, Rac1b, MMP3, Actin, Collagen, KRAS, tumor microenvironment, infiltrating immune cells, tumorigenesis
Introduction
Pancreatic cancer is one of the few cancers with rising numbers in cancer death rates, and has one of the worst prognoses of human cancers, with only 6% patient survival at 5 years after diagnosis (1). The patient rarely displays symptoms until the disease has progressed to a metastatic stage, and current standards of treatment are largely palliative (2). Despite extensive research efforts to dissect the mediators that underlie pancreatic cancer initiation and progression, it remains one of the more poorly understood human cancers due to its phenotypic complexity (3).
As in other cancers, malignancies of the pancreas arise and progress via the interplay between genetic alterations within tumor cells and collateral alterations of the surrounding stroma that shape a facilitative tumor microenvironment. Mutations in KRAS are found in more than 90% of pancreatic tumors as well as in many premalignant pancreatic intraepithelial neoplasias (3), implicating this oncogene as an early driver of pancreatic cell malignant transformation. Microenvironmental influences are also critical from the earliest stages of tumorigenesis, often involving tissue inflammation, infiltration of a variety of immune cell types, and activation of fibrosis and fibrotic responses (4,5). To some extent, it can be difficult to untangle the sequence of cause and effect in the coevolution of tumor and microenvironment, particularly in a disease like pancreatic cancer that almost universally presents at an advanced stage.
Matrix metalloproteinases (MMPs) have been implicated in many stages of tumor progression and metastasis (4,6). MMPs can facilitate tumor cell detachment and invasion through degradation of cell adhesion and extracellular matrix (ECM) molecules. They can directly induce genomic instability through disruption of tissue homeostasis (6,7). MMPs can also directly activate cellular invasive characteristics by induction of epithelial-mesenchymal transition (EMT), a programmed phenotypic transformation that is instrumental in developmental processes. Several MMPs can even initiate tumorigenesis in some circumstances; in particular, transgenic expression of MMP3 is capable of stimulating spontaneous tumor development in mammary gland and lung (8–10). MMP3 has also been linked to tumor growth and metastasis in human breast, colon, cervical, and lung cancers (8,9,11–13).
While many secreted MMPs are produced primarily by stromal cells in the tumor microenvironment in response to paracrine cytokine signals (14), MMP3 is expressed directly by pancreatic adenocarcinoma cells (15,16). As MMP3 has been found to drive tumorigenesis and tumor progression by directly stimulating the expression of Rac1b (7,9), a tumorigenic splice isoform of Rac1, and as recent findings indicate that pancreatic tumorigenesis is inhibited in mice with selective knockout of Rac1 (which would also block expression of Rac1b) (17), we hypothesized that MMP3 may play a role in pancreatic cancer tumorigenesis and progression via induction of Rac1b. We evaluated expression of these molecules in a large panel of human pancreatic adenocarcinomas, finding evidence of the involvement of this pathway throughout all stages of pancreatic cancer progression. Using transgenic mouse models, we found evidence that pancreatic acinar cell MMP3 interacts with mutant KRAS to initiate premalignant alterations in the surrounding stroma, including infiltration of immune cells. Using cultured pancreatic adenocarcinoma cells, we found that exposure to MMP3 and activation of Rac1b lead to comprehensive transcriptional and phenotypic alterations consistent with a central role in driving motility and protumorigenic responses. Our study identifies MMP3-induced Rac1b as a potential driving force in multiple stages of pancreatic cancer development.
Methods
Tissue microarray
Patient FFPE Biospecimen Samples mounted as tissue microarrays were obtained through the Mayo Clinic Pancreatic Cancer SPORE. Each slide contains up to 432 spots (16 rows × 27 columns) consisting of 12 process controls and 3 cores from each of the 140 unique patients. The slides were stained with human MMP3 (ProteinTech #17873-1-AP), human Rac1b (Millipore #09-271), human αSMA (Abcam #ab5694), and human Collagen (Abcam #ab34710). Tissue spots were analyzed by morphological analysis and protein expression was evaluated using TMA-lab (Image Scope Software, Aperio Technologies) and a color deconvolution algorithm to analyze intensity and positivity for the individual stains and to generate an H-score (scale 0-300) that was used for correlation and grade distribution analysis. H-scores are calculated as 1.0*(%weak positive)+2.0*(%median positive)+3.0*(%strong positive) (18). The morphological analysis was performed manually, differentiating the biopsies into two groups. One group contained tumors with baseline and polar Rac1b staining. Baseline staining was defined as diffuse, homogenous staining of the tumor cells, and polar staining showed in addition to baseline stain the accumulation of Rac1b in tight conglomerates that were very well organized at the luminal side of the nuclei. The second group included tumors that showed apolar Rac1b stain, defined as accumulation of Rac1b stain in a manner that is unsystematically distributed within the cytoplasm of the tumor cells. Investigators performing spot scoring and morphological analysis were blinded to patient characteristics and disease outcome.
Cell culture
PaTu8988T cells were a kind gift from Dr. Yaohe Wang, London. Cells were cultured in DMEM with 5% fetal bovine serum (FBS) and 5% horse serum (HS) at 37°C in 5% CO2 and grown to 80% confluency. PancTu1 cells were a kind gift from Drs. Christian Roeder and Holger Kalthoff, Kiel. Cells were cultured in RPMI-1640 with 10% FCS and Glutamax. For treatment with MMP3, PaTu8988T cells were plated at a density of 5×105 cells in 6-well plates. Experimental setup included two controls (complete media and serum free media). The test cells were incubated with recombinant MMP3 (rMMP3) at concentrations 25U or 100U in serum free media. Treatment with fresh rMMP3 was given on day 0, and day 2; RNA was harvested for further analysis on day 4. For knockdown of MMP3 in PancTu1 cells, we used Sigma Mission shRNA, catalog NM_002422.x-1512s1c1. Each study was performed at least two times in triplicate.
Transcriptional analysis
Samples of PaTu8988T cells were cultured in growth media (control) or growth media supplemented with 100U/ml recombinant MMP3 for three days. Isolated total RNA was assessed with Affymetrix U130A gene expression chips which were processed and normalized via GCRMA. Duplicate technical replicates were averaged and then analyzed using Genespring GX via t-tests using previously described methods (9,19). Thus, results presented are averages of two separate experiments performed in duplicate. Transcriptional profiles have been deposited in Gene Expression Omnibus (GEO Archive #GSE50931). Nextbio (www.nextbio.com) meta-analysis (20) was performed to identify similarities between our dataset and published datasets as previously described (21). Ingenuity Pathway Analysis (IPA) was performed using the web interface (www.ingenuity.com) to build gene interactions associated with response to MMP3.
Quantitative real-time PCR was performed on reverse-transcribed cDNA, using an ABI 7900HT Fast-Real Time PCR System. TaqMan assays were purchased from ABI: MMP3 (Rn00591740_m1), Rac1b (custom assay, sequence: CCGCAGACAGTTGGAGA), and GAPDH (Hs99999905_m1) as an endogenous control and tested over 40 cycles. The data was analyzed using SDS RQ Manager Software.
Invasion assay
The in vitro cell invasion assay was performed using BD BioCoat Cell culture inserts (8.0μm, 24-well format, BD Biosciences, Cat#354578) following the manufacturer's protocol with the following modifications. The cell culture inserts were evenly coated with diluted Matrigel (0.5 mg/ml dilution with serum free DMEM medium) and incubated at 37°C for 4 hours. During the last hour of incubation, the lower chamber was filled with 700 μl DMEM medium containing 10% horse serum. In triplicate, 2×105 PaTu8988T cells in DMEM medium with 0.1% BSA were added to the upper chamber with the addition of 100U of rMMP3 and incubated at 37°C for 18 hours. Cells on the top side of the membrane were removed with cotton-tipped swabs, and the inserts were fixed with 100% methanol at -20°C for 20 minutes, and stained with 0.5% crystal violet for 1 hour. Pictures of the stained inserts were taken using a 2× objective lens and counted using Image-Pro 6.3 software (Media Cybernetics). The results are presented as the relative invasion compared to untreated control cells.
Ethics Statement
Animal studies were conducted with the approval of the Mayo Clinic Institutional Animal Care and Use Committee (IACUC) (protocol # A511) and under strict accordance with the recommended guidelines.
Transgenic expression of MMP3
Transgenic mice used in this study were generated by crossing tetracycline-regulated MMP3 transgenic mice (tet-MMP3; (9)) with transgenic mice in which the tetracycline transactivator was driven by the human elastase promoter (Hsel-tTA; (22)) and tetracycline-regulated G12V KRAS mice (tet-KRAS; (22)) (Hsel-tTA and tet- KRAS mice were kind gifts from Eric Sandgren, University of Wisconsin, Madison; their characterization was described in Guerra et al. (22)) under negative Dox control. Female parents and nursing pups were maintained on doxycycline (in food 500mg/kg) from conception until weaning (21 days after birth). MMP3×KRAS×Hsel transgenic mice were sacrificed after 24 weeks, the pancreas and other organs were removed, formalin fixed and paraffin embedded for immunohistochemistry staining with H&E, MMP3, Rac1b, KRAS (Abcam #ab81075), and F4/80 (AbdSerotec #MCA497G).
Statistical analysis
Mean patient level H-score for MMP3 and Rac1b staining intensity for each of the patients with results available was compared across patient grade using a Kruskal-Wallis Test. The maximum H-score value across all cores for a patient was also considered. Patient samples expressing Rac1b (N=106) were classified by morphologic staining pattern by CM and DCR without knowledge of patient characteristics and disease outcome; comparison of patients expressing baseline and organized Rac1b expression vs apolar disorganized Rac1b expression were compared using Kaplan-Meier Curves and logrank tests. Patient demographics were summarized as median +/- interquartile range (IQR) for continuous variables (Age and Usual Adult BMI) and Frequency (n) and percentage for categorical variables. Statistical analysis related to the TMA scoring and relevant patient characteristics was performed using SAS/STAT software, version 9.2 of the SAS System for UNIX. Correlation analysis was conducted with the use of Prism version 4.0 (GraphPad Software, La Jolla, CA, USA), using Pearson's test.
Results
Correlation of Rac1b and MMP3 expression in human pancreatic cancer biopsies
We stained a tissue microarray of 140 patients with human pancreatic adenocarcinoma (Table 1) for expression of Rac1b and MMP3, as well as αSMA and Collagen-I, markers of fibrosis, a common comorbidity of pancreatic cancer and predisposing factor in cancer progression (23). We found that MMP3 and Rac1b were expressed specifically in the pancreatic cancer tumor cells (Fig. 1A; Supplemental Fig. 1A). We used TMA-lab to generate a numeric score for protein expression for each biomarker, producing an H-score (18) for each marker and each tissue spot. This method of quantification has been used to assess staining intensity of lung, ovarian, and prostate cancers (18), and is a fully automated method that reduces human bias. We found the expected association between the markers of the desmoplastic response, αSMA and Collagen-I (Supplemental Fig. 1B), and a much stronger association between MMP3 and Rac1b expression (Fig. 1B), but a much weaker association between MMP3 and either Collagen-I or αSMA (Supplemental Fig. 1C,D), or between Rac1b and either Collagen-I or αSMA (Supplemental Fig. 1E,F). Although expression of MMP3 in epithelial cells has been noted before in association with pancreatitis (15), the lack of an association here was not surprising, since MMP3 and Rac1b are both expressed in epithelial tumor cells while Collagen-I and αSMA are expressed in the stroma (Fig. 1A; Supplemental Fig. 1A), and correlations at the whole tumor level could have been masked by random variability in the tumor/stroma composition of the biopsy cores used in this study.
Table 1. Patient Demographic Characteristics.
| Characteristics | n | (%) |
|---|---|---|
| Number of Patients | 140 | |
| Age, Median (IQR) | 68.0 | (58.0, 74.0) |
| Gender – Male | 82 | 58.6 |
| Pancreas Cancer Stage | ||
| Resectable | 99 | 90.8 |
| Locally advanced | 6 | 5.5 |
| Metastatic | 4 | 3.7 |
| Grade | ||
| 1 & 2 | 20 | 14.3 |
| 3 | 92 | 65.7 |
| 4 | 28 | 20.0 |
| Usual Adult BMI, Median (IQR) | 27.5 | (24.9, 30.3) |
| Usual Adult BMI ≥ 30 | 17 | 27.0 |
| DM – Positive Self-Report | 22 | 18.6 |
| Pancreatitis – Positive Self-Report | 34 | 28.8 |
| Vital Status – Deceased | 111 | 79.3 |
Figure 1. MMP3 and Rac1b are expressed in pancreatic adenocarcinoma cells.
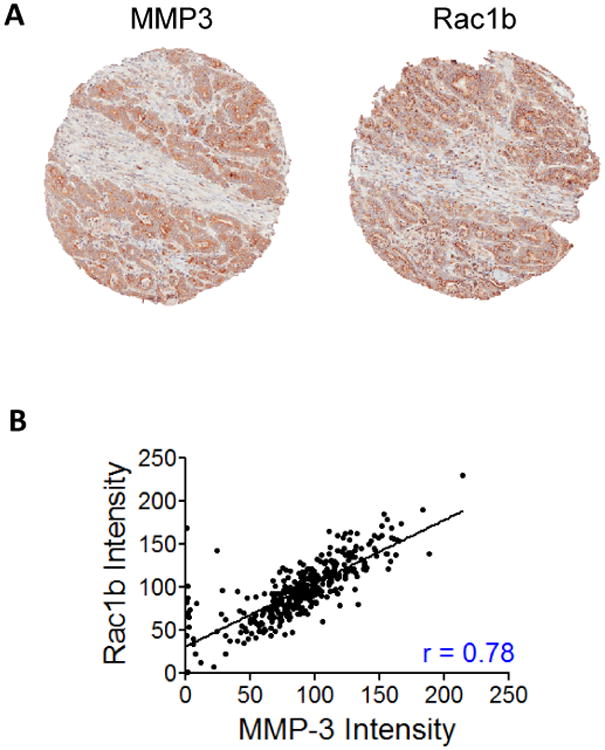
(A) Representative tissue biopsy section from pancreatic adenocarcinoma tissue microarray, stained with hematoxylin and eosin (H&E), α-smooth muscle actin (αSMA), Collagen-1 (Collagen), MMP3, and Rac1b. (B) Correlation plot of H-Score quantification for Rac1b vs MMP3. rvalues for Pearson's correlations is indicated.
We next assessed the relationship between staining intensity of MMP3 or Rac1b (Figure 2A,B) and patient tumor characteristics. The lowest scores for MMP3 and Rac1b were found in samples with very few tumor cells (Fig. 2A,B, upper left panels), consistent with expression of these markers primarily in the cancer cells themselves. We found a Gaussian distribution of expression values for both MMP3 and Rac1b (Fig. 2C,D). The MMP3 and Rac1b H-score values from each of the three tissue spots per patient were averaged to generate mean intensity scores; when these values were segregated by cancer grade at diagnosis, we found a significant, progressive increase in both MMP3 (p=0.0007, Kruskal-Wallis test; Fig. 2E) and Rac1b (p=0.0008, Kruskal Wallis test; Fig. 2F) expression in patients from grades 2-4 (full analysis in Supplemental Table 1). These results indicate that expression of MMP3 and Rac1b is increased as pancreatic cancers progress in grade.
Figure 2. Expression of MMP3 and Rac1b is correlated with tumor grade.
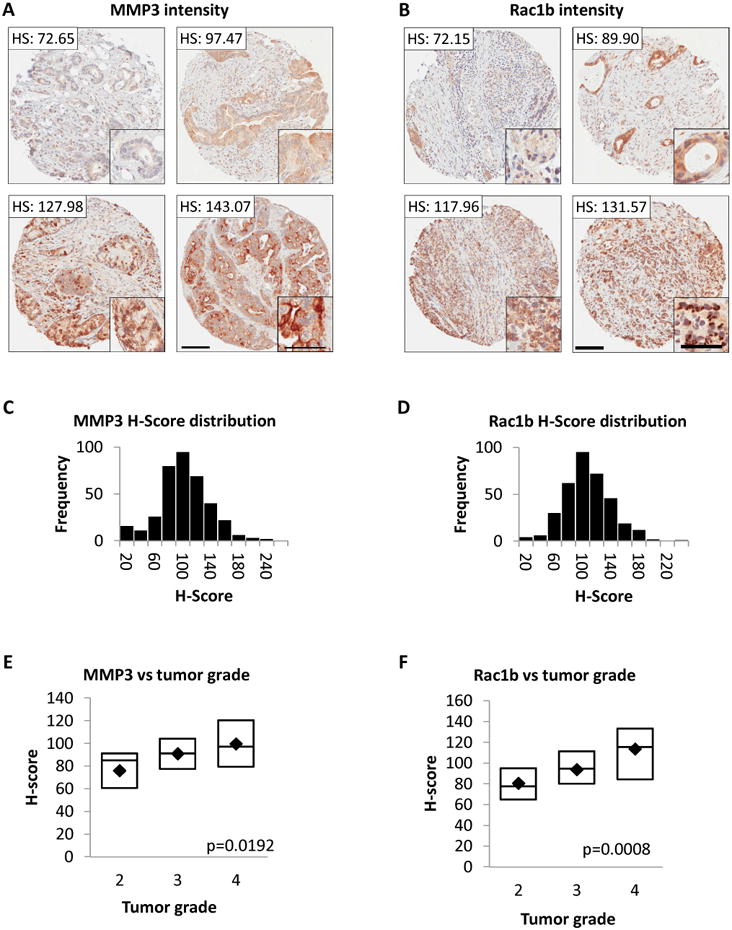
(A-B) H-scores (HS) for four different biopsy sections for MMP3 (A) or Rac1b (B); insets show protein staining in tumor cells. Scale bars 50 μm large images; 100 μm insets. (C-D) H-score distributions for MMP3 (C) or Rac1b (D). (E-F) Box plots of mean H-scores (average score of 3 spots per patient) for MMP3 (E) or Rac1b (F) vs tumors grade 2 (N=19), grade 3 (N=89), or grade 4 (N=28). Boxes indicate median, 25th and 75th quartiles; marker indicates mean. Indicated p values from Kruskal-Wallis test.
Some cancer biomarkers have been found to have different prognostic implications when localized to specific subcellular domains (21,24). In the course of sample analysis, we observed distinct morphological staining patterns in the patient samples staining positive for Rac1b (representing 106 of the total 120 patients). We identified three distinct staining patterns (Fig. 3A): diffuse cytoplasmic staining only (baseline; N=69), cytoplasmic staining with punctate polar staining (N=19), and cytoplasmic staining with punctate apolar staining (N=18). We compared outcomes for patients expressing baseline and polar Rac1b expression (N=88) vs apolar disorganized Rac1b expression (N=18), and found that the punctate apolar group showed significantly poorer survival with a median of 12.02 months compared to 21.06 months for the remaining patients (p=0.0175, logrank test; Fig. 3B). We observed no significant differences in quantified Rac1b staining levels between the different staining patterns (not shown); therefore, while Rac1b expression increases with tumor grade (Fig. 2F), it is the localization of Rac1b expression that is most important for prognostic outcome. Further multivariate analyses adjusting for stage or grade did not attenuate risk (Supplemental Fig. 2), suggesting that the poor prognosis associated with apolar expression of Rac1b was independent of patient stage or grade. We also found that apolar expression of Rac1b was associated with higher expression of both MMP3 and Rac1b, although the observed differences were only marginally significant (Supplemental Table 2).
Figure 3. Rac1b subcellular localization is associated with patient prognosis.
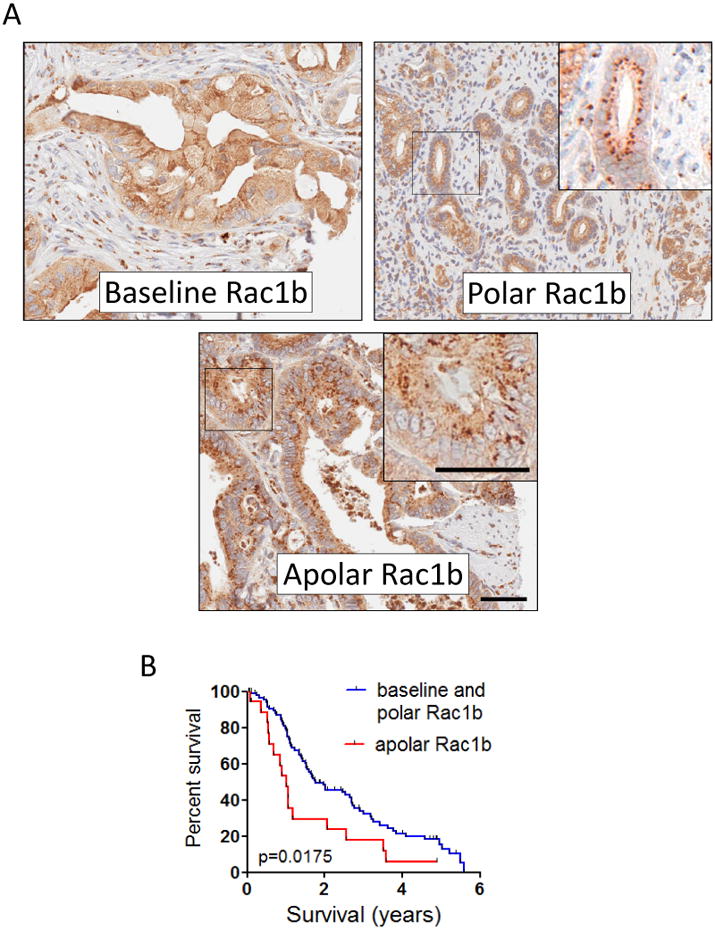
(A) Representative images of Rac1b IHC staining classified as cytoplasmic diffuse (baseline staining), punctate polar, and punctate apolar; insets show magnification of indicated region, highlighting punctate polar vs punctate apolar stating. Scale bar, 50 μm. (B) Significantly shorter survival for patients showing apolar Rac1b staining (N=18) vs patients showing baseline and polar (N=88). p=0.014, logranktest.
MMP3 expression combined with activated KRAS stimulates Rac1b expression, cellular metaplasia, and macrophage influx in transgenic mice
Our studies with human pancreatic adenocarcinoma tissue biopsies had shown that MMP3 was expressed at all stages of pancreatic cancer, where its expression levels were highly correlated with the tumorigenic signaling molecule Rac1b. A prior study had reported epithelial-associated MMP3 expression in 80% of pancreatitis patients (15); this inflammatory condition is a well-established risk factor for later development of pancreatic cancer (23). Together, these observations suggested the possibility that MMP3 expression may precede pancreatic cancer and represent a factor driving tumor development. Since nearly all pancreatic cancers arise in association with oncogenic KRAS mutations, we investigated the impact of MMP3 expression on premalignant tissue alterations in a pancreas-directed oncogenic KRAS mouse model.
We generated transgenic mice in which MMP3 and constitutively active KRASG12V were inducibly expressed by pancreatic acinar cells by combining mouse strains in which (a) the tetracycline transactivator is controlled by the human elastase promoter (Hsel-tTA; (22)), (b) KRASG12V is controlled by the tetracycline transactivator (tet-KRAS; (22)), and (c) auto-activated MMP3 is controlled by the tetracycline transactivator (tet-MMP3; (9)). Transgenes were activated at 3 weeks by removal of doxycycline, and mice were maintained for 21 additional weeks, at which time mice were sacrificed and pancreata were fixed and stained for expression of F4/80, a marker of macrophage influx that is an early indicator of pancreatitis and also is a risk factor for pancreatic cancer development (25), as well as MMP3, KRAS, and Rac1b. We saw a significant increase in incidence of F4/80-positive foci in mice which expressed both MMP3 and KRAS (n=5; p=0.035, Fisher's exact test) as compared with mice expressing no transgenes (n=5; tet-KRAS/tet-MMP3), only MMP3 (n=5; Hsel-tTA/tet-MMP3), or only activated KRAS (n=4; Fig. 4A). Parallel sections of Hsel-tTA/tet- KRAS/tet-MMP3 pancreata stained for MMP3 transgene and Rac1b (9), showed that F4/80-positive foci were associated with transgenic expression of MMP3 and induction of Rac1b and were uncommon in nontransgenic mice (Fig. 4B). Expression of MMP3 and activated KRAS was also sufficient to induce fibrotic changes, as indicated by expression of smooth muscle actin and collagen, accompanied by signs of early metaplasia, including acinus dilation and concomitant expression of cytokeratin 19 in the parenchyma (Supplemental Fig. 3). These results indicate that expression of MMP3 along with activated KRAS is sufficient not only to stimulate neoplastic phenotypic alterations in pancreatic acinar cells, but also to recruit infiltrating immune cells and to initiate fibrotic changes, priming the stromal microenvironment.
Figure 4. Expression of MMP3 and activated KRAS in pancreatic acinar cells stimulates metaplasia and immune cell invasion in transgenic mice.
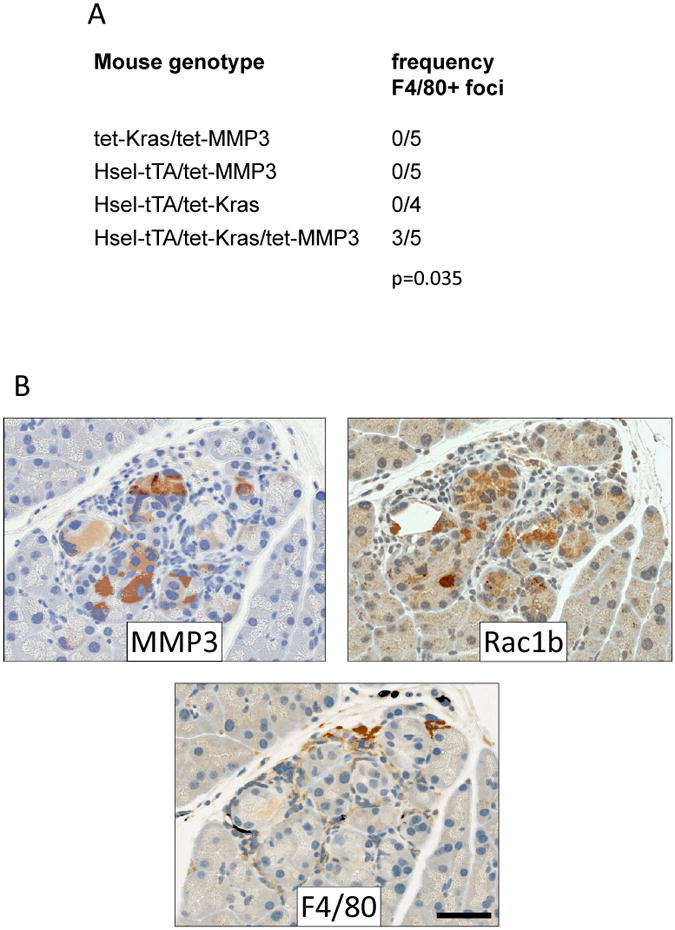
(A) Incidence of F4/80-positive foci in transgenic mice with indicated genotypes when induced for transgene expression for 21 weeks. p-value, Fisher's exact test. (B) Staining of MMP3 and Rac1b in representative F4/80 positive region in Hsel-tTA/tet- KRAS/tet-MMP3 pancreata. Scale bar, 50 μm.
MMP3-induced Rac1b is associated with a tumorigenic expression profile in cultured pancreatic adenocarcinoma cells
To directly assess the effect of activation of the MMP3/Rac1b pathway in pancreatic adenocarcinoma cells, we used the PaTu8988T and PancTu1 pancreatic cancer cells. PaTu8988T cells are invasive (26) and do not express detectible MMP3, while PancTu1 cells are more aggressive and express MMP3 (not shown). We tested the effect of exposure of PaTu8988T cells to recombinant MMP3, and found that 3 days of treatment with 100U/ml MMP3 stimulated increased significantly increased expression of Rac1b by QPCR (Fig. 5A). MMP3-treated cultured cells also showed increased cell spreading and separation from adjacent cells (not shown), morphological alterations associated with tumor progression and increased motility. Consistent with this, we found that MMP3-treated cells also showed increased invasiveness in Boyden chamber assays (Fig. 5B), suggestive of conversion to a more aggressive cancer phenotype. We also tested the effect of knockdown of MMP3 in the PancTu1 cells, finding that decreased expression of MMP3 (Fig. 5C) was associated with decreased expression of Rac1b (Fig. 5D) and decreased invasiveness (Fig. 5E), consistent with a direct role for MMP3-induced Rac1b in pancreatic cancer cell invasiveness.
Figure 5. MMP3 activates tumorigenic phenotypic characteristics.
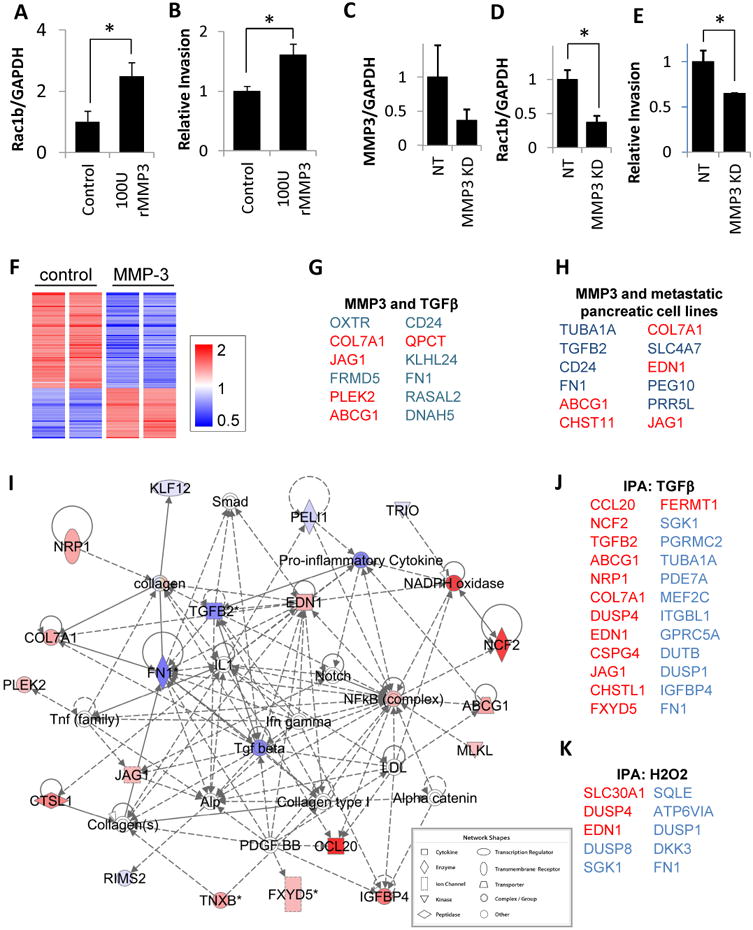
(A-B) In PaTu8988T cells, treatment with 100 U/ml recombinant MMP3 induces increased expression of Rac1b (A) and cellular invasiveness (B). (C-E) In PancTu1 cells, knockdown of endogenous MMP3 (C) leads to decreased expression of Rac1b (D) and decreased invasiveness (E). Graphs show means±SEM; *, p<0.05, unpaired t-test (p=0.030 for PaTu8988T Rac1b expression, p=0.039 for PaTu8988T invasion, p=0.022 for PancTu1 Rac1b expression, p=0.020 for PancTu1 invasion). (F) Heat map of 141 transcripts differentially regulated in PaTu8988T cells exposed to MMP3 (p<0.05, FC>1.5) (G-H) Top 12 coregulated transcripts between genes differentially regulated in response to MMP3 and (G) a dataset assessing response of Panc-1 cells to TGFβ, and (H) two different datasets assessing increased tumorigenic potential in pancreatic cancer cells; upregulated transcripts are red; transcripts downregulated are blue. (I) Ingenuity Pathway Analysis (IPA) network of response to MMP3 (intensity of color indicates degree of regulation by MMP3). Direct interactions are indicated by solid lines, indirect by dashed lines. (J-K) IPA also revealed enrichment for genes activated by TGFβ (J, overlap p-value 7.15E-16) and H2O2 (K, overlap p-value 9.28E-06).
We then performed transcriptional profiling of the untreated and MMP3-treated PaTu8988T cells, and found substantial alterations associated with exposure to MMP3 (Fig. 5F); 141 transcripts were differentially regulated (p<0.05, FC>1.5, annotated expression data in Supplemental Table 3). Consistent with previous studies implicating MMP3 in activation of cell motility and induction of cell shape change (7,27), gene ontology analyses showed regulation of transcripts associated with cell adhesion (p=1.59E-06), cellular morphogenesis (p=1.61E-4), cell migration (p=3.46E-4), and response to wounding (p=1.18E-3; all categories with significant overlap listed in Supplemental Table 4).
We subjected the list of differentially expressed genes to a NextBio meta-analysis (20), and found significant overlap with a dataset comparing Panc-1 cells treated with TGFß vs. untreated (28) (Fig. 5G; Supplemental Fig. 4A; Supplemental Table 5), and with differentially expressed genes in pancreas cancer cell datasets comparing profiles of metastatic AsPC-1 cells vs. nonmetastatic BxPC-3 cells (29) (Supplemental Fig. 4B) and of PaTu8988S cells vs. PaTu8988T cells (30) (Fig. 5H; Supplemental Fig. 4C; Supplemental Table 6). These results suggest that the MMP3 that is expressed in pancreatic cancer cells (Fig. 1, refs (15,16)) induces cell motility transcriptional processes and activates pro-tumorigenic responses; similar effects of MMP3 have been documented in breast and lung cancer (7,9). Consistent with these results, Ingenuity Pathway Analysis (IPA) of the differentially expressed genes identified a top-ranked interaction network that included a prominent nexus of molecules associated with TGFß (Fig. 5I), a well-known mediator of cell scattering that is induced by MMP3 (7). IPA analysis also identified significant enrichment of genes predicted to be regulated by TGFß (Fig. 5J; overlap p-value 7.15E-06), as well as genes predicted to be regulated by hydrogen peroxide (H2O2; Fig. 5K), which we have shown to be an effective inducer of MMP3-activated pathways (7,27,31). Significantly, TGFβ is a key signaling activator of tumor-associated macrophages and of fibrotic alterations (32,33), effects we observed in our transgenic mice (Fig. 4), and we found further evidence of TGFβ signaling in our transgenic mice through nuclear localization of pSMAD2 (Supplemental Fig. 5)
Discussion
Here we have dissected the relationship between MMP3 expression in pancreatic cancers, consequent induction of Rac1b, and the role of MMP3 and Rac1b in development and progression of pancreatic cancer. Our analysis of the pancreatic adenocarcinoma tissue biopsy samples demonstrated that MMP3 and Rac1b are expressed in pancreatic adenocarcinoma cells (Fig. 1A), that intensity of MMP3 and Rac1b staining are closely correlated (Fig. 1B), and that both show progressively increased staining in higher grade cancers (Fig. 2E,F). We also found that the localization of Rac1b in pancreatic adenocarcinoma cells is associated with patient survival (Fig. 3). We found that increased expression of MMP3 in transgenic mice by pancreatic acinar cells along with expression of KRAS was sufficient to recruit infiltrating immune cells, upregulate Rac1b, and stimulate a premalignant morphological phenotype (Fig. 4), and that exposure of human pancreatic carcinoma cells to MMP3 was sufficient to stimulate expression of Rac1b and to activate an invasive phenotype associated with tumor progression (Fig. 5A-C). These results identify a role for MMP3 first as a coconspirator of oncogenic KRAS in orchestrating pancreatic cancer tumorigenesis while priming the activated stroma, and later as a critical driver of tumor invasion and progression.
Multiple pathways are activated during cancer development; improving current standards of therapy will require a better understanding of which tumor-associated processes are specifically involved in driving tumor progression. Here, we have identified a mechanistic link between MMP3 and Rac1b in pancreatic cancer. Rac1b, a Rho-GTPase family member that has been identified in colon, breast, and lung cancer (9,34,35) has been implicated as a driver of progression and metastasis in these cancer types. MMPs have also been studied for their involvement in these processes (36), and MMP3 specifically has been shown to be expressed not only in pancreatic cancer, but also in chronic pancreatitis, a risk factor for later development of pancreatic cancer (15,16). Here we show that MMP3 and Rac1b are factors associated with patient disease stage and prognosis, are capable of inducing metaplastic changes in the pancreas, and can activate tumorigenic and invasive phenotypes in cultured pancreatic cells. While direct targeting of MMPs has not been successful clinically (37), our results suggest that targeting MMP3-induced Rac1b could be an effective strategy. One possible approach might be to modulate the splicing factors involved in Rac1b expression (38,39), although specific targeting of splicing factors has been challenging (40).
In our patient cohort we found a clear correlation between MMP3 and Rac1b staining intensity and a significant association between overall staining intensity of both proteins and tumor grade (Figs. 1 and 2). However, we found the strongest predictor of patient prognosis was Rac1b localization, not overall intensity (Fig. 3), in which the observed Rac1b staining patterns could be differentiated into baseline and polar staining, defined as polarized distribution at the luminal side of the nuclei, as compared with apolar staining, in which accumulated Rac1b showed a disorganized distribution throughout the cytoplasm of the cells. A correlation of staining localization with tumor progression has been found also for a number of other biomarkers (21,24), where subcellular relocalization is associated with transition to a more advanced stage of tumor development. In previous studies using breast tissue samples, polarized expression of Rac1b in normal breast epithelium was found to give way to depolarized Rac1b in breast cancer (31); similarly, we found here that an apolar staining pattern of Rac1b is associated with significantly decreased survival time in our patient cohort (Fig. 3B), and that this risk was not attenuated when patient stage or grade was incorporated into multivariate analyses (Supplemental Fig. 2). We also found that apolar distribution of Rac1b was associated with increased expression of both MMP3 and Rac1b (Supplemental Table 2). These results may be linked with the importance of tissue polarity as a barrier for tumor progression (41). Whether the apolar distribution of Rac1b found in our poor prognosis patient group is associated with a more general breakdown of tissue polarity or whether it is directly caused by Rac1b itself is unclear, although support for the latter possibility can be derived from the fact that Rac1b has been found to drive EMT, a developmental process associated with tumor malignancy (7,9,27). We found that treatment of pancreatic cancer cells with MMP3 led to increased expression of Rac1b, associated with cell spreading, migration away from colonial growth patterns, and increased cellular invasiveness (Fig. 5A-C). Defining a direct role for Rac1b depolarization these processes will require additional investigations using 3D tissue models in which the roles of Rac1b localization, tissue structure formation, and cellular invasiveness can be dissected.
Activating mutations of KRAS are found in most pancreatic cancers (3), and expression of activated KRAS is necessary for initiation and maintenance of pancreatic cancer in mouse models (42). However, expression of activated KRAS is not sufficient: additional alterations are required for the development of pancreatic lesions, such as inflammation-induced reprogramming (22) and activation of EGFR (43). We found that transgenic expression of MMP3 in pancreatic acinar cells induced expression of Rac1b, and MMP3 expressed in combination with activated KRAS in pancreatic acinar cells led to metaplasia and macrophage influx (Fig. 4). It may be that expression of Rac1b is the key step in this process: increased expression of Rac1 has been found in human pancreatic cancer biopsies (44), and selective knockout of Rac1 in pancreatic progenitor cells (which would also block expression of Rac1b) inhibited KRAS-associated tumor development (17).
The specific mechanism by which Rac1b may induce premalignant alterations in pancreatic tumor cells remains to be elucidated. Rac1b protein shows reduced intrinsic GTPase activity and altered effector protein binding (45), resulting in phenotypic alterations in cytoskeletal structure and cellular morphology (7,27). Rac1b also activates NADPH oxidase (31), which has been found to be a critical factor in Rac1-associated growth of pancreatic cancer (46). We have previously shown that MMP3/Rac1b-induced reactive oxygen species (ROS) can induce genomic instability in mammary epithelial cells (7); our identification here that MMP3/Rac1b-induced alterations in cultured pancreatic cancer cells include activation of hydrogen peroxide-associated pathways (Fig. 5I) suggests that MMP3/Rac1b could be driving pancreatic tumor progression through activation of genomic instability as well. Further experiments will be required to dissect the specific role of Rac1b in pancreatic cancer tumorigenesis and progression.
MMPs have been extensively investigated as effectors of cancer progression and facilitators of metastasis (47). Many of these studies have focused on MMPs as effectors of tumor spread through their role as ECM-degrading enzymes. Here, we have focused on MMP3, a protease that has been comparatively little studied in the context of pancreatic cancer. It has been found in well differentiated tumors (15,16) and in the serum of patients with pancreatic adenocarcinoma at later stages of tumor development (48). By contrast, MMP2, MMP7, MMP9 and MMP14 have been investigated for their roles in earlier stages of pancreatic cancer development (16). Here, we have implicated a potential role for MMP3 in the earliest stages of tumor development, as our transgenic mice show cellular metaplasia and influx of inflammatory cells associated with expression of MMP3 acting synergistically with activated KRAS (Fig. 4).
Influx of inflammatory cells serves to transform the stromal microenvironment to promote tumorigenesis and sustain tumor growth. Infiltrating immune cells supply paracrine growth factors and angiogenic factors that stimulate and feed proliferation of neoplastic cells, as well as a battery of diverse proteolytic enzymes that modify the structure and function of the ECM (49). Macrophages and neutrophils are particularly notable for their production of MMP9, an enzyme that serves critical functions early in carcinogenesis by stimulating hyperproliferative growth and triggering angiogenesis (49). MMP9 is secreted as a proenzyme and requires proteolytic activation in the tumor microenvironment; MMP3 is a potent activator of MMP9 of probable physiological significance (50). Thus, it is very plausible that pancreatic epithelial cell-produced MMP3 shapes the stromal microenvironment preliminary to and throughout tumorigenesis not only by stimulating immune cell influx, but also as a primary mediator of MMP9 activation. While further studies are needed to define mechanistic details, the present work identifies MMP3 as an epithelial produced mediator of multiple heterotypic interactions that induce a tumorigenic microenvironment.
Conclusions
In summary, we have used human tumor tissue samples to show that Rac1b is associated with MMP3 in pancreatic cancer and has predictive potential for patient survival. We have also found that MMP3 can directly induce Rac1b and tumorigenic alterations in transgenic mouse models and in cultured pancreatic tumor cells. While most tumor-associated MMPs have been identified as produced by surrounding stromal cells as a consequence of tumor cell-derived signals (16), we have found MMP3 to be expressed specifically in the epithelial cells of our patient samples, consistent with previous results (15). Our results suggest that production of MMP3 by pancreatic epithelial cells precedes tumor initiation and continues throughout tumor progression, and that MMP3 is a coconspirator of activated KRAS both in inducing tumorigenic changes in epithelial cells themselves, and in promoting the establishment of a tumorigenic microenvironment. These findings also emphasize the potential therapeutic value of targeting the specific downstream effects of MMP3 in pancreatic cancer.
Supplementary Material
Acknowledgments
Financial support: We acknowledge support from the NCI (D.C.R., CA122086; E.S.R, CA154387; H.C.C., R01CA159222 and R01CA136754), and from the Mayo Clinic SPORE in Pancreatic Cancer (P50 CA102701).
Abbreviations
- MMP
matrix metalloproteinase
- PDA
pancreatic ductal adenocarcinoma
- TMA
tissue microarray
- H&E
hematoxylin and eosin
- αSMA
α-smooth muscle actin
- ECM
extracellular matrix
- IHC
immunohistochemistry
- EMT
epithelial-mesenchymal transition
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests
Authors' contributions: Conception and design: C. Mehner, D.C. Radisky
Development of methodology: C. Mehner, E. Miller, D. Khauv, L. Zhang, D.C. Radisky
Acquisition of data (provided animals, acquired and managed patients, provided facilities, provided recombinant MMP3, etc.): C. Mehner, E. Miller, D. Khauv, A. Nassar, E. S. Radisky
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C. Mehner, A. Oberg, W.R. Bamlet, D.C. Radisky
Writing, review, and/or revision of the manuscript: C. Mehner, E.S. Radisky, H.C. Crawford, D.C. Radisky, A. Oberg
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): C. Mehner, A. Oberg, W.R. Bamlet, L. Zhang, D.C. Radisky
Study supervision: C. Mehner, E.S. Radisky, H.C. Crawford, D.C. Radisky
References
- 1.American Cancer Society. Cancer Facts & Figures 2013. Atlanta: American Cancer Society; 2013. [Google Scholar]
- 2.Costello E, Greenhalf W, Neoptolemos JP. New biomarkers and targets in pancreatic cancer and their application to treatment. Nat Rev Gastroenterol Hepatol. 2012;9:435–44. doi: 10.1038/nrgastro.2012.119. [DOI] [PubMed] [Google Scholar]
- 3.Jones S, Zhang X, Parsons DW, Lin JCH, Leary RJ, Angenendt P, et al. Core Signaling Pathways in Human Pancreatic Cancers Revealed by Global Genomic Analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Radisky DC, Bissell MJ. Matrix metalloproteinase-induced genomic instability. Curr Opin Genet Dev. 2006;16:45–50. doi: 10.1016/j.gde.2005.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sternlicht MD, Lochter A, Sympson CJ, Huey B, Rougier JP, Gray JW, et al. The Stromal Proteinase MMP3/Stromelysin-1 Promotes Mammary Carcinogenesis. Cell. 1999;98:137–46. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stallings-Mann ML, Waldmann J, Zhang Y, Miller E, Gauthier ML, Visscher DW, et al. Matrix Metalloproteinase Induction of Rac1b, a Key Effector of Lung Cancer Progression. Sci Transl Med. 2012;4:142ra95–142ra95. doi: 10.1126/scitranslmed.3004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kessenbrock K, Dijkgraaf GJP, Lawson DA, Littlepage LE, Shahi P, Pieper U, et al. A Role for Matrix Metalloproteinases in Regulating Mammary Stem Cell Function via the Wnt Signaling Pathway. Cell Stem Cell. 2013;13:300–13. doi: 10.1016/j.stem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis. 2005;22:237–46. doi: 10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- 12.Lièvre A, Milet J, Carayol J, Le Corre D, Milan C, Pariente A, et al. Genetic polymorphisms of MMP1, MMP3 and MMP7 gene promoter and risk of colorectal adenoma. BMC Cancer. 2006;6:270. doi: 10.1186/1471-2407-6-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagemann T, Bozanovic T, Hooper S, Ljubic A, Slettenaar VIF, Wilson JL, et al. Molecular profiling of cervical cancer progression. Br J Cancer. 2007;96:321–8. doi: 10.1038/sj.bjc.6603543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radisky ES, Radisky DC. Stromal induction of breast cancer: inflammation and invasion. Rev Endocr Metab Disord. 2007;8:279–87. doi: 10.1007/s11154-007-9037-1. [DOI] [PubMed] [Google Scholar]
- 15.Bramhall SR, Stamp GW, Dunn J, Lemoine NR, Neoptolemos JP. Expression of collagenase (MMP2), stromelysin (MMP3) and tissue inhibitor of the metalloproteinases (TIMP1) in pancreatic and ampullary disease. Br J Cancer. 1996;73:972–8. doi: 10.1038/bjc.1996.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardito CM, Briggs CD, Crawford HC. Targeting of extracellular proteases required for the progression of pancreatic cancer. Expert Opin Ther Targets. 2008;12:605–19. doi: 10.1517/14728222.12.5.605. [DOI] [PubMed] [Google Scholar]
- 17.Heid I, Lubeseder-Martellato C, Sipos B, Mazur PK, Lesina M, Schmid RM, et al. Early requirement of Rac1 in a mouse model of pancreatic cancer. Gastroenterology. 2011;141:719–730. 730.e1. doi: 10.1053/j.gastro.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Galgano MT, Hampton GM, Frierson HF. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol. 2006;19:847–53. doi: 10.1038/modpathol.3800612. [DOI] [PubMed] [Google Scholar]
- 19.Cichon MA, Gainullin VG, Zhang Y, Radisky DC. Growth of lung cancer cells in three-dimensional microenvironments reveals key features of tumor malignancy. Integr Biol (Camb) 2012;4:440–8. doi: 10.1039/c1ib00090j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kupershmidt I, Su QJ, Grewal A, Sundaresh S, Halperin I, Flynn J, et al. Ontology-based meta-analysis of global collections of high-throughput public data. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0013066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu B, Gonzalez A, Massion PP, Shyr Y, Shaktour B, Carbone DP, et al. Nuclear survivin as a biomarker for non-small-cell lung cancer. Br J Cancer. 2004;91:537–40. doi: 10.1038/sj.bjc.6602027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, et al. Chronic Pancreatitis Is Essential for Induction of Pancreatic Ductal Adenocarcinoma by K-Ras Oncogenes in Adult Mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Mehner C, Radisky DC. Triggering the landslide: The tumor-promotional effects of myofibroblasts. Exp Cell Res. 2013 doi: 10.1016/j.yexcr.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima Y, Akimoto K, Nagashima Y, Ishiguro H, Shirai S, Chishima T, et al. The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum Pathol. 2008;39:824–31. doi: 10.1016/j.humpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Gironella M, Calvo C, Fernández A, Closa D, Iovanna JL, Rosello-Catafau J, et al. Reg3β deficiency impairs pancreatic tumor growth by skewing macrophage polarization. Cancer Res. 2013;73:5682–94. doi: 10.1158/0008-5472.CAN-12-3057. [DOI] [PubMed] [Google Scholar]
- 26.Elsässer HP, Lehr U, Agricola B, Kern HF. Establishment and characterisation of two cell lines with different grade of differentiation derived from one primary human pancreatic adenocarcinoma. Virchows Arch, B, Cell Pathol. 1992;61:295–306. doi: 10.1007/BF02890431. [DOI] [PubMed] [Google Scholar]
- 27.Nelson CM, Khauv D, Bissell MJ, Radisky DC. Change in cell shape is required for matrix metalloproteinase-induced epithelial-mesenchymal transition of mammary epithelial cells. J Cell Biochem. 2008;105:25–33. doi: 10.1002/jcb.21821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maupin KA, Sinha A, Eugster E, Miller J, Ross J, Paulino V, et al. Glycogene Expression Alterations Associated with Pancreatic Cancer Epithelial-Mesenchymal Transition in Complementary Model Systems. PLoS ONE. 2010;5:e13002. doi: 10.1371/journal.pone.0013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers A, Smith MJ, Doolan P, Clarke C, Clynes M, Murphy JF, et al. Invasive markers identified by gene expression profiling in pancreatic cancer. Pancreatology. 2012;12:130–40. doi: 10.1016/j.pan.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Gangeswaran R, Zhao X, Wang P, Tysome J, Bhakta V, et al. CEACAM6 attenuates adenovirus infection by antagonizing viral trafficking in cancer cells. J Clin Invest. 2009;119:1604–15. doi: 10.1172/JCI37905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee K, Chen QK, Lui C, Cichon MA, Radisky DC, Nelson CM. Matrix compliance regulates Rac1b localization, NADPH oxidase assembly, and epithelial–mesenchymal transition. Mol Biol Cell. 2012;23:4097–108. doi: 10.1091/mbc.E12-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 33.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: Do myofibroblasts come also from epithelial cells via EMT? Journal of Cellular Biochemistry. 2007;101:830–9. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou C, Licciulli S, Avila JL, Cho M, Troutman S, Jiang P, et al. The Rac1 splice form Rac1b promotes K-ras-induced lung tumorigenesis. Oncogene. 2013;32:903–9. doi: 10.1038/onc.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matos P, Jordan P. Increased Rac1b expression sustains colorectal tumor cell survival. Mol Cancer Res. 2008;6:1178–84. doi: 10.1158/1541-7786.MCR-08-0008. [DOI] [PubMed] [Google Scholar]
- 36.Shuman Moss LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–9. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zucker S, Cao J, Chen WT. Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene. 2000;19:6642–50. doi: 10.1038/sj.onc.1204097. [DOI] [PubMed] [Google Scholar]
- 38.Pelisch F, Khauv D, Risso G, Stallings-Mann M, Blaustein M, Quadrana L, et al. Involvement of hnRNP A1 in the matrix metalloprotease-3-dependent regulation of Rac1 pre-mRNA splicing. J Cell Biochem. 2012;113:2319–29. doi: 10.1002/jcb.24103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonçalves V, Matos P, Jordan P. Antagonistic SR proteins regulate alternative splicing of tumor-related Rac1b downstream of the PI3-kinase and Wnt pathways. Hum Mol Genet. 2009;18:3696–707. doi: 10.1093/hmg/ddp317. [DOI] [PubMed] [Google Scholar]
- 40.David CJ, Manley JL. Alternative pre-mRNA splicing regulation in cancer: pathways and programs unhinged. Genes Dev. 2010;24:2343–64. doi: 10.1101/gad.1973010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L, Muthuswamy SK. Polarity protein alterations in carcinoma: a focus on emerging roles for polarity regulators. Curr Opin Genet Dev. 2010;20:41–50. doi: 10.1016/j.gde.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins MA, Bednar F, Zhang Y, Brisset JC, Galbán S, Galbán CJ, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–53. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, et al. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304–17. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crnogorac-Jurcevic T, Efthimiou E, Capelli P, Blaveri E, Baron A, Terris B, et al. Gene expression profiles of pancreatic cancer and stromal desmoplasia. Oncogene. 2001;20:7437–46. doi: 10.1038/sj.onc.1204935. [DOI] [PubMed] [Google Scholar]
- 45.Orlichenko L, Geyer R, Yanagisawa M, Khauv D, Radisky ES, Anastasiadis PZ, et al. The 19-amino acid insertion in the tumor-associated splice isoform Rac1b confers specific binding to p120 catenin. J Biol Chem. 2010;285:19153–61. doi: 10.1074/jbc.M109.099382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du J, Liu J, Smith BJ, Tsao MS, Cullen JJ. Role of Rac1-dependent NADPH oxidase in the growth of pancreatic cancer. Cancer Gene Ther. 2011;18:135–43. doi: 10.1038/cgt.2010.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 48.Pezzilli R, Corsi MM, Fabbri D, Imbrogno A, Barassi A, Morselli-Labate AM, et al. Circulating metalloproteinase-3 and tissue inhibitor of metalloproteinase-2 in patients with ductal pancreatic neoplasms. The Journal of Bioscience and Medicine. 2011;1:9. [Google Scholar]
- 49.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–22. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 50.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–4. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


