Abstract
Extracellular α-synuclein is important in the pathogenesis of Parkinson disease (PD) and also as a potential biomarker when tested in the cerebrospinal fluid (CSF). The performance of blood plasma or serum α-synuclein as a biomarker has been found to be inconsistent and generally ineffective, largely due to the contribution of peripherally derived α-synuclein. In this study, we discovered, via an intracerebroventricular injection of radiolabeled α-synuclein into mouse brain, that CSF α-synuclein was readily transported to blood, with a small portion being contained in exosomes that are relatively specific to the central nervous system (CNS). Consequently, we developed a technique to evaluate the levels of α-synuclein in these exosomes in individual plasma samples. When applied to a large cohort of clinical samples (267 PD, 215 controls), we found that in contrast to CSF α-synuclein concentrations, which are consistently reported to be lower in PD patients compared to controls, the levels of plasma exosomal α-synuclein were substantially higher in PD patients, suggesting an increased efflux of the protein to the peripheral blood of these patients. Furthermore, although no association was observed between plasma exosomal and CSF α-synuclein, a significant correlation between plasma exosomal α-synuclein and disease severity (r=0.176, p=0.004) was observed, and the diagnostic sensitivity and specificity achieved by plasma exosomal α-synuclein were comparable to those determined by CSF α-synuclein. Further studies are clearly needed to elucidate the mechanism involved in the transport of CNS α-synuclein to the periphery, which may lead to a more convenient and robust assessment of PD clinically.
Keywords: Parkinson disease, exosome, α-synuclein, biomarker, plasma
Introduction
One of the pathological hallmarks of Parkinson disease (PD), the second most common neurodegenerative disease, is the presence of Lewy bodies in surviving neurons [38]. Lewy bodies consist of insoluble aggregated proteins, with α-synuclein (α-syn) being the major component [60]. Mechanistically, α-syn clearly plays an important role in PD pathogenesis, as mutations or multiplications of the α-syn gene (SNCA) are known to be causative of some familial forms of PD [55,41]. More recently, genome-wide association studies have also confirmed that common variants of SNCA modulate risk for sporadic PD [61,41,29]. In addition, α-syn is readily secreted into extracellular spaces and has been identified in the cerebrospinal fluid (CSF), blood, and saliva [48]. The mechanisms of α-syn secretion are not fully understood, but studies have demonstrated at least a fraction of α-syn to be secreted in association with exosomes [20,2,12], the 40–100 nm membrane vesicles of endocytic origin [40,9]. Extracellular α-syn has been shown to activate microglia and astroglia, enhancing neurodegeneration [73,35]. The significance of extracellular α-syn is further indicated by recent studies showing that cell-to-cell transfer of α-syn within the central nervous system (CNS) is essential to the progression of synucleinopathies [15,30].
Beyond its implications in the pathogenesis of PD and related synucleinopathies, extracellular α-syn has also been studied extensively as a potential biomarker of diagnosis and/or indicator of disease progression [31,51,58,18,19,59,16]. In this regard, current clinical diagnosis of PD is typically made upon observation of its motor symptoms [69]. However, there is an appreciable misdiagnosis rate [69], particularly in the early disease stages, and definitive diagnosis can only be made upon autopsy. Reports on CSF α-syn concentrations have been largely consistent and generally accepted as being significantly lower in patients with PD when compared to controls [50,67,31,51,58,27], with moderate performance in aiding PD diagnosis [31,51,58,46]. In contrast, reports on α-syn concentrations within the blood, which is more readily accessible and, therefore, more clinically desirable, have been less consistent [19,42,39,59,17,24,23], largely because of an abundant production of the protein in the peripheral tissue, especially red blood cells and platelets [48,7,59,47].
Therefore, an unmet need centers on defining the mechanisms underlying α-syn secretion, transportation, and clearance as well as identification of CNS-derived α-syn in peripheral body fluids. In the current investigation, we began exploring whether CSF α-syn can be transported to blood, and then focused on the isolation of exosomes likely derived from the CNS and quantification of α-syn within this fraction in clinical plasma samples from patients with PD and healthy controls.
Participants and methods
1. Brain to blood trafficking in mice
CD-1 male (8 weeks old) mice (Charles River, Wilmington, MA, USA) were kept on a 12/12 h light/dark cycle with ad lib access to food and water. All animal studies were performed at a facility approved by AAALCC and under protocols approved by the local animal use committee. α-Syn (rPeptide, Athens, GA, USA) was radioactively labeled with Na125I (Perkin Elmer, Waltham, MA, USA) by the chloramine-T (Sigma-Aldrich, St Louis, MO, USA) method [6], and then purified using a Sephadex G-10 column (Sigma-Aldrich).
Mice were anesthetized with 0.15 mL of 40% urethane (Sigma-Aldrich) via i.p. injection. The scalp was removed and a hole was made 0.5 mm posterior to the bregma and 1.0 mm to the right of the sagittal suture. Using a 1.0 μL Hamilton syringe, 1 μL of lactated Ringer’s solution containing 1×106 CPM of the radioactively labeled α-Syn was slowly injected into the left ventricle of the brain. For the efflux-time curve, blood was collected from the carotid artery in 10% EDTA coated tubes (Sigma-Aldrich) at 2, 5, 10, 20 and 60 min after injection. For the efflux-exosome comparison, blood was collected at 60 min after injection, followed by exosome extraction from platelet-free plasma. The mouse was then decapitated and the whole brain was removed and weighed. Levels of radioactivity in the brain, the whole plasma, the exosome fraction, and the exosome-less fraction (supernatant after immunoaffinity capture) were counted using a gamma counter.
2. Human subjects and clinical sample collection
A total of 482 subjects were included in this study. 267 patients with PD and 215 age- and sex-matched healthy controls were recruited at the Pacific Northwest Udall Center of Excellence for Parkinson’s Disease Research (PANUC) and the Alzheimer’s Disease Research Centers (ADRCs) at the University of Washington (UW) and the University of California at San Diego (UCSD) as previously described [10,58,49], or were enrolled in the Parkinson’s Genetic Research Study (PaGeR) [10,49]. All subjects underwent evaluations consisting of medical history, physical and neurological examinations, laboratory tests, and neuropsychological assessments. The inclusion and exclusion criteria have been previously described [10,31,58,49]. Briefly, all PD patients met UK PD Society Brain Bank clinical diagnostic criteria for PD [25], except that having “more than one affected relative” was not considered an exclusion criterion. Control subjects were the patients’ spouses or community volunteers in good health. They had no signs or symptoms suggesting cognitive impairment or neurological disease; all subjects had a Mini Mental Status Examination (MMSE) score between 28 and 30, a Clinical Dementia Rating (CDR) score of 0, and New York University paragraph recall scores (immediate and delayed) of >6. All participants underwent detailed informed consent procedures and provided consent in writing in accordance with procedures approved by the institutional review boards at the UW, Veterans Affairs (VA) Puget Sound Health Care System at Seattle, Portland VA Medical Center, Oregon Health and Science University, and the UCSD. Demographic information is listed in Table 1 for all subjects.
Table 1. Demographic data of participating subjects.
| Whole Cohort | Subset with CSF | |||
|---|---|---|---|---|
| Con | PD | Con | PD | |
| Subject number | 215 | 267 | 100 | 100 |
| Age* (range) |
65.7±9.1 (40-88) |
66.3±9.1 (45-88) |
66.4±9.2 (51-84) |
65.8±8.1 (50-83) |
| Sex (male:female) |
1.2 (116:99) | 1.2 (145:119) | 1.4 (59:41) | 1.6 (62:38) |
| UPDRS motor* (range) |
28.4±12.6 (5-65) |
24.2±11.5 (6-61) |
||
| H&Y* (range) |
2.4±0.7 (1-5) |
2.2±0.6 (1-4) |
||
| MMSE* (range) |
28.0±2.6 (6-30) |
28.5±2.7 (6-30) |
||
| Disease duration, year* (range) |
9.6±6.6 (0-34) |
7.7±5.9 (0-27) |
||
Mean±SD. Con, healthy control; CSF, cerebrospinal fluid; H&Y, Hoehn & Yahr; MMSE, Mini Mental State Examination; PD, Parkinson disease; UPDRS, Unified Parkinson’s Disease Rating Scale.
All blood (including plasma) and CSF samples were obtained as previously described [58,59]. Blood samples were collected from all subjects, but matching CSF (collected within 6 months of the blood collection) samples were collected in only a subset of the subjects (100 patients with PD and 100 age- and sex-matched healthy controls). Similar sample collection protocols and quality control procedures were followed at all participating centers, in particular, use of polypropylene collection and storage tubes, rapid separation into single use aliquots, and freezing of plasma and CSF samples, to minimize potential site variability.
3. Exosome isolation
Exosomes were isolated from mouse or human plasma using antibody-coated superparamagnetic microbeads following a protocol adapted from Tauro et al. [66]. Briefly, 10 μg of anti-L1CAM antibodies (clone UJ127, abcam, Cambridge, MA, USA) or anti-CD63 antibodies (clone H5C6, BD Biosciences, San Diego, CA, USA) [or normal mouse IgGs (Santa Cruz Biotechnology, Dallas, TX, USA) as negative controls] were coated onto one set (1 mg) of M-270 Epoxy beads using a Dynabeads® Antibody Coupling Kit (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions. After thawing quickly (within 2 min) at 37°C, plasma samples (>300 μL) were centrifuged at 2,000 × g for 15 min followed by 12,000 × g for 30 min, and then the supernatant was diluted 1:3 with phosphate buffered saline (PBS) (pH7.4). One set of antibody-coated beads and 900 μL of diluted plasma were incubated for ~24 h at 4°C with gentle rotation. The beads were then washed four times with 1 mL of 0.1% bovine serum albumin (BSA)/PBS (pH7.4) and transferred into a new tube. Exosomes were eluted from the beads with 60 μL of a 1:1 mixture of 0.1% BSA/PBS (pH7.4) and a fixing buffer (4% paraformaldehyde/5% glutaraldehyde) for electron microscopy imaging or lysed by incubating the beads in 110 μL of 1% Triton X-100 plus 10% of a protease inhibitor cocktail (P2714, Sigma-Aldrich; prepared in 10 ml of H2O) in 0.1% BSA/PBS (pH7.4) for 1 h at room temperature with gentle shaking for Luminex measurements and other analyses.
Exosomes in clinical plasma samples were extracted in batches, and PD and control samples were distributed into each batch. Two reference plasma samples, pooled from 30 healthy controls, were added into each batch to help to eliminate batch variations.
For quality control and assay development, some reference plasma samples (pooled from healthy old controls) were also diluted in PBS and then subjected to ultracentrifugation (100,000 × g for 3 h at 4°C); the pellet was then resuspended, loaded onto an OptiPrep™ density gradient, and centrifuged at 100,000 × g for 18 h at 4°C to obtain exosomes as described previously [66]. Exosome-poor plasma samples were prepared by removing exosomes after a 2-step ultracentrifugation (180,000 × g for 3 h at 4°C × 2).
4. Luminex assays
Exosome preparations of 100 μL (extracted from 300 μL of plasma) were used to quantify α-syn with an established Luminex protocol [31]. Total α-syn concentrations in plasma and CSF were also measured as previously described [31,59]. Hemoglobin concentrations as an index of the blood contamination in CSF were measured using an ELISA kit [31].
5. Electron microscopy (EM)
Isolated exosome preparations mixed 1:1 with 4% (v/v) paraformaldehyde and 5% (v/v) glutaraldehyde were layered onto formvar/carbon-coated 300 mesh copper grids (Polysciences, Warrington, PA, USA), and allowed to dry for 20 min at room temperature. For direct imaging, grids were then washed twice with water for 2 min, and stained with 2% (w/v) uranyl acetate in water (Electron Microscopy Sciences, Hatfield, PA, USA) for 20 min at room temperature. For immunogold staining, grids were washed with PBS before blocking for 30 min using a 1%BSA/5% normal goat serum/PBS buffer. The grids were re-washed with PBS, incubated with or without a 1:50 dilution of the anti-L1CAM antibody (abcam) in blocking buffer for 2 h at room temperature, and then incubated with an 18-nm gold conjugated goat anti-mouse IgG antibody (abcam) for 60 min at room temperature. The grids were washed again and left at room temperature to dry before contrasting with uranyl acetate. Imaging was performed on a JEOL (Peabody, MA, USA) 1230 transmission electron microscope.
6. Western blot analysis
Western blotting was performed following a standard protocol [43]. Exosome samples (~10 μg proteins) were solubilized with Laemmli sample buffer and separated on a 1D SDS-PAGE gel before transfer to a polyvinylidene difluoride membrane. The membrane was probed with the following primary antibodies: mouse anti-human L1CAM (abcam, 1:500) and mouse anti-human Alix (Cat# ABC40, Millipore, Billerica, MA, USA; 1:1000). To examine the expression of L1CAM in human blood cells, cell lysates (100 μg proteins) from red blood cells and platelets were used, along with human cerebral cortex (100 μg proteins) homogenates as a control; membranes were probed with the anti-L1CAM antibody.
7. Mass spectrometry (MS) analysis
Human plasma exosomal proteins were digested sequentially with trypsin and ASP-N as previously described [16,43]. Digested samples were cleaned up with a HiPPR (High Protein and Peptide Recovery) Detergent Removal kit (Thermo Scientific, Rockford, IL, USA) followed by C18 MicroSpin columns (The Nest Group, Southborough, MA, USA) according to the manufacturers’ protocols. Peptides were then analyzed using a Q-Exactive Plus mass spectrometer (Thermo Scientific) with or without an inclusion list containing peptides derived from the top 25 proteins that are often identified in exosomes (http://exocarta.org/exosome_markers). Liquid chromatography was performed using a NanoAcquity UPLC (Waters, Milford, MA, USA); peptides were separated online with 75 mm i.d. × 20 cm home-packed fused silica columns (ReproSil-Pur C18-AQ, 3 μm, Dr Maisch GmBH, Ammerbuch-Entringen, Germany) with a 120-min 2-80% acetonitrile/water gradient containing 0.1 % formic acid. MS/MS spectral data were converted to the mgf format and searched using ProteinPilot v4.1 (AB SCIEX, Framingham, MA, USA) against a human International Protein Index (IPI) sequence database (version 3.87) for peptide and protein identification.
Digested samples were also subjected to targeted selected reaction monitoring (SRM) assay for α-syn peptides confirmation on a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific) coupled to a NanoAcquity UPLC (Waters). Reversed-phase chromatography was performed on capillary columns (75 mm × 20 cm; Polymicro Technologies, Phoenix, AZ, USA) packed with 100 Å Magic C18 (Michrom/Bruker, Auburn, CA, USA). All SRM mass spectral data were processed using the Skyline Targeted Proteomics Environment (v2.5) (McCoss Lab, University of Washington, Seattle, WA, USA) [45]. Typical settings applied were 0.055 Th match tolerance m/z and default peak boundary assignment informed by Savitzky-Golay smoothing. A spectral library containing α-syn peptides and transitions generated in our lab was used for MS1 filtering to identify precursor ions. Peak assignments were also manually inspected and the dot-product ratios generated in Skyline were considered.
8. Statistical analysis
All analyses were performed in SPSS 18.0 (IBM, Chicago, IL, USA) or Prism 4.0 (GraphPad Software, La Jolla, CA, USA). Luminex data (plasma exosomal and total α-syn, and their ratio) were Log10 transformed to generate a normally distributed dataset. The Student’s t test was used to examine median differences between PD and control groups. Receiver operating characteristic (ROC) curves for analytes were generated to evaluate their sensitivities and specificities in distinguishing PD from healthy control subjects. The “optimum” cutoff value for a ROC curve was defined as the value associated with the maximal sum of sensitivity and specificity. Additionally, relationships between the analytes and age, sex, and the Unified Parkinson’s Disease Rating Scale (UPDRS) motor scores were analyzed with bivariate correlation using Pearson’s correlation coefficients. Partial correlations between analyte levels and UPDRS scores were also conducted while controlling for potential confounding factors such as age and sex of subjects. Values with p<0.05 were regarded as significant.
Results
Transportation of α-syn from the brain to blood in mice
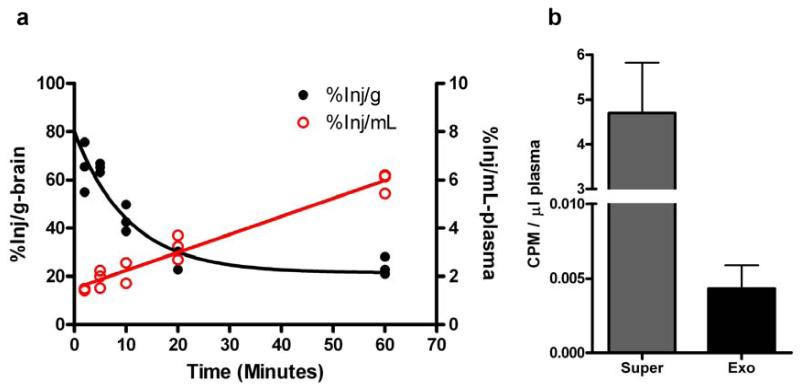
To determine whether α-syn could be transported from the CSF to blood, first we injected 125I-labeled α-syn intracerebroventricularly (icv) into mouse brain. While the radioactivity rapidly decreased in the brain, it continued to increase in the blood and reached ~6%/mL of the icv dose 60 min after injection (Fig. 1a), indicating that α-syn could be readily transported from the brain (CSF) into blood. Because α-syn can be secreted in exosomes [20,2,12], a practical way to capture CNS-derived α-syn in blood is to isolate exosomes originating from the CNS. Next, we isolated exosomes using an immunoaffinity capturing method (see below) from mouse plasma collected 60 min after injection. The labeled α-syn was detected in the exosome fraction; however, it appeared that most of the radioactivity was not in the exosomes (Fig. 1b and data not shown).
Fig. 1. Transportation of α-synuclein from the brain to blood.
Mice were intracerebroventricularly injected with 125I-labeled α-synuclein. (a) Brain and blood plasma were then collected at 2, 5, 10, 20 and 60 min after injection. Levels of radioactivity in the brain and the plasma were determined using a gamma counter. (b) Blood was collected at 60 min after injection, followed by L1CAM-containing exosome extraction from platelet-free plasma. Levels of radioactivity were measured in the whole plasma, the exosome fraction (Exo), and the exosome-less fraction (supernatant after immunoaffinity capture; Super). Data shown are mean ± S.D. from 5 mice. CPM, counts per minute.
Identification of α-syn in plasma exosomes likely derived from the CNS
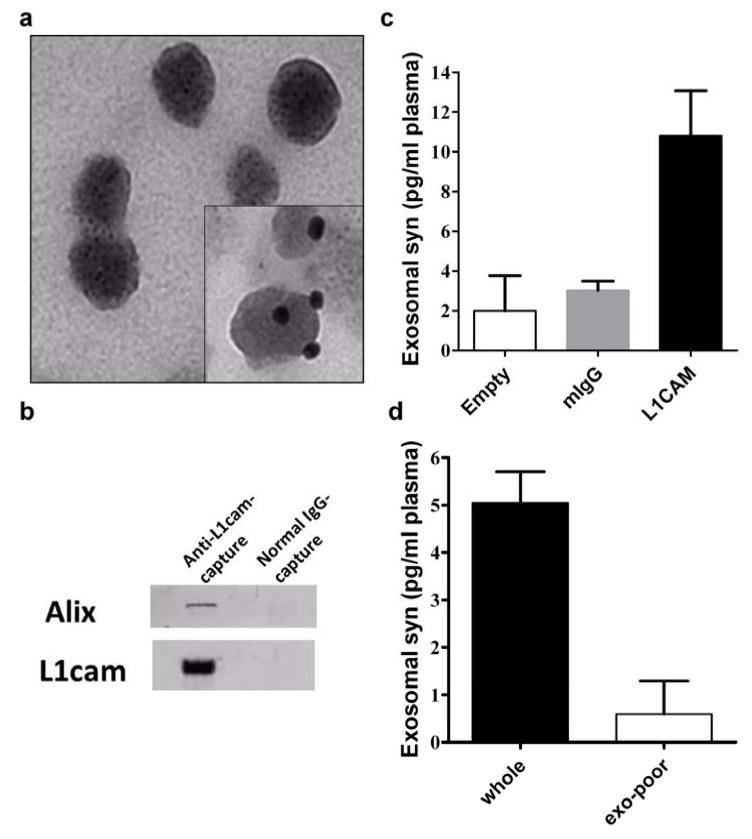
To enrich plasma exosomes that are likely derived from the CNS, we established an immunoaffinity capturing protocol to isolate L1 cell adhesion molecule (L1CAM)-containing exosomes in human or mouse plasma. L1CAM is the founding member of a subfamily of cell adhesion molecules that are primarily expressed in the nervous system and is believed to be a marker on the surface of exosomes derived from the CNS [64,33,22,37]. The quality of the anti-L1CAM-captured exosomes were examined with EM (Fig. 2a), and found to be similar to that of exosomes isolated using anti-CD63 (a more general exosomal marker [64,63]) antibodies or those acquired with ultracentrifugation followed by a density gradient separation (data not shown). Western blot analysis demonstrated that in these L1CAM-containing exosome preparations, Alix, another general exosomal protein [40,56], was also enriched (Fig. 2b). We also performed a proteomic profiling in anti-L1CAM-captured exosomes from pooled reference human plasma, while normal mouse IgG-captured samples were used as a negative control; a number of proteins that are believed to be exosomal markers (http://exocarta.org/exosome_markers), including CD9, enolase 1 (ENO1), 14-3-3 protein zeta/delta (YWHAZ), and cofilin-1 (CFL1), were identified in L1CAM-containing exosomes but not in normal mouse IgG preparations. At least three peptides derived from α-syn were also observed in these exosome preparations through a targeted MS analysis (Supplementary Fig. 1).
Fig. 2. Characterization of immunoaffinity-captured exosomes from human blood plasma.
(a) Electron micrograph of anti-L1CAM-captured plasma exosomes (inset: immunogold labeling of L1CAM). (b) Western blot showing that Alix, a common exosome marker, and L1CAM were enriched with anti-L1cam capture, but not with normal IgG capture. (c) α-Synuclein (syn) levels in anti-L1CAM-captured plasma exosomes were measured using a Luminex immunoassay, compared to the levels in normal mouse IgG-captured (mIgG) or “Empty” (no bead “capture”) samples. (d) Specificity was also confirmed by using exosome-poor plasma (supernatant after ultracentrifugation). Aliquots from the same pooled samples were used in these experiments (b-d) for comparison.
Using our established Luminex assay [31], we also observed a detectable α-syn signal in anti-L1CAM-captured exosomes from human plasma. The signal was unlikely to be from free α-syn contamination, because the signal from normal mouse IgG-captured or “empty” (no bead “capture”) samples was minimal (Fig. 2c). To further confirm the α-syn signal was from exosomes, we generated exosome-poor plasma using ultracentrifugation. Compared to regular plasma, the α-syn signal in L1CAM-containing exosomes in this exosome-poor plasma was reduced more than tenfold (Fig. 2d). Given that the specificity of our α-syn Luminex assay was confirmed previously [31], we are confident that the Luminex signal we observed was from the α-syn in anti-L1CAM-captured exosomes.
As a further assurance, we examined the L1CAM expression in blood cells. We found that L1CAM was not detectable in red blood cells and platelets (Supplementary Fig. 2), where >99% of α-syn in whole blood resides [59], suggesting the α-syn in anti-L1CAM-captured exosomes is not from these blood cells and is likely to be CNS or at least nervous system derived.
Evaluation of plasma exosomal α-syn in clinical samples
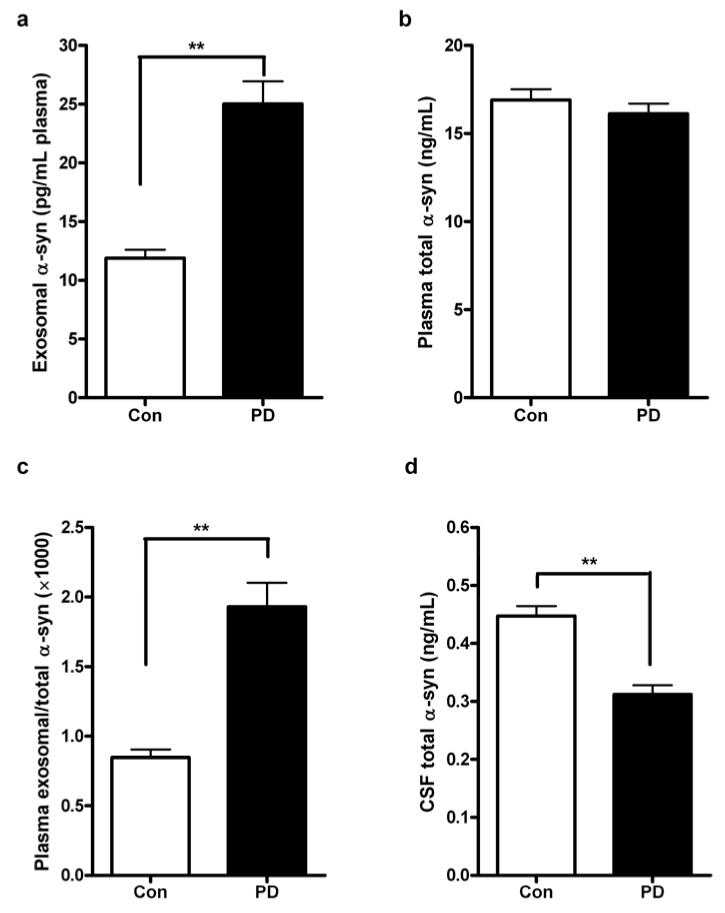
We collected a large cohort of 267 PD and 215 age- and sex-matched healthy control plasma samples (Table 1), and measured α-syn concentrations in plasma L1CAM-containing exosomes and in whole plasma using Luminex assays. Similar to our previous findings [59], there was no significant difference in the total plasma α-syn concentrations in PD versus controls (p=0.134, t test), but the α-syn concentrations in plasma L1CAM-containing exosomes were significantly higher in patients with PD compared to healthy controls (p<0.0001) (Fig. 3a, b). The plasma exosomal α-syn/total α-syn (exo/total) ratio was also significantly higher in patients with PD than in the controls (p<0.0001) (Fig. 3c).
Fig. 3. Evaluation of α-synuclein concentrations in clinical samples.
α-Synuclein (α-syn) concentrations were measured using Luminex and comparisons were performed for α-syn in L1CAM-containing exosomes isolated from plasma (a), total α-syn in plasma (b), or the plasma exosomal α-syn/total α-syn ratio (c) in patients with Parkinson disease (PD; n=267) and healthy controls (Con; n=215). CSF total α-syn concentrations were also evaluated in a subset of the subjects (100 PD and 100 Con) with CSF samples available (d). Data shown are mean ± S.D. **, p<0.001.
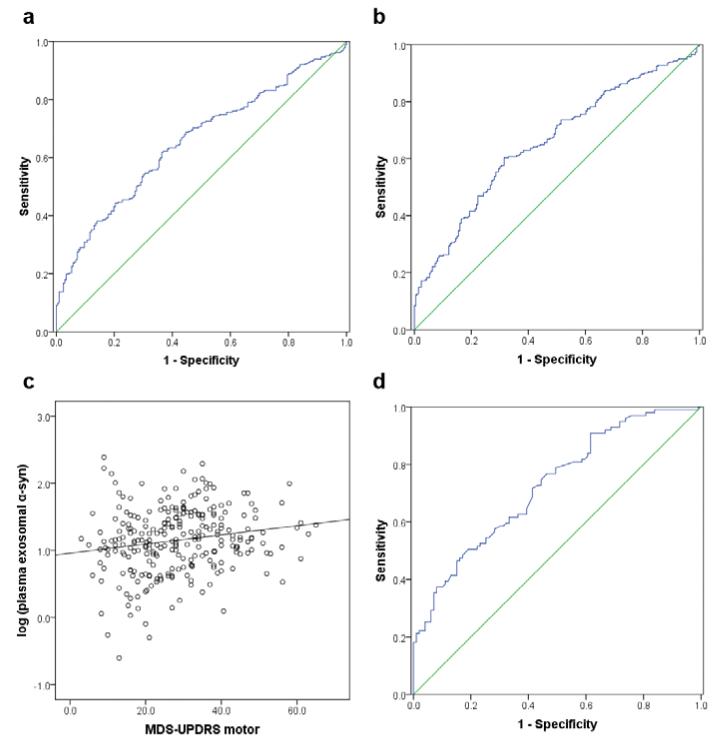
To further evaluate the potential for plasma α-syn in L1CAM-containing exosomes to aid in PD diagnosis, we performed ROC analysis to characterize its sensitivity and specificity. The performance of plasma exosomal α-syn was found to be only moderate (AUC = 0.654, sensitivity = 70.1%, specificity = 52.9%) (Fig. 4a). The plasma exo/total α-syn ratio generated similar results (AUC = 0.657, sensitivity = 71.2%, specificity = 50.0%) (Fig. 4b). Furthermore, we examined the correlation between plasma exosomal α-syn and disease severity. Significant correlations between the plasma α-syn in L1CAM-containing exosomes (r=0.176, p=0.004, Pearson correlation; Fig. 4c) or the plasma exo/total α-syn ratio (r=0.130, p=0.035) with the UPDRS motor scores were observed.
Fig. 4. ROC analysis of biomarker candidates for PD diagnosis and correlation with disease severity.
(a) In the whole cohort (267 patients with PD and 215 healthy controls), the plasma exosomal α-syn provided an AUC of 0.654 (sensitivity=70.1%, specificity=52.9%) for PD versus controls. (b) The exosomal α-syn/total α-syn ratio in plasma performed similarly (AUC=0.657, sensitivity=71.2%, specificity=50.0%) in the whole cohort. (c) A significant correlation between the plasma exosomal α-syn and the disease severity indexed by the UPDRS motor score was observed in PD patients (r=0.176, p=0.004, Pearson correlation). (d) In a subset of subjects with CSF samples available (100 PD and 100 controls), the CSF total α-syn could produce an AUC of 0.724 (sensitivity=76.8%, specificity=53.5%). No correlation between CSF total α-syn and the UPDRS motor scores could be found (not shown). AUC, area under curve; CSF, cerebrospinal fluid; exo, exosomal; MDS-UPDRS, the Movement Disorder Society (MDS) Unified Parkinson’s Disease Rating Scale; PD, Parkinson disease; ROC, receiver operating characteristic; α-syn, α-synuclein.
In a subset of the cohort (100 PD and 100 age-, sex-matched healthy controls), matching CSF samples from the same subjects were also collected, allowing us to evaluate the performance of CSF α-syn. Consistent with our previous results [31,58], CSF α-syn concentrations were significantly lower in patients with PD versus healthy controls (p<0.0001) (Fig. 3d), with a moderate diagnostic value (AUC = 0.724, sensitivity = 76.8%, specificity = 53.5%) (Fig. 4d) close to that of the plasma exosomal α-syn or the plasma exo/total α-syn ratio. In addition, no correlation between CSF α-syn and the UPDRS motor scores were observed (r=0.012, p=0.905, Pearson correlation), similar to what we reported before [31,58]. Interestingly, there was no significant correlation between the α-syn concentrations in plasma L1CAM-containing exosomes and those in CSF (r=-0.053, p=0.465); the plasma total α-syn concentrations (r=0.197, p=0.006) and the exo/total α-syn ratio (r=-0.161, p=0.027), however, significantly correlated with CSF α-syn concentrations.
Discussion
The results of this study demonstrate several major advances with regard to extracellular α-syn. First, we have, for the first time, demonstrated that CSF α-syn can be readily transported into blood via an in vivo study of radiolabeled α-syn administered to mouse brain by icv injection. This finding established our foundation to search for CNS-derived α-syn in blood. It also raised a concern regarding the source of α-syn in the CSF. Because α-syn is highly expressed in blood cells [7,59,48] and its concentrations in plasma or serum are much higher than those in CSF [31,59], it’s quite likely that the blood α-syn could also enter the CSF through a similar mechanism. Thus, at least a portion of the CSF α-syn may be from the blood, as implied by the significant correlation between CSF and plasma α-syn observed in this study. This may help to explain the limitations CSF α-syn has faced as a biomarker to date.
The second major advance made in the study is to define a method for isolating plasma exosomes likely originating from the CNS (i.e., anti-L1CAM immunoaffinity-captured exosomes) that can be analyzed effectively. Evidence suggests exosomes may cross multiple layers of the blood-brain barrier [3], possibly by jumping from cell-to-cell via the multivesicular body (MVB) compartment [56]. Exosomes carrying unique, disease-specific, functionally important cargo, including those likely originating from the CNS [65], are known to be well detectable in vivo in blood and other body fluids [40,63], and thus represent a potentially valuable source of biomarkers for neurodegenerative diseases such as PD. Our immune-capturing method is also of significance because despite recent advances in our understanding of exosomes and other extracellular microvesicles, much of the published information has been obtained from impure preparations [62]. Currently, differential (ultra-)centrifugation is the most commonly used method, but the resulting pellet is usually contaminated with co-sedimenting vesicles and protein aggregates rendering a very crude preparation [62]. The L1CAM-containing exosomes produced by immuno-capture in this study were of similar quality, as demonstrated by EM and other measurements, to those obtained by other robust exosome isolation techniques, including the current “gold standard” - ultracentrifugation plus density gradient, which has been employed by us as well as others [66,32,22,37]. More direct evidence of exosome purity may be obtained as our knowledge of exosomes becomes more advanced; for example, methods to further reliably determine the potential contribution of non-exosomal fractions in our preparation can be developed when “markers” exclusively absent from exosomes are identified. Nonetheless, since no ultracentrifugation is involved in our protocol, this methodology may be more practical for use in a routine clinical setting after further optimization.
Next, we employed this isolation methodology to evaluate α-syn levels within L1CAM-containing exosomes in clinical blood plasma samples collected from PD patients and healthy controls. We found a robust elevation of L1CAM-exosomal α-syn in PD patients as compared to controls, with ROC analysis demonstrating essentially equivalent diagnostic performance (sensitivity and specificity) as has been achieved using CSF α-syn (Fig. 4), which is the best molecular biomarker of PD described to date [50,67,31,51,58,27]. There is currently significant interest in the development of objective biomarkers that reliably indicate PD diagnosis, or monitor the disease progression, in readily obtainable biological specimens. The markers that have been found to exhibit the best performance are measured in the CSF, which is not an ideal specimen for routine monitoring due to the invasive nature of sample collection by lumbar puncture.
Furthermore, CSF biomarkers described to date have been unable to reproducibly achieve sufficient sensitivity and specificity to warrant widespread clinical application [46]. Although plasma exosomal α-syn exhibited similar limited performance (specificity in particular) which CSF α-syn currently suffers from, the comparable diagnostic performance indicates we may bridge the gap between the more effective, but less accessible, CSF α-syn measurements, and the less effective, but more accessible, blood α-syn measurements by focusing on α-syn species in the blood likely originating in the CNS. More significantly, plasma exosomal α-syn displayed a significant, though weak, correlation with disease severity (UPDRS motor), while CSF α-syn did not show such correlation, meaning that plasma exosomal α-syn but not CSF α-syn has the potential to help in monitoring or predicting disease progression. Given that most α-syn in blood resides in blood cells [59] and that total α-syn in plasma cannot differentiate PD patients and controls [59,23], our findings demonstrate a substantial improvement over the current state of PD biomarker evaluation in blood and other peripheral body fluids. A caveat is that we could not exclude the possibility that the L1CAM-containing exosomes in blood may be partially secreted from the peripheral nervous system, because synuclein pathology has been reported in the sympathetic ganglia and autonomic nerves in PD [13,72,70]. Even so, our findings of the potential usage of plasma α-syn in L1CAM-containing exosomes as PD biomarkers are still of significance. Further study of other proteins (e.g., DJ-1, tau, etc) in CNS- or nervous system-derived exosomes in blood may be needed to form an “ideal” panel of PD biomarkers, which provide high diagnostic sensitivity and specificity.
It is unclear why the concentrations of plasma α-syn in L1CAM-containing exosomes were significantly higher in patients with PD compared to healthy controls. One possible explanation is that it results from the clearance of excess, potentially toxic α-syn species from the PD brain. Such α-syn species are known to require the lysosome for its degradation [54], and lysosomal function has been reported to be decreased in PD patients [11,1]. The proteins designated for lysosomal degradation are usually sequestered by endocytic MVBs; however, an alternative destination of MVBs is their exocytic fusion with the plasma membrane leading to the release of intraluminal vesicles (i.e., exosomes) into the extracellular environment [40,9]. This clearance of α-syn is supported by a recent study reporting that lysosomal inhibition dramatically increased exosomal α-syn release in a cellular model [2]. The increased efflux of CNS α-syn may also contribute to the decreased CSF total α-syn levels in PD and other synucleinopathies (e.g., dementia with Lewy bodies and multiple system atrophy), which is generally thought to be due to the deposition of α-syn in the brain [31,58,51,27]. In contrast, the CSF total α-syn concentrations in Alzheimer disease (AD) have been reported to be significantly higher compared to controls [31,58,51,27,36,68,71], while up to 50% of familial and sporadic AD patients also show evidence of α-syn-positive Lewy bodies upon autopsy [44,28,4]. Although lysosome dysfunction may be common in neurodegenerative disorders [53], there may be multiple mechanisms for neurons to degrade or remove α-syn in normal and different pathological conditions. Increased exosome-associated α-syn in PD may reflect a mechanism to release α-syn under conditions of cellular stress and relatively increased α-syn concentrations. Therefore, it is possible that CNS α-syn secretion and clearance is regulated differently in PD and AD, even if the same pathway is involved in both diseases. Further studies are needed to investigate the underlying mechanisms and the utility of plasma exosomal α-syn in differential diagnosis of PD and related disorders.
In addition to the advances described above, we gained further insight into the mechanisms of CNS-to-blood efflux of α-syn. While we did find evidence of α-syn transport from the brain (ventricle) to the blood, most of the injected α-syn was found to be in a presumably free form within blood plasma rather than being contained within L1CAM-containing exosomes. This indicates that only a portion of the CNS-derived α-syn in plasma resides in L1CAM-containing exosomes, even if the CSF-blood pathway/barrier is not the only pathway for CNS α-syn efflux (further discussed below). This may partially explain why the performance of plasma exosomal α-syn was only moderate for PD diagnosis.
It is quite obvious that the source(s) of plasma α-syn in L1CAM-containing exosomes needs to be further defined. Although the efflux of CSF proteins into blood can be very fast (appearing within minutes in rodents), most likely through facilitated or active transport, and with consideration given to the perspective that the commonly accepted flow model for CSF circulation may need to be revised [26,57,5,52,21], it is unclear how fast α-syn could be incorporated into exosomes in cells and transported from brain to blood. Our observation may suggest that although α-syn does cross from the brain (CSF) to the blood, the α-syn measured within L1CAM-containing exosomes in clinical plasma samples enters the blood via a mechanism upstream of the CSF-containing lateral ventricles. Alternatively, the majority of CNS-derived L1CAM-containing exosomes may enter the blood through pathways other than the CSF-blood barrier, such as a dysfunctional or damaged blood-brain barrier as has been suggested in PD patients [14,8]. We speculate that direct α-syn injection to the brain tissue or overexpression of α-syn in brain cells (e.g., in α-syn knock-out mouse brain) may provide more direct evidence for the origin of L1CAM-containing exosome α-syn species. A caveat here is that although primarily expressed in the nervous system, L1CAM may also be found in other specialized cells, particularly a few cancer cells [33,34]. While our findings regarding the performance of plasma exosomal α-syn on correlation with PD severity suggest that such non-CNS-derived L1CAM-containing exosomes, if present, might not represent the majority of existent species in plasma, the potential confounding effects need to be further investigated.
In summary, we found α-syn administered via icv injection is transported from the brain (CSF) to the blood in mice, and demonstrated the presence of α-syn-containing L1CAM exosomes in the blood plasma of both PD patients and healthy controls. Importantly, we found the quantity of α-syn within these exosomes to be significantly higher in PD patients as compared to controls and correlated with disease severity (UPDRS motor), and further that the performance of these α-syn species in differentiating subjects with PD from controls to be close to that of CSF α-syn in terms of sensitivity and specificity. Further investigations are needed to yield more insight into the underlying mechanisms of α-syn-containing CNS-derived exosome efflux into the blood and the utility of α-syn and other proteins in these exosomes as PD biomarkers.
Supplementary Material
Acknowledgments
We thank Drs. Honglian Li and Sangwoo Jung for their kind help on EM method development, and Dr. Ane Korff for her assistance in Western blot confirmation. We also deeply appreciate the patients and participants for their generous participation and donation of samples. This study was supported by generous grants from the National Institutes of Health (NIH) (U01 NS082137, P42 ES004696-5897, P30 ES007033-6364, R01 AG033398, R01 ES016873, R01 ES019277, R01 NS057567, and P50 NS062684-6221 to JZ, R01 NS065070 to CPZ, and P50 AG005131 to DRG), and partially by a pilot study award from the NIH-sponsored ADRC at the UW (P50 AG003156-30) and a National Institute of Neurological Disorders and Stroke/NIH award R21 NS085425 to MS. It was also supported in part by the University of Washington’s Proteomics Resource (UWPR95794). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH. Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol. 2010;67(12):1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Erviti L, Seow Y, Schapira AH, Gardiner C, Sargent IL, Wood MJ, Cooper JM. Lysosomal dysfunction increases exosome-mediated alpha-synuclein release and transmission. Neurobiol Dis. 2011;42(3):360–367. doi: 10.1016/j.nbd.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 4.Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y. Alpha-synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res. 2001;888(2):287–296. doi: 10.1016/s0006-8993(00)03082-1. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Broadwell RD. Blood to brain and brain to blood passage of native horseradish peroxidase, wheat germ agglutinin, and albumin: pharmacokinetic and morphological assessments. J Neurochem. 1994;62(6):2404–2419. doi: 10.1046/j.1471-4159.1994.62062404.x. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Kumar VB, Farr SA, Nakaoke R, Robinson SM, Morley JE. Impairments in brain-to-blood transport of amyloid-beta and reabsorption of cerebrospinal fluid in an animal model of Alzheimer’s disease are reversed by antisense directed against amyloid-beta protein precursor. J Alzheimers Dis. 2011;23(4):599–605. doi: 10.3233/JAD-2010-100021. [DOI] [PubMed] [Google Scholar]
- 7.Barbour R, Kling K, Anderson JP, Banducci K, Cole T, Diep L, Fox M, Goldstein JM, Soriano F, Seubert P, Chilcote TJ. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener Dis. 2008;5(2):55–59. doi: 10.1159/000112832. [DOI] [PubMed] [Google Scholar]
- 8.Bartels AL. Blood-brain barrier P-glycoprotein function in neurodegenerative disease. Curr Pharm Des. 2011;17(26):2771–2777. doi: 10.2174/138161211797440122. [DOI] [PubMed] [Google Scholar]
- 9.Chivet M, Hemming F, Pernet-Gallay K, Fraboulet S, Sadoul R. Emerging role of neuronal exosomes in the central nervous system. Front Physiol. 2012;3:145. doi: 10.3389/fphys.2012.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Edwards KL, Montine TJ, Leverenz JB. Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis. 2013;3(2):205–214. doi: 10.3233/JPD-130189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35(3):385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Danzer KM, Kranich LR, Ruf WP, Cagsal-Getkin O, Winslow AR, Zhu L, Vanderburg CR, McLean PJ. Exosomal cell-to-cell transmission of alpha synuclein oligomers. Mol Neurodegener. 2012;7:42. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derkinderen P, Rouaud T, Lebouvier T, Bruley des Varannes S, Neunlist M, De Giorgio R. Parkinson disease: the enteric nervous system spills its guts. Neurology. 2011;77(19):1761–1767. doi: 10.1212/WNL.0b013e318236ef60. [DOI] [PubMed] [Google Scholar]
- 14.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood-brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16(3):285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 15.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devic I, Hwang H, Edgar JS, Izutsu K, Presland R, Pan C, Goodlett DR, Wang Y, Armaly J, Tumas V, Zabetian CP, Leverenz JB, Shi M, Zhang J. Salivary alpha-synuclein and DJ-1: potential biomarkers for Parkinson’s disease. Brain. 2011;134(Pt 7):e178. doi: 10.1093/brain/awr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duran R, Barrero FJ, Morales B, Luna JD, Ramirez M, Vives F. Plasma alpha-synuclein in patients with Parkinson’s disease with and without treatment. Mov Disord. 2010;25(4):489–493. doi: 10.1002/mds.22928. [DOI] [PubMed] [Google Scholar]
- 18.El-Agnaf OM, Salem SA, Paleologou KE, Cooper LJ, Fullwood NJ, Gibson MJ, Curran MD, Court JA, Mann DM, Ikeda S, Cookson MR, Hardy J, Allsop D. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17(13):1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 19.El-Agnaf OM, Salem SA, Paleologou KE, Curran MD, Gibson MJ, Court JA, Schlossmacher MG, Allsop D. Detection of oligomeric forms of alpha-synuclein protein in human plasma as a potential biomarker for Parkinson’s disease. FASEB J. 2006;20(3):419–425. doi: 10.1096/fj.03-1449com. [DOI] [PubMed] [Google Scholar]
- 20.Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci. 2010;30(20):6838–6851. doi: 10.1523/JNEUROSCI.5699-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA. Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation. 2012;9:150. doi: 10.1186/1742-2094-9-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, Grange J, Schoehn G, Goldberg Y, Boyer V, Kirchhoff F, Raposo G, Garin J, Sadoul R. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31(4):642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 23.Foulds PG, Diggle P, Mitchell JD, Parker A, Hasegawa M, Masuda-Suzukake M, Mann DM, Allsop D. A longitudinal study on alpha-synuclein in blood plasma as a biomarker for Parkinson’s disease. Sci Rep. 2013;3:2540. doi: 10.1038/srep02540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foulds PG, Mitchell JD, Parker A, Turner R, Green G, Diggle P, Hasegawa M, Taylor M, Mann D, Allsop D. Phosphorylated alpha-synuclein can be detected in blood plasma and is potentially a useful biomarker for Parkinson’s disease. FASEB J. 2011;25(12):4127–4137. doi: 10.1096/fj.10-179192. [DOI] [PubMed] [Google Scholar]
- 25.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greitz D, Greitz T, Hindmarsh T. A new view on the CSF-circulation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr. 1997;86(2):125–132. doi: 10.1111/j.1651-2227.1997.tb08850.x. [DOI] [PubMed] [Google Scholar]
- 27.Hall S, Ohrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F, Nilsson C, Hakan W, Decraemer H, Nagga K, Minthon L, Londos E, Vanmechelen E, Holmberg B, Zetterberg H, Blennow K, Hansson O. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol. 2012;69(11):1445–1452. doi: 10.1001/archneurol.2012.1654. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10(3):378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamza TH, Zabetian CP, Tenesa A, Laederach A, Montimurro J, Yearout D, Kay DM, Doheny KF, Paschall J, Pugh E, Kusel VI, Collura R, Roberts J, Griffith A, Samii A, Scott WK, Nutt J, Factor SA, Payami H. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42(9):781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121(2):715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong Z, Shi M, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Leverenz JB, Baird G, Montine TJ, Hancock AM, Hwang H, Pan C, Bradner J, Kang UJ, Jensen PH, Zhang J. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson’s disease. Brain. 2010;133(Pt 3):713–726. doi: 10.1093/brain/awq008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalra H, Adda CG, Liem M, Ang CS, Mechler A, Simpson RJ, Hulett MD, Mathivanan S. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13(22):3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 33.Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9(6):879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 34.Kiefel H, Bondong S, Hazin J, Ridinger J, Schirmer U, Riedle S, Altevogt P. L1CAM: a major driver for tumor cell invasion and motility. Cell Adh Migr. 2012;6(4):374–384. doi: 10.4161/cam.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klegeris A, Giasson BI, Zhang H, Maguire J, Pelech S, McGeer PL. Alpha-synuclein and its disease-causing mutants induce ICAM-1 and IL-6 in human astrocytes and astrocytoma cells. FASEB J. 2006;20(12):2000–2008. doi: 10.1096/fj.06-6183com. [DOI] [PubMed] [Google Scholar]
- 36.Korff A, Liu C, Ginghina C, Shi M, Zhang J. alpha-Synuclein in Cerebrospinal Fluid of Alzheimer’s Disease and Mild Cognitive Impairment. J Alzheimers Dis. 2013;36(4):679–688. doi: 10.3233/JAD-130458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46(2):409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 39.Lee PH, Lee G, Park HJ, Bang OY, Joo IS, Huh K. The plasma alpha-synuclein levels in patients with Parkinson’s disease and multiple system atrophy. J Neural Transm. 2006;113(10):1435–1439. doi: 10.1007/s00702-005-0427-9. [DOI] [PubMed] [Google Scholar]
- 40.Lee TH, D’Asti E, Magnus N, Al-Nedawi K, Meehan B, Rak J. Microvesicles as mediators of intercellular communication in cancer--the emerging science of cellular ‘debris’. Semin Immunopathol. 2011;33(5):455–467. doi: 10.1007/s00281-011-0250-3. [DOI] [PubMed] [Google Scholar]
- 41.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum Mol Genet. 2009;18(R1):R48–59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 42.Li QX, Mok SS, Laughton KM, McLean CA, Cappai R, Masters CL, Culvenor JG, Horne MK. Plasma alpha-synuclein is decreased in subjects with Parkinson’s disease. Exp Neurol. 2007;204(2):583–588. doi: 10.1016/j.expneurol.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Lin X, Cook TJ, Zabetian CP, Leverenz JB, Peskind ER, Hu SC, Cain KC, Pan C, Edgar JS, Goodlett DR, Racette BA, Checkoway H, Montine TJ, Shi M, Zhang J. DJ-1 isoforms in whole blood as potential biomarkers of Parkinson disease. Sci Rep. 2012;2:954. doi: 10.1038/srep00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lippa CF, Schmidt ML, Lee VM, Trojanowski JQ. Antibodies to alpha-synuclein detect Lewy bodies in many Down’s syndrome brains with Alzheimer’s disease. Ann Neurol. 1999;45(3):353–357. doi: 10.1002/1531-8249(199903)45:3<353::aid-ana11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 45.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magdalinou N, Lees AJ, Zetterberg H. Cerebrospinal fluid biomarkers in parkinsonian conditions: an update and future directions. J Neurol Neurosurg Psychiatry. 2014 doi: 10.1136/jnnp-2013-307539. doi:10.1136/jnnp-2013-307539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malek N, Swallow D, Grosset KA, Anichtchik O, Spillantini M, Grosset DG. Alpha-synuclein in peripheral tissues and body fluids as a biomarker for Parkinson’s disease - a systematic review. Acta Neurol Scand. 2014 doi: 10.1111/ane.12247. doi:10.1111/ane.12247. [DOI] [PubMed] [Google Scholar]
- 48.Marques O, Outeiro TF. Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis. 2012;3:e350. doi: 10.1038/cddis.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mata IF, Shi M, Agarwal P, Chung KA, Edwards KL, Factor SA, Galasko DR, Ginghina C, Griffith A, Higgins DS, Kay DM, Kim H, Leverenz JB, Quinn JF, Roberts JW, Samii A, Snapinn KW, Tsuang DW, Yearout D, Zhang J, Payami H, Zabetian CP. SNCA variant associated with Parkinson disease and plasma alpha-synuclein level. Arch Neurol. 2010;67(11):1350–1356. doi: 10.1001/archneurol.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mollenhauer B, Cullen V, Kahn I, Krastins B, Outeiro TF, Pepivani I, Ng J, Schulz-Schaeffer W, Kretzschmar HA, McLean PJ, Trenkwalder C, Sarracino DA, Vonsattel JP, Locascio JJ, Agnaf OM, Schlossmacher MG. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol. 2008;213(2):315–325. doi: 10.1016/j.expneurol.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Döring F, Trenkwalder C, Schlossmacher MG. alpha-Synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol. 2011;10(3):230–240. doi: 10.1016/S1474-4422(11)70014-X. [DOI] [PubMed] [Google Scholar]
- 52.Nagaraja TN, Patel P, Gorski M, Gorevic PD, Patlak CS, Fenstermacher JD. In normal rat, intraventricularly administered insulin-like growth factor-1 is rapidly cleared from CSF with limited distribution into brain. Cerebrospinal Fluid Res. 2005;2:5. doi: 10.1186/1743-8454-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nixon RA, Yang DS, Lee JH. Neurodegenerative lysosomal disorders: a continuum from development to late age. Autophagy. 2008;4(5):590–599. doi: 10.4161/auto.6259. [DOI] [PubMed] [Google Scholar]
- 54.Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J Neurosci. 2001;21(20):8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 56.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81(10):1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Reed DJ, Woodbury DM. Kinetics of Movement of Iodide, Sucrose, Inulin and Radio-Iodinated Serum Albumin in the Central Nervous System and Cerebrospinal Fluid of the Rat. J Physiol. 1963;169:816–850. doi: 10.1113/jphysiol.1963.sp007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Zabetian CP, Kim HM, Leverenz JB, Montine TJ, Ginghina C, Kang UJ, Cain KC, Wang Y, Aasly J, Goldstein DS, Zhang J. Cerebrospinal Fluid Biomarkers for Parkinson Disease Diagnosis and Progression. Ann Neurol. 2011;69(3):570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi M, Zabetian CP, Hancock AM, Ginghina C, Hong Z, Yearout D, Chung KA, Quinn JF, Peskind ER, Galasko D, Jankovic J, Leverenz JB, Zhang J. Significance and confounders of peripheral DJ-1 and alpha-synuclein in Parkinson’s disease. Neurosci Lett. 2010;480(1):78–82. doi: 10.1016/j.neulet.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shults CW. Lewy bodies. Proc Natl Acad Sci U S A. 2006;103(6):1661–1668. doi: 10.1073/pnas.0509567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, Paisan-Ruiz C, Lichtner P, Scholz SW, Hernandez DG, Kruger R, Federoff M, Klein C, Goate A, Perlmutter J, Bonin M, Nalls MA, Illig T, Gieger C, Houlden H, Steffens M, Okun MS, Racette BA, Cookson MR, Foote KD, Fernandez HH, Traynor BJ, Schreiber S, Arepalli S, Zonozi R, Gwinn K, Brug M, Lopez G, Chanock SJ, Schatzkin A, Park Y, Hollenbeck A, Gao J, Huang X, Wood NW, Lorenz D, Deuschl G, Chen H, Riess O, Hardy JA, Singleton AB, Gasser T. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simpson R, Mathivanan S. Extracellular Microvesicles: The Need for Internationally Recognised Nomenclature and Stringent Purification Criteria. J Proteomics Bioinform. 2012;5:2. [Google Scholar]
- 63.Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8(19):4083–4099. doi: 10.1002/pmic.200800109. [DOI] [PubMed] [Google Scholar]
- 64.Simpson RJ, Kalra H, Mathivanan S. ExoCarta as a resource for exosomal research. Journal of Extracellular Vesicles. 2012;1:18374. doi: 10.3402/jev.v1i0.18374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr., Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56(2):293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Tokuda T, Salem SA, Allsop D, Mizuno T, Nakagawa M, Qureshi MM, Locascio JJ, Schlossmacher MG, El-Agnaf OM. Decreased alpha-synuclein in cerebrospinal fluid of aged individuals and subjects with Parkinson’s disease. Biochem Biophys Res Commun. 2006;349(1):162–166. doi: 10.1016/j.bbrc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 68.Toledo JB, Korff A, Shaw LM, Trojanowski JQ, Zhang J. CSF alpha-synuclein improves diagnostic and prognostic performance of CSF tau and Abeta in Alzheimer’s disease. Acta Neuropathol. 2013;126(5):683–697. doi: 10.1007/s00401-013-1148-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006;5(1):75–86. doi: 10.1016/S1474-4422(05)70285-4. [DOI] [PubMed] [Google Scholar]
- 70.Wang N, Gibbons CH, Lafo J, Freeman R. alpha-Synuclein in cutaneous autonomic nerves. Neurology. 2013;81(18):1604–1610. doi: 10.1212/WNL.0b013e3182a9f449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wennstrom M, Surova Y, Hall S, Nilsson C, Minthon L, Bostrom F, Hansson O, Nielsen HM. Low CSF levels of both alpha-synuclein and the alpha-synuclein cleaving enzyme neurosin in patients with synucleinopathy. PLoS ONE. 2013;8(1):e53250. doi: 10.1371/journal.pone.0053250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winkler J, Ehret R, Buttner T, Dillmann U, Fogel W, Sabolek M, Winkelmann J, Kassubek J. Parkinson’s disease risk score: moving to a premotor diagnosis. J Neurol. 2011;258(Suppl 2):S311–315. doi: 10.1007/s00415-011-5952-x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.