Abstract
Infusion of blood cells from a donor can induce humoral tolerance in a recipient and increase the probability of successful organ transplant; a clinical method defined as donor-specific transfusion (DST). Despite the clinical success of DST, the immunological mechanism(s) by which blood cells displaying a foreign antigen induce tolerance remain poorly understood. Based on recent findings showing that the B cell siglecs, CD22 and Siglec-G, can promote tolerance to antigens presented on the same surface as their ligands, we speculated that the B cell siglecs are key players in tolerance induced by DST. Using a variety of chemical and genetic approaches, we show that the B cell siglecs mediate tolerance to cell surface antigens by initiating an inhibitory signal that culminates in elimination of the antigen-reactive B cell. CD22 and Siglec-G are recruited to the immunological synapse by sialic acid ligands on the antigen-bearing cells, producing a tolerogenic signal involving Lyn and the pro-apoptotic factor BIM that promotes deletion of the B cell and failure of mice to develop antibodies to the antigen upon subsequent challenge. We speculate that this tolerogenic mechanism is a contributing factor in DST and a mechanism of peripheral B cell tolerance to cell surface autoantigens.
Keywords: B cells, siglecs, tolerance, antigen, sialic acid, apoptosis, BIM, Lyn, CD22, Siglec-G
Introduction
Classic experiments performed over 40 years ago demonstrated that whole blood or lymphocytes displaying a foreign antigen could induce immunological tolerance in rodents, facilitating successful transplantation of donor tissue(1-3). These results stimulated the evaluation of donor-specific transfusion (DST) for organ transplantation in humans, with numerous clinical trials reporting a reduced rate of transplant rejection(4-7). Although the success of broad immunosuppressive drugs for transplantation curtailed the use of DST, interest has resurfaced as a result of improved long-term organ survival over treatment with immunosuppressive drugs alone(8, 9). A lack of clear understanding of the cellular mechanism(s) mediating tolerance, however, has hampered the refinement of DST and its adoption for routine use in human transplantation(6, 10).
A major objective of DST-induced tolerance is elimination of a humoral response to donor-specific antigens. Several studies suggest that lymphocytes displaying a foreign antigen can directly induce depletion of the antigen-reactive B cells to achieve tolerance(11-13). Other studies suggest that Tregs are induced by the combination of DST and transplantation, which could work synergistically with B cell-intrinsic mechanisms to blunt a humoral response(14-16). Intrinsic mechanisms of peripheral B cell tolerance include those mediated by inhibitory co-receptors that modulate B cell receptor (BCR) signaling(17). Among these are CD22 and Siglec-G, two sialic acid-binding immunoglobulin-type lectins (siglecs), that participate in peripheral B cell tolerance, as evidenced by the development of autoimmune antibodies in mice deficient in these siglecs(18-22).
The siglecs are a subfamily of the Ig superfamily expressed in cells of the immune system that recognize sialic acid-containing glycans, which are present on the cell surface of all cells(22). B cell siglecs dampen BCR signaling by a mechanism involving phosphorylation of their cytoplasmic ITIM motifs and recruitment of phosphatases, such as Shp-1, that in turn dephosphorylate BCR signaling components and set a threshold for B cell activation(23-25). Glycan ligands of siglecs on the same cell in cis, or on opposing cells in trans, modulate their activity as inhibitory receptors by regulating their proximity to the BCR(22, 26, 27).
In a landmark study involving B cells reactive to a cell surface antigen, Lanuoe et al. found that B cell activation was suppressed if antigen-expressing cells were transfected with the gene encoding ST6Gal1(26), the enzyme that creates α2-6 linked sialosides, which serve as ligands for CD22(28). The further demonstration that trans ligands cause CD22 to redistribute to the site of cell contact suggest that ligands participate in suppression of BCR signaling to cell surface antigens by recruiting CD22 to the synapse between the two cells(26, 29, 30). More recent studies from our group and others have investigated the in vitro and in vivo consequences of ligating CD22 or Siglec-G to the BCR using polymers or liposomes displaying both an antigen and high affinity analogs of siglec ligands(31-34). In all cases, co-presentation of siglec ligands with the antigen induces a profound suppression of BCR signaling. Moreover, we further showed that the siglecs induce an apoptotic signal that results in antigen-specific tolerance in mice by elimination of the antigen-reactive B cells(32-34).
In our studies with antigenic liposomes, we found that natural sialoside ligands of CD22 or Siglec-G also induced B cell tolerance, albeit with reduced activity compared to the high affinity ligands(33, 34). This suggested to us, that the co-presentation of antigen and siglec ligands on such artificial scaffolds are mimicking and exploiting an intrinsic tolerogenic mechanism in B cells, whereby tolerance to cell surface autoantigens can be induced by B cell siglecs that are recruited to the immunological synapse by natural ligands on the cells displaying antigen. We further reasoned that B cell tolerance induced by DST might similarly invoke apoptosis of antigen-reactive B cells through a mechanism involving the B cell siglecs.
Using transfer of lymphocytes bearing a foreign antigen as a model of DST, we show here that antigen-reactive B cells are deleted through a siglec-mediated mechanism, rendering the mouse tolerant to subsequent challenge with antigen. CD22 and Siglec-G are independently recruited in a ligand-dependent manner to an immunological synapse formed between a B cell and a lymphocyte bearing its cognate antigen. Subsequent deletion of the B cell requires both Lyn kinase to initiate the apoptotic signal and the downstream pro-apoptotic factor BIM. The results suggest that the B cell siglecs co-operate to delete B cells reactive to cell surface antigens. We propose that DST exploits this natural mechanism of peripheral B cell tolerance by donor-specific antigens displayed on blood cells that express siglecs ligands.
Methods
Animal studies
The Scripps Research Institute IACUC approved all experimental procedures involving mice. CD22-/- and Siglec-G-/- mice were obtained from L. Nitschke (University of Erlangen) and Y. Liu (University of Michigan), respectively. ST6Gal1-/- mice were obtained from the Consortium for Functional Glycomics. BIM-/-, Bcl2 transgenic, Lyn-/-, Blk-/-, Fyn-/-, MD4, and KLK4 mice were obtained from Jackson laboratories. The TSRI rodent breeding colony provided WT C57BL/6J mice.
Immunization and Blood Collection
Blood was collected via retro-orbital bleed and stored at -20 °C. Cells or liposomes were delivered via the lateral tail vein in a volume of 200 μL. Protein emulsified in Complete Freund's Adjuvant (CFA) used to immunize mice via an intraperitoneal injection in a total volume of 200 μL.
Flow cytometry
An LSR-II flow cytometer (BD) was used with up to eight colors. Dead cells were gated out with 1 μg/mL of propidium iodide.
B cell purification
B and T cells were purified by negative selection using magnetic beads (Miltenyi). Adoptively transferred IgMHEL B cells were defined as CD19+CD45.1+IgMa+.
Fluorescent Labeling of B cells
Purified IgMHEL B cells (10×106 cells/ml) were fluorescently labeled with 1 μM Cell Trace Violet (CTV; Invitrogen) in HBSS for 7 minutes at RT and washed twice before resuspension at the appropriate concentration.
Mild periodate oxidation of B cells
Cells (10×106 cells/ml) were washed twice with PBS and cooled on ice for 10 min. Sodium periodate (4 mM) was added and following incubation on ice for 20 min, glycerol (10 mM) and an equal volume of media (RPMI + 10% FCS) were added. Cells were centrifuged (270 rcf, 7 min) and washed once more in the appropriate assay buffer. To verify destruction of sialic acids, cells were analyzed by flow cytometry for staining with SNA.
Insertion of pegylated-lipids into cells
Preparation of the high affinity CD22 ligand (6′BPANeuAc-PEG-DSPE) and Siglec-G ligand (3′BPANeuAc-PEG-DSPE) has been described previously(33, 34). Compounds were incubated with periodate-treated mHEL (Per-mHEL) cells in HBSS buffer at a concentration of 1 μM for one hour at 37 °C. Cells were washed twice and used immediately in assays.
In Vitro B Cell Assays
Purified IgMHEL B cells (0.2×106) were plated in U-bottom 96-well culture plates. Liposomes (5 μM lipid final concentration) or mHEL cells were added and cells were incubated at 37 °C for 24 hr.
Adoptive transfers
IgMHEL cells (5×106, 200 μl) in HBSS were injected into host mice via the tail vein. The following day, liposomes or mHEL cells were injected via the tail vein. Host spleens were harvested on the appropriate day, stained with antibodies, and analyzed by flow cytometry.
Calcium flux and ELISAs
Experimental procedures were carried out identically as described previously(33). Liposomes were added to cells 10 seconds after starting acquisition.
Western blotting
Purified IgMHEL B cells (10×106) were stimulated with either liposomes (5 μM lipid final concentration) or mHEL B cells (1×106) for 30 min at 37 °C in 200 μL of media, briefly centrifuged (13,000 rcf, 8 sec), washed (1 mL cold PBS), and lysed in 100 μL (20 mM Tris, 150 NaCl, 1 mM EDTA, 1% Triton-X 100, 10 mM NaF, 2 mM Sodium orthovanadate, protease inhibitor cocktail (Roche), pH 7.5) on ice for 30 min. The remaining procedures for SDS-PAGE and Western blotting are identical to a previous protocol(33).
Microscopy
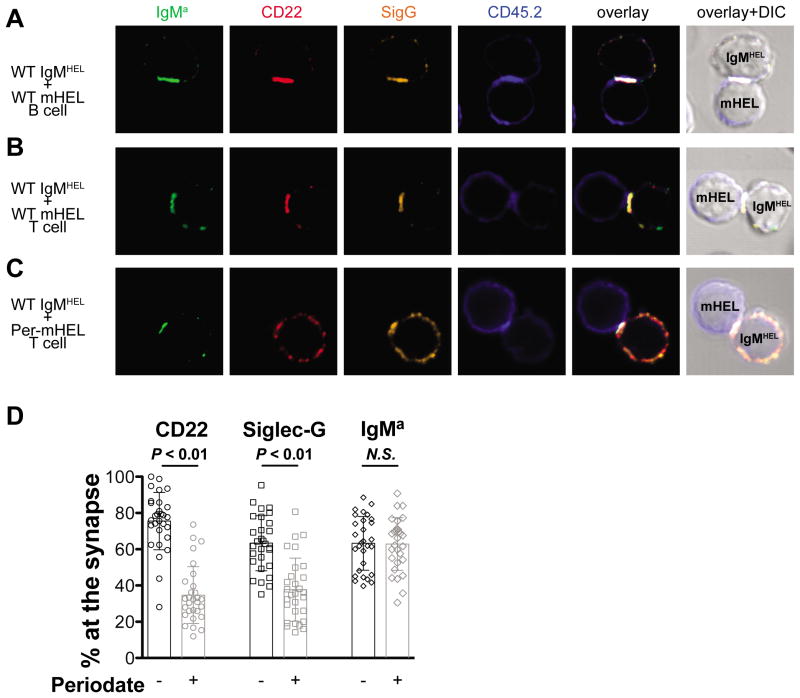
Purified IgMHEL B cells (20×106/ml) and mHEL B or T cells (20×106/ml) were mixed at 1:1 ratio (IgMHEL:mHEL) and incubated at 37 °C for 30 min. Cells (1×106; 100 μl) were plated onto poly-lysine cover slips (BD). After 5 min, the media was gently removed, and chilled 3% paraformaldehyde (PFA) was added for 5 min at 4 °C. Cells were washed twice with PBS and blocked with 5% normal goat serum (NGS) in PBS for 30 min at RT. Slides were probed with pacific blue-labeled anti-CD45.1 (1:100), FITC-labeled anti-IgMa (1:200), biotinylated anti-CD22 (1:500; clone cy34.1), and AF647-labeled anti-Siglec-G (20 μg/mL final) in 1% NGS overnight at 4 °C. The following day, slides were washed with PBS and probed with AF488-conjugated anti-FITC (1:500; Invitrogen), AF555-strepavidin (1:1000; Invitrogen), and AF647-labeled goat-anti rat IgG (1:500; Invitrogen). Slides were mounted in anti-fade medium and images acquired on a Zeiss confocal microscope at 60x magnification using oil emersion. To quantify the percentage of CD22, Siglec-G, and IgMa at the synapse, 30 images of each condition were captured. The IgMHEL B cells were divided into four quadrants and the amount of each component in the quadrant containing the synapse was determined as a percentage of the total amount of each component in all four quadrants. Using Image Pro Plus 7 software, the amount of each component in a particular area was calculated by the total area of fluorescent signal above a threshold set by the signal on T cells, where these components are not expressed. Under conditions where there is equal distribution around the cell, the percentage at the synapse would be expected to be 25%.
Protein crosslinking
Hen egg lysozyme-ovalbumin (HEL-OVA) and ovalbumin-chicken gamma globulin (OVA-CGG) conjugates were prepared by crosslinking with glutaraldehyde. Each protein was dissolved in 5 mL at a concentration of 5 mg/mL in PBS. The proteins were combined and crosslinked by the addition of 10 μL of 25% glutaraldehyde (Sigma). After 1 hr (RT), crosslinked proteins were desalted and emulsified in CFA (BD Difco).
Liposomes
A protocol for the preparation of liposomes is described in detail elsewhere(33, 34).
Statistical analyses
Statistical significance was determined using an unpaired two-tailed Student's t-test.
Results
Glycan ligands recruit siglecs to an immunological synapse
Intense CD22 staining is observed at the site of cell contact between two lymphocytes and redistribution is dependent on glycan ligands expressed on the opposing cell(29, 30). To determine if Siglec-G exhibits similar behavior, primary mouse B cells were stained with a newly developed anti-Siglec-G monoclonal antibody(34). Similar to CD22, Siglec-G redistributed to the cellular interface between two B cells, while CD45 remained uniformly distributed (Fig. 1A). This is ligand-dependent since mild periodate oxidation, which destroys sialic acids(35), prevents redistribution of both siglecs (Fig. 1B).
Fig. 1.
Ligand-dependent redistribution of CD22 and Siglec-G to the site of cell contact between two B cells. (A) Purified splenic B cells from a WT mouse were plated, fixed, stained with the indicated markers, and analyzed by confocal microscopy. (B) Purified splenic B cells from the spleen of a WT CD45.1+CD45.2- mouse and CD45.1+CD45.2+ mouse were independently purified and periodate (Per) treated to destroy sialic acid. The two samples were mixed for an hour, plated on cover slips, fixed, stained with the indicated markers, and analyzed by confocal microscopy. The width of each image is 12.5 μm.
To study this redistribution in the context of an immunological synapse formed between a B cell and a second cell bearing its cognate antigen, we used HEL-reactive (IgMHEL) B cells from transgenic MD4 mice(36) mixed with splenocytes expressing membrane-bound HEL (mHEL) from KLK4 transgenic mice(37). Intense and overlapping staining of the HEL-reactive BCR (IgMa), CD22, and Siglec-G was clearly observed at the interface between a WT IgMHEL B cell and WT mHEL B cell (Fig. 2A) or mHEL T cell (Fig. 2B). Recruitment of both siglecs was abrogated when sialic acid was destroyed on the mHEL T cells by periodate-treatment (Fig. 2C). The amount of each component recruited to the synapse was quantified as a percentage of the total fluorescence in the quadrant comprising the synapse for 30 individual pairs of WT IgMHEL B cells contacting mHEL T cells that were either untreated, or treated with periodate prior to mixing with the IgMHEL B cells (Fig. 2D). Destroying sialic acid on the mHEL cells resulted in significantly less CD22 and Siglec-G at the synapse, but had no effect on recruitment of the HEL specific IgMa to the synapse. These results demonstrate that the siglecs are recruited to an immunological synapse through interactions with sialoside ligands on the antigen-expressing cell.
Fig. 2.
Recruitment of CD22 and Siglec-G to the immunological synapse by ligands on cells displaying cell surface antigen. WT HEL-reactive B cells (IgMHEL; CD45.1+CD45.2-) were mixed with (A) WT B cells expressing membrane HEL, (B) WT mHEL T cells, or (C) periodate (Per) treated mHEL cells. mHEL cells are CD45.1-CD45.2+. After 30 minutes of incubation, cells were plated onto cover slips, fixed, stained with the indicated markers, and analyzed by confocal microscopy. The width of each image is 12.5 μm.
To determine if Siglec-G used different ligands than CD22, we made use of mHEL-expressing lymphocytes deficient in ST6Gal1(38), which lack α2-6 linked sialosides that are the preferred ligands of CD22(28, 39). At the synapse formed between an IgMHEL B cell and ST6Gal1-/- mHEL B cell, the CD22 from the IgMHEL B cell is uniformly distributed, while the CD22 from the ST6Gal1-/- mHEL B cell is strongly enriched at the interface (Supplemental Fig. 1A), consistent with α2-6 linked sialoside ligands being depleted only on the cell bearing mHEL, and their requirement for recruiting CD22 to the immunological synapse. Conversely, Siglec-G was recruited to the synapse on both cells, demonstrating that ligands on the ST6Gal1-/- B cells other than α2-6 linked sialoside ligands can support recruitment of Siglec-G. Similarly, other combinations of B ant T cells (Supplemental Fig. 1B-D) support the conclusion that CD22 and Siglec-G exhibit different ligand specificities and can be differentially recruited to the immunological synapse by sialoside ligands on the antigen-bearing cell.
Recruitment of siglecs inhibits B cell activation
To investigate the functional consequence of ligand-mediated recruitment of CD22 and Siglec-G to the immunological synapse, IgMHEL B cells and mHEL B or T cells were co-cultured for 24 hr and B cell activation was assessed by CD86 upregulation. Although WT mHEL B cells elicited only weak activation of IgMHEL B cells, mHEL B cells treated with periodate to destroy ligands (Per-mHEL) or ST6Gal1-/- mHEL B cells elicited strong activation (Fig. 3A). In contrast to weak activation of WT IgMHEL B cells by mHEL cells, IgMHEL B cells deficient in CD22 (CD22‐/-) or both siglecs (CD22‐/-SigG-/-) were strongly activated, while SigG-/- IgMHEL B cells showed intermediate activation. These differences could be assessed more quantitatively by assessing the number of WT or ligand-deficient mHEL cells required to activate IgMHEL B cells (Fig. 3B, Supplemental Fig. 2), which more clearly revealed that SigG-/- IgMHEL B cells required 2-fold fewer mHEL B cells for half-maximal activation than WT IgMHEL B cells.
Fig. 3.
Recruitment of CD22 and Siglec-G to the immunological synapse inhibits B cell activation. (A) WT, CD22-/-, Siglec-G-/-, or CD22-/-Siglec-G-/- IgMHEL B cells were mixed with WT (blue), periodate-treated (Per; cyan), or ST6Gal1-/- (red) mHEL B cells at a 1:1 ratio. IgMHEL B cells incubated without mHEL B cells (grey) were used as resting B cells. Activation of the IgMHEL B cells was assessed 24 hr later by CD86 upregulation using flow cytomery. (B) Summary of the EC50 values for CD86 upregulation as a function of the mHEL:IgMHEL ratio. Values and errors are derived from non-linear fits. (C) Periodate-treated mHEL B cells were reconstituted with a CD22-specific ligand (6′BPANeuGc; blue) or a Siglec-G-specific ligand (3′BPANeuGc; cyan) attached to a pegylated-lipid (PEG-DSPE) for insertion into the cell membrane. mHEL B cells incubated with PEG-DSPE (red) served as a control for insertion of no ligands. mHEL B cells were incubated with IgMHEL B cells and CD86 staining of the IgMHEL B cells was analyzed by flow cytometry 16 hr later. IgMHEL B cells incubated without mHEL cells (grey) represent unstimulated cells.
To confirm that recruitment of either siglec can inhibit B cell activation, ligand-deficient Per-mHEL B cells were reconstituted with synthetic lipid-linked ligands selective for either CD22 (6′BPANeuGc) or Siglec-G (3′BPANeuGc)(33, 34). Cells reconstituted with either ligand strongly suppressed activation of WT IgMHEL B cells (Fig. 3C), whereas activation of CD22-/-or SigG-/- IgMHEL B cells were inhibited only by cells carrying their cognate ligands. Taken together, these results demonstrate that recruitment of either CD22 or Siglec-G to the immunological synapse can independently inhibit B cell activation.
Siglec-dependent deletion of B cells reactive to mHEL
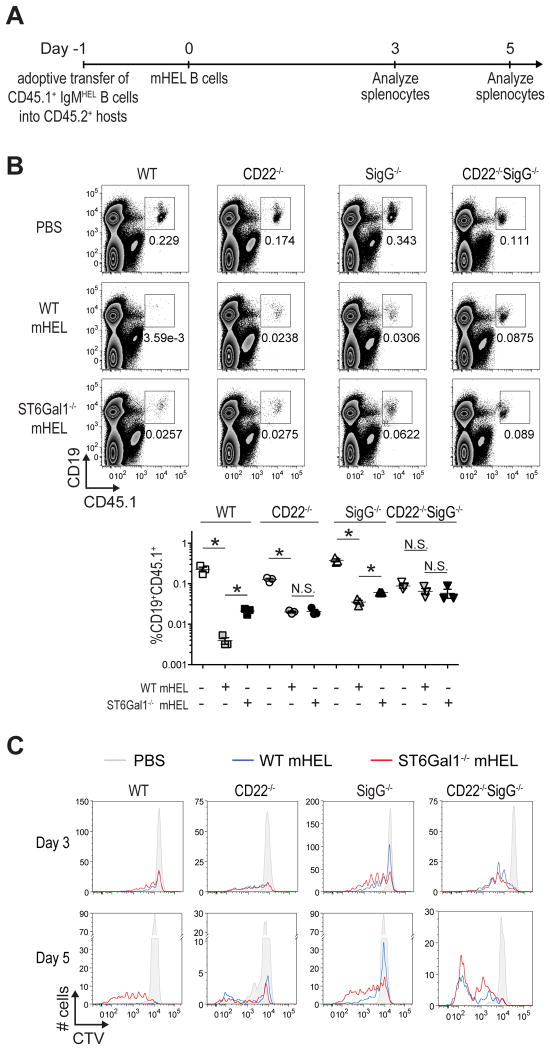
The fate of IgMHEL B cells encountering mHEL cells in vivo was examined by adoptively transferring CD45.1+IgMHEL B cells into non-transgenic CD45.2+ WT hosts, followed by a second adoptive transfer of mHEL B cells the following day (Fig. 4A). Using optimized parameters, WT IgMHEL B cells were dramatically depleted by mHEL B cells at day 5 and 12(Fig. 4B; Supplemental Fig. 3). While IgMHEL B cells lacking either CD22 or Siglec-G were depleted to a lesser extent, there was no significant depletion of IgMHEL B cells deficient in both siglecs (CD22-/-SigG-/-), demonstrating that depletion was siglec-dependent. Ligands on the mHEL B cells are also required since ST6Gal1-/-mHEL B cells, deficient in CD22 ligands, induced significantly less depletion of WT and SigG-/- deficient IgMHEL B cells (Fig. 4B). Siglec-dependent depletion of IgMHEL B cells was also observed using mHEL T cells (Supplemental Fig. 4).
Fig. 4.
Siglec-dependent inhibition and depletion of IgMHEL B cells encountering mHEL B cells in vivo. (A) Scheme for adoptive transfer experiments of CTV labeled IgMHEL B cells and mHEL cells into host mice. On day -1, CD45.1+ IgMHEL B cells are adoptive transferred into CD45.2+CD45.1- host mice. The following day (day 0), mHEL B or T cells are adoptively transferred into the same host mice. Three or five days later, the spleens of host mice were analyzed for HEL-reactive B cells (CD19+CD45.1+). (B) CTV dilution was used to monitor proliferation of the IgMHEL B cells (5×106) in host mice 5 days after the mice received 5×106 WT mHEL B cells (blue), 5×106 ST6Gal1-/- mHEL B cells (red), or PBS (grey). For each condition, 1×106 total splenocytes were analyzed. (C) Upper: flow cytometry analysis of 5v106 adoptively transferred IgMHEL B cells (CD19+CD45.1+) in the spleen of host mice five days after adoptive transfer of PBS, 2×106 WT mHEL B cells, or 2×106 ST6Gal1-/- mHEL B cells. Lower: Numeration of the CD19+CD45.1+ cells in host mice. Values represent the average ± SEM of three replicates and are representative of three independent experiments.
To better understand the depletion of IgMHEL B cells in response to mHEL cells, the proliferation of CTV-labeled IgMHEL cells was analyzed on day 3 and 5 after transfer of WT and ST6Gal1-/- mHEL B cells (Fig. 4C). WT IgMHEL B cells showed dramatically impaired proliferation in response to WT mHEL cells relative to ST6Gal1-/- mHEL cells. In contrast, IgMHEL B cells from CD22-/-SigG-/- mice showed substantial proliferation in response to either WT or ST6Gal1-/-mHEL cells, while CD22-/- or SigG-/- IgMHEL B cells exhibited intermediate levels of proliferation. These in vivo results support and extend the in vitro results, suggesting that activation of B cells to membrane antigens is suppressed by CD22 and Siglec-G, as a result of their recruitment to the immunological synapse, by trans sialoside ligands on the antigen-expressing cell.
Depletion of antigen-reactive B cells requires BIM and Lyn
Based on our previous work on the mechanism of induction of B cell tolerance by siglec tolerizing antigenic liposomes (STALs)(33, 34), we hypothesized that depletion of antigen-reactive B cells may be mediated through inhibition of the Akt survival pathway. Since BIM is downstream of Akt and plays an essential role in B cell apoptosis(40, 41), we investigated its impact using IgMHEL B cells derived from BIM-deficient mice(42). In contrast to WT IgMHEL B cells, BIM-/- IgMHEL B cells were not deleted by mHEL cells (Fig. 5A). A modest inhibitory effect of the siglecs was still evident since ST6Gal1-/- mHEL cells induced stronger proliferation and expansion of the BIM-/-IgMHEL B cells relative to WT cells (Fig. 5A,B), whereas triple-deficient BIM-/-CD22-/-SigG-/- IgMHEL B cells responded equally to both WT and ST6Gal1-/- mHEL cells. Supporting a critical role for BIM in mediating apoptosis, IgMHEL B cells from Bcl-2 transgenic mice(43), which overexpress the anti-apoptotic Bcl-2, also failed to be depleted by WT mHEL B cells (Fig. 5A,B).
Fig. 5.
BIM and Lyn are required for Siglec-dependent inhibition and depletion. (A) Left: Flow cytometry analysis of adoptively transferred IgMHEL B cells (CD19+CD45.1+) on a WT, BIM-/-, Bcl-2 transgenic, or Lyn-/- background in the spleen of host mice five days after adoptive transfer of PBS, WT mHEL, or ST6Gal1-/- mHEL. Right: Numeration of the CD19+CD45.1+ cells in host mice. Values represent the average ± SEM of three replicates and are representative of two independent experiments. (B) CTV dilution was used to monitor proliferation of WT, BIM-/-, BIM-/-CD22-/-SigG-/-, Bcl-2 transgenic, and Lyn-/- IgMHEL B cells in host mice that also received WT mHEL B cells (blue), ST6Gal1-/- mHEL B cells (red), or PBS (grey). For each condition, 1×106 total splenocytes were analyzed. Results are representative of three replicates. (C) Analysis of Akt and Erk activation in IgMHEL B cells stimulated with mHEL B cells. WT, CD22-/-SigG-/-, or Lyn-/- IgMHEL B cells were incubated with non-mHEL or mHEL B cells, with and without periodate-treatment, for 30 minutes at a mHEL:IgMHEL ratio of 1:9. Cell lysates were analyzed by Western blot for activation of Akt and Erk using phospho-specific antibodies. Results are representative of two independent experiments.
Dampening of BCR signaling through CD22 is initiated by phosphorylation of tyrosine residue(s) within ITIM motif(s) on the cytoplasmic domain of CD22, which recruit Shp-1(23). Lyn has been implicated as the dominant kinase that phosphorylates CD22(44). Siglec-G inhibition also requires Shp-1(34), but the kinase(s) have not been established. To determine if Lyn is required for the siglec-dependent depletion of IgMHEL B cells encountering mHEL cells, in vivo adoptive transfer studies were repeated with IgMHEL B cells on a Lyn-/- background(45). Lyn-/-B cells were not depleted by WT mHEL cells and showed equivalent proliferation with ST6Gal1-/- mHEL cells (Fig. 5A,B). To analyze the role of CD22, Siglec-G, and Lyn in mediating the inhibition of Akt and Erk1/2 phosphorylation, central mediators of signaling pathways upstream of BIM expression, we performed Western blot analyses following a 30-minute incubation of IgMHEL B cells with mHEL B cells (Fig. 5C). In WT IgMHEL B cells, Akt and Erk were not phosphorylated when exposed to WT mHEL cells, but were phosphorylated with Per-mHEL cells. In contrast, in CD22-/-SigG-/- or Lyn-/- IgMHEL B cells, Akt and Erk were phosphorylated when stimulated with either WT or Per-mHEL cells, demonstrating that Lyn is required for siglec-mediated inhibition of Akt and Erk activation.
STALs induce B cell apoptosis by a similar mechanism
STALs induce antigen-specific tolerance through a siglec-dependent mechanism(33, 34). To determine if STALs induce apoptosis through the same mechanism as mHEL cells, we probed the requirement of BIM and Lyn in B cell apoptosis and inhibition assays. For initial experiments, we used liposomes displaying a surrogate antigen (anti-IgM Fab fragment) to probe the in vitro activation of B cells by calcium flux or Western blotting (Fig. 6A,B). Antigenic liposomes displaying ligands of either CD22 (6′BPCNeuGc) or Siglec-G (3′BPCNeuGc) inhibit calcium flux in WT B cells, while CD22-/- or SigG-/-B cells are activated only by liposomes containing the ligand for the other siglec (Fig. 6A). Inhibition by STALs was largely abrogated in Lyn-/- B cells. B cells deficient in the other two major B cell Src kinases, Fyn or Blk(46), had no effect on STAL inhibition, indicating that Lyn is the major player mediating inhibition through CD22 and Siglec-G. The results observed in the calcium signaling experiments were recapitulated by monitoring phosphorylation of Akt and Erk by Western blotting (Fig. 6B). Taken together, the results confirm the dominant role of Lyn kinase in inhibition of BCR signaling by CD22, and show that Lyn is similarly responsible for inhibition of BCR signaling mediated by Siglec-G.
Fig. 6.
Antigenic liposomes displaying a CD22 or Siglec-G ligand support a critical role for BIM and Lyn in siglec-mediated apoptosis and inhibition. (A) Co-presentation of a CD22-specific ligand (6′BPANeuGc) or Siglec-G-specific ligand (3′BPANeuGc) on antigenic liposomes inhibits B cell activation in a Lyn-dependent manner. B cells on the indicated background were stimulated with liposomes displaying anti-IgM alone (red), anti-IgM + 6′BPANeuGc (blue) or anti-IgM + 3′BPANeuGc (green), and calcium flux was monitored by flow cytometry. (B) Co-presentation of 6′BPANeuGc or 3′BPANeuGc on antigenic liposomes inhibits Akt and Erk activation in a Lyn-dependent manner. IgMHEL B cells were stimulated for 3 minutes and cell lysates were probed by Western blot. (C) Liposomes displaying HEL and 6′BPANeuGc induce apoptosis of IgMHEL B cells in a CD22-, BIM-, and Lyn-dependent manner. IgMHEL B cells were incubated with liposomes displaying either HEL alone or HEL and 6′BPANeuGc, or PBS as a control. Upper: cells were stained with AnnexinV and PI. Lower: numeration of the percentage of PI+AnnexinV+ cells in three replicates. (D) Liposomes displaying HEL and 6′BPANeuGc deplete adoptively transferred IgMHEL B in a CD22-, BIM-, and Lyn-dependent manner. IgMHEL B cells (CD45.1+) were adoptively transferred into host mice (CD45.2+CD45.1-). The following day, mice received liposomes displaying either HEL alone or HEL and 6′BPANeuGc, or PBS as a control. Upper: twelve days later, the number of IgMHEL B cells (CD19+CD45.1+) in the spleen of host mice was analyzed. Lower: the percentage of CD19+CD45.1+ cells was numerated from three replicates in each condition.
The involvement of BIM and Lyn in siglec-mediated apoptosis of B cells by STALs was also was assessed. Using PI versus AnnexinV staining, liposomes displaying HEL and the CD22 ligand (6′BPANeuGc) induce robust apoptosis of IgMHEL B cells in vitro after 24 hours of incubation, but not in B cells deficient in CD22, BIM, or Lyn (Fig. 6C). In an adoptive transfer experiment, IgMHEL B cells were depleted by STALs displaying HEL and the CD22 ligand in a CD22-, BIM-, and Lyn-dependent manner (Fig. 6D), although STALs still inhibited B cell expansion of BIM-/- B cells relative to liposomes displaying HEL alone.
Cell surface antigens induce humoral tolerance
Based on these results, we considered that adoptive transfer of mHEL B cells is analogous to donor-specific transplantation (DST) used to induce humoral tolerance to transplant antigens. Accordingly, to determine if induction of humoral tolerance in non-transgenic B cells is siglec-mediated, mHEL B cells were transferred into host mice followed by immunization 15 days later to analyze anti-HEL antibodies (Fig. 7A). WT mice were strongly tolerized to HEL, as were mice lacking CD22 or Siglec-G. However, tolerance was lost in mice lacking both CD22 and Siglec-G (Fig. 7B), demonstrating that tolerance is siglec-mediated, and can be independently mediated by either siglec. A similar experiment was carried out with B cells from mice that express membrane ovalbumin (mOVA)(47) (Fig. 7C). In this case, tolerance was observed in WT mice that received mOVA B cells, but the degree of tolerance was weaker than with mHEL B cells. A break in tolerance was observed in mice that lacked either CD22 or Siglec-G, and CD22-/-SigG-/-mice appeared sensitized because they mounted a significantly larger anti-OVA antibody response than control mice.
Fig. 7.
CD22 and Siglec-G help to maintain tolerance and prevent sensitization of transferred B cells. (A) Scheme for testing tolerance induction to adoptively transferred mHEL or mOVA B cells. mHEL or mOVA B cells (2×106) were adoptively transferred into non-transgenic host mice. B cells that do not express mHEL or mOVA were used in control mice. To test for tolerance induction, mice were immunized with either HEL-OVA/CFA or OVA-CGG/CFA 15 days later and antibody titers were determined after another 15 days. (B,C) Adoptive transfer of mHEL or mOVA B cells induces siglec-dependent B cell tolerance. WT (squares), CD22-/- (circles), SigG-/- (triangles), or CD22-/-SigG-/- (inverted triangles) mice were administered adoptively transferred WT B cells (open symbol), (B) mHEL B cells or (C) mOVA B cells. Anti-HEL and anti-OVA antibody responses to the challenge were determined by ELISA. (D) IgMHEL B cells lacking CD22 and Siglec-G that encounter mHEL B cells develop a germinal center phenotype. CD45.1+ WT, CD22-/-SigG-/-, or BIM-/- IgMHEL B cells were adoptively transferred into host mice. The following day, host mice received PBS, WT mHEL B cells, or ST6Gal1-/- B cells. Twelve days later, the spleens of host mice were analyzed. Upper: adoptively transferred cells were gated on CD19+CD45.1+IgMa+ to assess CD95 and GL7 staining. Lower: the percentage of CD95+GL7+ cells was numerated from three replicates of each condition (lower panel).
To investigate this sensitization effect in more detail, we analyzed IgMHEL B cells for a germinal center (GC) phenotype, by CD95 and GL7 staining, 12 days after adoptive transfer with mHEL in host mice (Fig. 7D). The very few WT IgMHEL B cells that remained in mice administered mHEL cells showed no sign of a GC phenotype. On the other hand, a large percentage of CD22-/-SigG-/- IgMHEL B cells stained positive for GL7 and CD95 in mice that received either WT or ST6Gal1-/- mHEL B cells. BIM-/- IgMHEL only developed a GC phenotype when ST6Gal1-/- mHEL B cells were used, reinforcing the preservation of an inhibitory function for siglecs in BIM-/- B cells. These results strongly suggest that a contributing mechanism to tolerance induction by adoptively transferred blood cells expressing a foreign antigen involves siglec-dependent B cell tolerization.
Discussion
We have shown that CD22 and Siglec-G induce tolerance to cell surface antigens by a mechanism that results in deletion of the antigen-reactive B cells. This intrinsic mechanism is initiated by recruitment of the siglecs to an immunological synapse by natural sialic acid-containing ligands on the antigen-bearing cell, resulting in phosphorylation of the siglecs by Lyn, and inhibition of BCR signaling. Since expression of the pro-apoptotic factor BIM is influenced by the activated forms of Akt and Erk(48, 49), the net effect of siglec-mediated inhibition of BCR is predicted to be induction of BIM expression and apoptosis of the B cell. Indeed, B cells lacking BIM failed to be deleted both in vitro and in vivo through siglec-mediated inhibition. Consequently, mice fail to mount an antibody response to that antigen in a subsequent challenge. When siglec-ligand interactions are abrogated, siglecs are not recruited to the synapse, and the highly multivalent nature of cell surface antigens induces strong B cell activation, proliferation, and acquisition of a GC phenotype.
We suggest that this B cell-intrinsic mechanism is directly relevant to induction of humoral tolerance to transplant antigens by DST. Studies in mice have documented that lymphocytes expressing foreign antigens induce antigen-specific tolerance(12, 50, 51), with several reports demonstrating that the antigen-reactive B cells are deleted(11-13). Lack of T cell help has been suggested to be a mechanism contributing to B cell depletion, but cell surface antigens are highly multivalent and can, by themselves, extensively crosslink the BCR similar to T-independent type 2 (TI-2) antigens that are capable of inducing B cell memory and long-lived plasma cells(52). We have previously reported that strong T cell help can counteract siglec-mediated tolerance with STALs(25, 33). This may explain why mHEL cells induced more robust tolerance than mOVA cells, since HEL elicits weak T cell help in C57BL/6J mice(53). In this regard, it is of interest whether dampening T cell help with immunosuppressive drugs or co-stimulatory blockade enhances the effectiveness of DST(9, 54) and tolerance induction with lymphocytes expressing foreign antigen in mice(11, 55).
Mechanisms that enforce B cell tolerance prevent an autoimmune response by self-reactive B lymphocytes that reach the periphery due to incomplete central tolerance or through hypermutation of the BCR in GCs(56). A systemic autoimmune phenotype has been documented to arise in mice deficient in both CD22 and Siglec-G, which has implicated these siglecs in maintaining B cell tolerance(57). Our results are consistent with these genetic studies since each siglec can operate independently and a complete break in tolerance occurs only when the functions of both are compromised. We suggest that the overlapping, but distinct, ligand specificities of CD22 and Siglec-G enable the two siglecs to maintain peripheral B cell tolerance to cell surface antigens on a wide variety of cell types that display different sialoside structures(32, 34, 58, 59). These considerations also apply to human B cells since CD22 and Siglec-10, the human ortholog of Siglec-G, also exhibit different specificities for sialoside ligands(22, 60).
In summary, CD22 and Siglec-G promote deletion of B cells encountering their cognate antigen on a cell surface displaying sialoside ligands through a BIM-dependent mechanism. As documented here, the ability of STALs to induce B cell tolerance exploits this naturally occurring mechanism. We believe that this mechanism is a basis for tolerance to a foreign antigen on the surface of blood cells, as occurs in DST. A deeper understanding of these basic mechanisms of tolerance should lead to more robust methods for induction of B cell tolerance for treatment in transplantation and autoimmunity.
Supplementary Material
Acknowledgments
We thank Britni Arlian and Jessica Lu for technical assistance and Anna Tran-Crie for assistance in preparation of the manuscript.
Footnote: This work is funded by grants from the NIH R01AI050143 and R01AI099141. M.S.M. is supported by a fellowship from the Human Frontiers Scholarship Program.
References
- 1.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 2.Stuart FP, Saitoh T, Fitch FW. Rejection of renal allografts: specific immunologic suppression. Science. 1968;160:1463–1465. doi: 10.1126/science.160.3835.1463. [DOI] [PubMed] [Google Scholar]
- 3.Fabre JW, Morris PJ. The mechanism of specific immunosuppression of renal allograft rejection by donor strain blood. Transplantation. 1972;14:634–640. doi: 10.1097/00007890-197211000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Opelz G, Terasaki PI. Improvement of kidney-graft survival with increased numbers of blood transfusions. N Engl J Med. 1978;299:799–803. doi: 10.1056/NEJM197810122991503. [DOI] [PubMed] [Google Scholar]
- 5.van Twuyver E, Mooijaart RJ, ten Berge IJ, van der Horst AR, Wilmink JM, Kast WM, Melief CJ, de Waal LP. Pretransplantation blood transfusion revisited. N Engl J Med. 1991;325:1210–1213. doi: 10.1056/NEJM199110243251704. [DOI] [PubMed] [Google Scholar]
- 6.Brennan DC, Mohanakumar T, Flye MW. Donor-specific transfusion and donor bone marrow infusion in renal transplantation tolerance: a review of efficacy and mechanisms. Am J Kidney Dis. 1995;26:701–715. doi: 10.1016/0272-6386(95)90432-8. [DOI] [PubMed] [Google Scholar]
- 7.Newton WT, Anderson CB. Planned Preimmunization of Renal-Allograft Recipients. Surgery. 1973;74:430–436. [PubMed] [Google Scholar]
- 8.Opelz G, Vanrenterghem Y, Kirste G, Gray DW, Horsburgh T, Lachance JG, Largiader F, Lange H, Vujaklija-Stipanovic K, Alvarez-Grande J, Schott W, Hoyer J, Schnuelle P, Descoeudres C, Ruder H, Wujciak T, Schwarz V. Prospective evaluation of pretransplant blood transfusions in cadaver kidney recipients. Transplantation. 1997;63:964–967. doi: 10.1097/00007890-199704150-00010. [DOI] [PubMed] [Google Scholar]
- 9.Marti HP, Henschkowski J, Laux G, Vogt B, Seiler C, Opelz G, Frey FJ. Effect of donor-specific transfusions on the outcome of renal allografts in the cyclosporine era. Transpl Int. 2006;19:19–26. doi: 10.1111/j.1432-2277.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 10.Cowan ML, Sciammas R, Chong AS. Experimental models of B cell tolerance in transplantation. Semin Immunol. 2012;24:77–85. doi: 10.1016/j.smim.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci U S A. 2007;104:12093–12098. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen P, Geiger TL. Induction of B-cell immune tolerance by antigen-modified cytotoxic T lymphocytes. Transplantation. 2010;89:667–676. doi: 10.1097/TP.0b013e3181ca9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohiuddin MM, Ogawa H, Yin DP, Galili U. Tolerance induction to a mammalian blood group-like carbohydrate antigen by syngeneic lymphocytes expressing the antigen, II: tolerance induction on memory B cells. Blood. 2003;102:229–236. doi: 10.1182/blood-2002-11-3515. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong HE, Bolton EM, Mcmillan I, Spencer SC, Bradley JA. Prolonged Survival of Actively Enhanced Rat Renal-Allografts Despite Accelerated Cellular Infiltration and Rapid Induction of Both Class-I and Class-Ii Mhc Antigens. Journal of Experimental Medicine. 1987;165:891–907. doi: 10.1084/jem.165.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roelen D, Brand A, Claas FH. Pretransplant blood transfusions revisited: a role for CD(4+) regulatory T cells? Transplantation. 2004;77:S26–28. doi: 10.1097/01.TP.0000106469.12073.01. [DOI] [PubMed] [Google Scholar]
- 16.Pirenne J, Kitade H, Kawai M, Koshiba T, Van Damme B, Mathieu C, Waer M. Regulatory cells, TH1/TH2 unbalance, and antibody-induced chronic rejection in operational tolerance induced by donor-specific blood transfusion. Transplantation. 2005;79:S25–27. doi: 10.1097/01.tp.0000153295.51565.f1. [DOI] [PubMed] [Google Scholar]
- 17.Tsubata T. Role of inhibitory BCR co-receptors in immunity. Infect Disord Drug Targets. 2012;12:181–190. doi: 10.2174/187152612800564455. [DOI] [PubMed] [Google Scholar]
- 18.Poe JC, Tedder TF. CD22 and Siglec-G in B cell function and tolerance. Trends in Immunology. 2012;33:413–420. doi: 10.1016/j.it.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annu Rev Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Ann N Y Acad Sci. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellusova J, Nitschke L. Regulation of B Cell Functions by the Sialic Acid-Binding Receptors Siglec-G and CD22. Front Immunol. 2011;2:96. doi: 10.3389/fimmu.2011.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 23.Doody GM, Justement LB, Delibrias CC, Matthews RJ, Lin J, Thomas ML, Fearon DT. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 24.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. J Immunol. 2013;191:1724–1731. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lanoue A, Batista FD, Stewart M, Neuberger MS. Interaction of CD22 with alpha2,6-linked sialoglycoconjugates: innate recognition of self to dampen B cell autoreactivity? Eur J Immunol. 2002;32:348–355. doi: 10.1002/1521-4141(200202)32:2<348::AID-IMMU348>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Poe JC, Fujimoto Y, Hasegawa M, Haas KM, Miller AS, Sanford IG, Bock CB, Fujimoto M, Tedder TF. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 28.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268:7019–7027. [PubMed] [Google Scholar]
- 29.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci U S A. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramya TN, Weerapana E, Liao L, Zeng Y, Tateno H, Yates JR, 3rd, Cravatt BF, Paulson JC. In situ trans ligands of CD22 identified by glycan-protein photocross-linking-enabled proteomics. Mol Cell Proteomics. 2010;9:1339–1351. doi: 10.1074/mcp.M900461-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Courtney AH, Puffer EB, Pontrello JK, Yang ZQ, Kiessling LL. Sialylated multivalent antigens engage CD22 in trans and inhibit B cell activation. Proc Natl Acad Sci U S A. 2009;106:2500–2505. doi: 10.1073/pnas.0807207106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duong BH, Tian H, Ota T, Completo G, Han S, Vela JL, Ota M, Kubitz M, Bovin N, Paulson JC, Nemazee D. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. J Exp Med. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, von Drygalski A, Paulson JC. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123:3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of Antigen and Ligands of Siglec-G Induces B Cell Tolerance Independent of CD22. J Immunol. 2013;191:1724–1731. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci U S A. 1998;95:7469–7474. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K, et al. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. [DOI] [PubMed] [Google Scholar]
- 37.Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid tissues of self-reactive B lymphocytes recognizing membrane-bound antigens. Nature. 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 38.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci U S A. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blixt O, Han S, Liao L, Zeng Y, Hoffmann J, Futakawa S, Paulson JC. Sialoside analogue arrays for rapid identification of high affinity siglec ligands. J Am Chem Soc. 2008;130:6680–6681. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Enders A, Bouillet P, Puthalakath H, Xu Y, Tarlinton DM, Strasser A. Loss of the pro-apoptotic BH3-only Bcl-2 family member Bim inhibits BCR stimulation-induced apoptosis and deletion of autoreactive B cells. J Exp Med. 2003;198:1119–1126. doi: 10.1084/jem.20030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliver PM, Vass T, Kappler J, Marrack P. Loss of the proapoptotic protein, Bim, breaks B cell anergy. J Exp Med. 2006;203:731–741. doi: 10.1084/jem.20051407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 43.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornall RJ, Goodnow CC, Cyster JG. Regulation of B cell antigen receptor signaling by the Lyn/CD22/SHP1 pathway. Curr Top Microbiol Immunol. 1999;244:57–68. doi: 10.1007/978-3-642-58537-1_5. [DOI] [PubMed] [Google Scholar]
- 45.Hibbs ML, Tarlinton DM, Armes J, Grail D, Hodgson G, Maglitto R, Stacker SA, Dunn AR. Multiple defects in the immune system of Lyn-deficient mice, culminating in autoimmune disease. Cell. 1995;83:301–311. doi: 10.1016/0092-8674(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 46.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 47.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. American Journal of Transplantation. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 48.Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr Biol. 2000;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- 49.O'reilly LA, Kruse EA, Puthalakath H, Kelly PN, Kaufmann T, Huang DCS, Strasser A. MEK/ERK-Mediated Phosphorylation of Bim Is Required to Ensure Survival of T and B Lymphocytes during Mitogenic Stimulation. Journal of Immunology. 2009;183:261–269. doi: 10.4049/jimmunol.0803853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa H, Yin DP, Shen J, Galili U. Tolerance induction to a mammalian blood group-like carbohydrate antigen by syngeneic lymphocytes expressing the antigen. Blood. 2003;101:2318–2320. doi: 10.1182/blood-2002-07-2151. [DOI] [PubMed] [Google Scholar]
- 51.Smarr CB, Hsu CL, Byrne AJ, Miller SD, Bryce PJ. Antigen-fixed leukocytes tolerize Th2 responses in mouse models of allergy. J Immunol. 2011;187:5090–5098. doi: 10.4049/jimmunol.1100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Good-Jacobson KL, Tarlinton DM. Multiple routes to B-cell memory. International immunology. 2012;24:403–408. doi: 10.1093/intimm/dxs050. [DOI] [PubMed] [Google Scholar]
- 53.Hill SW, Sercarz EE. Fine specificity of the H-2 linked immune response gene for the gallinaceous lysozymes. Eur J Immunol. 1975;5:317–324. doi: 10.1002/eji.1830050506. [DOI] [PubMed] [Google Scholar]
- 54.Anderson CB, Tyler JD, Sicard GA, Anderman CK, Rodey GE, Etheredge EE. Pretreatment of renal allograft recipients with immunosuppression and donor-specific blood. Transplantation. 1984;38:664–668. doi: 10.1097/00007890-198412000-00023. [DOI] [PubMed] [Google Scholar]
- 55.Sandner SE, Clarkson MR, Salama AD, Sanchez-Fueyo A, Yagita H, Turka LA, Sayegh MH. Mechanisms of tolerance induced by donor-specific transfusion and ICOS-B7h blockade in a model of CD4(+) T-cell-mediated allograft rejection. American Journal of Transplantation. 2005;5:31–39. doi: 10.1111/j.1600-6143.2004.00640.x. [DOI] [PubMed] [Google Scholar]
- 56.Basten A, Silveira PA. B-cell tolerance mechanisms and implications. Curr Opin Immunol. 2010;22:566–574. doi: 10.1016/j.coi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 57.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 58.Martin LT, Marth JD, Varki A, Varki NM. Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J Biol Chem. 2002;277:32930–32938. doi: 10.1074/jbc.M203362200. [DOI] [PubMed] [Google Scholar]
- 59.Kitagawa H, Paulson JC. Differential expression of five sialyltransferase genes in human tissues. J Biol Chem. 1994;269:17872–17878. [PubMed] [Google Scholar]
- 60.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.