Summary
Lipids presented by the major histocompatibility complex (MHC) class I-like molecule, CD1d, are recognized by natural killer T (NKT) cells, which can be broadly categorized into two subsets. The well-characterized type I NKT cells, express a semi-invariant T cell receptor (TCR) and can recognize both α- and β-linked glycolipids, whereas type II NKT cells are less well studied, express a relatively diverse TCR repertoire, and recognize β-linked lipids. Recent structural studies have shown a distinct mode of recognition of a self-glycolipid sulfatide bound to CD1d by a type II NKT TCR. To further characterize antigen recognition by these cells we have used the structural data and screened other small molecules able to bind to CD1d and activate type II NKT cells. Using plate-bound CD1d and APC-based antigen presentation assay we found that phospholipids such as lysophosphatidylcholine (LPC) can stimulate the sulfatide-reactive type II NKT hybridoma Hy19.3 in a CD1d-dependent manner. Using plasmon resonance studies we found that this type II NKT TCR binds with CD1d-bound LPC with micromolar affinities similar to that for sulfatide. Furthermore LPC-mediated activation of type II NKT cells leads to anergy induction in type I NKT cells and affords protection from ConA-induced hepatitis. These data indicate that, in addition to self-glycolipids, self-lysophospholipids are also recognized by type II NKT cells. Since lysophospholipids are involved during inflammation our findings have implications for not only understanding activation of type II NKT cells in physiological settings but also for the development of immune intervention in inflammatory diseases.
Keywords: CD1d, phospholipids, sulfatide, natural killer T cells, glycolipids, hepatitis, liver disease
Introduction
Natural killer T (NKT) cells are innate-like and generally reactive to lipid antigens presented by CD1d MHC class I like molecules (1–3). NKT cells can play an important immunoregulatory role in inflammatory conditions, including autoimmune diseases, infectious diseases, and cancer (4–8). NKT cells are comprised of two main subsets, type I and type II. Type I NKT cells express a semi-invariant TCR encoded predominantly by a germline invariant Vα gene (Vα14-Jα18 in mice and Vα24-JαQ in humans), and a more diverse non-germline Vβ chain genes (Vβ8.2/7/2 in mice and Vβ11 in human) (1, 3). Owing to their predominance in mice as well as the ability of type I NKT cells to recognize a marine sponge-derived glycolipid, αGalCer, this subset has been well studied. In contrast, type II NKT cells that use a relatively diverse TCR repertoire are less abundant in mice and are less well studied with regard to their physiological role and antigen recognition.
Recently, one of the major subsets of type II NKT cells has been shown to be reactive to a self-glycolipid sulfatide (9, 10). The sulfatide/CD1d-tetramer+ cells express an oligoclonal TCR repertoire with predominant usage of Vα3/Vα1-Jα7/Jα9 and Vβ8.1/Vβ3.1-Jβ2.7 gene segments (9). Only about 14% of TCR Vα and 13–27% of TCR Vβ chains in sulfatide-reactive type II NKT cells are exclusively encoded by germline gene segments. The semi-invariant TCR on type I NKT cells binds to CD1d in a parallel configuration that mainly involves the α-chain. At least one type II NKT TCR contacts its ligands primarily via its β chain rather than α chain, suggesting that the TCR Vβ chain contributes significantly to antigen fine specificity (11, 12). The mechanism of binding of type II NKT TCRs to antigens uses features of TCR binding shared by both type I NKT cells and conventional T cells (9, 11, 12). Thus type I and type II NKT cell subsets display distinct modes of recognition.
Type I NKT cells respond to both α- and β-linked glycolipids whereas type II NKT cells have been shown to recognize β-linked glycolipids. Unlike αGalCer, most microbial lipids and other self-antigens, including isoglobotrihexosylceramide (iGb3), do not stimulate type I NKT cells very effectively. Similarly, lipids recognized by the type II NKT cells, including sulfatides, βGlcCer and βGalCer, as well as some pollen-derived lipids, are not as potent in activating them, as αGalCer is in activating type I NKT cells. In this regard it is notable that while the binding affinity of the TCR of type I NKT cells to CD1d-presented αGalCer is very high (Kd of 11–30 nM) (13), the affinity to microbial ligands is in the micromolar range (0.7–6 μM) (14) comparable to that of typical peptide-MHC I interactions (1–100 μM) (15). Recently it has been shown that lysophosphatidylethanolamine (LPE) induced following hepatitis B viral infection may be a self-antigen for a subset of type II NKT cells (16). Type II NKT cells have been shown to be regulatory, as their activation with the self-glycolipid sulfatide results in protection from autoimmune diseases by down-regulation of inflammatory responses elicited by type I NKT cells as well as conventional MHC-restricted CD4+ and CD8+ T cells (7, 10, 17–19). In order to further characterize the type II NKT subset and their physiological role in immune regulation it is important to identify additional lipid antigens recognized by them. Here we have found that murine type II NKT cells are also reactive to self-phospholipids, including lysophosphatidylcholine (LPC), lysosphingomyelin (LSM) and lyso platelet activating factor (LPAF). Furthermore, as with sulfatide, LPC-mediated activation of type II NKT cells induces anergy in type I NKT cells. Since LPC levels are physiologically controlled during inflammatory conditions these findings have important implications in understanding biology of type II NKT cells as well as in the development of potential therapeutics for the inflammatory diseases.
Materials and Methods
Animals
C57BL/6J female mice (7–10 wk) were purchased from The Jackson Laboratory (Bar Harbor, ME). CD1d−/− and Jα18−/− BL/6 mice, originally generated in the laboratories of Drs. Luc Van Kaer (Vanderbilt Univ., Nashville, TN) and M. Taniguchi (Chiba Univ., Chiba, Japan), were provided by Dr. Mitch Kronenberg (La Jolla Institute for Allergy and Immunology, La Jolla, CA). All mice were bred and maintained in specific pathogen free-conditions in the TPIMS animal facility. Treatment of animals was in compliance with federal and institutional guidelines and approved by the TPIMS Animal Care and Use Committee.
Lipid antigens
Synthetic lipids were acquired from different sources as follows: LPC (C16:0), LSM), LPAF (C18:0) and N-palmitoyl dopamine from Cayman Chemicals, Ann Arbor, MI; LPC (C18:0), LPC (C18:1 9Z), LPC (C18:1 1oleyl), LPC (C24:0) or 1-lignoceroyl-2-hydroxy-sn-glycero3-phosphocholine (Lign. A) from Avanti Polar Lipids (Alabaster, AL); (+/−)-erythro-aleuritic acid from Alfa Aesar (Ward Hill, MA); LSF and lysoβGluCer from Matreya (Pleasant Gap, PA). Synthetic αGalCer was provided by Kirin Brewery Co., (Tokyo, Japan). All the lipids were dissolved in vehicle (0.5% Tween 20 and 0.9% NaCl solution) and further diluted in PBS.
Antigen presentation assay using APCs to stimulate NKT cells
APC based presentation assays were performed essentially as described earlier (10) with a Vα1+ sulfatide-reactive (Hy19.3 or XV19)(20) as well as non-sulfatide-reactive Vα3+ (Hy24 or VIII24, HyIF4 or IF4)(20) or Vα8+ (HyB11 or TCB11, Hy49 or VII49)(21) type II NKT hybridomas or an αGalCer-reactive type I NKT hybridoma (Hy1.2) in the presence of different lipids and irradiated splenocytes. Type II NKT hybrids Hy19.3, Hy24, HyIF4, Hy49 and HyB11 were kindly provided by laboratories of Drs. S. Cardell and A. Bendelac. The type I NKT Hy1.2 was kindly provided by Dr. M. Kronenberg. T cell hybridoma cells (5 × 104/well) were cocultured with irradiated splenocytes (4 × 105/well) in triplicates from 7 to 10 wk old WT BL/6, or CD1d1−/− mice in the presence of graded concentrations of glycolipids. Supernatants from these 16-h cultures were used in a typical sandwich ELISA to measure IL-2 production. Proliferative responses in splenic cell proliferation assays (6 ×105 cells/well) from naïve mice were measured in RPMI with 10% Fetal bovine serum (Atlas Biologicals, Fort Collins, CO) and by addition of 1 μCi 3H-thymidine (MP Biomedicals, Santa Ana, CA) per well for the final 18 hours of a 5-day in vitro culture in the presence of different lipids and CD1d molecules.
Antigen presentation assay using CD1d-coated plates to stimulate NKT cells
Flat bottom 96-well plates were coated for 18 h with 1 μg/well of soluble mouse CD1d or CD1d mutants isolated and purified using a baculovirus expression system at the concentration of 1.0 μg at 4°C in 50 mM Na-acetate buffer (pH 5), essentially as described earlier (22). Coated plates were washed three times with the same buffer and then incubated for another 4h at 37°C with graded concentrations of lipids. After washing three times with PBS, 5 × 104/well T cell hybridomas (Hy19.3 or Hy1.2) were added in triplicate. Supernatants were collected after 16 h to measure IL-2 release by sandwich ELISA (eBioscience, San Diego, CA).
Surface plasmon resonance analysis for LPC-CD1d binding to the Hy19.3 TCR
Surface plasmon resonance measurements were performed with purified proteins essentially as described earlier (11). Briefly, recombinant mouse CD1d and β2m were co-expressed in Sf9 cells and purified by affinity, ion exchange, and size exclusion chromatography. The Hy19.3 TCR was expressed as V/mouse-C/human chimeric constructs in E.coli. The expressed inclusion bodies were then refolded and purified by ion exchange and size exclusion chromatography. Lipid antigens were loaded O/N in the presence of 10 mM Hepes pH 7.5 and 150 mM NaCl and further purified by size exclusion chromatography. Biotinylated Hy19.3 TCR (300–500 RUs) was immobilized on a CAPture chip on a Biacore 3000 instrument (GE Healthcare). A range of concentrations of each mCD1d-lipid complex was then passed on the surface to measure association and dissociation rates and equilibrium constants by fitting with a 1:1 Langmuir model in the Biaevaluation software.
Flowcytometry analysis for monitoring type I NKT cells
Splenocytes were suspended in FACS buffer (PBS containing 0.02% NaN3 and 2% FCS), blocked (anti-mouse FcR-γ, BD Biosciences, San Diego, CA) and stained with α GalCer-loaded mCD1d-tetramer-PE, and FITC-labeled anti-TCRβ mAb (eBioscience, San Diego, CA) as described earlier (18). Flowcytometric analysis was performed using FlowJo (version 9.3.1) software (Ashland, OR).
Induction of ConA-induced hepatitis, serum ALT, histopathology and cytokine analysis
To induce autoimmune hepatitis, mice were injected i.v. with 10 mg/kg of ConA. Lipids, including LPC (100 μg), were administered i.p. immediately after ConA injection. Control mice were injected with PBS/vehicle (200 μl/mouse). Mice were sacrificed and serum was collected 24 hours after the injections. ALT serum levels were measured using ALT (GPT) Infinity Liquid Stable Reagent from Thermo Scientific (TR71121). For histopathology, liver tissues were fixed in 10% formaldehyde solution and kept at room temperature until use. H&E staining of the liver sections was performed by The Scripps Research Institute Histology Core Laboratory. Serum cytokines were determined in different groups of mice 24 hr after Con A injection using BD cytometric Bead Array (CBA) mouse Th1/Th2/Th17 cytokine kit (BD Biosciences) per manufacturer’s protocol.
Determination of anergy induction in type I NKT cells
Groups of BL/6 mice were injected with LPC (C18:0) or LPC (C16:0) 100 μg/mouse, or LSF 80 μg/mouse, or PBS. One day later all treated mice were administered a single injection of αGalCer (2 μg/ml). Mice were sacrificed on day 5 (three days after αGalCer injection), and single cell suspensions of spleen cells were stained with anti-TCRβ FITC and αGalCer/CD1d-tetramer PE, ex vivo and analyzed as mentioned above.
In another experiment groups of BL/6 mice injected as above with LPC (C18:0) or LPC (C16:0), LSF, or PBS were sacrificed after 19 hours, and splenocytes were cultured (6 × 105 cells/well) in 96-well plates in the presence of αGalCer (10 ng/ml). After an initial 72 hours of in vitro culture, 3H Thymidine was added and after another 18 hours cells were harvested, and incorporated radioactivity was measured using a beta plate counter as described earlier (18, 23).
Statistical Analysis
Data are expressed as mean ± SEM for each group. Statistical differences between groups were evaluated by the unpaired one-tailed Student’s t test using GraphPad Prism software (version 5.0a, GraphPad Software Inc., La Jolla, CA).
Results
Activation of a sulfatide-reactive type II NKT hybridoma by lysophospholipids using a CD1d plate-bound assay
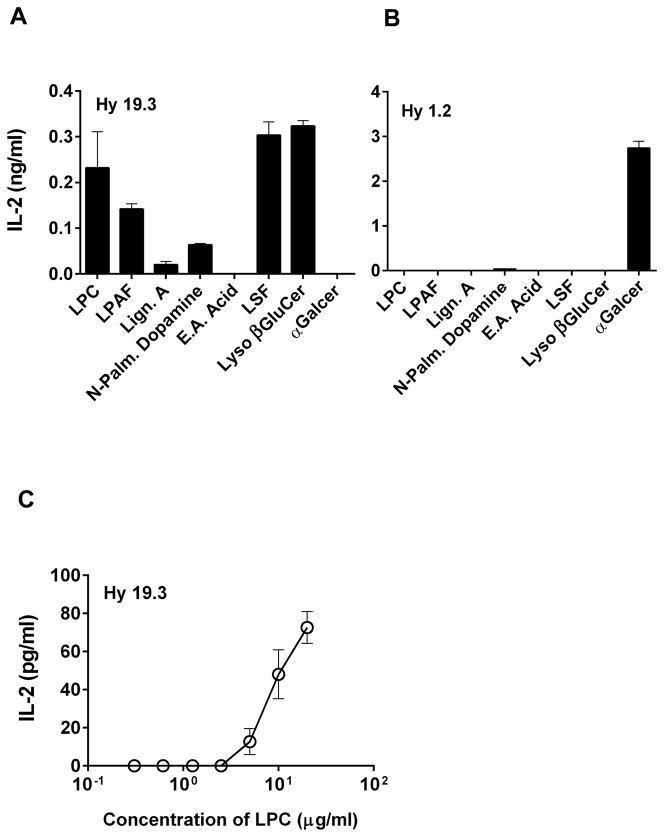
Recently we and others have provided a snapshot of type II NKT TCR-mediated recognition of sulfatide or LSF by determining the ternary structure of a type II NKT TCR (Vα1Jα26-Vβ16Jβ2.1) in complex with LSF-CD1d or C24:1 sulfatide-CD1d (11, 12). Based upon the similarities of these structures we have used computer-based lead generation technology (ScieIntl, Greensboro, NC) to predict small molecules other than glycolipids that may bind to CD1d in a similar manner and could be potentially recognized by the type II NKT cells. Here we have used a plate-bound CD1d assay to determine whether any of the six molecules (for structures see Figure 1) can stimulate a type II NKT hybridoma (Hy19.3). As shown in Figure 2a, two synthetic lysophospholipids: LPC and LPAF are efficient in stimulating type II NKT cells. Interestingly, in earlier experiments (11), purified egg lysolecithin, a mixture of LPC isoforms was unable to stimulate Hy19.3 effectively owing to perhaps sub-optimal loading/presentation of a single species. Additionally N-palmitoyl dopamine also stimulated Hy19.3, albeit less efficiently compared to lysophospholipids. As expected and shown earlier (11, 24), both LSF and lyso βglucosylceramide were able to stimulate Hy19.3 and were used as positive controls. Two other molecules, 1-lignoceroyl-2-hydroxy-sn-glycero-3-phosphocholine (Lign. A or LPC C24:0) and (+/−)-erythro-aleuritic acid (E.A. acid), were unable to activate the type II NKT Hy19.3. In contrast none of these molecules were able to stimulate a type I NKT hybridoma Hy1.2 (Fig. 2b). However αGalCer, a ligand for the type I NKT cells, stimulated Hy1.2 but not Hy19.3. Here we have chosen to further analyze the activity of LPC to activate type II NKT cells both in vitro as well as in in vivo assays. A dose titration of curve of stimulation of Hy19.3 with LPC in a typical CD1d-plate bound assays is shown in Figure 2c. It is interesting that the concentration of LPC required to stimulate Hy19.3 is similar to that of LSF (22).
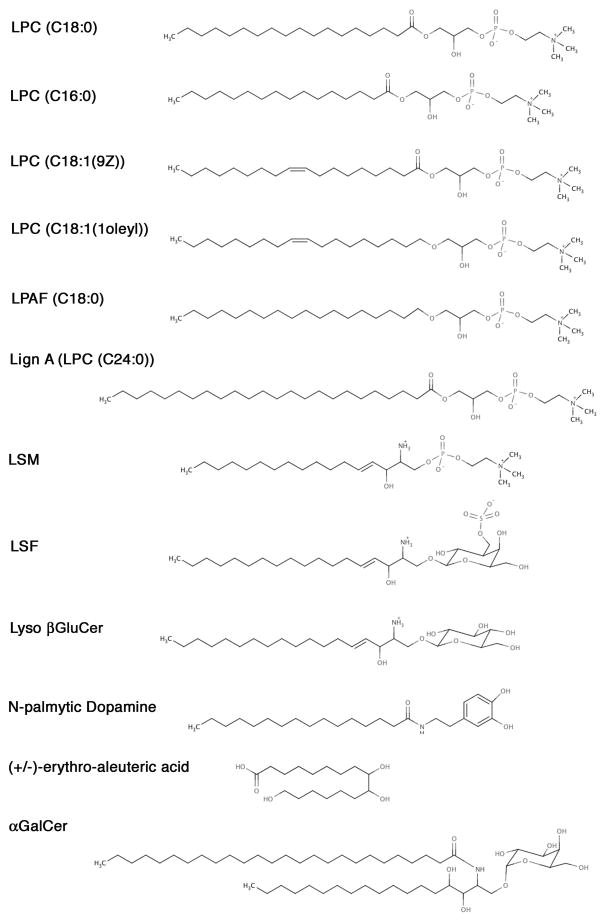
Figure 1.
Detailed chemical structures of different lysophospholipids as well as glycolipids used in this study
Figure 2. Stimulation of a type II NKT cell Hy19.3 by self-lysophospholipids in an APC-free CD1d-coated antigen presentation assay.
Plates were coated with the recombinant CD1d protein in PBS and incubated at 4°C overnight. Next day plates were washed and loaded with indicated lipids for 5–6 hr at 37°C. IL-2 release at an optimum concentration (5 μg/ml) following incubation with 5 × 104 cells/well of a type II NKT cell Hy19.3 (A) or a type I NKT cell Hy1.2 (B) is shown. A typical dose titration curve of stimulation of Hy19.3 with various concentrations (0.6 – 20 μg/ml) of LPC is shown in Figure 2C. There was no significant amount of IL-2 secretion (0.01–0.03 ng/ml) with 1 μg/well of plate coated CD1d only in these assays. These data are representative of 4 different experiments.
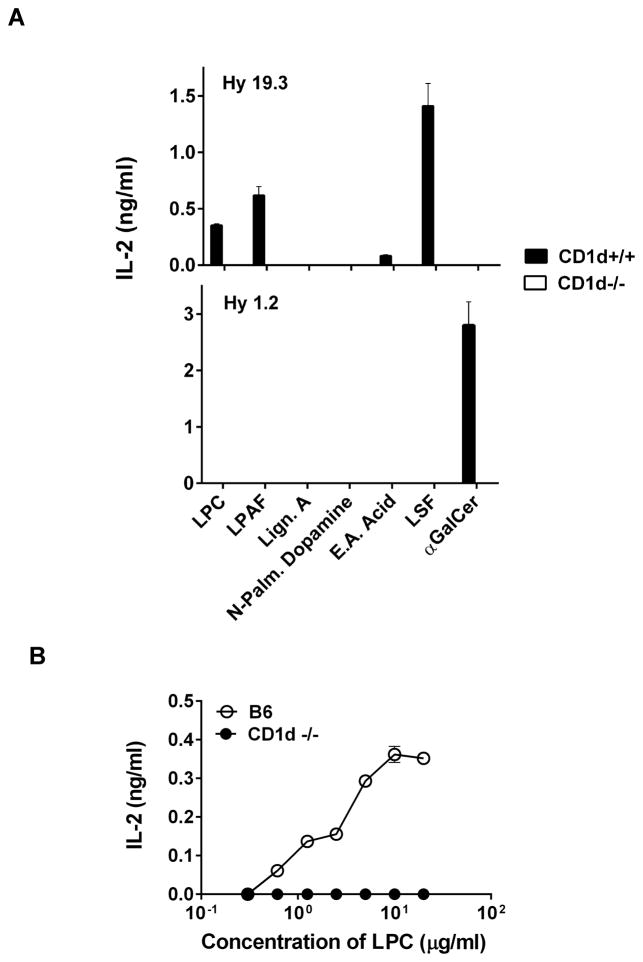
Stimulation of a type II NKT cell hybridoma by lysophospholipids in the presence of CD1d+/+ but not CD1d−/− antigen-presenting cells
Antigen presentation of lipid molecules by CD1d may involve endocytic pathways as well as processing and loading with the help of the lipid-binding proteins. Here we determined whether in a typical antigen presentation assay with irradiated splenocytes from the wild type C57BL/6 (CD1d+/+) or the CD1d-deficient (CD1d−/−) mice, lysopohospholipids can be appropriately presented to stimulate Hy19.3. Figure 3a clearly shows that both LPC and LPAF are able to stimulate the type II NKT Hy19.3 in the presence of CD1d+/+ but not CD1d−/− splenocytes. Other lipids, including N-palmitoyl dopamine, did not stimulate type II NKT cells. As expected, Hy19.3 and Hy1.2 were stimulated by their respective ligands LSF and αGalCer respectively, in the presence of CD1d+/+ splenocytes but not CD1d−/− APCs. A typical dose titration curve of Hy19.3 stimulation with LPC is shown in Figure 3b and is similar to that of LSF (22).
Figure 3. CD1d-dependent stimulation of a sulfatide-reactive type II NKT cell by self-lysophospholipids.
(A) A type II NKT Hy19.3 or a type I NKT Hy1.2 were stimulated in the presence of APCs (irradiated splenocytes) from either the wild-type C57BL/6 (CD1d+/+) or CD1d-deficient (CD1d−/−) mice in the presence of different concentration of lipids (0.6 – 20 μg/ml). IL-2 release at optimum concentration (5 μg/ml) of indicated lipids is shown. (B) A typical dose titration of Hy19.3 stimulation in the presence of APC and LPC (0.6 – 20 μg/ml) is shown. These data are representative of at least 3 independent experiments.
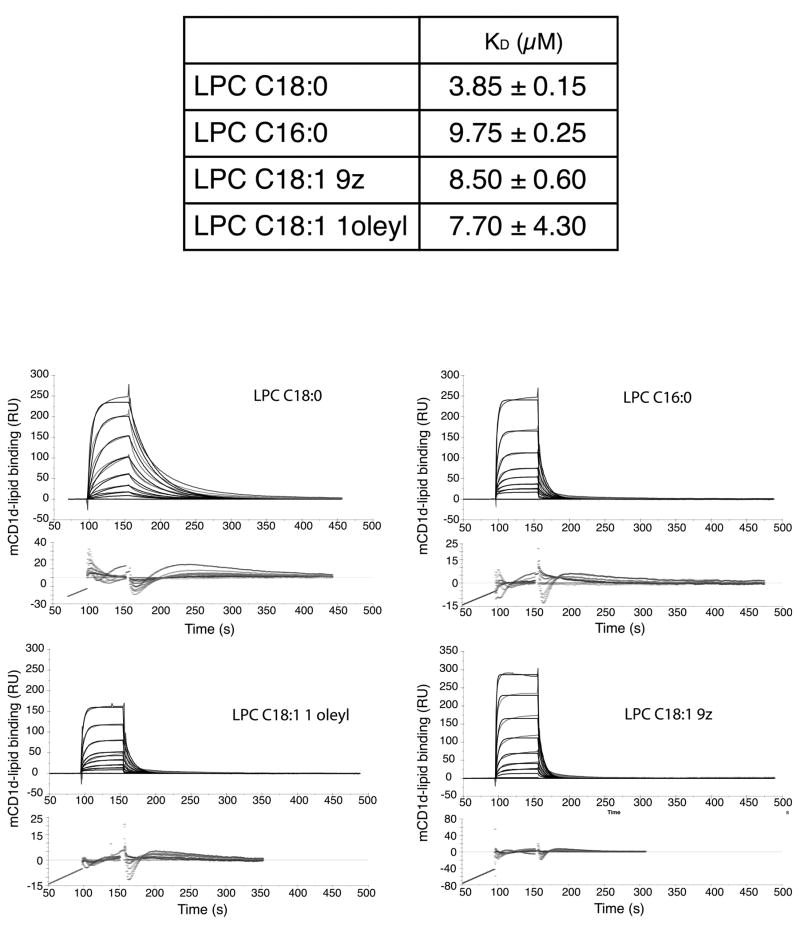
Binding of different forms of LPC with a type II NKT TCR
We recently reported that the Hy19.3 TCR binds to the mouse CD1d-LSF complex with micromolar affinities (11). Here we determined the binding affinities of different isoforms of LPC-CD1d complexes (LPC C18:0, LPC C16:0, LPC C18:1 9Z, LPC C18:1 1oleyl) to the Hy19.3 TCR using surface plasmon resonance. As shown in Figure 4, this type II NKT TCR binds to CD1d-LPC with micromolar affinities (~ 3–9 μM) similar to CD1d-LSF binding (~ 6 μM). The binding was characterized by relatively slow on rates followed by fast off rates, similar to that in the case of LSF-CD1d (11). This is also similar to what has been reported for the weak type I NKT cell lipid ligands, such as microbial glycolipids or self-antigens (25). Interestingly LPC C18:0 appears to bind with the higher affinity in comparison to other LPC isoforms examined here (Fig. 4).
Figure 4. Binding of a type II NKT TCR to mCD1d/LPC complexes.
(A) Sensograms showing mCD1d-LPC complexes binding to the Hy19.3 TCR. The association and dissociation constants measured show a slightly higher binding affinity of this ligand for the TCR, compared to mCD1d-LSF complexes. (B) Affinity measurements for the lysophospholipid variants measured for this study. Average and SEM of two independent measurements are reported.
Ability of different LPC isoforms to stimulate type II NKT cells
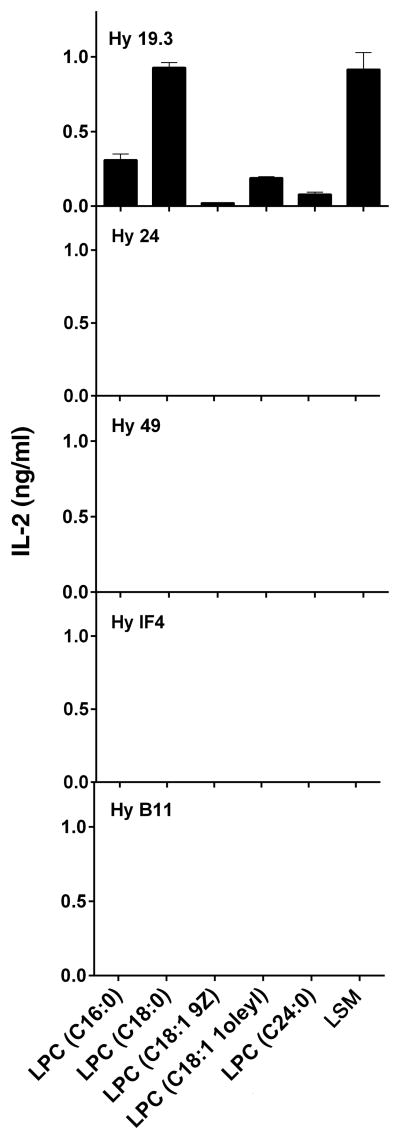
Different isoforms of LPC are predominantly enriched in different tissues and among them C16 and C18 are the most abundant forms (26). Here we have examined the ability of different LPC isoforms to stimulate the sulfatide-reactive Vα1+ Hy19.3 as well as a panel of other non-sulfatide-reactive Vα3+ and Vα8+ type II NKT hybridomas in a typical antigen-presentation assay. Figure 5 clearly shows that LPC stimulated only the sulfatide-reactive Hy19.3 but none of the other type II NKT cell hybridomas. Furthermore LPC C18:0 is most efficient in stimulating Hy19.3 compared to other LPC isoforms, such as C16:0, C18:1 9Z, C18:1 1oleyl, or C24:0 (Fig. 5). Notably another phospholipid, LSM, similar in structure to that of LPC, was also able to stimulate the sulfatide-reactive Hy19.3 hybridoma in this assay.
Figure 5. Recognition of different isoforms of LPC and LSM by a sulfatide-reactive type II NKT cell but not other type II NKT hybridomas.
A sulfatide-reactive type II NKT Hy19.3 as well as other non-sulfatide-reactive type II NKT hybridomas were used in a typical IL-2 release assay in the presence of APC and indicated lipids. IL-2 release at an optimum concentration (5 μg/ml) of lipids is shown. All other hybridomas showed IL-2 release (ranges from 0.7 – 1.3 ng/ml) in response to the plate-bound anti-CD3 stimulation. These data are representative of two independent experiments.
Substitutions of the A′ pocket on CD1d results in loss of recognition by the type II NKT Hy19.3
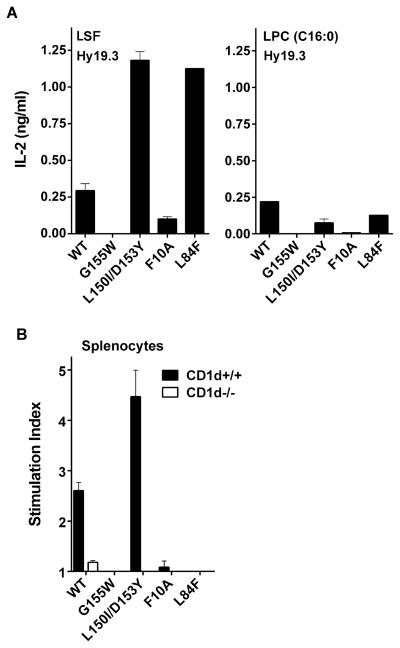
From two recent structural studies it is clear that the docking of the Hy19.3 type II NKT TCR on the surface of CD1d molecule is distinct from that of the type I NKT TCR (11, 12). Here we determined whether the some of the key residues on CD1d involved in the binding of the LSF-CD1d complex to the type II NKT TCR are also important for LPC recognition using a CD1d plate-bound antigen-presentation assay. Figure 6a shows that perturbations of the area around the A′ pocket (Phe10Ala, Gly155Trp) of CD1d resulted in the loss of recognition of LPC by the type II NKT Hy19.3. However substitutions around the F′ pocket (Asp153Y/Leu150Ile, Leu84Phe) did not result in loss of stimulation of the Hy19.3 hybridoma. These residues are involved in the recognition of lipid antigens by the type I NKT TCR (11, 12). These data suggest that residues in the CD1d molecule critically involved in the recognition of LSF are also similar for the recognition of LPC by this type II NKT TCR.
Figure 6. Modification of residues in CD1d molecules around the A′ but not F′ pocket inhibits recognition of LPC by a type II NKT cell hybridoma.
(A) In a CD1d coated plate assay Hy19.3 was stimulated in the presence of wild-type CD1d or mutant CD1d molecules with modifications in residues as indicated. IL-2 release at an optimum concentration of either 2 μg/ml of LSF or 5 μg/ml of LPC is shown. These data are representative of 3 independent experiments. (B) A proliferative response of spleen cells from naïve wild-type C57BL/6 (CD1d+/+) or CD1d-deficient (CD1d−/−) mice in response to a typical CD1d coated plate assay as above in the presence of an optimum concentration of LPC (5 μg/ml) is shown. Stimulation index was calculated by dividing CPM in the presence vs. absence of the lipid. These data are representative of 2 independent experiments.
It is clear from Figure 5 that none of the other non-sulfatide-reactive type II NKT hybridomas examined here were reactive to LPC. Thus we were not able to determine reactivity of LPC to other type II NKT cells at the monoclonal level. Here we determined LPC recognition at the polyclonal level using a plate bound CD1d antigen presentation assay with polyclonal spleen cells from the wild type (CD1d+/+) or CD1d-deficient (CD1d−/−) BL/6 mice in the presence of plate-bound CD1d molecules (Fig. 6b, WT or mutant CD1d as in Fig. 6a). As shown in Figure 6b, although the reactivity is low at the polyclonal level using naïve spleen cells, the stimulation pattern (stimulation index more than 2) of LPC with the WT CD1d as well as some of the mutant CD1d molecules was similar to that of the Hy19.3. These data collectively suggest the presence of LPC reactive type II NKT cells in a polyclonal population with an overlapping repertoire similar to that of sulfatide-reactive type II NKT cells.
Induction of type I NKT anergy following LPC administration
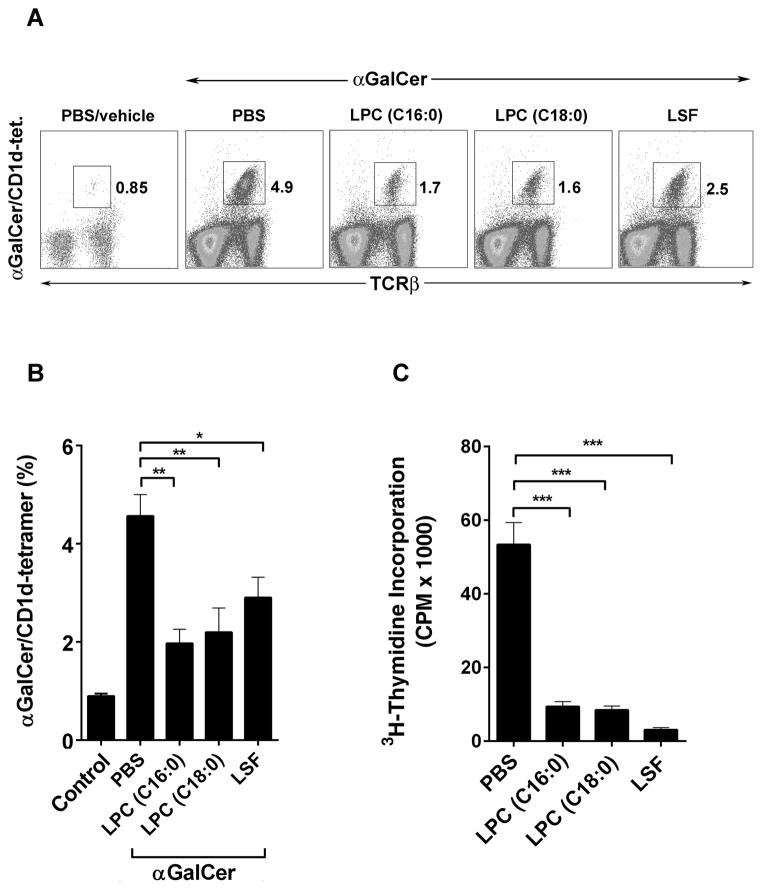
Recently we have identified a dominant immunoregulatory pathway in which activation of CD1d-restricted type II NKT cells by sulfatide leads to anergy induction in type I NKT cells (17, 18). Here we determined whether type I NKT cells are also anergized following activation of type II NKT cells by LPC. Groups of BL/6 mice were administered LPC, and the following day anergy induction in type I NKT cells was examined by in vivo expansion of αGalCer/CD1d-tetramer+ cells (Figs. 7a and 7b) as well as by proliferation (as measured 3H-thymidine incorporation) in response to an in vitro challenge with αGalCer (Fig. 7c). As shown in Figures 7a and 7b, type I NKT cells (αGalCer/CD1d-tetramer+) expand in spleen following αGalCer administration in PBS/vehicle treated mice (0.8% to 4.9%). However in mice treated with LPC (C16:0 or C18:0) or LSF, the ability of type I NKT cells to expand in vivo in response to αGalCer injection was significantly inhibited (Figs. 7a and 7b). In another assay shown in Figure 7c, a significant inhibition of the proliferative response of type I NKT cells was found in splenocytes from LPC or LSF-treated mice in comparison to that in control or PBS-injected mice. This inhibition of proliferation of type I NKT cells can be reversed by the addition of IL-2 ((17, 18), and data not shown). These data suggest that type I NKT cells are anergized following activation of type II NKT cells by LPC in C57BL/6 mice.
Figure 7. Induction of anergy in type I NKT cells following LPC administration in C57BL/6 mice.
(A) Flow cytometric analysis of splenocytes from groups of C57BL/6 mice (n = 3–5) treated i.p. with PBS/vehicle or indicated lipids, LPC (C16:0), LPC (C18:0) or LSF, at 100 μg/mouse. An in vivo expansion of type I NKT cells (αGalCer/CD1d-tetramer+) was measured in splenocytes following administration with αGalCer (2 μg) using two-color staining with αGalCer/CD1d-tetramer and anti-TCRβand flow cytometry. Numbers indicate percentage tetramer+ positive cells in total spleen lymphocytes. (B) A summary of the data from 2 independent experiments indicating LPC-mediated inhibition of type I NKT cell expansion in BL/6 mice (n = 3–5) is shown. Average percentage of αGalCer/CD1d-tetramer positive cells for each group from figure A (mean +/− SEM) is shown. *P value <0.05, **P values <0.01 (C) Inhibition of type I NKT cells in response to an in vitro challenge with αGalCer in splenocytes isolated from the groups (n=3) of control (PBS/vehicle) or different lipids-injected mice. Proliferation following a 90 hr culture in the presence of αGalCer among indicated groups is shown as 3H Thymidine incorporation. The frequency of αGalCer-tetramer+ cells in the beginning of the culture was similar in all the groups. ***P values <0.001
LPC administration leads to inhibition of ConA-induced hepatitis
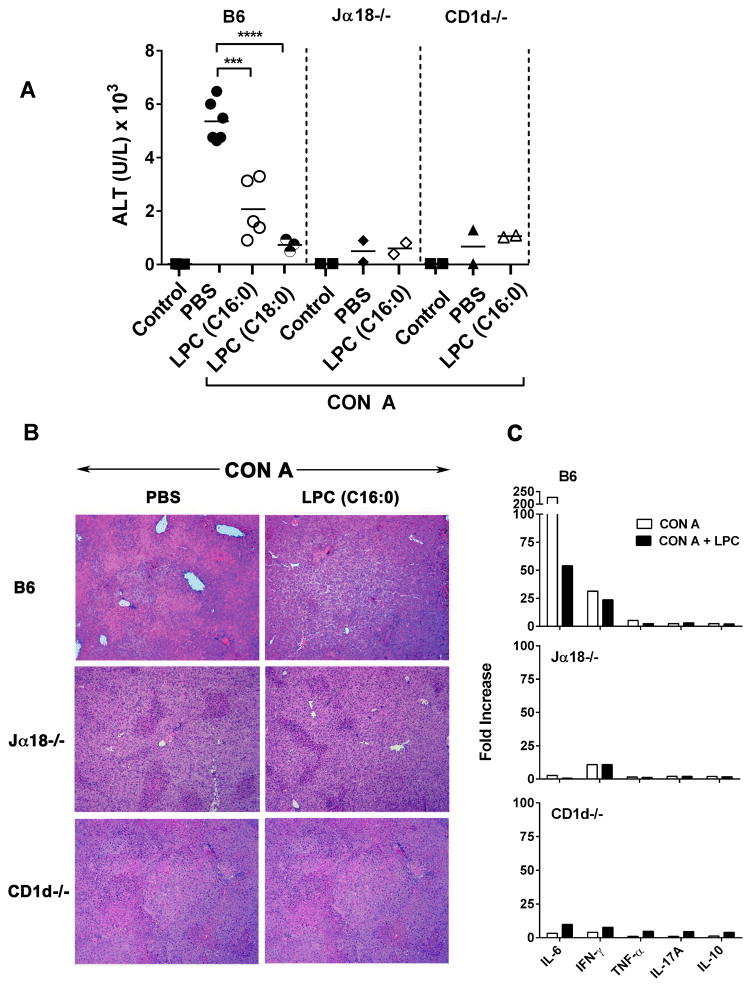
Anergy induction in type I NKT cells following activation of type II NKT cells with sulfatide has been recently shown to limit inflammation-induced damage in liver diseases mediated by these cells (17, 18). Here we examined whether LPC-mediated anergy induction in type I NKT cells is able to prevent Con A-induced hepatitis in C57BL/6 mice. As shown in Figure 8a, treatment of BL/6 mice with LPC C16:0 or LPC C18:0 significantly inhibited liver damage as determined by the serum ALT levels. Furthermore H&E staining of liver sections (Fig. 8b) showed that liver damage or necrosis was significantly blunted in BL/6 mice treated with LPC C16:0 compared to those treated with PBS/vehicle in the control group. Since Con A-induced liver injury is mediated by type I NKT cells (27–29), Jα18−/− animals deficient in type I NKT cells or CD1d−/− mice lacking both type I and type II NKT cells are protected. Accordingly, LPC C16:0 administration did not further alter liver damage in Jα18−/− or CD1d−/− mice as indicated by liver histology (Fig. 8b) as well as by serum levels of ALT (Fig 8a) and proinflmmatory cytokines (Fig. 8c). These data suggest LPC-mediated regulation of type I NKT cells can be used to regulate inflammatory liver diseases
Figure 8. Inhibition of ConA-induced hepatitis in mice treated with LPC.
(A) Serum ALT levels from groups of BL/6, Jα18−/− or CD1d−/− mice immunized i.v. with ConA (10 mg/kg of body weight) and treated i.p. with PBS or indicated LPC C:16 (100 μg/mouse) or naïve control are shown. ***P value <0.001, ****P value <0.0001. (B) Inhibition of histological severity of ConA-induced liver damage following treatment with LPC as in A. Groups of BL/6, Jα18−/− or CD1d−/− mice were sacrificed 24 hours after the injection and livers were removed and fixed in 10% formalin solution until H & E staining. H & E stained histological sections at 100 x magnification are shown. (C) A fold increase in serum cytokines in Con A or Con A plus LPC-treated BL/6, Jα18−/− or CD1d−/− mice as in A is shown. Basal values (pg/ml) for IL-6, IFN-γ, TNF-α, IL-17A, and IL-10 were 11.0, 2.6, 2.9, 2.3, and 2.1 respectively.
Discussion
The data presented here demonstrate that lysophospholipids are recognized by CD1d-restricted type II NKT cells. Accordingly, a self-glycolipid sulfatide-reactive type II NKT hybridoma is activated by LPC, LPAF and LSM in a CD1d-dependent manner. The CD1d-bound LPC binds to a type II NKT TCR with affinity similar to that observed for the CD1d-LSF. A similar recognition mode of LPC and LSF is also reflected by the finding that modification of critical residues around the A′ but not the F′ pocket in CD1d has a relatively similar influence on the ability of both lipids to stimulate type II NKT cells at the monoclonal and polyclonal level. Notably, similar to the immunoregulatory pathway following CD1d-dependent activation of sulfatide-reactive type II NKT cells, LPC administration also leads to anergy induction in type I NKT cells and protection from type I NKT-mediated ConA-induced hepatitis. Collectively our data suggest that at least a portion of type II NKT cells is reactive to lysophospholipids and their activation can play an important role in immune regulation.
Lysophospholipids are generated from their respective phospholipids by the action of phospholipases that cleave the acyl-ester bonds at sn-1 and sn-2 positions leaving only a single fatty acid chain. Recent studies have shown that LPC and LPE are associated with the CD1d molecules (30, 31). Human type II NKT cells from myeloma patients (32) and some of the human type I NKT cell clones can also recognize LPC bound to CD1d (33). Furthermore LPE generated in hepatocytes following hepatitis B infection can also stimulate type II NKT cells in murine liver (16). Interestingly although mouse type I NKT cells do not recognize lysophospholipids (16, 34), type I NKT-mediated lipid presentation is compromised in mice deficient in the lysosomal phosphatase A2 (PLA2) (35). These data suggest that lysophospholipids in addition to being directly recognized by type II NKT cells may also be involved in antigen loading or CD1d-mediated presentation. Additionally both microbial and mammalian phospholipids, such as phosphatidylglycerol (PG), phosphatidylinositol and cardiolipin have been shown to activate murine type II NKT cell hybridomas (36). Thus both self-lysophospholipids and phospholipids are potential antigens for type II NKT cells.
The levels of self-lysophospholipids, including LPC and lysophosphatidic acid (LPA) are altered during inflammation, viral infections and in cancer (26, 37). Additionally the enzymes that participate in PLC/LPA metabolism play a central role in regulating their levels and form important signaling axes, the PLA2/LPC and autotaxin/LPC for regulating inflammation (26, 37). Accordingly decreased level of LPC correlates with sepsis-related mortality and the beneficial effects of synthetic LPC administration in experimental sepsis has recently been shown (38). A similar therapeutic effect of an LPC analogue has been shown in the treatment of chronic-relapsing EAE (39). It is interesting that a decrease in serum levels of C16 and C18 isoforms of LPC also has been found in inflammatory liver diseases, including patients following alcoholic consumption, chronic HBV infection as well as nonalcoholic steatohepatitis (40, 41). It will be important to examine whether LPC-reactive CD1d-restricted T cells shown to be potentially anti-inflammatory here (Figure 8) also play a regulatory role in these inflammatory conditions and whether these cells can be targeted for disease intervention.
Recent insights from the crystal structure of a type II NKT cell TCR that recognizes sulfatide and LSF suggest the presence of a distinct recognition motif for TCR recognition between the type I and type II NKT cell subsets (11, 12). While the type I NKT TCR binds above the CD1d F′ pocket, the Hy19.3 Type II NKT TCR binds above the A′ pocket. This results in the TCRs approaching the antigen, which is generally bound at the center of the binding groove between the two pockets, from opposite directions. It therefore appears that two different binding strategies evolved to allow recognition of ligands, e.g. LPC, by NKT cells (11, 12, 42). In particular, the type II NKT TCR binds sulfatide and LSF exclusively with its β chain by pinning them against the CD1d surface. This binding mode would allow binding of different lipid moieties, such as LSF/sulfatide and LPC (See Supplement Fig. 1), while maintaining a conserved TCR docking footprint on CD1d. It is interesting that though LSF- and isoforms of LPC-CD1d complexes bind to TCR (Fig. 4) with similar binding affinities, (~6 mM and 3–9 mM respectively), two of them LPC (C18:1(9Z)) and LPC (C18:1(1oleyl)) containing unsaturation do not stimulate Hy19.3 effectively (Fig. 5). Further studies are needed to address whether unsaturation may result in differential binding, e.g., in A′ pocket and an altered orientation resulting in loss of TCR signaling.
Understanding antigen specificity and TCR repertoire of NKT cell subsets is critical for the manipulation of their function. Although our data suggest some redundancy and overlap in TCR repertoires among type II NKT cells that recognize self-glycolipids as well as self-phospholipids, a detailed structural study of the TCR repertoire from several type II NKT cell clones/hybidomas is needed to further clarify this. It is becoming clear that type I NKT cells can also recognize self-lipids (43–45) and some of these can activate both type I and type II NKT cells. For example βGluCer as well as LPC and LSM can activate at least a portion of cells in both subsets (43–45). Although only a small number of human type I NKT cell clones can be in vitro stimulated with LPC (33, 42), frequency of LPC-reactive type II NKT cell clones has been shown to be increased significantly in lymphoma patients (32). As shown here (Fig. 2) and earlier (11, 24), βGluCer is also recognized by sulfatide-reactive type II NKT hybridomas. It is important to point out that, while some antigens are able to activate both type I and type II NKT cells, the two lineages are not completely redundant as αGalCer as well as some microbial lipids (46) are not recognized by the type II NKT cells. It is not yet known whether the semi-invariant TCR bias the recognition of microbial antigens by type I NKT cells?
Generally it seems that self-glycolipids with a long acyl chain are better at activating type II NKT cells (47, 48). For example, sulfatide isoforms with long acyl chains are more efficient in activating type II NKT cells and accordingly in regulating EAE and T1D in NOD mice. Thus C24:1 and C24:0 sulfatides were better than C16:0 or C18:0 isoforms in treating EAE and T1D respectively (47–49). Interestingly, self-phospholipid LPC C18:0 is better than other isoforms, including LPC C16:0 in activating type II NKT cells (Fig. 5). Surprisingly, LPC C24:0 is less efficient than LPC C18:0 in stimulating the Hy19.3. It is not yet known whether this is due to a higher binding affinity to CD1d or the TCR, or both or whether C24:0 binds in the A′ pocket. Interestingly, unlike the synthetic C24:1 sulfatide (48), both LSF and LPC (C16:0, C18:0) are inefficient in forming stable CD1d tetramers that do not stain Hy19.3.
It is clear that activation of type II NKT cells with sulfatide controls inflammatory liver or kidney diseases, antigen-induced and spontaneously arising autoimmune diseases, as well as anti-tumor immunity in a CD1d-dependent manner (4, 6, 7, 50). Mechanisms by which NKT cell subsets modulate immunity depend on their interactions with other immune cells following activation by their respective lipid antigens, e.g. αGalCer and sulfatide for type I and type II NKT cell subsets, respectively. It is notable that dendritic cells (DCs) play a crucial role not only in the activation of NKT cells but also are central to their role in the regulation of immune responses (6, 50). This type II NKT-mediated immunoregulatory pathway results in (i) inactivation of type I NKT cells that now function as regulatory T cells; (ii) tolerization of conventional DC; (iii) tolerization of microglia in the CNS and (iv) inhibition of the effector functions of pathogenic MHC-restricted CD4+ T cells (6, 10, 17–19, 49). Consistent with this, LPC-mediated activation of type II NKT cells also leads to inactivation of type I NKT cells (Fig. 7) and ultimately to regulation of inflammatory liver disease. Thus activation of type II NKT cells with sulfatide or LPC appears to follow a similar mechanism of immune regulation. Recently CpG- (51), alum- (52) or IL-25- (53) activated type II NKT cells also have been shown to play important roles in anti-tumor, or humoral immunity or in regulating inflammation in adipose tissue/obesity. It is becoming clear that activation of type II NKT cells has major consequences in regulating immune responses in a variety of conditions.
The CD1d-dependent antigen recognition pathway is highly conserved from mice to humans, and several key features of NKT cell subsets are shared between them. Interestingly, type II NKT cells occur more frequently than type I NKT cells in humans, thereby facilitating their further characterization using appropriate lipid ligands such as LPC. A detailed characterization of LPC-reactive type II NKT cells in mice and in humans will have important implications for their appropriate manipulation for intervention in inflammatory diseases, including autoimmunity and cancer.
Supplementary Material
Acknowledgments
Grant support: This work was supported by grants from the National Institutes of Health, USA, R01 CA100660 and R01 AA020864 (VK) and R01 AI074952 (DMZ).
Authors would like to thank other members of Kumar’s laboratory and Dr. Randle Ware for a critical reading of the manuscript.
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nat Immunol. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 4.Arrenberg P, Halder R, Kumar V. Cross-regulation between distinct natural killer T cell subsets influences immune response to self and foreign antigens. J Cell Physiol. 2009;218:246–250. doi: 10.1002/jcp.21597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kronenberg M, Rudensky A. Regulation of immunity by self-reactive T cells. Nature. 2005;435:598–604. doi: 10.1038/nature03725. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Delovitch TL. Different subsets of natural killer T cells may vary in their roles in health and disease. Immunology. 2014 doi: 10.1111/imm.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhost S, Sedimbi S, Kadri N, Cardell SL. Immunomodulatory type II natural killer T lymphocytes in health and disease. Scand J Immunol. 2012;76:246–255. doi: 10.1111/j.1365-3083.2012.02750.x. [DOI] [PubMed] [Google Scholar]
- 8.Terabe M, Berzofsky JA. NKT cells in immunoregulation of tumor immunity: a new immunoregulatory axis. Trends Immunol. 2007;28:491–496. doi: 10.1016/j.it.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Arrenberg P, Halder R, Dai Y, Maricic I, Kumar V. Oligoclonality and innate-like features in the TCR repertoire of type II NKT cells reactive to a beta-linked self-glycolipid. Proc Natl Acad Sci U S A. 2010;107:10984–10989. doi: 10.1073/pnas.1000576107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jahng A, Maricic I, Aguilera C, Cardell S, Halder RC, Kumar V. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J Exp Med. 2004;199:947–957. doi: 10.1084/jem.20031389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Girardi E, Maricic I, Wang J, Mac TT, Iyer P, Kumar V, Zajonc DM. Type II natural killer T cells use features of both innate-like and conventional T cells to recognize sulfatide self antigens. Nat Immunol. 2012;13:851–856. doi: 10.1038/ni.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel O, Pellicci DG, Gras S, Sandoval-Romero ML, Uldrich AP, Mallevaey T, Clarke AJ, Le Nours J, Theodossis A, Cardell SL, Gapin L, Godfrey DI, Rossjohn J. Recognition of CD1d-sulfatide mediated by a type II natural killer T cell antigen receptor. Nat Immunol. 2012;13:857–863. doi: 10.1038/ni.2372. [DOI] [PubMed] [Google Scholar]
- 13.Pellicci DG, Patel O, Kjer-Nielsen L, Pang SS, Sullivan LC, Kyparissoudis K, Brooks AG, Reid HH, Gras S, Lucet IS, Koh R, Smyth MJ, Mallevaey T, Matsuda JL, Gapin L, McCluskey J, Godfrey DI, Rossjohn J. Differential recognition of CD1d-alpha-galactosyl ceramide by the V beta 8.2 and V beta 7 semi-invariant NKT T cell receptors. Immunity. 2009;31:47–59. doi: 10.1016/j.immuni.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Girardi E, Wang J, Yu ED, Painter GF, Kronenberg M, Zajonc DM. The Valpha14 invariant natural killer T cell TCR forces microbial glycolipids and CD1d into a conserved binding mode. J Exp Med. 2010;207:2383–2393. doi: 10.1084/jem.20101335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudolph MG, Stanfield RL, Wilson IA. How TCRs bind MHCs, peptides, and coreceptors. Annu Rev Immunol. 2006;24:419–466. doi: 10.1146/annurev.immunol.23.021704.115658. [DOI] [PubMed] [Google Scholar]
- 16.Zeissig S, Murata K, Sweet L, Publicover J, Hu Z, Kaser A, Bosse E, Iqbal J, Hussain MM, Balschun K, Rocken C, Arlt A, Gunther R, Hampe J, Schreiber S, Baron JL, Moody DB, Liang TJ, Blumberg RS. Hepatitis B virus-induced lipid alterations contribute to natural killer T cell-dependent protective immunity. Nat Med. 2012;18:1060–1068. doi: 10.1038/nm.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arrenberg P, Maricic I, Kumar V. Sulfatide-mediated activation of type II natural killer T cells prevents hepatic ischemic reperfusion injury in mice. Gastroenterology. 2011;140:646–655. doi: 10.1053/j.gastro.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halder RC, Aguilera C, Maricic I, Kumar V. Type II NKT cell-mediated anergy induction in type I NKT cells prevents inflammatory liver disease. J Clin Invest. 2007;117:2302–2312. doi: 10.1172/JCI31602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian L, Blumenfeld H, Tohn R, Ly D, Aguilera C, Maricic I, Mansson JE, Buschard K, Kumar V, Delovitch TL. NKT cells stimulated by long fatty acyl chain sulfatides significantly reduce the incidence of type 1 diabetes in nonobese diabetic mice [corrected] PLoS One. 2012;7:e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SH, Weiss A, Benlagha K, Kyin T, Teyton L, Bendelac A. The mouse CD1d-restricted repertoire is dominated by a few autoreactive T cell receptor families. J Exp Med. 2001;193:893–904. doi: 10.1084/jem.193.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy KC, Maricic I, Khurana A, Smith TR, Halder RC, Kumar V. Involvement of secretory and endosomal compartments in presentation of an exogenous self-glycolipid to type II NKT cells. J Immunol. 2008;180:2942–2950. doi: 10.4049/jimmunol.180.5.2942. [DOI] [PubMed] [Google Scholar]
- 23.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, Kumar V, Wu L, Kaer LV. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–2826. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhost S, Lofbom L, Rynmark BM, Pei B, Mansson JE, Teneberg S, Blomqvist M, Cardell SL. Identification of novel glycolipid ligands activating a sulfatide-reactive, CD1d-restricted, type II natural killer T lymphocyte. Eur J Immunol. 2012;42:2851–2860. doi: 10.1002/eji.201142350. [DOI] [PubMed] [Google Scholar]
- 25.Joyce S, Girardi E, Zajonc DM. NKT cell ligand recognition logic: molecular basis for a synaptic duet and transmission of inflammatory effectors. J Immunol. 2011;187:1081–1089. doi: 10.4049/jimmunol.1001910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sevastou I, Kaffe E, Mouratis MA, Aidinis V. Lysoglycerophospholipids in chronic inflammatory disorders: the PLA(2)/LPC and ATX/LPA axes. Biochim Biophys Acta. 2013;1831:42–60. doi: 10.1016/j.bbalip.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Diao H, Kon S, Iwabuchi K, Kimura C, Morimoto J, Ito D, Segawa T, Maeda M, Hamuro J, Nakayama T, Taniguchi M, Yagita H, Van Kaer L, Onoe K, Denhardt D, Rittling S, Uede T. Osteopontin as a mediator of NKT cell function in T cell-mediated liver diseases. Immunity. 2004;21:539–550. doi: 10.1016/j.immuni.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko Y, Harada M, Kawano T, Yamashita M, Shibata Y, Gejyo F, Nakayama T, Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeda K, Hayakawa Y, Van Kaer L, Matsuda H, Yagita H, Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci U S A. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan W, Kang SJ, Evans JE, Cresswell P. Natural lipid ligands associated with human CD1d targeted to different subcellular compartments. J Immunol. 2009;182:4784–4791. doi: 10.4049/jimmunol.0803981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox D, Fox L, Tian R, Bardet W, Skaley M, Mojsilovic D, Gumperz J, Hildebrand W. Determination of cellular lipids bound to human CD1d molecules. PLoS One. 2009;4:e5325. doi: 10.1371/journal.pone.0005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang DH, Deng H, Matthews P, Krasovsky J, Ragupathi G, Spisek R, Mazumder A, Vesole DH, Jagannath S, Dhodapkar MV. Inflammation-associated lysophospholipids as ligands for CD1d-restricted T cells in human cancer. Blood. 2008;112:1308–1316. doi: 10.1182/blood-2008-04-149831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, Trott DL, Ndonye RM, Veerapen N, Besra GS, Howell AR, Cook ME, Adams EJ, Hildebrand WH, Gumperz JE. Recognition of lyso-phospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei B, Speak AO, Shepherd D, Butters T, Cerundolo V, Platt FM, Kronenberg M. Diverse endogenous antigens for mouse NKT cells: self-antigens that are not glycosphingolipids. J Immunol. 2011;186:1348–1360. doi: 10.4049/jimmunol.1001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paduraru C, Bezbradica JS, Kunte A, Kelly R, Shayman JA, Veerapen N, Cox LR, Besra GS, Cresswell P. Role for lysosomal phospholipase A2 in iNKT cell-mediated CD1d recognition. Proc Natl Acad Sci U S A. 2013;110:5097–5102. doi: 10.1073/pnas.1302923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tatituri RV, Watts GF, Bhowruth V, Barton N, Rothchild A, Hsu FF, Almeida CF, Cox LR, Eggeling L, Cardell S, Rossjohn J, Godfrey DI, Behar SM, Besra GS, Brenner MB, Brigl M. Recognition of microbial and mammalian phospholipid antigens by NKT cells with diverse TCRs. Proc Natl Acad Sci U S A. 2013;110:1827–1832. doi: 10.1073/pnas.1220601110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Knowlden S, Georas SN. The Autotaxin-LPA Axis Emerges as a Novel Regulator of Lymphocyte Homing and Inflammation. J Immunol. 2014;192:851–857. doi: 10.4049/jimmunol.1302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murch O, Collin M, Sepodes B, Foster SJ, Mota-Filipe H, Thiemermann C. Lysophosphatidylcholine reduces the organ injury and dysfunction in rodent models of gram-negative and gram-positive shock. British journal of pharmacology. 2006;148:769–777. doi: 10.1038/sj.bjp.0706788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabannes D, Ryffel B, Borel JF. SRI 62–834, a cyclic ether analogue of the phospholipid ET-18-OCH3, displays long-lasting beneficial effect in chronic relapsing experimental allergic encephalomyelitis in the Lewis rat. Comparison with cyclosporin and (Val2)-dihydrocyclosporin effects in clinical, functional and histological studies. J Autoimmun. 1992;5:199–211. doi: 10.1016/0896-8411(92)90200-a. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118–129. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lehmann R, Franken H, Dammeier S, Rosenbaum L, Kantartzis K, Peter A, Zell A, Adam P, Li J, Xu G, Konigsrainer A, Machann J, Schick F, Hrabe de Angelis M, Schwab M, Staiger H, Schleicher E, Gastaldelli A, Fritsche A, Haring HU, Stefan N. Circulating lysophosphatidylcholines are markers of a metabolically benign nonalcoholic fatty liver. Diabetes care. 2013;36:2331–2338. doi: 10.2337/dc12-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopez-Sagaseta J, Sibener LV, Kung JE, Gumperz J, Adams EJ. Lysophospholipid presentation by CD1d and recognition by a human Natural Killer T-cell receptor. The EMBO journal. 2012;31:2047–2059. doi: 10.1038/emboj.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan PJ, Tatituri RV, Brigl M, Kim EY, Tuli A, Sanderson JP, Gadola SD, Hsu FF, Besra GS, Brenner MB. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–1211. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gapin L, Godfrey DI, Rossjohn J. Natural Killer T cell obsession with self-antigens. Curr Opin Immunol. 2013;25:168–173. doi: 10.1016/j.coi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–857. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kinjo Y, Kitano N, Kronenberg M. The role of invariant natural killer T cells in microbial immunity. J Infect Chemother. 2013;19:560–570. doi: 10.1007/s10156-013-0638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian L, Blumenfeld H, Tohn R, Ly D, Aguilera C, Maricic I, Mansson JE, Buschard K, Kumar V, Delovitch TL. NKT cells stimulated by long Fatty acyl chain sulfatides significantly reduces the incidence of type 1 diabetes in nonobese diabetic mice. PLoS One. 2012;7:e37771. doi: 10.1371/journal.pone.0037771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zajonc DM, Maricic I, Wu D, Halder R, Roy K, Wong CH, Kumar V, Wilson IA. Structural basis for CD1d presentation of a sulfatide derived from myelin and its implications for autoimmunity. J Exp Med. 2005;202:1517–1526. doi: 10.1084/jem.20051625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maricic I, Halder R, Bischof F, Kumar V. Dendritic Cells and Anergic Type I NKT Cells Play a Crucial Role in Sulfatide-Mediated Immune Regulation in Experimental Autoimmune Encephalomyelitis. J Immunol. 2014 doi: 10.4049/jimmunol.1302898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kumar V. NKT-cell subsets: Promoters and protectors in inflammatory liver disease. J Hepatol. 2013;59:618–620. doi: 10.1016/j.jhep.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Weng X, Bagchi S, Wang CR. Polyclonal type II natural killer T cells require PLZF and SAP for their development and contribute to CpG-mediated antitumor response. Proc Natl Acad Sci U S A. 2014;111:2674–2679. doi: 10.1073/pnas.1323845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah HB, Devera TS, Rampuria P, Lang GA, Lang ML. Type II NKT cells facilitate Alum-sensing and humoral immunity. J Leukoc Biol. 2012;92:883–893. doi: 10.1189/jlb.0412177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hams E, Locksley RM, McKenzie AN, Fallon PG. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.