Abstract
Intratumoral immune activation can induce local and systemic anti-tumor immunity. Imiquimod is a cream-formulated, TLR-7 agonist that is FDA-approved for the treatment of non-melanoma skin cancers, but has limited activity against melanoma. We studied the anti-tumor activity and mechanism of action of a novel, injectable, tissue-retained TLR 7/8 agonist, 3M-052, which avoids systemic distribution. Intratumoral administration of 3M-052 generated systemic anti-tumor immunity, and suppressed both injected and distant, uninjected wild-type B16.F10 melanomas. Treated tumors showed increased level of CCL2 chemokines and infiltration of M1 phenotype-shifted macrophages, which could kill tumor cells directly through production of nitric oxide and CCL2, was essential for the anti-tumor activity of 3M-052. CD8+ T cells, B cells, Type I IFN, IFN-γ, and pDC were contributed to efficient tumor suppression whereas perforin, NK cells and CD4 T cells were not required. Finally, 3M-052 therapy potentiated checkpoint blockade therapy with anti-CTLA-4 and anti-PD-L1 antibodies, even when checkpoint blockade alone was ineffective. Our findings suggest that intratumoral treatment with 3M-052 is a promising approach for the treatment of cancer and establish a rational strategy and mechanistic understanding for combination therapy with intratumoral, tissue-retained TLR7/8 agonist and checkpoint blockade in metastatic cancer.
Keywords: TLR7/8, intratumoral, melanoma, CD8+ T cells, M1/M2 macrophages, PD-1/PD-L1
Introduction
Toll like receptor (TLR) agonists activate innate immune cells through the TLR–MyD88-NFkβ and IRF3/7 pathways (1). We and others have shown that intratumoral TLR9 ligation in mice induces activation of innate immune cells including plasmacytoid and conventional dendritic cells (pDCs and cDCs), followed by induction of tumor-specific, CD8+ T cell responses (2, 3). The synthetic TLR7 agonist, imiquimod, is FDA-approved in a cream formulation for the treatment of cutaneous basal cell carcinoma, actinic keratosis and genital warts, and has limited activity against cutaneous melanoma and breast tumors (4–7). Controversy exists as to whether TLR7 (e.g. imiquimod) and TLR7/8 dual agonists (e.g. resiquimod), generate tumor-specific T cell immunity and/or kill tumor directly by activation of innate immunity (8, 9). The cream formulation of imiquimod limits its application for deep, non-cutaneous tumors, and systemic administration of TLR agonists is limited by severe toxicity, including cytokine storm (10). Therefore, development of injectable, local-release formulations of TLR7 and TLR7/8 agonists are an area of intense study and drug development.
Growing evidence suggests that tumor associated macrophages (TAM) play an important role in tumor growth. TAM can assume M1 or M2 phenotypes, with M1 TAM producing interleukin (IL)-12 to promote tumoricidal responses, whereas M2 TAM produce IL-10 and promote tumor progression (11),(12). One of the factors that may drive M1/M2 TAM ratios in tumors is the chemokine, Chemokine (C-C motif) ligand 2/macrophage chemotactic protein-1 (CCL2/MCP-1). Low CCL2 concentrations can promote accumulation of M2 TAM and tumor growth while high CCL2 secretion results in predominant M1 TAM infiltration and tumor destruction (13). Therefore, shifting TAM phenotype from M2 towards M1 could be an important therapeutic strategy (14–17).
Here we report therapeutic activity of a novel TLR7/8 dual agonist, 3M-052, in a preclinical model of melanoma. 3M-052 is an injectable, lipid modified imidazoquinoline that forms a tissue depot with gradual, sustained release, allowing local TLR triggering activity without systemic cytokine release (18). 3M-052 is currently under clinical development by 3M Drug Delivery Systems Division for use in vaccines and cancer therapy. Intratumoral 3M-052 monotherapy induced local innate immune activation as well as systemic, antigen-specific CD8+ T cell responses which suppressed distant, uninjected tumors. Mechanistically, the intratumoral macrophages shifted from a M2-dominant to M1 dominant phenotype, while CCL2 blockade or macrophage depletion abolished therapeutic activity. 3M-052 has promise as monotherapy or in combination with checkpoint blockades, anti-PD-L1 or anti-CTLA-4, for the treatment of metastatic melanoma and other cancers.
Materials and Method
Mice and cell lines
All animal experiments were performed in accordance with NIH guidelines and approved by the MDACC IACUC. C57BL/6 mice were purchased from the NCI. Rag2 KO, B cell KO (IgH), Prf KO, IFN-γ KO and Bdca2-DTR mice were from Jackson laboratory and IFNAR KO mice were provided by Dr. Paul W. Dempsey at University of Zürich, Switzerland (19). All mice were used at 6–12 weeks of age. B16.F10, B16.OVA, and BP (brafV600E × pten−/−) melanoma cell line (20) were cultured in RPMI 1640 supplemented with 10% heat-inactivated FBS, L-glutamine, sodium pyruvate, nonessential amino acids, and penicillin-streptomycin (all from Invitrogen/Life Technologies Inc.). TRAMP-C2 cell line were cultured in DMEM-HG (Life Technologies Inc.) supplemented with 5% Nu-serum IV, 5% FBS, 5ug/ml insulin, 10nM dihydrotestosterone (DHT) and penicillin-streptomycin.
3M-052 and its vehicle
An injectable formulation of 3M-052 and the vehicle were provided by 3M Drug Delivery Systems, 3M Center, St. Paul, MN 55144, USA (18). Briefly, 3M-052 was formulated in sesame oil (Sesame Oil, NF NP, Super Refined®, Croda Europa Ltd, UK)/ethanol. The formulation was filtered through a polyethersulfone 0.2 µm filter (Millipore, Bellerica, MA). Drug content was determined by HPLC, and the final concentration was 0.4 to 0.5 mg/ml.
Tumor induction, treatment and monitoring
C57BL/6 or KO mice were subcutaneously inoculated with 3×105 B16.F10 or B16.OVA melanoma cells on day −7 in the left flank and for contralateral tumor experiments, 3×105 B16.F10 or B16.OVA tumor cells were also inoculated in to right flank on day −3. Only left flank palpable tumors were treated i.t. on day 0 and 4 with 28–35 ug of 3M-052 or vehicle and right flank tumors were left untreated. For BP and TRAMP-C2 models tumor were implanted by injection of 5×105 cells in the left flank by subcutaneously on day −9. Tumor size is expressed as the product of perpendicular diameters of tumors measured with calipers. Mice were sacrificed when tumor size reached ≥200 mm2 in diameter.
Cell depletion, chemokine neutralization and checkpoint blockade experiments
Mouse Abs against CCL2 (2H5), CD4 (GK1.5), CD8 (2.43), NK1.1, Ly6C cells, PDL-1(10F.9G20), CTLA-4 (9H10) and respective isotype control were purchased from Bio-X-cell. Anti-PD-L1 mAb and anti-CTLA-4 were injected i.p. (200 µg) 1 hr after 3M-052 treatment and repeated every 4 days. Others Abs were injected i.p. (200µg) on days −1 and 0 and antibody injections were repeated every 4 days thereafter to maintain depletion or neutralization. Cell depletion efficiency was confirmed by flow cytometry on peripheral blood or tumor 2–3 days after antibody injection. In vivo depletion of pDCs was induced and maintained by DT injection (i.p.; 5 ng DT/g body weight every other day) in Bdca2-DTR mice harboring B16 melanoma one day before the start of 3M-052 treatments and repeated every other day until the end of experiment. Clodronate and control liposome (Anionic) were purchased from FormuMax and injected i.t. (40 ul of 1:10 dilution in PBS) 2 days before the start of 3M-052 treatment and repeated every week.
Flow Cytometric analysis
Leukocytes were isolated from mechanically disrupted tumors by lymphocytes separation medium (Corning, Cellgro). Red blood cells lysis was performed on spleens and blood. Antigen-specific T cell responses were evaluated by OVA-tetramer (Beckman Culter, Inc.) and CD8 (Bio-legend) staining. Intracellular CD206 (Bio-legend) and IFN- γ staining was performed using the cytofix/cytoperm kit (BD Biosciences). Cells were stained with antibodies against CD45,CD19,B220,CD11b,CD11C,B220,Siglec-H,Ly6C,F4\80,CD40 from Bio-legend and CD4,CD3, NK1.1,CD86,CD69 from BD Pharmingen. Data were acquired on a LSR-II Flow Cytometer (BD Biosciences) and analyzed using FlowJo (Treestar).
Cytokine/Chemokine Assay
24 hrs after 3M-052 or vehicle treatment, tumors were mechanically disrupted, centrifuged and supernatant was collected. Cytokines/chemokines were measured using MILLIPLEX mouse Cytokine/Chemokine Panel (Millipore) according to the manufacturer's instructions. Fluorescence signal was measured on Luminex® 100/200™ System and data were analyzed using Excel software.
Cytolytic assay
Macrophages (CD11b+F4/80+) were sorted from mouse splenocytes and cultured with 3M-052 (3 ug/ml) or vehicle for 48 hrs. B16-F10 melanoma cells (6×103) were cultured with stimulated macrophages at a 10:1 effector to target ratio or with 200 ul macrophage culture supernatant for 96 hrs. Cytotoxicity was measured in 96-well plates using EZ4U nonradioactive cell proliferation and cytotoxicity assay (Biomedica) according to the manufacturer’s manual. Data were expressed as the percent lysis, or percentage of live cells calculated by the following formula.
% Lysis = (1− (Aof coculture cells − Aof effector cells) / Aof target cells) × 100
% live cells=(AB16 cells with 3M /V stimulated macrophages supernatant − blank) / AB16 cells with 3M/V − Blank) × 100
Statistics
All results are expressed as the means ±SEM. Mouse and sample group sizes were n = 5, unless otherwise indicated. Statistical analysis was performed with Graph Pad Prism 4 software. Data were analyzed using unpaired two-tailed t tests, and differences were considered significant at P < 0.05. Figures denote statistical significance of p <0.05 as *, p <0.01 as **, and p<0.001 as ***. Survival experiments utilized log- rank Mantel Cox test for survival analysis. All experiments were performed at least twice with comparable results.
Results
Intratumoral administration of 3M-052 suppresses local injected and distant uninjected melanoma growth
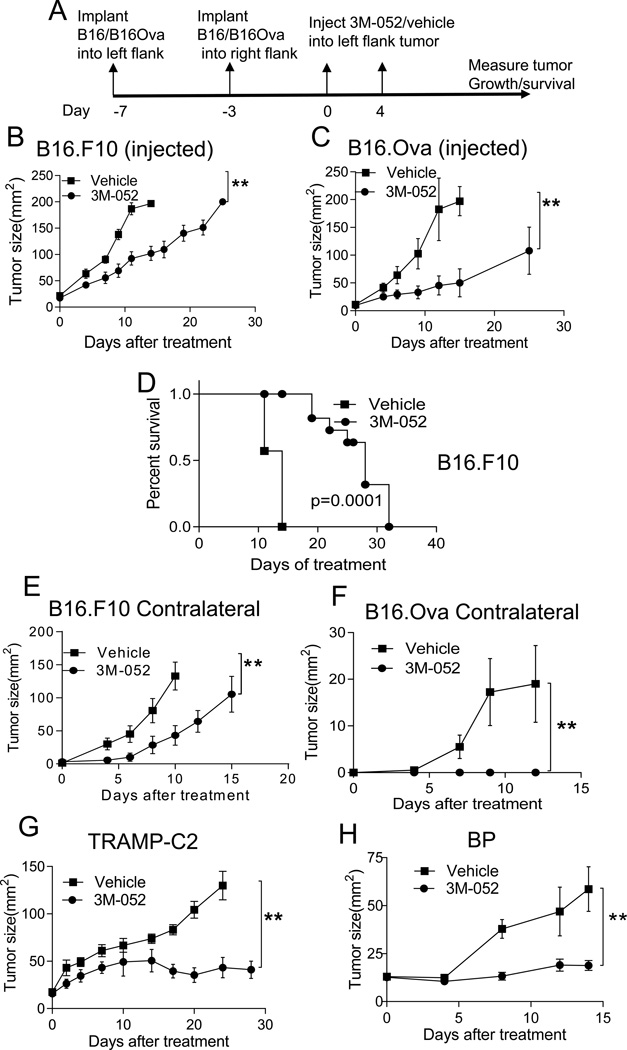
Most innate immune cells, including antigen presenting cells (APCs) in mouse and man express TLR7 and/or TLR8 (21, 22). In C57BL/6 mice, TLR8 is non-responsive to imidazoquinolines like resiquimod and 3M-052, but both pDCs, mDCs and macrophages in mice express TLR7 and respond to TLR7 agonists (10, 23). Thus, activation of tumor-associated TLR7+ APCs with 3M-052 could generate a range of innate and adaptive anti-tumor immune responses. We tested the anti-tumor effect of 3M-052 against the poorly immunogenic, wild-type B16.F10 melanoma and the more immunogenic version B16.OVA, engineered to express the chicken ovalbumin protein. Palpable 7-day tumors (~20 mm2) were treated with intratumoral 3M-052 or vehicle on day 0 and 4 (treatment schematic; Fig 1A). Growth of both B16.F10 and B16.OVA tumors were suppressed after 3M-052 treatment, resulting in prolonged survival (Fig. 1B, C and D). However, the treatment efficacy of 3M-052 was more profound with B16.Ova than B16.F10 tumor. Although the majority of the tumors that were treated with 3M-052 never reached a size of 200 mm2 during the observation period, they developed dry ulceration (necrosis), requiring euthanasia. Since the goal of cancer therapy is typically the treatment of metastatic cancer, we tested the activity of i.t. 3M-052 on the growth of distant, uninjected tumors. 3M-052 treatment of an established tumor effectively suppressed the growth of distant, uninjected B16.F10 and B16.OVA tumors, suggesting this local approach can have systemic efficacy (Fig. 1E and F). To ensure anti-tumor activity of 3M-052 was not limited to B16 melanoma, we also tested activity against established BP melanoma, derived from the Tyr::CreER; BrafCA; Ptenlox/lox mouse (20), and against TRAMP-C2 prostate cancer, and found anti-tumor activity against these tumors as well (Fig. 1G and H).
Fig. 1. Intratumoral 3M-052 suppresses local and distant melanoma growth.
(A) Treatment Scheme. Mice bearing subcutaneous B16.F10 (B, E) or B16.Ova (C,F) tumors in left (B&C; 7–day tumor) and right (E&F; 3-day tumor) flanks were treated only in the left tumor on day 0 and 4 with 35ug 3M-052 or vehicle. (D) Survival of mice with 7-day old B16.F10 tumors were treated with 3M-052 or vehicle. Mice bearing 9 day-TRAMP-2C (G) or BP (H) tumor were treated with 3M-052 or vehicle, graphs show tumor growth over time. Tumor growth is plotted as mean ± SEM, n=5 in each group. Data is representative of at least 2 independent experiments or cumulative data of 2 experiments (B, D, E). *P<0.05, **P<0.005(unpaired two-tailed t test).
Migration and activation of innate immune cells
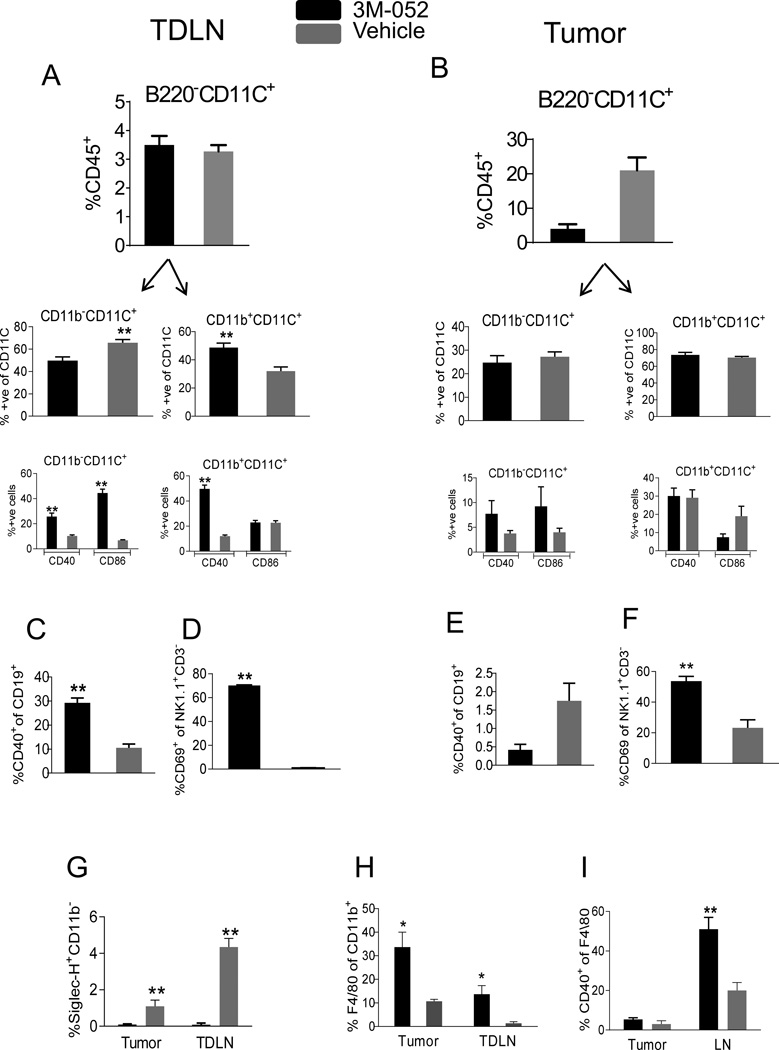
TLR7/8 triggering by 3M-052 is expected to activate murine TLR7+ innate leukocytes including macrophages, pDCs and cDCs, possibly resulting in tumor-specific T cell responses. We studied leukocytes (CD45+) in tumor and tumor draining lymph node (TDLN) 24 hours after i.t. administration of 3M-052 or vehicle control. Significant accumulation of myeloid DCs (CD11C+CD11b+B220−), and decreased numbers of lymphoid DCs (CD11C+CD11b−B220−) were found in TDLN of 3M-052 treated mice, and both DC types had upregulated CD40 and CD86 activation markers. We did not find expansion and activation of DCs in the tumor (Fig 2A, B). Similarly, activated B cells (CD19+CD40+) were present in TDLN but not in the tumor (Fig 2C, E), suggesting that i.t. 3M-052 induces APC activation and migration from tumor to TDLN. NK cells were reduced in both tumor and TDLN (data not shown) but showed increased expression of CD69 activation marker (Fig 2 D, F). Interestingly, we found greatly reduced numbers of pDCs (CD11C+SiglecH+ and CD11C+SiglecH+CD40+) in tumor and in TDLN (Fig 2G and data not shown). In contrast, macrophages (CD11b+F4/80+) were significantly increased in tumor and TDLN 24 hrs after i.t. 3M-052 administration (Fig 2H); however CD40 upregulation on macrophages was seen only in TDLN (Fig 2I). These data indicate potent innate immune activation by 3M-052 demonstrated by accumulation, activation and migration of innate immune cells.
Fig. 2. 3M-052 activates innate immune cells.
7-day B16.F10 tumors were treated 35 ug 3M-052 or vehicle by i.t. and after 24 hrs, CD45+ cells in tumor and TDLN were analyzed for innate immune cells. Activated (CD40+/CD86+) myeloid (CD11b+CD11C+B220−) and lymphoid (CD11b−CD11C+B220−) DCs in (A) TDLN and (B) tumor. Activated (CD40+) B cells in (C) TDLN and (E) tumor. Activated (CD69+) NK cells in (D) TDLN (F) and tumor. (G) pDCs in tumor and TDLN. (H) Total TAMs and (I) Activated (CD40+) TAMs in tumor and TDLN. Data is representative of at least 2 independent experiments. N=4 in each group. Error bars are SEM*P<0.05, **P<0.005(unpaired two-tailed t test).
Induction of tumor-specific CD8+ T cells and mechanism of tumor suppression
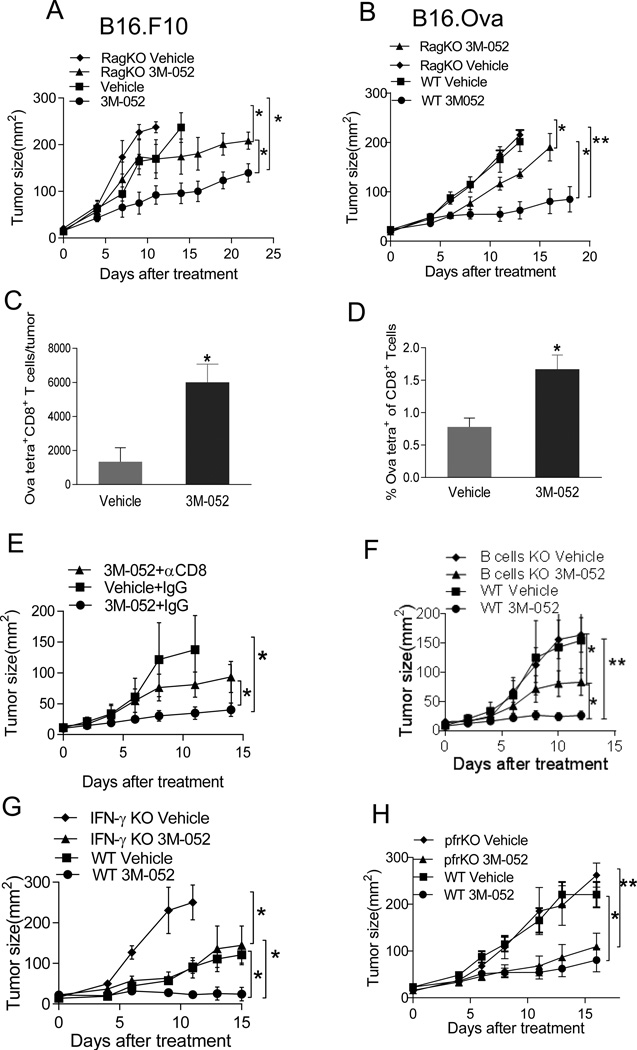
To investigate whether adaptive immunity is required for 3M-052-mediated tumor suppression, we treated both B16.F10 and B16.OVA tumors in C57BL/6 and Rag2 KO mice, deficient in T and B cells. Therapeutic efficacy of 3M-052 was significantly but not completely abrogated in Rag2 KO mice (Fig. 3A and B). Indeed, 8 days after i.t. treatment, we found more OVA257–264-specific CD8+ T cells in 3M-052 vs. vehicle-treated tumors (Fig 3C) and spleens (Fig 3D). Depletion of CD4+ or CD8+ T cells in C57BL/6 mice bearing B16.Ova tumors revealed that tumor killing partially dependent on CD8+ (Fig 3E) but not CD4+ T cells (data not shown) while B cell KO mice revealed a contribution of B cells as well (Fig 3F). We also confirmed that CD8+ T cells were required for therapeutic efficacy against wild-type B16.F10 tumors (Fig. S1A). The CD8+ T cell effector molecules, IFN-γ, contributed to the therapeutic efficacy in both B16.Ova and B16.F10 tumor models (Fig.3G and data not shown) while perforin did not contribute (Fig. 3H). Thus, while adaptive immunity contributed to the anti-tumor activity of i.t. 3M-052, additional mechanisms also mediate tumor suppression.
Fig. 3. Mechanism of tumor suppression by 3M-052.
(A,B) 3M-052 treatment of B16.F10 and B16.OVA in Rag2 KO mice, (C) Absolute numbers of OVA-tetramer+ CD8+ T cells in tumor (D) Percentage of OVA-tetramer+ CD8+ T cells in spleen cells 8 days after 3M-052 treatment. 3M-052 treatment of B16.OVA in (E) CD8-depleted mice (F) B cell KO mice, (G) IFN- γ KO mice (H) perforin KO mice or WT mice. Tumor growth is plotted as mean ± SEM, n=5 in each group. Data is representative of at least 2 independent experiments. *P<0.05, **P<0.005 (unpaired two-tailed t test).
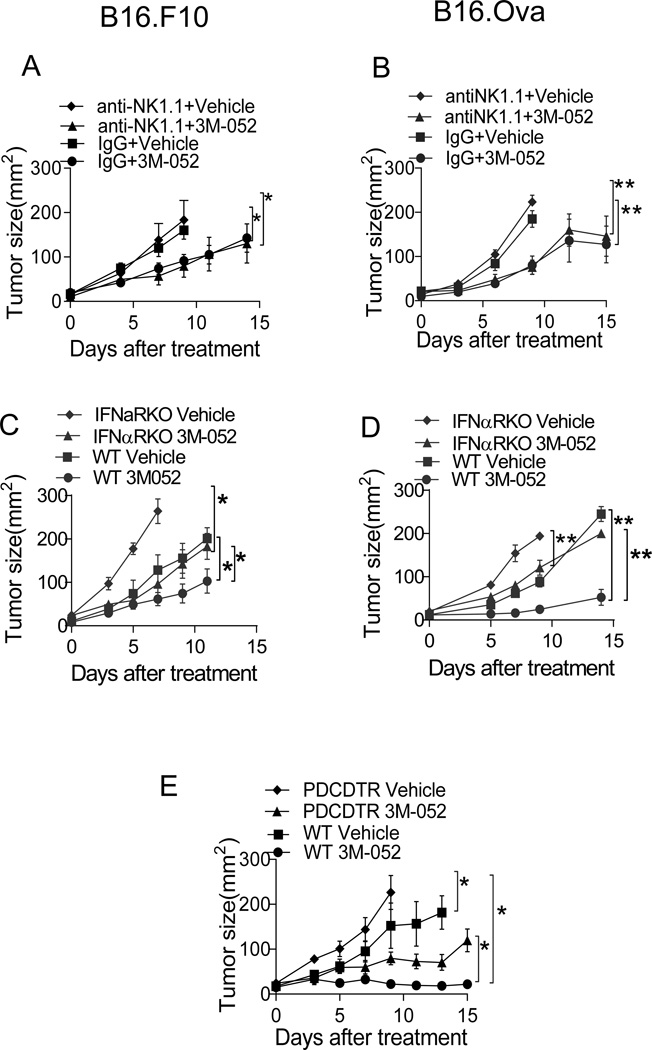
Type I IFN, pDCs and NK cells have been shown to play an important role in TLR9 and TLR7/8 agonist-mediated tumor suppression (8, 24); we evaluated their importance in 3M-052 mediated B16.F10 and B16.Ova tumor suppression. NK cells, while suppressing tumor growth even without treatment, were not required for 3M-052 mediated anti-tumor activity (Fig. 4A and B). To establish the contribution of pDCs and type I IFNs, we treated conditionally pDC-deficient (Bdca-2-DTR) mice and type I IFN receptor (IFNAR) KO mice. Similar to results observed in IFN-γ KO mice, growth of vehicle-treated tumors was accelerated in mice lacking type I IFN or pDCs. However, i.t. 3M-052 still reduced tumor size in these settings (Fig.4C, D and E), suggesting pDCs and type I IFN are not major mediators of the anti-tumor activity of 3M-052, however required for efficient tumor suppression.
Fig. 4. Anti-tumor activity of 3M-052 partially requires Type I IFN and pDC but not NK cells.
Intratumoral treatment with 3M-052 of B16.F10 tumors in mice depleted of (A) NK cells or (C) deficient in type I IFN receptor. Treatment of B16.OVA tumors in mice depleted of (B) NK cells or (D) deficient in type I IFN receptor. (E) Treatment of B16.OVA tumors in Bdca-2-DTR or WT mice. Tumor growth is plotted as mean ± SEM, n=5 in each group. Data is representative of at least 2 independent experiments. *P<0.05, **P<0.005(unpaired two-tailed t test).
Tumor suppression requires CCL2 and tumor-associated macrophages
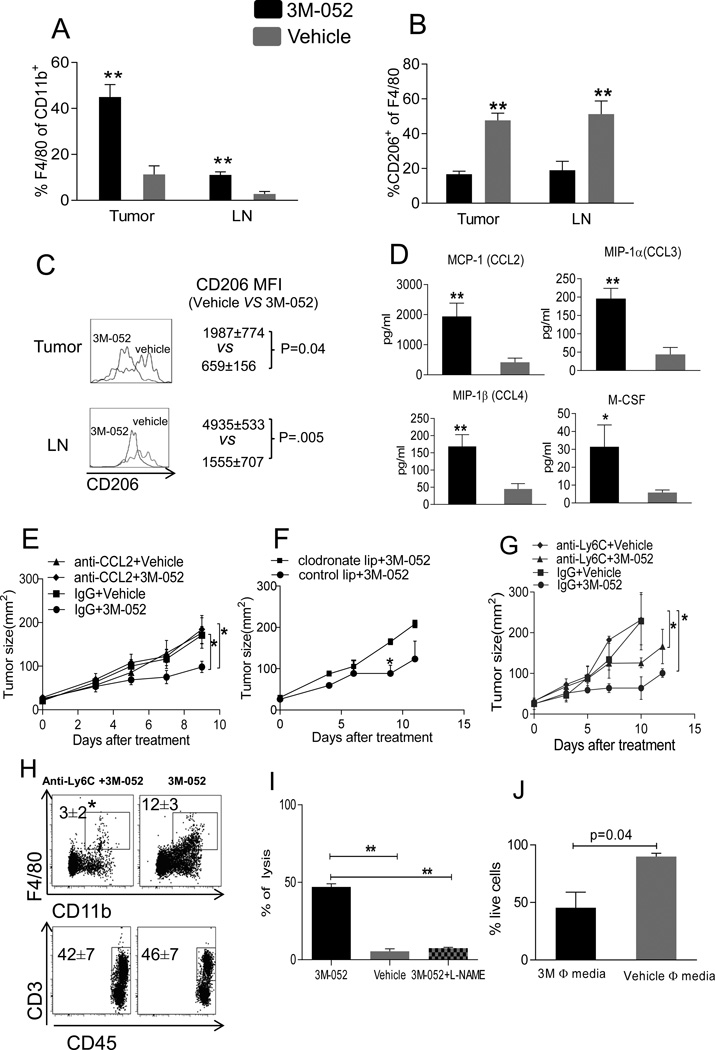
Suppression of B16.F10 and B16.Ova melanoma in Rag2 KO mice in response to 3M-052 indicate the importance of innate immune cells. The relative abundance of TAM 1 day after i.t. 3M-052 pointed to a possible role in the anti-tumor activity of i.t. 3M-052. Indeed, 3 days later we found more TAM (CD11b+F4/80+) in tumor and TDLN (Fig 5A). TAM can play a dual role in tumor development; M1 TAM can suppress and M2 TAM can promote tumor growth (13,17, 25). Using CD206 (mannose binding receptor), a definitive marker for M2 macrophages (26), we found that i.t. 3M-052 induced the accumulation of M1 over M2 macrophages in tumor and TDLN (Fig. 5B, C). Thus, 3M-052 treatment increases the M1/M2 TAM ratio in tumor, possibly implicating them in its mechanism of tumor control.
Fig. 5. CCL2 and macrophages mediate tumor suppression by 3M-052.
B16.F10 tumors were treated with 3M-052. (A) Total macrophages and (B, C) CD206 expression in macrophages (CD11b+F4/80+CD206+) were measured in tumor and TDLN after 3 days of treatment by flow cytometry. (D) CCL2, CCL3, CCL4 and M-CSF in tumor lysate after 1 day of treatment. (E) B16.F10 tumor growth after 3M-052 treatment and CCL2 neutralization. (F) B16.F10 tumor growth after 3M-052 treatment and macrophage depletion with clodronate liposomes. (G) B16.F10 tumor growth after 3M-052 treatment and Ly6C+ cells depletion. (H) Ly6C antibody treatment effect on macrophages and T cells in tumor; tumor were analyzed after 1 week of Ly6C antibody treatment for indicated cell types (I) 3M-052 or vehicle activated macrophages and B16.F10 tumor cells were co-cultured for 96 hrs in the presence or absence of L-NAME (J) B16.F10 tumor cells were cultured in the presence of 3M-052 or vehicle activated macrophage supernatant for 96 hrs. MTT assay was performed to evaluate tumor cell lysis. Tumor growth is plotted as mean ± SEM, n=5 in each group. Data is representative of at least 2 independent experiments. *P<0.05, **P<0.005(unpaired two-tailed t test).
Macrophage-related chemokines like CCL2/MCP-1, CCL3/MIP-1α, CCL4/MIP-1β and M-CSF were also highly upregulated in the tumor after 1 day of treatment (Fig 5D), with CCL2 being the most dramatically increased. Since we had found strong induction of the macrophage chemotactic chemokine CCL2 in 3M-052-injected tumors, we studied the relationship between CCL2 and TAM accumulation by systemically neutralizing CCL2 with CCL2-specific antibody. Mice bearing B16.F10 or B16.Ova tumors were treated with 3M-052 or vehicle with or without repeated anti-CCL2 treatments. Remarkably, CCL2 neutralization completely abolished the therapeutic effect of 3M-052 against B16.F10 or B16.Ova tumors (Fig. 5E & Fig. S1B) with reduced infiltration of TAM (data not shown). These data indicate that the anti-tumor activity of 3M-052 against B16.F10 was dependent on CCL2. To further confirm that TAM are responsible for tumor suppression, we depleted more than 80% of TAM in tumor-bearing mice by clodronate liposome and found that the anti-tumor effect of 3M-052 was lost (Fig. 5F). However, clodronate liposomes also appeared to deplete small fractions of other leukocytes (data not shown). To confirm the direct importance of TAM for the anti-tumor effect of 3M-052, we depleted the macrophage precursor subset (27), CD11bLy6Chi monocytes, which were enriched in tumor and TDLN after 3M-052 treatment (data not shown). Systemic administration of anti-Ly6C antibody completely abrogated therapeutic efficacy of 3M-052 (Fig. 5G), and greatly reduced the number of TAMs but not T cells (Fig. 5H).
3M-052 activated macrophages induce direct tumor killing via nitric oxide
We next analyzed whether macrophages could directly kill tumor cells in response to 3M-052 treatment. Since a major mechanism of tumor killing by macrophages is through production of large quantities of nitric oxide (28), we determined the involvement of nitric oxide in 3M-052-mediated tumor killing. B16.F10 tumor cells were co-cultured with 3M-052-activated splenic macrophages in the presence or absence of L-NAME (a nitric oxide synthase inhibitor). 3M-052 increased macrophage cytotoxicity against tumor cells, and this killing was completely abolished by addition of L-NAME (Fig 5I) indicating that nitric oxide from 3M-052 activated macrophages was a major mediator of direct tumor killing. Indeed, we found that culture supernatants from 3M-052 activated macrophages killed B16.F10 tumor cells (fig 5J).
3M-052 enhances anti-tumor activity of checkpoint blockade
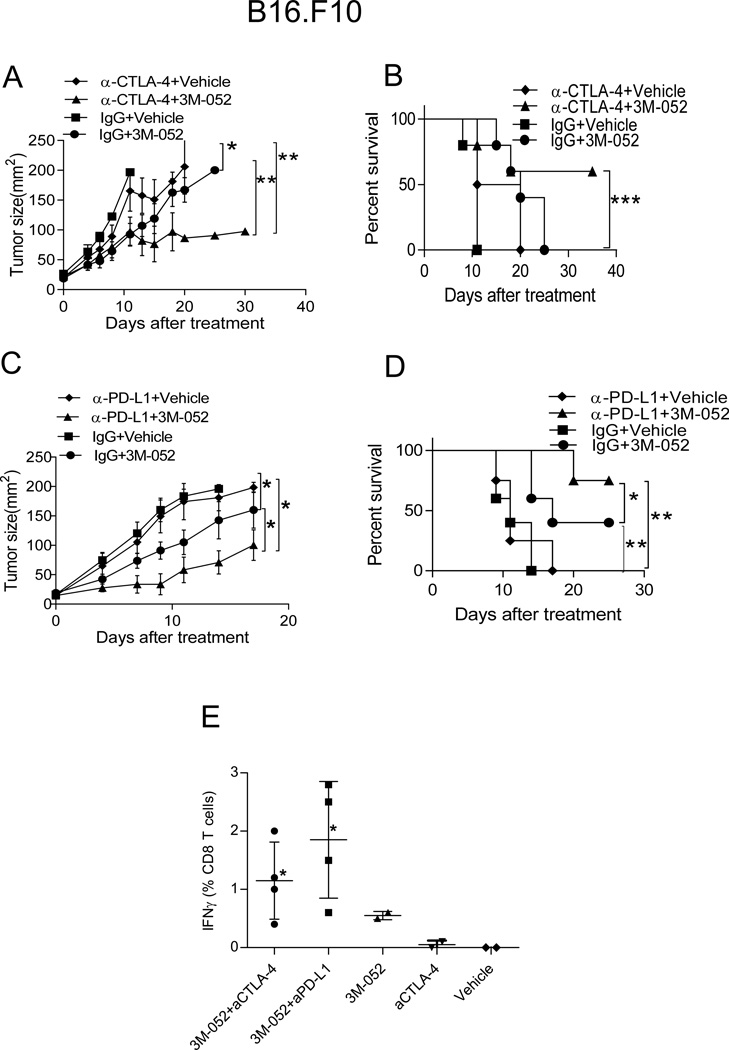
Anti-CTLA-4 and anti-PD-(L)1 blocking antibodies have shown impressive T cell-mediated clinical efficacy against human melanoma; yet a sizeable fraction of patients does no respond to these therapies (29, 30). It is possible that induction of tumor-specific CD8+ T cells, for example by intratumoral TLR ligation, could provide a better T cell substrate for checkpoint blockade to act on. To test this hypothesis, we combined 3M-052 therapy with checkpoint blockade in a setting of established, rapidly progressing wild-type B16.F10 melanoma where checkpoint blockade therapy is ineffective. Interestingly, addition of 3M-052 therapy converted therapeutic failure to PD-L1 or CTLA-4 blockade into tumor suppression that was stronger than suppression observed after 3M-052 monotherapy (Fig. 6 A and C), resulting in prolonged survival. (Fig 6B and D). Although 90% tumors that were treated with anti-CTLA-4 plus 3M-052 or anti-PD-L1 plus 3M-052 never reached a size of 200 mm2 during the observation period, they developed dry ulceration, requiring euthanasia. Next, we determined whether combination therapy is also superior for contralateral tumor growth suppression. Anti-CTLA-4 plus 3M-052 or anti-PD-L1 plus 3M-052 were more effective than anti-CTLA-4 or anti-PD-L1 alone in inhibiting the growth of the injected tumor. Triple combination of 3M-052, anti-CTLA-4 and anti-PD-L1 showed superior activity against injected (data not shown) and distant, uninjected tumors (Fig.S2A), and anti-tumor activity required CD8+ T cells (Fig.S2B). To evaluate systemic immunity, B16.Ova tumors were treated with 3M-052 alone or with combination therapy and PBMC were analyzed for the presence of tumor antigen-specific CD8+ T cells. Indeed, mice treated with 3M-052 plus anti-CTLA-4/anti-PD-L1 had more circulating Ova-specific IFN-γ+ CD8+ T cells than mice treated with either agent alone (Fig 6E). Together these data suggest that 3M-052 synergizes with checkpoint blockades in the T cell-mediated, systemic suppression of established B16.F10 melanoma.
Fig. 6. Synergic effect of 3M-052 plus checkpoint blockade on B16.F10 tumor growth.
Mice bearing subcutaneous B16.F10 tumors were treatedon day 0 and 4 with 35ug 3M-052 or vehicle i.t. and received 200 ug anti-CTLA-4 (A, B) and anti-PD-L1 (C, D) i.p. for every 4 days Graphs depict tumor growth (A, C) and mouse survival (B, D). Tumor growth is plotted as mean ± SEM, n=5 in each group. (E) after 21 days of indicated treatment; PBMC were isolated and cultured with OVA peptide for 4 hrs, surface and intracellular staining for CD8 T cells and IFN- γ were performed and cells were analyzed by flow cytometry. * p<0.05 vs. aCTLA-4. (all aPD-L1-treated mice were dead from tumor at this time). Data is representative of at least 2 independent experiments. *P<0.05, **P<0.005, ***P<0.005 (unpaired two-tailed t test).
Discussion
This is the first report on the anti-tumor activity and mechanism of action of 3M-052, a new dual TLR7/8 agonist designed to overcome the limitation of imiquimod (TLR7) and resiquimod (TLR7/8) agonists by forming a depot at the site of injection and preventing systemic immune activation and toxicity (18). We and others have previously shown that i.t. administration of the TLR9 agonist, CpG oligonucleotide, is more effective than systemic administration, due to the induction of local innate immune activation, resulting in systemic adaptive anti-tumor immunity (2, 31). Here, we found that i.t. 3M-052 exerts anti-tumor activity against the aggressive wild-type B16.F10 melanoma through a dual mechanism of local activation of innate immunity, primarily through TAMs, as well as systemic, adaptive immunity. We also found systemic suppression of uninjected, distant B16.F10 as well as B16.OVA tumors however this effect was more profound against B16.OVA which caused tumor rejection, likely due to the presence of the highly immunogenic model antigen, chicken egg ovalbumin. We suspect that 3M-052 will be more potent in activating immune cells from humans than from mice, since in mice, TLR8 does not signal in response to imidazoquinolines such as 3M-052. Since this non-functional TLR8 (and not the functional TLR7) is expressed on CD8α+ cDCs, the most efficient cross-primers of CD8+ T cells in mice (32), 3M-052 is unable to activate CD8α+ cDCs, and therefore cross-priming is inefficient unless the antigen is highly potent, such as OVA. However, BDCA3+ cDCs, the human counterpart of CD8α+ cDCs, do express TLR8 and respond to imidazoquinolines. We therefore speculate that 3M-052 will be more efficient at inducing cross-priming of tumor-specific CD8+ T cells in humans than in mice. The dual mechanism we here report of direct tumor killing by macrophage and T and B cell-dependent tumor killing after treatment with TLR7 and 7/8 agonists may help explain earlier controversies regarding the contributions of innate and adaptive immunity to the anti-melanoma activity of (res) imiquimod (8, 9, 33–35). Broomfield et al injected imiquimod into malignant mesothelioma tumors, and showed tumor suppression that included distant, uninjected tumors when anti-CD40 mAb was added to the regimen (36). These results, together with our data on the use of i.t. TLR7/8 agonist with anti-CTLA-4 and anti-PD-L1 checkpoint blockade, suggest several combination approaches to the treatment of metastatic cancer.
Nesbit et al. found that low concentrations of CCL2 recruit M2 TAMs and promote tumor growth while high concentrations of this chemokine mostly attract tumoricidal M1 TAMs (13). Likewise, we found that B16 tumor produced low levels of CCL2 and contained mostly M2 TAMs, while i.t. 3M-052 increased levels of CCL2 and M1 TAMs, and induced tumor destruction. 3M-052 also increased numbers of CD11b+Ly6Chi monocytes, which are known precursors for TAM; indeed their depletion strongly reduced TAMs and anti-tumor activity. We show for the first time, that intratumoral TLR7/8 agonist shifts the intratumoral M1/M2 macrophage ratio, and importantly, that these macrophages and their chemokine CCL2 are important for tumor control. M2 to M1 polarization of TAMs by 3M-052 holds promise for clinical application, since high M2/M1 TAM ratios are considered as a poor prognostic factor in multiple cancers including cutaneous and uveal melanoma and lung cancer (15, 37–40). In contrast, O'Sullivan et. al reported that even in the absence of adaptive immunity, M1 TAMs can still mediate immunoediting and regression of sarcomas (41), underlining the importance of TAMs as therapeutic targets. Since M2 TAM can also mediate resistance to other therapeutic modalities such as chemotherapy, the manipulation of the M1/M2 ratio in tumors with TLR agonists such as 3M-052 may hold promise beyond its direct anti-tumor activity (14, 42, 43).
It is interesting that 3M-052 converted non-responsiveness to PD-L1 or CTLA-4 checkpoint blockade into T cell-dependent responsiveness. This suggests that 3M-052 induces tumor-specific T cells, which then benefit from PD-L1 and CTLA-4 blockade (44). It will be interesting to see whether melanoma patients who do not respond to checkpoint blockade alone will benefit from the addition of intratumoral 3M-052 therapy.
Several reports show that TAM infiltration and tumor growth can be reduced by CCL2 inhibition (45–48). However, these reports evaluated the role of TAM in untreated tumors. We found that upon immunotherapy with 3M-052, M1 TAMs increased in the tumor, and these TAMs were critical for tumor control. Similarly, inactivation of CCL2 by TAM-derived free nitric oxide radicals severely inhibited the activity of T cell-based immunotherapy due to the dependency of tumoricidal CD8+ T cells on CCL2 to infiltrate the tumors. Therefore, before instituting anti-CCL2 therapy, it is critical to determine whether CCL2 plays a tumor-promoting or tumor-suppressing role, depending on the types of pre-existing or treatment-induced innate and adaptive immunity.
We were surprised to find that pDC and type I IFN were not indispensable for the anti-tumor activity of 3M-052, in contrast to previous studies on TLR7/8 agonists (8, 33, 34). This difference may be due to the difference between the widely used cream-based formulation which would primarily impact the skin, vs. our intratumorally injected and retained formulation, which mostly impacts the tumor tissue.
We found abundantly more TAMS than pDCs in B16 tumors both before and after therapy with 3M-052, possibly explaining the dominance of macrophages in the anti-tumor effect. Our results are in accordance with earlier findings where pulmonary infection with Newcastle disease virus (a natural TLR7 agonist) resulted in increased numbers of alveolar macrophages and cDCs but not pDCs. IFN-α production by pDCs was only initiated when alveolar macrophages were depleted suggesting a possible competition or cross-inhibition between macrophages and pDCs (49).
Clinically, the TLR7 agonist, imiquimod, is formulated as a cream and applied on the skin where it is partially absorbed. In contrast, 3M-052 is directly injected into the tumor, making dose comparisons difficult. In this study, 0.06 mg 3M-052 was injected, compared to 6.25 mg imiquimod gel topically applied in a previous mouse study (8). On a mg/kg basis, 3M-052 was used at ~6× greater amount than imiquimod is typically used in humans.
Consistent with previous report on imiquimod (8), NK cells were not involved in 3M-052 mediated tumor killing, however it has been demonstrated that NK cells are indispensable in CpG-mediated anti-tumor immunity (24) suggesting that TLR9 and TLR7/8 agonist suppress tumor growth by partially different immune mechanisms. B cells did contribute, raising the possibility of contribution of antibodies to the anti-tumor effect of 3M-052 (50).
We and others previously reported systemic induction of CD8+ T cell immunity after TLR9 agonist therapy (2, 31). This is important, since a major goal of i.t. therapy is systemic tumor regression including distant, uninjected lesions. Here we show that systemic CD8+ T cell immunity is also induced after TLR7/8 treatment, and this immunity is strong enough to reject distant, uninjected tumors. Tumor specific CD8+ T cells can have potent anti-tumor effects, however recent studies indicate that CD8+ T cells and IFN-γ induce a T cell-resistant environment within the tumor (51, 52). It will be interesting to see whether in such settings, macrophages or/and other innate immune cells activated by TLR agonists, including 3M-052, may be less affected by these mechanisms and could continue to kill tumor cells.
In summary, 3M-052 not only generated systemic tumor-specific CD8+ T cell immunity but also modified the tumor microenvironment from tumor-promoting to tumor-inhibiting by shifting the phenotype of intratumoral macrophages from a predominant M2 to M1 phenotype. M1 macrophages, T cells and B cells all contributed to suppression of tumor growth, which was further enhanced by combination with PD-L1 or CTLA-4 checkpoint blockade. The effective induction of both innate and adaptive immunity makes 3M-052 useful for the treatment of both poorly and strongly immunogenic tumors. Our findings suggest that local intratumoral treatment with immunomodulatory compounds such as 3M-052 is a promising approach for the treatment of metastatic cancer.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the National Institutes of Health (NIH) grants R01 1CA143077 (WWO), P01 CA128913 (PH/WWO) and The UT MDACC SPORE in Melanoma P50 CA093459 (PH)
Footnotes
Conflict of interest: John P Vasilakos is an employee of 3M and is a beneficiary of the 3M's employee stock plan
References
- 1.Hoffmann J, Akira S. Innate immunity. Current opinion in immunology. 2013;25:1–3. doi: 10.1016/j.coi.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Lou Y, Liu C, Lizee G, Peng W, Xu C, Ye Y, Rabinovich BA, Hailemichael Y, Gelbard A, Zhou D, Overwijk WW, Hwu P. Antitumor activity mediated by CpG: the route of administration is critical. Journal of immunotherapy. 2011;34:279–288. doi: 10.1097/CJI.0b013e31820d2a05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R. In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4324–4332. doi: 10.1200/JCO.2010.28.9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodmansee C, Pillow J, Skinner RB., Jr The role of topical immune response modifiers in skin cancer. Drugs. 2006;66:1657–1664. doi: 10.2165/00003495-200666130-00001. [DOI] [PubMed] [Google Scholar]
- 5.Alessi SS, Sanches JA, Oliveira WR, Messina MC, Pimentel ER, Festa Neto C. Treatment of cutaneous tumors with topical 5% imiquimod cream. Clinics (Sao Paulo) 2009;64:961–966. doi: 10.1590/S1807-59322009001000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garland SM, Sellors JW, Wikstrom A, Petersen CS, Aranda C, Aractingi S, Maw RD, Imiquimod Study G. Imiquimod 5% cream is a safe and effective self-applied treatment for anogenital warts--results of an open-label, multicentre Phase IIIB trial. International journal of STD & AIDS. 2001;12:722–729. doi: 10.1258/0956462011924218. [DOI] [PubMed] [Google Scholar]
- 7.Ooi T, Barnetson RS, Zhuang L, McKane S, Lee JH, Slade HB, Halliday GM. Imiquimod-induced regression of actinic keratosis is associated with infiltration by T lymphocytes and dendritic cells: a randomized controlled trial. The British journal of dermatology. 2006;154:72–78. doi: 10.1111/j.1365-2133.2005.06932.x. [DOI] [PubMed] [Google Scholar]
- 8.Drobits B, Holcmann M, Amberg N, Swiecki M, Grundtner R, Hammer M, Colonna M, Sibilia M. Imiquimod clears tumors in mice independent of adaptive immunity by converting pDCs into tumor-killing effector cells. The Journal of clinical investigation. 2012;122:575–585. doi: 10.1172/JCI61034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aranda F, Llopiz D, Diaz-Valdes N, Riezu-Boj JI, Bezunartea J, Ruiz M, Martinez M, Durantez M, Mansilla C, Prieto J, Lasarte JJ, Borras-Cuesta F, Sarobe P. Adjuvant combination and antigen targeting as a strategy to induce polyfunctional and high-avidity T-cell responses against poorly immunogenic tumors. Cancer research. 2011;71:3214–3224. doi: 10.1158/0008-5472.CAN-10-3259. [DOI] [PubMed] [Google Scholar]
- 10.Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W. The use of Toll-like receptor-7 agonist in the treatment of basal cell carcinoma: an overview. The British journal of dermatology. 2003;149(Suppl 66):53–56. doi: 10.1046/j.0366-077x.2003.05626.x. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 12.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature immunology. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 13.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. Journal of immunology. 2001;166:6483–6490. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 14.Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour-associated macrophages. Immunology. 2013;138:93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herwig MC, Bergstrom C, Wells JR, Holler T, Grossniklaus HE. M2/M1 ratio of tumor associated macrophages and PPAR-gamma expression in uveal melanomas with class 1 and class 2 molecular profiles. Experimental eye research. 2013;107:52–58. doi: 10.1016/j.exer.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heusinkveld M, van der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. Journal of translational medicine. 2011;9:216. doi: 10.1186/1479-5876-9-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2013 doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 18.Smirnov D, Schmidt JJ, Capecchi JT, Wightman PD. Vaccine adjuvant activity of 3M-052: an imidazoquinoline designed for local activity without systemic cytokine induction. Vaccine. 2011;29:5434–5442. doi: 10.1016/j.vaccine.2011.05.061. [DOI] [PubMed] [Google Scholar]
- 19.Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 20.Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr, You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nature genetics. 2009;41:544–552. doi: 10.1038/ng.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Applequist SE, Wallin RP, Ljunggren HG. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. International immunology. 2002;14:1065–1074. doi: 10.1093/intimm/dxf069. [DOI] [PubMed] [Google Scholar]
- 22.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature immunology. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 23.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. Journal of immunology. 2012;188:1592–1599. doi: 10.4049/jimmunol.1101304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Lou Y, Lizee G, Qin H, Liu S, Rabinovich B, Kim GJ, Wang YH, Ye Y, Sikora AG, Overwijk WW, Liu YJ, Wang G, Hwu P. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. The Journal of clinical investigation. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Liu L, Che G, Yu N, Dai F, You Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC cancer. 2010;10:112. doi: 10.1186/1471-2407-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ezekowitz RA, Gordon S. Alterations of surface properties by macrophage activation: expression of receptors for Fc and mannose-terminal glycoproteins and differentiation antigens. Contemporary topics in immunobiology. 1984;13:33–56. doi: 10.1007/978-1-4757-1445-6_2. [DOI] [PubMed] [Google Scholar]
- 27.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van den Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter JA. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer research. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 28.Rahat MA, Hemmerlein B. Macrophage-tumor cell interactions regulate the function of nitric oxide. Frontiers in physiology. 2013;4:144. doi: 10.3389/fphys.2013.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, Sanmamed MF, Melero I. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Current opinion in immunology. 2014 doi: 10.1016/j.coi.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Song W, Czerwinski DK, Varghese B, Uematsu S, Akira S, Krieg AM, Levy R. Lymphoma immunotherapy with CpG oligodeoxynucleotides requires TLR9 either in the host or in the tumor itself. Journal of immunology. 2007;179:2493–2500. doi: 10.4049/jimmunol.179.4.2493. [DOI] [PubMed] [Google Scholar]
- 32.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunological reviews. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 33.Inglefield JR, Dumitru CD, Alkan SS, Gibson SJ, Lipson KE, Tomai MA, Larson CJ, Vasilakos JP. TLR7 agonist 852A inhibition of tumor cell proliferation is dependent on plasmacytoid dendritic cells and type I IFN. J Interferon Cytokine Res. 2008;28:253–263. doi: 10.1089/jir.2007.0097. [DOI] [PubMed] [Google Scholar]
- 34.Kalb ML, Glaser A, Stary G, Koszik F, Stingl G. TRAIL(+) human plasmacytoid dendritic cells kill tumor cells in vitro: mechanisms of imiquimod- and IFN-alpha-mediated antitumor reactivity. Journal of immunology. 2012;188:1583–1591. doi: 10.4049/jimmunol.1102437. [DOI] [PubMed] [Google Scholar]
- 35.Prins RM, Craft N, Bruhn KW, Khan-Farooqi H, Koya RC, Stripecke R, Miller JF, Liau LM. The TLR-7 agonist, imiquimod, enhances dendritic cell survival and promotes tumor antigen-specific T cell priming: relation to central nervous system antitumor immunity. Journal of immunology. 2006;176:157–164. doi: 10.4049/jimmunol.176.1.157. [DOI] [PubMed] [Google Scholar]
- 36.Broomfield SA, van der Most RG, Prosser AC, Mahendran S, Tovey MG, Smyth MJ, Robinson BW, Currie AJ. Locally administered TLR7 agonists drive systemic antitumor immune responses that are enhanced by anti-CD40 immunotherapy. Journal of immunology. 2009;182:5217–5224. doi: 10.4049/jimmunol.0803826. [DOI] [PubMed] [Google Scholar]
- 37.Hansen BD, Schmidt H, von der Maase H, Sjoegren P, Agger R, Hokland M. Tumour-associated macrophages are related to progression in patients with metastatic melanoma following interleukin-2 based immunotherapy. Acta Oncol. 2006;45:400–405. doi: 10.1080/02841860500471798. [DOI] [PubMed] [Google Scholar]
- 38.Byrne SN, Knox MC, Halliday GM. TGFbeta is responsible for skin tumour infiltration by macrophages enabling the tumours to escape immune destruction. Immunol Cell Biol. 2008;86:92–97. doi: 10.1038/sj.icb.7100116. [DOI] [PubMed] [Google Scholar]
- 39.Bronkhorst IH, Jager MJ. Uveal melanoma: the inflammatory microenvironment. J Innate Immun. 2012;4:454–462. doi: 10.1159/000334576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clinical & experimental metastasis. 2013;30:393–405. doi: 10.1007/s10585-012-9545-6. [DOI] [PubMed] [Google Scholar]
- 41.O'Sullivan T, Saddawi-Konefka R, Vermi W, Koebel CM, Arthur C, White JM, Uppaluri R, Andrews DM, Ngiow SF, Teng MW, Smyth MJ, Schreiber RD, Bui JD. Cancer immunoediting by the innate immune system in the absence of adaptive immunity. The Journal of experimental medicine. 2012;209:1869–1882. doi: 10.1084/jem.20112738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fridlender ZG, Jassar A, Mishalian I, Wang LC, Kapoor V, Cheng G, Sun J, Singhal S, Levy L, Albelda SM. Using macrophage activation to augment immunotherapy of established tumours. British journal of cancer. 2013;108:1288–1297. doi: 10.1038/bjc.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Brahmer JR. PD-1-targeted immunotherapy: recent clinical findings. Clinical advances in hematology & oncology : H&O. 2012;10:674–675. [PubMed] [Google Scholar]
- 45.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. The Journal of investigative dermatology. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 46.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. Journal of neuro-oncology. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi O, Akira S. Innate immunity to virus infection. Immunological reviews. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DiLillo DJ, Yanaba K, Tedder TF. B cells are required for optimal CD4+ and CD8+ T cell tumor immunity: therapeutic B cell depletion enhances B16 melanoma growth in mice. Journal of immunology. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Science translational medicine. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. The Journal of experimental medicine. 2013;210:1389–1402. doi: 10.1084/jem.20130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.