Abstract
Introduction
Symptomatic hip osteoarthritis (OA) is a disabling condition with up to a 25% cumulative lifetime risk. Total hip arthroplasty (THA) is effective in relieving patients’ symptoms and improving function. It is, however, associated with substantial risk of complications, pain and major functional limitation before patients can return to full function. In contrast, hip arthroscopy (HA) is less invasive and can postpone THA. However, there is no evidence regarding the delay in the need for THA that patients would find acceptable to undergoing HA. Knowing patients’ values and preferences (VP) on this expected delay is critical when making recommendations regarding the advisability of HA. Furthermore, little is known on the optimal amount of information regarding interventions and outcomes needed to present in order to optimally elicit patients’ VP.
Methods and analysis
We will perform a multinational, structured interview-based survey of preference in delay time for THA among patients with non-advanced OA who failed to respond to conservative therapy. We will combine these interviews with a randomised trial addressing the optimal amount of information regarding the interventions and outcomes required to elicit preferences. Eligible patients will be randomly assigned (1 : 1) to either a short or a long format of health scenarios of THA and HA. We will determine each patient's VP using a trade-off and anticipated regret exercises. Our primary outcomes for the combined surveys will be: (1) the minimal delay time in the need for THA surgery that patients would find acceptable to undertaking HA, (2) patients’ satisfaction with the amount of information provided in the health scenarios used to elicit their VPs.
Ethics and dissemination
The protocol has been approved by the Hamilton Integrated Research Ethics Board (HIREB13-506). We will disseminate our study findings through peer-reviewed publications and conference presentations, and make them available to guideline makers issuing recommendations addressing HA and THA.
Keywords: Patients' values and preference, Total Hip Arthroplasty, Hip Arthroscopy, Patient Written Information, Decision Making
Background
Osteoarthritis and surgical options
Osteoarthritis
Osteoarthritis (OA) is the most common form of chronic arthritis. Approximately 15% of men and women suffer from symptomatic OA,1 representing a large burden on patients, the healthcare system and society. Symptomatic hip OA is a particularly disabling condition with a cumulative lifetime risk of up to 25%. Conservative management of hip OA includes exercise, weight reduction, physical therapy and medications focusing on relieving symptoms, improving joint function and optimising the quality of life.2 Pharmacological and non-pharmacological interventions for severe OA are, however, substantially less effective than surgical treatment.3 Consequently, most patients with severe hip OA eventually need total hip arthroplasty (THA).3
Total hip arthroplasty
With an ageing population increasingly interested in staying physically active,4 the frequency and cost of THA continues to grow. Currently, more than a half million THA procedures are performed annually in the UK and USA alone, and in 2010 the global market was estimated to be as high as US$4.7 billion. 5
After the failure of conservative treatment, THA is usually effective in relieving patients’ symptoms and improving function, with more than 95% prosthesis survivorship at 10-year follow-up and more than 80% survivorship at 25-year follow-up.6 7 However, THA is also a major procedure, associated with a substantial risk of complications, and with weeks of pain and major functional limitations before patients can return to full function. Therefore, patients and caregivers are interested in less invasive interventions that could postpone THA.
Hip arthroscopy
Less invasive interventions include arthroscopy, partial replacements and bone-preserving techniques. They have shown varying success rates among patients with OA.8 Hip arthroscopy (HA) is a new and also the fastest growing procedure within orthopaedic surgery. 8 Despite the lack of high-quality evidence, the number of HAs performed is expected to double in the USA in 2013 compared with 2011.9 HA is used to treat intra-articular pathology of the hip, including mild hip OA. Compared with THA, it has the advantages of being minimally invasive and having fewer complications.10 Compared with THA, arthroscopy may help patients achieve a higher level of function more quickly with, over the short term, less restriction on exercise. The expectation, however, is that patients’ underlying OA will progress and THA will ultimately become necessary. The question then arises: what delay in the need for THA would warrant a patient undergoing HA? This is a question of values and preferences.
Measuring patients values and preferences
There are a number of techniques available for eliciting patients’ direct choices of which the probabilistic version of the threshold technique, also called the probability trade-off exercise, is widely used.11 Following descriptive and probabilistic information regarding the benefits and harms associated with treatment choices—for example, treatment A and B—in which the relative benefits of treatment A versus B are large, the respondent is asked to choose one option. Typically, patients will choose treatment A. The interviewer then presents an alternative situation in which the relative benefits of A versus B are very small, and patients typically choose B. The interviewer then presents a small reduction in the probability of benefits, relative to the first scenario, for option A. If the patient continues to choose A, the next scenario presents a small increase in the benefits of A versus B relative to the second scenario. The process is repeated until the indifference point between A and B is established (ping-pong approach).12
Utility elicitation uses a very different approach, presenting health states and using one of a variety of techniques to elicit the respondent's rating of the value of the health state on a scale between death (typically 0) and full health (typically 1.0 or 100). The patients’ responses are used to build a decision model that calculates the treatment option that, given the patient's utilities, achieves the maximum utility-adjusted outcome.13 14
Complementary approaches to assess patients’ decision-making integrate emotional aspects of the process. One such approach focuses on regret, an aversive emotion people experience when they believe their current situation would have been better had they acted differently in the past.15 16 In theory, regret is influenced by intuitive, affect-based, and analytical, deliberative processes.17 18 Reflecting on the anticipated regret of particular decisions (eg, choosing A vs B in the example above) may alert people to the choice that would be most likely to avoid this aversive emotion.19 20 The anticipated regret theory-based approach preserves a rational decision-making framework, while allowing anticipation of the effect of the decision on emotions.21
Using both (direct choice) trade-off and anticipated regret exercises, our study will provide empirical evidence regarding the delay in the need for THA that patients would find acceptable to undergoing HA.
Amount of information presented to elicit patients’ values and preferences
The choices patients make are critically dependent on how the health scenario (HS) that characterises the processes and outcomes of the alternative management options (A and B in the above—THA and HA in the current project) is presented. Research in marketing has addressed some of the relevant issues. The information-processing framework22 suggests that that there are limits to the human ability to assimilate and process information, and that once these limits are surpassed, behaviour becomes confused and dysfunctional.23 Evidence suggests an inverted U-shaped relationship between information available and decision quality, in which individuals with too little or too much information made poorer decisions than those with an intermediate amount of information.24 25
Other indirect evidence comes from research on written consent forms.26 27 Individuals often skim over consent forms for clinical trials in oncology if they are longer than 1000 words or four pages.28 Twenty-seven oncology trials showed that patients obtained significantly higher objective knowledge when the consent form page count was seven or less.29
In the area of pharmaceutical product choice, participants have had a better understanding of shorter and easier information presentations.25 One might expect, however, that if the information becomes limited, the decision quality will deteriorate.
Patients’ values and preferences on OA surgical options
Given the existing evidence, both HA and THA represent reasonable choices for patients with non-advanced OA. The choice may, however, be challenging. On the one hand, HA is likely to achieve only transient improvement in function. On the other hand, the morbidity associated with THA is substantial.
Therefore, one of the key aspects in the choice between HA and THA is the duration of delay in the need for THA that patients may achieve with HA. If patients demand a delay time much greater than HA can realistically achieve, the procedure should seldom be considered. On the other hand, if patients would be satisfied with a much shorter delay time, the procedure should be frequently considered. There is currently no empirical evidence addressing patients’ values and preferences regarding the delay they would demand to undertake HA. Knowing typical patients’ values and preferences regarding this expected delay is likely to be helpful for patients and healthcare providers in the clinical encounter and for guideline panellists when making recommendations regarding the advisability of HA.
The assessment of patients’ values and preferences will be valid only to the extent patients receive sufficiently accurate information on the outcomes of available treatment options presented in ways that they can easily process. Thus far, only limited indirect evidence informs us on the optimal amount of information to provide in scenarios when eliciting patients’ preferences.
Our study will provide direct empirical evidence on the optimal amount of information to provide when eliciting patients’ values and preferences. It may also provide insight into the amount of information to provide in shared decision-making, although our study only indirectly addresses that issue.
Objectives
The purpose of this study is to improve the management of patients with non-advanced symptomatic hip OA who failed conservative treatment by determining their values and preferences regarding the choice between immediate THA versus HA.
In the Pilot stage of our study, we will assess the following feasibility issues: (1) recruitment rate; (2) length of time to conduct the interview and fill out all the study measurements; (3) potential personnel and data management issues.
In Study 1—our primary objective is to determine the minimal delay time in the need for THA surgery that patients would find acceptable to undertake HA (which we will refer to as the ‘delay time’). Secondary objectives include assessing patients’ anticipated regret if the delay would differ from their expectations, as well as potential determinants of their preference (eg, age, gender, educational level and socioeconomic status).
In Study 2—our objective is to assess the ease of understanding, optimal quantity of information and patients’ satisfaction regarding alternative formats of the HSs used to elicit their preferences.
Methods
Study design
In a pilot study, we will assess the following feasibility issues: (1) recruitment rate; (2) length of time to conduct the interview and fill out all the study measurements; (3) potential personnel and data management problems in a real-life setting. We will perform this study at the outpatient orthopaedic clinic of the McMaster University Medical Center (Hamilton, Ontario, Canada).
Study 1: We will perform a multinational, cross-sectional, interview-study to assess the delay in THA that patients would demand to choose HA.
Study 2: Within Study 1, we will conduct a randomised trial comparing a short version versus a long version of HA and THA HSs.
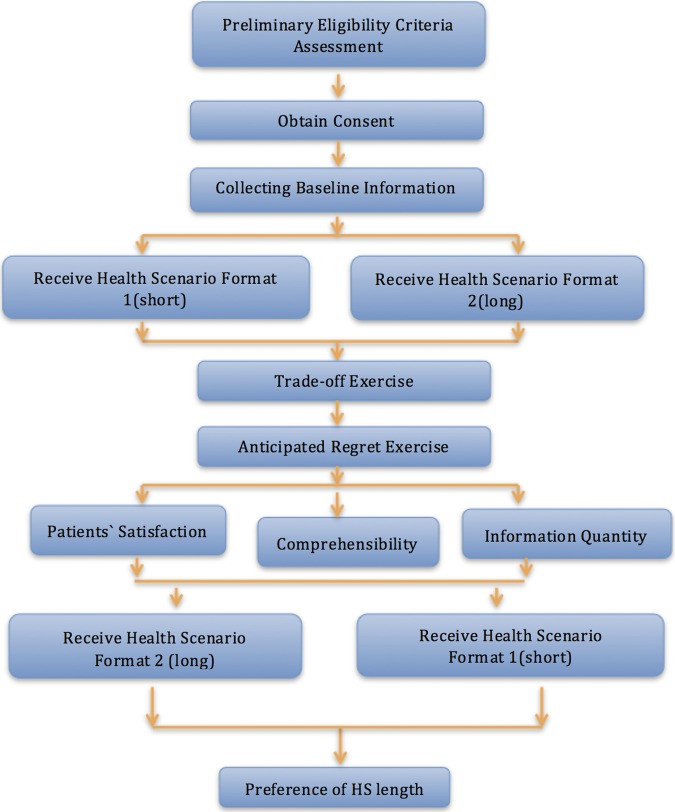
Figure 1 shows the study flow.
Figure 1.
Flow chart of study design (HS, health scenario).
Setting: The study will take place at McMaster University Medical Center, Hamilton, Canada; St. Michael’s Hospital, Toronto, Canada; Hospital de Sant Pau, Barcelona, Spain; and Sorocaba Hospitals, São Paulo, Brazil.
Study population
The population of interest consists of adults diagnosed with non-advanced hip OA. Table 1 presents the detailed inclusion and exclusion criteria.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| (1) Patient is at least 40 years old | (1) Patient has a history of prior hip surgery |
| (2) Patient diagnosed by X-ray or MRI with mild or moderate (grades 1 and 2) OA based on the Tonnis classification of OA51 Grade 0: no signs of OA Grade 1: mild: increased sclerosis, slight narrowing of the joint space, no or slight loss of head sphericity Grade 2: moderate: small cysts, moderate narrowing of the joint space, moderate loss of head sphericity Grade 3: severe: large cysts, severe narrowing of obliteration of the joint space, severe deformity of the head |
(2) Patient is unable to complete the research tasks due to cognitive impairment or language barriers |
| (3) Patient has a history of failed conservative management | (3) Patient is unwilling or unable to provide informed consent |
| (4) Patient provides a written informed consent |
OA, osteoarthritis.
Recruitment strategy
We will prospectively identify consecutive patients confirmed with non-advanced hip OA referred for consideration of HA. The orthopaedic surgeon will send a letter in advance of their visit to inform patients about our research project and the possibility of being approached by our research assistant (RA) for this study. The RA will then make initial contact with all the patients by phone to explain the purpose of the study. When the patients come to the orthopaedic clinic, we will ask them for their written informed consent.
Participants’ interview
Baseline information
We will document patients’ age, gender, ethnicity, educational level (not completed high school; completed high school only; some college/university; completed college or university), yearly income, and their impression of the experience of close relatives or friends who have undergone HA or THA (categorised as extremely dissatisfied; dissatisfied; neutral; satisfied; extremely satisfied, or differing across individuals).
Health scenarios
The HSs are designed to inform patients of the surgical options. On the basis of the available evidence,30 we will include the following five sections in the HSs for THA and HA26: (1) brief introduction to the surgery31; (2) description of the surgical procedure; (3) postoperative recovery and rehabilitation32; (4) expected benefits; (5) risks and potential complications (see online supplementary appendix: Script #1: Health scenarios).
The short versions have approximately 850 words and the long versions approximately twice the number of words; both versions use the same subheadings.
To ensure that we present accurate estimates of the benefits and risks of THA and HA to patients,33 conveyed in the most simple and easy-to-understand way possible, we applied a rigorous process to develop these HSs.
First, we performed a search on PubMed to retrieve relevant content from systematic reviews, randomised control trials (RCT) and observational studies. Evidence from systematic reviews was preferred if available.
Second, we reviewed THA booklets from the Brant Community Healthcare System, Hamilton Health Sciences, Joseph Brant Memorial Hospital, Niagara Health System, St. Joseph's Healthcare Hamilton, National Institute of Arthritis and Musculoskeletal, and Skin Diseases (NIAMS) to inform SCENARIO design and content. We also reviewed information from other sources such as the Informed Medical Decisions Foundations (IMDF)34 and National Institute of Health for both THA and HA HSs (when available).
Third, we considered the following strategies to increase the ease of understanding and readability of our scenarios.35 We focused the material on key concepts with consistent and simple words aiming for 1–2 syllables.32 36 A clear topic sentence was used at the beginning of each subheading with the following details and examples.37 We used a conversational style from the second person point of view (ie, ‘you’).37 We also used the Flesch-Kincaid Grade Level test in Microsoft Word 2011 to ensure that the English was understandable for people with a grade 10 level education.
Finally, we revised our scenarios based on feedback from 15 orthopaedic surgeons (8 of them commented on THA, and 7 of them commented on HA); from two focus groups (3 patients in each group) and four individual interviews with a total of 10 patients (5 for each surgery) who had undergone THA or HA; and five physiotherapists.
For the Spanish and Portuguese part of the study, an experienced medical translator will undertake the initial translation. In each language, one clinical epidemiologist and one orthopaedic surgeon, native in the non-English language and fluent in English, will check the translation and discuss potential revisions with the translator. After we obtain the Spanish and Portuguese versions, back translations will be performed and checked by the epidemiologist and the orthopaedic surgeon, with further revisions to the Spanish and Portuguese versions if necessary.
Randomisation of the HSs
Participants will be randomised to receive the short or long format of the scenarios in coded packages that the interviewer will open at the start of the interview. We will perform central randomisation using a computer-generated randomised system at McMaster University with an allocation ratio of 1:1 and random blocks size (2,4,8).
We will ask participants to read hard copies of the corresponding HSs (short or long). At the end of the interview—that is, after the trade-off exercise, anticipated regret exercise and a check for consistency and understanding that we will describe subsequently—the RA will show patients in each group the version they have not yet seen and ask about their preferred format. If participants have more content questions regarding the scenarios, the RA will instruct the patient to ask the orthopaedic surgeon for further assistance in the patient–doctor consultation after the interview.
Trade-off exercise
After participants have read the initial HS (short or long version), we will assess the minimum acceptable delay (delay time) in THA that patients would find acceptable to undergo HA. We will use the following generic questions: “By how much longer should the arthroscopy postpone the need for hip replacement surgery for you to consider the hip arthroscopy worthwhile? Would you choose hip arthroscopy if it would delay the need for total hip replacement by [delay time in months/year]?” We will offer a range of delay times, alternating between short and long times in a ping-pong strategy, for example, 3 months—12 years—6 months—10 years, etc. We will progressively narrow the range of the alternatives offered as we repeat the exercise.
The lower bound of delay time offered (ie, 3 months) is just below the anticipated least stringent participants’ demand and also corresponds to the shortest follow-up time in studies that evaluate the efficacy of HA.38 For the upper bound initially offered, the literature suggests that the most optimistic estimate of the time by which HA may delay THA is approximately 10 years.39 If patients are not satisfied with the upper boundary of the delay time—that is, they would demand a delay of more than 12 years before they would undergo HA—there will be provision for them to express this preference.
Anticipated regret exercise
Following the trade-off exercise, we will assess participants’ anticipated regret associated with choosing or not choosing a treatment alternative. We will measure anticipated regret using a 100 mm visual analogue scale (VAS) called the feeling thermometer,40 anchored at no regret (0) to maximum regret (100; figure 2 anticipated regret VAS).
Figure 2.
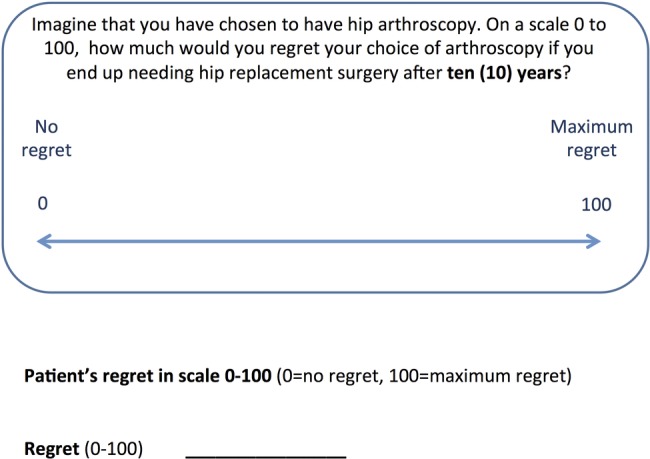
Anticipated regret visual analogue scale.
We will assess anticipated regret at five different time points (the patient personal threshold determined during the trade-off exercise, as well as two shorter and two longer options). For example, if the patient chose 2 years as their shortest delay time, we would ask her: “How much regret would you feel about choosing HA if you need to have a total hip replacement surgery after 12 months/1.5 years/2 years/3 years/4 years?” This process allows us to check for inconsistent answers (see below).
Blinding
Since this is a patient educational trial, the interviewers (data collectors) cannot be blinded. The orthopaedic surgeons, patients (outcome assessors) and data analysts will be blinded to the sequence of giving HSs.
Outcomes
Our primary outcome measures for the pilot stage regarding feasibility issues are the recruitment rate, as well as the length of time to conduct the interview and fill out all the outcome measurements. We will explore the potential personnel and data management problems in the McMaster Medical Center to ensure the quality of the definitive stage of our study. We will note the number of participants enrolled each week. The mean and SE of the centre’s recruitment rate over the recruitment period will be our study recruitment rate. We will also calculate the percentage of eligible patients who agree to participate. We will time the length of the interview, as well as the length of finishing interviewers’ administrated or patients’ administrated questions.
We will consider recruitment feasible for a large study if we will be able to recruit two patients at McMaster Medical Center per week (ie, 100 participants over 50 weeks). We will consider the pilot stage (approximately 2 months) to be successful, and a large multicentre RCT to be feasible if: (1) we successfully recruit 20% of the patients according to our estimated sample size in 2 months; (2) we will be able to finish the interview and all the outcome assessments in approximately 1 h (see table 2 for outcomes and corresponding objectives).
Table 2.
Summary of analysis plan
| Study | Objectives | Outcomes | Predictors | Hypothesis | Outcome measure | Methods of analysis |
|---|---|---|---|---|---|---|
| Pilot stage | ||||||
| Determine feasibility | (A) Recruitment rate | 2 Participants/week | Participants per week | |||
| (B) Time to conduct the interview and finish all the measurements | 1 h would be optimal | Interview duration | ||||
| (C) Patients’ attrition | Less than 5% | Patients’ attrition rate | ||||
| Study 1: interview study |
Primary | (A) Delay time | Age, gender, ethnicity, educational level, social economics status and medical history | Trade-off exercise | Normally distributed: mean delay time +SD; mean delay time and CI If data are skewed: mode, median and IQR |
|
| Secondary | (A) Patients’ anticipated regret scores | 100 mm visual analogue scale | t test | |||
| Study 2: RCT |
Primary | (A) Patients’ satisfaction on the HSs | Higher satisfaction on the short version | 7-point Likert-type scale | t test | |
| Secondary | (A) Understandability | Both have rated as 5/7 | 7-point Likert-type scale | t test | ||
| (B) Information quantity | Short will be rated at 4; long will be rated at 5 | 7-point Likert-type scale | t test | |||
| (C) Patients’ preference on the length of format | Prefer the short version | 7-point Likert-type scale | t test or Mann-Whitney U test | |||
| Sensitivity analyses | Patients’ satisfaction on the HSs | Higher satisfaction on the short version | 7-point Likert-type scale | Mann-Whitney U test | ||
| Comprehensibility | Both have 5/7 | 7-point Likert-type scale | Mann-Whitney U test | |||
| Information quantity | Short will be 4; long will be 5 | 7-point Likert-type scale | Mann-Whitney U test | |||
| Patients’ preference on the length of format | Prefer the short version | 7-point Likert-type scale | Mann-Whitney U test |
HS, health scenario; RCT, randomised controlled trial.
We will modify our protocols in response to limitations with respect to excessive length of the interview, difficulties with comprehension or ambiguities in the questions, and personnel or data management problems identified in the pilot.
For Study 1, our outcomes are:
Primary outcome: the minimal delay in the need for THA surgery that patients would find acceptable to undertake HA (which we will refer to as the ‘delay time’).
Secondary outcomes:
Independent predictors of the primary outcome include age, gender, educational level, socioeconomic status and family/friends’ experiences with previous THA and/or HA.
Patients’ anticipated regret scores on a 100 mm VAS at five different time points (the one patients chose in the trade-off exercise, and two shorter and two longer options).
For Study 2, our outcomes are:
Primary outcome: patients’ satisfaction on the scenarios after reading the initial scenarios. Interviewers will determine the degree of satisfaction participants place in the scenarios using a seven-point Likert-type scale with response options: completely dissatisfied, mostly dissatisfied, somewhat dissatisfied, neither satisfied nor dissatisfied, somewhat satisfied, mostly satisfied, completely satisfied.
Secondary outcomes:
Ease of understanding: we will assess participants' impression of understanding of each scenario using a seven-point Likert-type scale with response options: extremely hard, very hard, hard, not easy not hard, easy, very easy, extremely easy.
Information quantity: we will ask participants to rate the quantity of the information displayed in the initial presented scenario by a seven-point Likert-type scale with response options: much too little, somewhat too little, slightly too little, about right amount of information, slightly too much, somewhat too much, much too much.
Patients’ preference on length of format: after patients finish reading both the long and short versions of scenarios, we will ask them about their preference for the short or long version, using a seven-point Likert-type scale with response options: short version much better, short version somewhat better, short version little better, no preference, long version little better, long version somewhat better, long version much better.
Data collection
A trained interviewer will collect all the outcomes by completing the case-report forms at the end of the interview. No follow-up and further data collection will be involved.
Sample size calculation
Study 1
Owing to the paucity of similar studies in the literature, we are unable to estimate the SE of delay time precisely. If the data are normally distributed, 99.7% of the area under the normal distribution curve lies within 3 SDs.41 We assume that the range of delay time (12 years) will be normally distributed. Therefore, we anticipate an SD of approximately 2 years. We developed the sample size estimation table using the SD and varying the CI around the mean to obtain a sample size using the formula below42 (table 3).
Table 3.
Sample size estimation tables
| Study | α |
SD | (width of CI, years) | Sample size | |
|---|---|---|---|---|---|
| Study 1 | 0.05 | 2 | 0.5 | 246 | |
| 1 | 62 | ||||
| 2 | 16 | ||||
| Study | α | β | SD | Difference | Sample size (per arm) |
|---|---|---|---|---|---|
| Study 2 | 0.05 | 0.8 | 1 | 0.5 | 62 |
| 1 | 16 | ||||
| 2 | 4 |
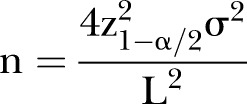 |
N represents the sample size, σ represents the SE, and L represents the CI around the mean.
At the end of the pilot stage, we will calculate the SE of delay time in the 20 patients as a reference point to modify our earlier sample size estimation for the definite study.
Study 2
Based on Cohen’s rule of sums,43 we used ‘SD=0.5’ to calculate the sample size to achieve a medium effect size. With a sample size of 62 in each group, the trial is powered to detect a medium effect size of mean=0.5 or a larger given 80% power level and α=0.05 in a two-sided test. Considering that the result will be obtained immediately after the assessment and all outcomes will be interviewer administrated, we anticipate no loss to follow-up. We also made a sample size estimation table with different CIs around the mean (table 3). Sample size calculation is performed using SPSS (Statistical Package for the Social Sciences) V.21.0 for Windows.
After finishing the pilot stage of our study, we will compare the estimated sample size for studies 1 and 2 and take the larger number as our final sample size for the combined studies.
Data analysis and interpretation
Study 1
Description of baseline characteristics
We will present patients’ age, gender, ethnical/cultural group, educational level, socioeconomic status and medical history.44 Means and SDs will be used to present continuous variables and a two-tailed t test (or Mann-Whitney U test for non-normal distributions) to detect significant differences (p<0.05) between group means. We will use proportions and frequency tables to present categorical variables and a two-tailed Fisher's Exact test will be used to detect statistically significant (p<0.05) differences between two groups.
Primary and secondary outcome(s)
We will assess the distribution of the mean delay time and represent it graphically using histogram(s). If the data are normally distributed, we will present the mean delay time and SD. We will also estimate 95% CIs of the mean. If the data are skewed, we will present the mode, median and IQR.
Multiple variable linear regressions will be undertaken to determine statistically independent predictors of the threshold of delay time. In this analysis, the delay time will be the dependent variable and the independent variables will be the previous experience of THA and HA in friends and family, age, gender, socioeconomic status and educational level.
After presenting the HSs and recording participants’ response with both ‘trade-off’ and anticipated regret exercise, we will compare results between these two measurements of participants’ values and preferences.
We have defined three possible patterns of inconsistent response (see table 4 Inconsistency checking). If the participants’ answers fall into any of these patterns, the interviewers will review the participants’ original answers without, however, implying that they must modify their original choices. If participants confirm their original answers, interviewers will determine and record the reasons for the participants’ inconsistent choices based on the participants’ explanation. If patients, following the review of the relation between their trade-off and regret choices, desire to modify their chosen delay time, interviewers will repeat the trade-off exercise.
Table 4.
Inconsistency checking
| Definitions/criteria of inconsistencies | Explanations and examples |
|---|---|
| (1) Participants anticipate that the regret score is higher when delay in need for THA is longer than it is at their threshold of delay time | In the example we give that measures anticipated regret scores: we set the 5 time points as A (12 months), B (1.5 years), C (2 years), D (3 years) and E (4 years). The participant chose 2 years as the shortest delay time at which he/she can accept for processing HA. Then they placed scores 60 to represent their regret on VAS at 12 months but scores 90 to represent his/her regret at 1.5 years. In other words, the regret scores ‘r’ on VAS show: rA<rB OR rB<rC OR rC<rD OR rD<rE |
| (2) Participants anticipate substantial regret, although HA would delay THA longer than their threshold of delay time | We define substantial as the anticipated regret score on VAS at the time point that they chose in the ‘trade-off’ exercise, or any longer delay time point is bigger than (30) on the 100 VAS scale The participant chose 2 years as the shortest delay time at which they can accept for processing HA. Then they still placed scores 60 to represent their regret on VAS at 2, 3 or 4 years In other words, the regret scores ‘r’ on VAS show: rC>0 OR rD>0 OR rE>0 |
| (iii) Patients do not anticipate any regret when delay in THA ends up being shorter than what their threshold of delay time | Compared to the time point that participants chose in the ‘trade-off’ exercise, the anticipated regret score on VAS at any shorter delay time point is equal to (0) For example, the participant chose 2 years as the shortest delay time at which they can accept for processing HA. Then they place scores 0 to represent their regret on VAS at 12 months and 1.5 years In other words, the regret scores ‘r’ on VAS show: rA=0 OR rB=0 |
HA, hip arthroscopy; THA, total hip arthroplasty; VAS, visual analogue scale.
For the analyses above, we will determine whether the delay time differs between those with an apparently high level of understanding and those who demonstrate any of the inconsistencies depicted in table 4. If we find an important discrepancy between the results of patients categorised as understanding and not understanding, we will focus our primary analysis on the group of patients who apparently have a high level of understanding.
Study 2
Baseline characteristics description
We will summarise patients’ age, gender, ethnical/cultural group, educational level and social economics status in a table.
Primary and secondary outcome(s)
Our primary outcome will be participants’ satisfaction of the HSs assessed by a seven-point Likert scale. We will also visualise it by using histogram(s). We will conduct a two-sided Student t test will to compare mean satisfaction scores and ease of understanding between the short and long scenarios. We will also calculate the mean difference and 95% CIs.
For information quantity, we will present a histogram depicting the proportion of participants’ choice in each category. We will apply two approaches to analyse information quantity at the first assessment. First, we will determine if the distribution between the two groups differs by greater than chance with a two-sided Student t test (if it is normally distributed) or a Mann-Whitney U test (if it is not normally distributed). Second, using a χ2 test, we will determine if the proportions of participants who choose ‘about the right amount of information’, in comparison to those who choose other response options, differ between groups.
We will use two approaches to compare participants’ preferences for the short versus long formats after showing patients both scenarios. First, we will treat the outcomes on the seven-point scale as multinomial ordered outcomes. We will analyse the result using the Mann-Whitney U test. Second, we will use a more conservative approach and compare the proportions of participants who prefer the short format to the proportion of participants who have either no preference or prefer the long format by using a χ2 test (table 2: Summary of analysis plan).
Ethical and Dissemination
This study will be performed in accordance with established guidelines for research involving human patients. The proposed study does not pose any safety risks to participating patients. The research objectives and study intervention will be explained to the patient verbally and in writing in easily comprehensible language. Written informed consent will be obtained from all patients. Patients will be informed of their right to ask for further information at any time and to withdraw from the study without prejudice to their future care. In the unlikely event that participants find considering the above scenarios upsetting, the interview will be immediately stopped and support offered. We will ensure confidentiality of patient data by anonymising patients by a unique numerical identifier. Records will be stored in a secure database. Access to the database will be restricted to those directly involved in the design, implementation and analysis of the data. No patient will be identifiable in any publication arising from the study.
The reporting of Study 1 will conform to the STROBE statement,45 and that of Study 2 to the CONSORT statement.46 We will disseminate our study findings widely through peer-reviewed publications and conference presentations, and make them available to guideline makers issuing recommendations on HA and THA.
Discussion
Strengths and weaknesses
The design of our study has several strengths. First, we have incorporated the anticipated regret model as a new method in the exploration of patients’ values and preferences. Based on considerable previously published theoretical works by members of our research team,47 48 our study will be the first to evaluate and compare its results with other methodologies. The comparison will include differences in decisions, inconsistencies and understanding.
Second, in developing HSs, we obtained input from patients who have undergone both total hip replacement and HA and from surgeons who have expertise on total hip replacement and HA. These processes ensured the accuracy of our HSs that will be used in the study.
Third, we will be the first to explore the association between influence from family and friends’ previous experience on patients’ values and preferences on the minimal delay time in the need for THA surgery that patients would find acceptable to undertake HA. Indirect evidence suggests that friends or family members’ medical advice may influence patients’ preference on medical decisions.49 Men, African-American men in particular, are more inclined to discuss their medication concerns and to seek medical advice from trusted friends more frequently than women.50 Women are more often inclined to solicit medical advice from their family members. Identifying the factors that may influence patients’ preferences could provide valuable explanations for the variation on patients’ values and preferences in future research. Knowing patients’ previous perception on certain treatment options can help clinicians to explain certain things more clearly and makes clinical consultation more efficient.
Fourth, we will check for consistencies in the minimal delay patients with HA find THA surgery acceptable. If participants have discrepant answers between the trade-off and anticipated regret exercise, we will provide them the opportunity to change their responses. Interviewers will test patients’ understanding of the information presented using standardised questions and rate respondents’ understanding based on their judgement. This ensures the validity of patients’ values and preference elicitation.
Our study plan also has limitations. There are conceptual limitations to the anticipated regret exercise. For instance, no one has studied the relationship between anticipated regret and actual regret subsequently experienced. If we find important discrepancies between anticipated regret and the trade-off exercise, the interpretation may be challenging; in particular, it may remain uncertain which method better represents patients’ real preference. Subsequent studies may be needed to address such questions arising from our results.
Implications
Although there is increasing awareness regarding shared decision-making and patient centred care, the explicit consideration of values and preferences in the care of individual patients and in the recommendations made by clinical practice guidelines remains limited.31–36
Given the existing evidence, the choice between HA and THA for patients with non-advanced OA is challenging. The research outlined in this protocol will provide explicit, quantitative expressions of patients’ valuations of their expected delay of HA on THA. This information will alert clinicians to this issue and may provide guidance in their interactions with patients. It will certainly provide crucial information for guideline developers making recommendations for clinical practice. Identifying the factors that may influence patients’ preferences could provide insight into variations in the broader perspective of patients’ values and preferences in future research.
Our protocol also addresses some of the limitations of the previous studies in the field of written medical information regarding using an adequate amount of information in patients’ values and preference assessment. Results will have implications for clinical practice in terms of providing patients with the right amount of information in the shared decision-making process.
Supplementary Material
Acknowledgments
The authors would like to thank Matti Seppänen, Teppo Järvinen, Daniel Nightingale, Darryl Yardley, Gloria Preston and Colleen Cupido for their feedbacks on the Health Scenarios. They would also like to thank Qi Zhou (Department of Clinical Epidemiology and Biostatistics, McMaster University) for her help on planning the statistical analyses and Peggy Austin, CCRA for her help on operating the study.
Footnotes
Contributors: YZ, KAOT, TA and GHG designed the study. YZ drafted the manuscript, with substantial inputs from TA, KAOT and GHG. DY, AT and KAOT drafted the trade-off exercise and regret exercises. BD and GHG provided feedback on the regret exercises. MI and YZ drafted the HSs. PA, KAOT and YZ conducted the interview to test HSs. All authors contributed to the refinement of the study protocol and approved the final manuscript. GHG is the principal investigator of the study.
Competing interests: None.
Ethics approval: The protocol has been approved by the Hamilton Integrated Research Ethics Board (HIREB13–506).
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646–56 [PMC free article] [PubMed] [Google Scholar]
- 2.American College of Rheumatology Subcommittee on Osteoarthritis Guidelines. Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum 2000;43:1905–15 [DOI] [PubMed] [Google Scholar]
- 3.Brandt KD. Non-surgical treatment of osteoarthritis: a half century of “advances”. Ann Rheum Dis 2004;63:117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rath E, Tsvieli O, Levy O. Hip arthroscopy: an emerging technique and indications. Isr Med Assoc J 2012;14:170–4 [PubMed] [Google Scholar]
- 5.Chen A, Gupte C, Akhtar K, et al. The global economic cost of osteoarthritis: how the UK compares. Arthritis 2012;2012:698709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5 [DOI] [PubMed] [Google Scholar]
- 7.National Joint registry: 7th annual report for England and Wales 2010. http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf
- 8.Kenney NA, Farmer KW. Minimally invasive versus conventional joint arthroplasty. PM R 2012;4(5 Suppl):S134–40 [DOI] [PubMed] [Google Scholar]
- 9.Dean KM. Hip arthroscopy: update, advances, and future applications. AAOS Now, 2011:5 [Google Scholar]
- 10.Griffiths EJ, Khanduja V. Hip arthroscopy: evolution, current practice and future developments. Int Orthop 2012;36:1115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llewellyn-Thomas HA. Threshold technique. In: Kattan MW, ed. Encyclopedia of medical decision making. Thousand Oaks, CA: Sage Publications,2009:4 [Google Scholar]
- 12.Llewellyn-Thomas HA, Williams JI, Levy L, Naylor CD, et al. Using a trade-off technique to assess patients’ treatment preferences for benign prostatic hyperplasia. Med Decis Making 1996;16:262–82 [DOI] [PubMed] [Google Scholar]
- 13.Ubel PA, Loewenstein G. The role of decision analysis in informed consent: choosing between intuition and systematicity. Soc Sci Med 1997;44:647–56 [DOI] [PubMed] [Google Scholar]
- 14.George WT. Social preferences for health states: an empirical evaluation of three measurement techniques. Socio Econ Planning Sci 1976;10:36 [Google Scholar]
- 15.Zeelenberg M. The use of crying over spilled milk: a note on the rationality and functionality of regret. Philos Psychol 1999;12:325–40 [Google Scholar]
- 16.Zeelenberg M. Anticipated regret, expected feedback and behavioral decision making. J Behav Decis Making 1999;12:93–106 [Google Scholar]
- 17.Daniel K. Maps of bounded rationality: psychology for behavioral economics. Am Econ Rev 2003;(93):1449–75 [Google Scholar]
- 18.Jonathan SBTE. Hypothetical thinking: dual processes in reasoning and judgement. New York: Psychology Press, 2007 [Google Scholar]
- 19.Itamar S. The influence of anticipating regret and responsibility on purchase decisions. J Consum Res 1992;19:105–18 [Google Scholar]
- 20.Zeelenberg MPR. A theory of regret regulation 1·0. J Consum Psychol 2007;17:3–18 [Google Scholar]
- 21.Tsalatsanis A, Hozo I, Vickers A, et al. A regret theory approach to decision curve analysis: a novel method for eliciting decision makers’ preferences and decision-making. BMC Med Inform Decis Mak 2010;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bettman J. Information processing models of consumer behavior. J Mark Res 1970;7:6 [Google Scholar]
- 23.Jacoby J, Speller DE, Kohn CA. Brand Choice Behavior As a Function of Information Load: Study Ii (Abstract). In: NA - Advances in Consumer Research Volume 01, eds. Scott Ward and Peter Wright, Ann Abor, MI: Association for Consumer Research, pp 381–3. [Google Scholar]
- 24.Hahn M, Lawson R, Lee YG. The effects of time pressure and information load on decision quality. Psychol Mark 1992;9:365–78 [Google Scholar]
- 25.Young DR, Hooker DT, Freeberg FE. Informed consent documents: increasing comprehension by reducing reading level. IRB 1990;12:1–5 [PubMed] [Google Scholar]
- 26.Comprehensive Working Group on Informed Consent in Cancer Clinical Trials for the National Cancer Institute: Recommendations for the Development of Informed Consent Documents for Cancer Clinical Trials. 1998 [cited 9 April 2013]
- 27.OHRP Informed Consent Frequently Asked Questions. 2008. [cited 9 April 2013]; http://www.hhs.gov/ohrp/informconsfaq.html
- 28.Sharp SM. Consent documents for oncology trials: does anybody read these things? Am J Clin Oncol 2004;27:570–5 [DOI] [PubMed] [Google Scholar]
- 29.Beardsley E, Jefford M, Mileshkin L. Longer consent forms for clinical trials compromise patient understanding: so why are they lengthening? J Clin Oncol 2007;25:e13–14 [DOI] [PubMed] [Google Scholar]
- 30.Soever LJ, Mackay C, Saryeddine T, et al. Educational needs of patients undergoing total joint arthroplasty. Physiother Can 2010;62:206–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alonso-Coello P, Ebrahim S, Guyatt GH, et al. Evaluating patient values and preferences for thromboprophylaxis decision making during pregnancy: a study protocol. BMC Pregnancy Childbirth 2012;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monsivais D, Reynolds A. Developing and evaluating patient education materials. J Contin Educ Nurs 2003;34:172–6 [DOI] [PubMed] [Google Scholar]
- 33.Schünemann HJ, Ståhl E, Austin P, et al. A comparison of narrative and table formats for presenting hypothetical health states to patients with gastrointestinal or pulmonary disease. Med Decis Making 2004;24:53–60 [DOI] [PubMed] [Google Scholar]
- 34.Informed Medical Decisions Foundations. 2013. http://www.informedmedicaldecisions.org/
- 35.Pierce LL. How to choose and develop written educational materials. Rehabil Nurs 2010;35:99–105 [DOI] [PubMed] [Google Scholar]
- 36.Aldridge MD. Writing and designing readable patient education materials. Nephrol Nurs J 2004;31:373–7 [PubMed] [Google Scholar]
- 37.Turnbull A. How nurses can develop good patient information leaflets. Nurs Times 2003;99:26–7 [PubMed] [Google Scholar]
- 38.Stevens MS, Legay DA, Glazebrook MA, et al. The evidence for hip arthroscopy: grading the current indications. Arthroscopy 2010;26:1370–83 [DOI] [PubMed] [Google Scholar]
- 39.McCarthy JC, Jarrett BT, Ojeifo O, et al. What factors influence long-term survivorship after hip arthroscopy? Clin Orthop Relat Res 2011;469:362–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Green C, Brazier J, Deverill M. Valuing health-related quality of life. A review of health state valuation techniques . Pharmacoeconomics 2000;17:151–65 [DOI] [PubMed] [Google Scholar]
- 41.Bluman GA. Elementary statistics: a step by step approach. 5th edn. McGraw Hill, 2010 [Google Scholar]
- 42.Blalock HM. Social statistics. New York: McGraw-Hill, 1979 [Google Scholar]
- 43.Cohen J. A power primer. Psychol Bull 1992;112:155–9 [DOI] [PubMed] [Google Scholar]
- 44.Hawker GA. Who, when, and why total joint replacement surgery? The patient's perspective. Curr Opin Rheumatol 2006;18:526–30 [DOI] [PubMed] [Google Scholar]
- 45.Vandenbroucke JP, Von Elm E, Altman DG, et al. [Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration]. Gac Sanit 2009;23:158. [DOI] [PubMed] [Google Scholar]
- 46.Tfelt-Hansen PC. CONSORT recommendations in abstracts of randomised, controlled trials on migraine and headache. J Headache Pain 2011;12:505–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alolabi B, Shirali J, Bajammal S, Karanicolas PJ, et al. The development of a decision aid to elicit treatment preferences for displaced femoral neck fractures. Indian J Orthop 2012;46:22–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bryant D, Bednarski E, Gafni A. Incorporating patient preferences into orthopaedic practice: should the orthopaedic encounter change? Injury 2006;37:328–34 [DOI] [PubMed] [Google Scholar]
- 49.Rapley T. Distributed decision making: the anatomy of decisions-in-action. Sociol Health Illn 2008;30:429–44 [DOI] [PubMed] [Google Scholar]
- 50.Vieder JN, Krafchick MA, Kovach AC, et al. Physician-patient interaction: what do elders want? J Am Osteopath Assoc 2002;102:73–8 [PubMed] [Google Scholar]
- 51.Tönnis D. Congenital dysplasia and dislocation of the hip in children and adults. New York: Springer, 1987 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.