Abstract
Introduction
Pulmonary hypertension (PH) is a devastating, progressive disease with increasingly debilitating symptoms and usually shortened overall life expectancy due to a narrowing of the pulmonary vasculature and consecutive right heart failure. Little is known about PH in Africa, but limited reports suggest that PH is more prevalent in Africa compared with developed countries due to the high prevalence of risk factors in the region.
Methods and analysis
A multinational multicentre registry-type cohort study was established and tailored to resource-constraint settings to describe disease presentation, disease severity and aetiologies of PH, comorbidities, diagnostic and therapeutic management, and the natural course of PH in Africa. PH will be diagnosed by specialist cardiologists using echocardiography (right ventricular systolic pressure >35 mm Hg, absence of pulmonary stenosis and acute right heart failure), usually accompanied by shortness of breath, fatigue, peripheral oedema and other cardiovascular symptoms, ECG and chest X-ray changes in keeping with PH as per guidelines (European Society of Cardiology and European Respiratory Society (ESC/ERS) guidelines). Additional investigations such as a CT scan, a ventilation/perfusion scan or right heart catheterisation will be performed at the discretion of the treating physician. Functional tests include a 6 min walk test and the Karnofsky Performance Score. The WHO classification system for PH will be applied to describe the different aetiologies of PH. Several substudies have been implemented within the registry to investigate specific types of PH and their outcome at up to 24 months. Data will be analysed by an independent institution following a data analyse plan.
Ethics and dissemination
All local ethics committees of the participating centres approved the protocol. The data will be disseminated through peer-reviewed journals at national and international conferences and public events at local care providers.
Keywords: pulmonary hypertension, Africa, HIV/AIDS
Strengths and limitations of this study.
The Pan African Pulmonary hypertension Cohort (PAPUCO) study is the first multinational registry on pulmonary hypertension in Africa.
PAPUCO centres have different levels of medical infrastructure and not all patients underwent the same rigorous workup.
Introduction
Pulmonary hypertension (PH) is a devastating, progressive disease with increasingly debilitating symptoms and usually shortened overall life expectancy due to a narrowing of the pulmonary vasculature and consecutive right heart failure (RHF).1 The epidemiology of PH in Africa and the distribution of its multitude of aetiologies have not yet been described, but limited reports suggest that the incidence of PH in Africa is higher than that reported from developed countries, owing to the pattern of diseases prevalent in the region.2–5 Many known risk factors for PH are hyperendemic in this part of the world, including HIV/AIDS, rheumatic heart disease (RHD), hereditary haemoglobinopathies, schistosomiasis and other parasitic infections, and chronic hepatitis B and C infection. On the other hand, a high prevalence of tuberculosis (TB), poorly treated asthma, high levels of pollution in urban areas and exposure to mining may subsequently lead to various forms of pulmonary disease, PH and often to RHF with premature death. Also, a lack of specialist paediatric services to diagnose and manage congenital heart disease (CHD) potentially also leads to PH.6 Furthermore, the high prevalence of RHD and endomyocardial fibrosis, the latter possibly also due to the high burden of parasitic infections, may contribute to secondary PH.6–8 However, Africa is in epidemiological transition with increased prevalence of non-communicable forms of heart disease such as hypertensive and ischaemic heart diseases. Left heart disease is the most common cause of heart failure in Africa and most likely a major contributor to PH and consecutive RHF.4 9 Thus, within the registry, we expect the entire spectrum of aetiologies of PH. In this manuscript, we aim to provide an overview of PH in Africa and the epidemiology of risk factors prevalent in the region, and answer the question why Africa needs a registry for PH just as much as most other parts of the world and how we implemented it in the setting of scarce resources.
Preliminary evidence of a significant burden of PH and its risk factors in Africa
In recent years, there has been an increasing awareness of the clinical significance of PH and cor pulmonale in Africa. This applies equally to the recognition of the importance of PH and RHF as a primary diagnosis and as a poor prognostic marker for those primarily affected by left-sided heart failure. Data on the precursors and risk factors of those conditions are limited to a few reports. The largest study on PH/RHF in Africa has been conducted within the Heart of Soweto Study in South Africa; 2505 patients presented with de novo heart failure between 2006 and 2008.5 Of those, 697 (28%) were diagnosed with PH/RHF. PH/RHF was the primary diagnosis in 50% of those patients. The majority presented with dyspnoea and a mean right ventricular systolic pressure (RVSP) above 50 mm Hg. Left heart disease (31%), chronic lung disease due to chronic obstructive pulmonary disease and TB (26%), and pulmonary arterial hypertension (PAH; 20%) due to HIV (HIV-PAH), CHD or idiopathic PAH were the most common causes of PH. Sani et al10 described the prevalence of PH in patients with RHD at a tertiary centre in Nigeria. Of 1312 Echocardiography (ECHO) studies, 10% had evidence of RHD; secondary PH was present in 80% of patients with RHD. The same centre reported on 53 patients with PH on ECHO among 80 patients admitted with heart failure, with hypertensive heart disease (25%), peripartum cardiomyopathy (25%), dilated cardiomyopathy (17%) and RHD (13%) being the most common causes of PH.11 A study from Uganda showed a prevalence of PH in patients with newly diagnosed RHD (n=309) of 33%.12 A smaller survey from Lagos, Nigeria, investigated the prevalence of cardiovascular disease in an HIV-positive cohort and found 1 patient of 100 patients to have HIV-PAH. 13 An ECHO study on 102 HIV patients presenting with cardiac symptoms in Tanzania revealed PH in 13%.14 PH with an RVSP>30 mm Hg was present in 4% of long-term survivors in a Zimbabwe cohort of vertically acquired HIV infection.15 Haemolytic anaemia is a known risk factor for PH; a screening study of patients with sickle-cell disease (SCD) in Nigeria showed a PH prevalence of 25%16; a study from Egypt indicates that patients with β-thalassaemia are at risk of PH.17 Another study from Egypt found a PH prevalence of 9% in patients seropositive for schistosomal antibodies. Ahmed et al18 from Sudan described 14 consecutive cases of PH with previously treated pulmonary TB and concluded that PH can even occur after resolution of TB, most likely due to persistent lung destruction. These data suggest that left heart disease, chronic lung disease, RHD, HIV, schistosomiasis and SCD might be the most common underlying diseases to cause PH within the spectrum of aetiologies of PH that we will find within the Pan African Pulmonary hypertension Cohort (PAPUCO)
Rationale and objectives
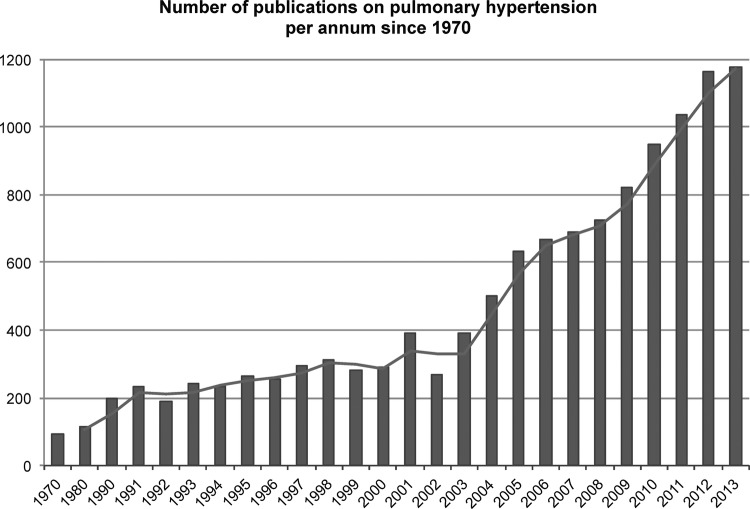
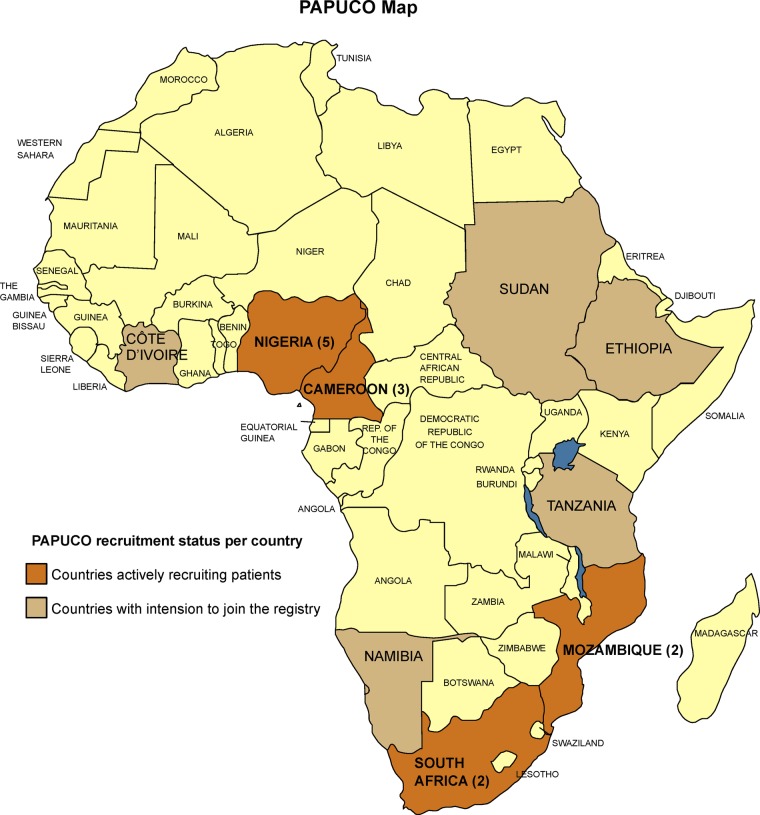
Worldwide, PH has received a great increase in awareness at the beginning of the 21st century, but less than 1% of the publications are from Africa (figure 1). It is expected that PH is more prevalent in Africa compared with developed countries, but little information is available about African patients with PH. Exposure to risk factors, human genetic variation, different socioeconomic backgrounds, lifestyles, comorbidities, nutrition and disparities in access to health services makes the African population unique but heterogeneous at the same time. Therefore, most research data and clinical guidelines from high-income countries cannot be translated into the African context. It is within the context described above that PAPUCO, a Pan Africa registry-type cohort study, was established (figure 2).
Figure 1.
Number of publications on pulmonary hypertension per annum since 1970 (grey bars) with moving average (blue line). Less than 1% (0.7%) of the publications were from Africa (not displayed). Title search terms in PubMed were ‘pulmonary hypertension’, ‘pulmonary arterial hypertension’ or ‘pulmonary venous hypertension’ and Africa and African country names; results are displayed annually between 1970 and 2013.
Figure 2.
The Pan African Pulmonary hypertension Cohort (PAPUCO) map displays countries currently participating in the PAPUCO registry (number of centres per country in brackets).
Scientific and clinical research has been fundamental in improving human health.19 In fact, research has been demonstrated to be an excellent vehicle to implement new technologies and to facilitate training for its use, as well as to develop new systems and establish the services around it—jointly leading to technical improvement, sharpening of skills and capacity development.20 21 Besides the main objectives of the PAPUCO registry to define and understand PH in Africa (box 1), this multinational and multicentre research project therefore aims to also develop sustainable clinical and research capacity across the African continent as well as raising awareness for PH and its risk factors.
Box 1. Pan African Pulmonary hypertension Cohort (PAPUCO) objectives.
Primary objective
To describe disease presentation, disease severity and aetiologies of pulmonary hypertension (PH), comorbidities, diagnostic and therapeutic management, and the natural course of PH in Africa.
Secondary objectives
To describe the overall 6-month survival rate.
To describe the 24-month survival rate in patients with HIV-pulmonary arterial hypertension.
To compare 6-month survival rates between different groups of PH.
To determine the predictors of mortality access the different groups.
The prevalence of PH in Africa varies geographically, according to the underlying risk factors and diseases, and the diagnosis of PH is most likely often missed, not only during the early stages due to the subtle nature of presentation of PH, but also at a more advanced stage of the disease, due to a lack of awareness by primary care doctors and low index of suspicion, limited access to ECHO services and tertiary care. However, it is crucial to diagnose PH in Africa early and reliably to ensure better care for these patients (box 2).
Box 2. The significance of pulmonary hypertension (PH) in Africa: Why we need to diagnose?
The right to know.
Prognostic implications.
Access to tertiary care: heart failure management, anticoagulation, home oxygen, chronic obstructive pulmonary disease management, access to PH-specific drugs (sildenafil).
Access to cardiothoracic surgery: valve repair/replacement, correction of congenital heart disease, heart transplantation.
Access to social and disability grants.
To raise awareness of PH and its risk factors.
Methods and analysis
Study design and setting
The PAPUCO research group was established in 2011 with the aim of implementing a registry-type cohort study on PH in Africa. The registry aims to recruit consecutive patients (1) with newly diagnosed PH-based clinical and ECHO criteria, (2) who are able or likely to return for a 6-month follow-up, (3) who are at least 18 years old (except for those in paediatric centres in Mozambique and Nigeria) and (4) who consented in writing to participate in the registry. Centre eligibility includes (1) availability of ECHO and training in assessing right heart function, (2) experience in diagnosing PH according to the WHO classification, (3) experience in clinical management of patients with RHF and (4) resources to review patients at 6-month follow-up. Participating centres will be invited to join the registry at African cardiac meetings and conferences.
Definition of PH, diagnostic algorithm and data points
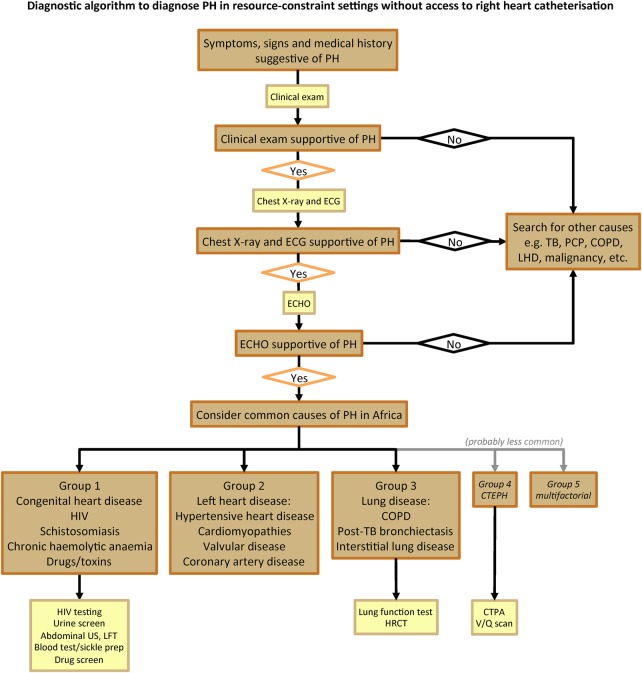
As shown in box 3, PH is defined as documented elevated RVSP>35 mm Hg on transthoracic ECHO in the absence of pulmonary stenosis and acute RHF, usually accompanied by shortness of breath, fatigue, peripheral oedema and other cardiovascular symptoms, and possibly ECG and chest X-ray (CXR) changes in keeping with PH as per the European Society of Cardiology and European Respiratory Society (ESC/ERS) guidelines on PH.5 22 Right heart catheterisation is optional. The updated WHO classification system for PH (Dana Point 2008) will be applied to describe the different aetiologies of PH.22 23 Once definitive assessment and treatment has been applied, the following specific data will be documented for each individual: (A) all major cardiovascular diagnoses according to the International Classification of Diseases (ICD) 10 coding, and (B) up to five non-cardiovascular diagnoses according to ICD 10 coding.24 Figure 3 shows the diagnostic algorithm to diagnose PH in resource-constraint settings without access to right heart catheterisation that has been developed following the ESC/ERS guidelines for the diagnosis of PH.22 Key information collected will include information on demographic profile, socioeconomic background, medical history, comorbidities, cardiac risk factors and environmental exposures. The clinical aspects of the assessment include symptom scoring, a full clinical examination, physical and clinical status; functional tests include the WHO functional class (WHO FC), a 6 min walk test (6MWT) and the Karnofsky Performance Score. Technical procedures include ECHO, CXR and a 12-lead ECG. Further investigations are at the discretion of the treating physician and typically include lung function tests, ventilation/perfusion lung scans, high resolution CT and CT pulmonary angiography, and right heart catheterisation, if available (figure 3). Data on heart failure treatment and co-medication, hospitalisation and death, and 6-month outcome will also be collected.
Box 3. Evidence to diagnose pulmonary hypertension in resource-constraint settings.
Symptoms: shortness of breath, fatigue, cough, chest pain, palpitations.
Clinical examination: raised jugular vein pressure, peripheral oedema, ascites, loud p2, systolic murmur, central cyanosis.
ECG: sinus tachycardia, p-pulmonale, right axis deviation, right bundle branch block, right ventricular hypertrophy.
Chest X-ray: cardiomegaly, pleural effusion, prominent pulmonary arteries.
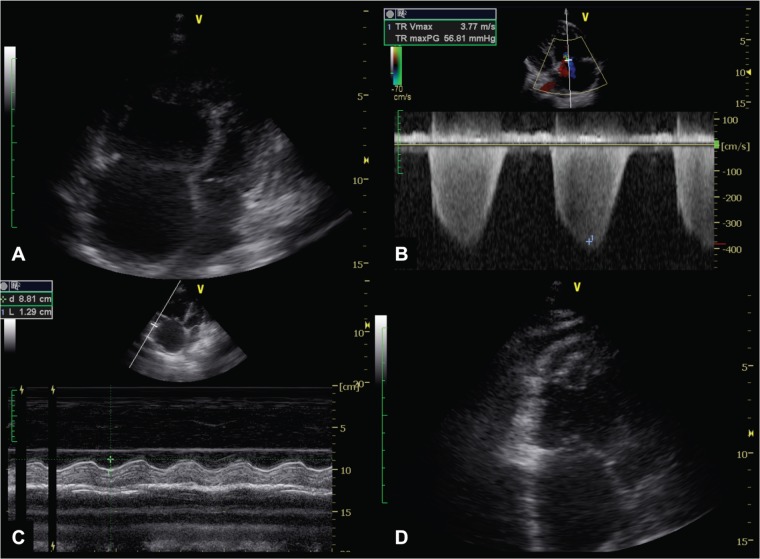
Echocardiography: dilated right atrium and ventricle, interventricular septal flattening, right ventricular systolic pressure >35 mm Hg, tricuspid annular plane systolic excursion <15 mm (figure 4).
Figure 3.
Diagnostic algorithm to diagnose pulmonary hypertension in resource-constraint settings. PH, pulmonary hypertension; TB, tuberculosis; PCP, pneumocystis pneumonia; COPD, chronic obstructive pulmonary disease; LHD, left heart disease; ECHO, echocardiography; US, ultrasound; LFT, liver function tests; HRCT, high-resolution CT; CTEPH, chronic thromboembolic pulmonary hypertension; CTPA, CT pulmonary angiography; V/Q, ventilation/perfusion lung scan.
Echocardiography
Echocardiography in adults
ECHO modalities applied included M-mode, two-dimensional (2D) and Doppler studies. ECHO will be performed with 3–5 MHz sector transducer. Complete 2D ECHO examination will be performed according to the recommendations of the American Society of ECHO (ASE).25 M-mode ECHO will be derived from 2D images. The M-mode cursor on the 2D scan will move to specific areas of the heart to obtain measurements according to the recommendation of the committee on M-mode standardisation of the ASE. Doppler indices of left ventricular (LV) diastolic filling will be obtained. A complete Doppler study will be carried out according to the recommendations of the ASE. From the M-mode measurements, indices of LV function will be derived. These include shortening fraction, ejection fraction (EF), and LV mass, cardiac output and relative wall thickness. ECHO examinations include (A) valvular assessment, (B) left atrial size, (C) LV size and function, (D) a semiquantitative estimate of the severity of valvular regurgitation, (E) size and function of the right atrium and the right ventricle (RV; figure 4A) and (F) evidence of PH. Doppler will be used to examine the valves on suspicion of any valvular lesion. ECHO findings associated with PH include a dilated pulmonary artery and dilation and hypertrophy of the RV, diastolic flattening of the interventricular septum and Doppler evidence of PH.26 Doppler ECHO estimates the pulmonary artery systolic pressure (PASP). By measuring the maximum velocity of the tricuspid regurgitant jet (v, figure 4B), the transtricuspid pressure gradient will be calculated using the modified Bernoulli equation (4v²).27 The PASP is approximated by adding the transtricuspid gradient to the right atrial pressure (RAP) as applied in the formula [PASP=4v2+RAP]. The PASP is equivalent to RVSP in the absence of pulmonary outflow obstruction. The RAP is estimated by the respiratory variation size of the vena cava inferior in M-mode.28 In our study, the systolic regurgitant tricuspid flow will be assessed in the parasternal RV inflow, parasternal short-axis and apical four-chamber views to determine the highest velocity, which reflects PASP/RVSP.29 PH is defined as mild if RVSP was 36–50 mm Hg, moderate if RVSP was 51–60 mm Hg and severe if RVSP was >60 mm Hg.30 In addition, we further assessed RV function by measuring the systolic displacement of the lateral portion of the tricuspid annular plane excursion on the M-mode tracing under the 2D-echo guidance (figure 4C).31 Peak mitral early diastolic velocity (E in m/s), the E-wave deceleration time (DCT in ms) and the velocity at atrial contraction (A in m/s) are measured using pulsed-wave Doppler. LV filling pressure classes were defined in accordance with the ASE 2009 guidelines.32 In patients with heart failure and reduced EF (HFrEF), raised LV filling pressure is defined as E/A ≥2 if in sinus rhythm or DCT <150 ms if in atrial fibrillation (AF); normal LV filling pressure is defined as E/A <1 in patients in sinus rhythm or DCT ≥200 ms for those in AF; patients between these limits will be classified as undetermined. In heart failure and preserved EF (HFpEF), LV filling pressure is classified as follows: raised LV filling pressure if the left atrium is dilated and normal LV filling pressure if the left atrium is of normal size.
Figure 4.
Diagnosing pulmonary hypertension using transthoracic two-dimensional Doppler echocardiography. (A) Four-chamber view showing grossly enlarged RV and RA; with right ventricularisation of the LV, and bulging of the RA into the LA; (B) Colour-wave and continuous-wave Doppler across the TV in the four-chamber view showing severe TR, despite the deceivingly less impressive colour-flow jet seen across the valve; (C) M-mode measurement of TAPSE depicting right ventricular systolic dysfunction; (D) Long-axis view of right ventricular apical thrombus. RA, right atrium; RV, right ventricle; LA, left atrium; TV, tricuspid valve; TR, tricuspid regurgitation; TAPSE, tricuspid annular plane excursion.
Echocardiography in infants and children
Translating the definitions of PH in adults to children, especially to infants, is controversial. Within the paediatric cardiology community, experts suggest that the ratio of the PASP to the systemic systolic blood pressure >0.4 should be the diagnostic criterion.33 The fact that the threshold of pulmonary vascular resistance (PVR) increase has not been included in the aforementioned definition is another limitation of its use in children or infants, since paediatric PH is mainly caused by left-to-right shunt CHD; and if PVR shows no significant increase in such cases, the patient may be considered only as a dynamic PH case led by increased pulmonary blood flow, rather than pulmonary vascular disease. Paediatric guidelines suggest that mPAP >25 mm Hg and PVR>3 Wood units remain in the definition of PAH.34 In our cohort of children with congenital heart malformations, we used not only tricuspid and pulmonary regurgitation envelopes, but also the flow across the ventricular septal defect or persistent ductus arteriosus to measure the gradient between the two circulations and therefore estimate the pulmonary pressure. Indirect signs of PH, such as dilation of the RV, RV-hypertrophy and dilation of the main pulmonary artery, were added to Doppler data to confirm the diagnosis.
Accuracy of echocardiography in the diagnosis of PH
Diagnosing PH is challenging when relying on bedside tests. RHC is the gold standard to diagnose and confirm PH, but performing RHC on all patients with dyspnoea would bear excessive risks and be impractical in any cost-constrained environment. Also, RHC is only available at very few centres in Africa. A viable device to diagnose PH in Africa needs to be widely available, accurate, safe and cost-effective. ECHO has become increasingly available in Africa and reliably allows the measurements to describe functional and morphological features of PH and to indirectly measure the pressures in the pulmonary artery; hence, to diagnose PH. Nonetheless, the exact measurements of the mean and systolic pulmonary artery are not possible, the PASP remains an estimate, and the use of ECHO to diagnose PH questionable.35 36 A number of possible explanations for this ‘inaccuracy’ merit attention: (1) conditions for a reproducible calculation of PASP/RVSP include the presence of sufficient tricuspid regurgitation (TR) to produce a Doppler envelope and appropriate gain adjustments. An ‘undergained’ spectral signal will tend to result in underestimated PASP, whereas an ‘overgained’ spectral signal might overestimate the measurements; (2) careful adjustment of the transducer position and the use of colour flow Doppler are critical in order to reduce the Doppler angle and to obtain the maximal regurgitant flow velocity and severe TR will cause a laminar flow which invalidates the Bernoulli equation; (3) volume status and systemic blood pressure are other factors that potentially influence the measurement of PASP and (4) the highest value of RAP in the ASE guidelines is 15 mm Hg, but RAP measured by RHC can exceed 15 mm Hg. In spite of all these shortcomings, several studies have been published in the literature demonstrating the good sensitivity and specificity of ECHO versus RHC.37–39 In the REVEAL registry, there was a good correlation in PASP between ECHO and RHC at baseline, even if repeated ECHO measurements alone were not sufficient to monitor changes in PASP or progression of PH.40 In the first systematic review and meta-analysis addressing the diagnostic accuracy of ECHO in PH by Janda et al41, the authors concluded that the correlation of PASP by ECHO compared with PASP by RHC was good with a summary correlation coefficient of 0.70 (95% CI 0.67 to 0.73). Also, the authors showed that the diagnostic accuracy of ECHO in PH was also acceptable with a summary sensitivity and specificity of 83% (95% CI 73% to 90%) and 72% (95% CI 53% to 85%), respectively. Additionally, Damy et al42 demonstrated in their recent work that PASP when measured in ‘good ECHO hands’ is a strong predictor of mortality. Last but not the least, easily obtainable ECHO parameters like the E/A ratio, DCT and LA size can reliably distinguish between PH due to lung disease and PH due to left heart disease, allowing for the rapid triage of patients with a need for RHC. In summary, ECHO is far beyond a good modality to confirm both the presence and aetiology of PH in the majority of suspected cases when performed and interpreted by the requisite expertise under incorporation of all information obtained during a detailed ECHO assessment. ECHO will always provide incremental information in PH that cannot be obtained using RHC, whether in developed or developing countries.
Data cleaning process and statistical analysis
All study data will be collected on electronic case report forms and stored on a dedicated secure central database. Cases will be reviewed by at least two investigators (FT, AD, KS, AOM) for completeness and data validated. Query reports will be sent to the sites and resolved by the site investigators. If consensus on the WHO classification of PH cannot be reached, a third investigator's opinion will be requested. Data will then be verified and transferred to SPSS Statistics V.17.0 for all analyses by an independent team at Preventative Health, Baker IDI Heart and Diabetes Institute, Melbourne, Australia. Normally distributed continuous data will be presented as mean±SD and non-Gaussian distributed variables as median+IQR. Categorical data will be presented as percentages with 95% CI where appropriate. For patient group comparisons, we will use χ2 analysis with calculation of ORs and 95% CI (where appropriate) for discrete variables and Student t test and analysis of variance for normally distributed continuous variables. Multiple logistic regression analyses (entry model) will be performed on age, sex and baseline risk factors to derive adjusted ORs for the risk of presenting with PH/RHF overall, chronic lung disease and PH WHO Group 1. Significance will be accepted at the two-sided level of 0.05. ECG analysis will be subject to blinded coding according to the published Minnesota criteria to determine any pathological abnormalities.43 We aim to adhere to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for this type of study wherever possible.44
PAPUCO substudies
Several substudies have been established within the PAPUCO registry. The ECHO substudy aims to describe in detail the ECHO characteristics of RV function in PH. The HIV-PAH substudy aims to describe the phenotype of HIV-PAH in Africa. The CHD and RHD substudies aim to describe the contribution of CHD and RHD to PH in Africa. In addition, serum will be collected at selected centres for studies on the biomarker profile. The HIV.INFLAME substudy aims to examine the role of inflammatory markers and oxidative stress on the development of HIV-PAH. We hypothesise that HIV-positive patients with PH have a proinflammatory state and raised markers of oxidative stress compared with healthy HIV-positive controls and that increased markers of inflammation and oxidative stress and decreased antioxidant capacity are predictors of outcome and indicators of disease severity.
Ethics and dissemination
All PAPUCO centres require ethical approval from their local ethics committee review board. Written informed consent must be obtained from every patient participating in the registry.
Study results will be disseminated in peer-reviewed journals. The first publication will include baseline and 6-month follow-up data from all centres. Substudy publications on the HIV cohort with a 24-month follow-up and the cohort of patients with PH due to left heart disease will be published after recruitment and follow-up has been completed. Laboratory-based research will be published after the work is completed.
The online data collection platform
A tailor-made database has been developed by integerafrica research and development to fulfil the study requirements. Open-source technology was used to develop the web-based system that allows investigators to collect, store, analyse, report on and export clinical research data. The interface is simple and user-friendly and leads the user through the data entry process. It anonymises personal patient data: data are stored as electronic case report forms on a secure, encrypted and backed-up server. It provides hierarchical permissions and validation at the point of data entry. Multimedia data formats, that is, scans of ECGs, CXRs and ECHO images, can be uploaded on the platform, allowing the storage of complete clinical records and data together. Tools for education, training and communication are installed within the web-portal. Frequently Asked Questions serve as a guide on how to use the platform. After secure login, documents such as paper case report forms, informed consent forms, study information sheets and patient education sheets on PH are available for download. All data can be exported in various formats for further analysis. The platform has been developed to cater for mobile internet connectivity available in most parts of Africa and a unique research platform far beyond a simple web-based database.
Recruitment process
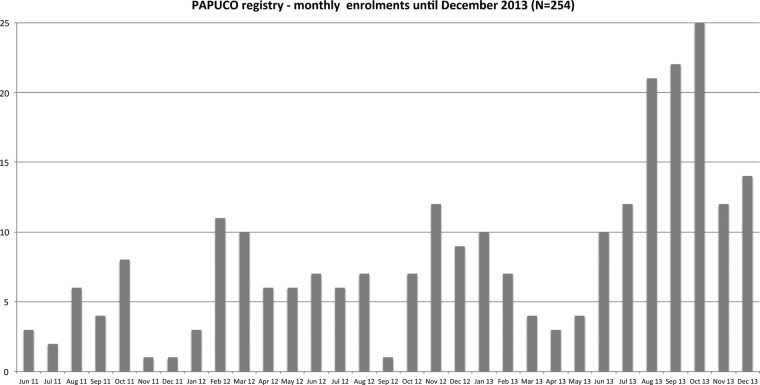
Twelve centres have received ethics approval and are currently recruiting actively; an additional six countries are showing interest in joining the registry (figure 2). All centres are government-run public healthcare institutions with different medical infrastructure profiles, and all but one are cardiac centres within tertiary care hospitals (table 1); one centre is an infectious diseases referral clinic in South Africa. A total of 254 patients have been recruited until December 2013 (figure 5).
Table 1.
Medical infrastructure at PAPUCO centres—diagnostic equipment and medical services
| Centre | ECG | CXR | ECHO | RHC | CS | HRCT | CTPA | LFT | V/Q | Lab |
|---|---|---|---|---|---|---|---|---|---|---|
| CM01 | X1 | X1 | X1 | X1 | X1 | X1 | X1 | |||
| CM02 | X1 | X1 | X1 | X1 | X1 | X1 | ||||
| CM03 | X1 | X1 | X1 | X1 | ||||||
| MZ01 | X | X | X | X | ||||||
| MZ02 | X | X | X | X | X | X | ||||
| NG01 | X1 | X1 | X1 | X1 | X1 | X1 | ||||
| NG02 | X1 | X1 | X1 | X1 | X1 | X1 | X1 | |||
| NG03 | X1 | X1 | X1 | X1 | X1 | X1 | X1 | |||
| NG04 | X1 | X1 | X1 | X1 | ||||||
| NG05 | X1 | X1 | X1 | X1 | X1 | X1 | X1 | |||
| SA01 | X | X | X | X | X | X | X | X | X | X |
| SA02 | X | X | X | X | X | X |
CM01, Douala General Hospital, Douala, Cameroon; CM02, Cardiac Centre, Shisong Hospital, Kumbo, Cameroon; CM03, Douala Cardiovascular Centre, Douala, Cameroon; CS, cardiac surgery; CTPA, CT pulmonary angiography; CXR, chest X-ray; ECHO, echocardiography; HRCT, high-resolution CT; Lab, laboratory facility for haematology, chemistry, and microbiology; LFT, lung function tests; MZ01, Instituto Nacional de Saúde, Maputo, Mozambique; MZ02, Division of Cardiology, Maputo Central Hospital, Maputo, Mozambique; NG01, Department of Medicine, Federal Medical Centre, Abeokuta, Nigeria; NG02, Department of Medicine, Bayero University and Aminu Kano Teaching Hospital, Kano, Nigeria; NG03, Department of Medicine, College of Medicine, Lagos, Nigeria; NG04, Department of Medicine, University of Uyo Teaching Hospital, Uyo, Nigeria; NG05, Federal Medical Centre, Umuahia, Nigeria; RHC, right heart catheterisation; SA01, Division of Cardiology, Department of Medicine, Groote Schuur Hospital, University of Cape Town, Cape Town, South Africa; SA02, Infectious Diseases Referral Clinic, GF Jooste Hospital, Mannenberg, South Africa and Rapid Assessment Unit, Khayelitsha District Hospital, Khayelitsha, South Africa; V/Q, ventilation/perfusion lung scan; X, equipment/service available at the centre and free-of-charge for the patients; X1, patients have to cover the costs for the service (out of pocket payments).
Figure 5.
Pan African Pulmonary hypertension Cohort (PAPUCO) registry—monthly enrolment until December 2013. Number of patients recruited per month since the launch of the registry in May 2011.
Limitations
The PAPUCO registry is an example of a functioning registry in low-resource settings. Limited resources should therefore not be seen as a limitation, but rather as a development opportunity. PAPUCO centres have different levels of medical infrastructure but not all patients will undergo the same rigorous workup in all centres, especially when compared with developed countries. Therefore, the clinical skills of the treating physician are often more important (and valuable!) than an additional diagnostic test in order to pin down the aetiology of PH in a patient. Moreover, patients often have to pay out of their pocket for diagnostic tests, and therefore accessibility to tests is limited due to the financial means of the patient. Although we aim for consecutive patient enrolment at each centre, we understand that this is often not feasible due to the high patient volume and workload of doctors.
Summary
PAPUCO is a contemporary registry on PH in Africa using high international standards to diagnose PH. It will provide new insight on PH in Africa and ultimately improve diagnosis and care of patients. PAPUCO is already a showcase for registry activities in Africa and a vehicle to capacity generation and sustainable development in the healthcare sector. It interconnects health centres far beyond PH.
Supplementary Material
Footnotes
Contributors: KS, AOM and FT were responsible for the initial idea, literature review, and study design and planning. All authors have contributed not only to the set-up of the PAPUCO registry, but also to various aspects of the study design with input relating to their specific expertise in the field. FT, AD, AOM, LB, MUS, KMK, OSO, AM, PU, ASI, AD and KS have developed the study protocol. FT and KS wrote the study protocol. FT developed the database. FT, AD, AOM and KS cleaned the database. LB trained the doctors in echocardiography of the heart and developed the Echo protocol. AD trained doctors in Cameroon. OSO trained doctors in Nigeria. SS developed the statistical analysis plan. FT, AD, AOM, MUS, KMK, OSO, IM, AM, PU, KT, RB, AD and KS were substantially involved in data acquisition. All authors read and approved the final manuscript.
Funding: Financial support for this research was provided by unconditional research grants from the Pulmonary Vascular Research Institute (PVRI), Bayer Health Care, and the University of Cape Town, and the Hatter Institute for Cardiovascular Research in Africa of the University of Cape Town provided institutional support. The electronic research platform was developed by integerafrica research and development.
Competing interests: None.
Ethics approval: All centres received ethics approval from their local ethic committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–41 [DOI] [PubMed] [Google Scholar]
- 2.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 2013;34:3538–46 [DOI] [PubMed] [Google Scholar]
- 3.Sliwa K, Carrington MJ, Becker A, et al. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012;33:866–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet 2008;371:915–22 [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, Mocumbi AO, Carrington MJ, et al. A not-so-rare form of heart failure in urban black Africans: pathways to right heart failure in the Heart of Soweto Study cohort. Eur J Heart Fail 2011;13:1070–7 [DOI] [PubMed] [Google Scholar]
- 6.Mocumbi AO, Lameira E, Yaksh A, et al. Challenges on the management of congenital heart disease in developing countries. Int J Cardiol 2011;148:285–8 [DOI] [PubMed] [Google Scholar]
- 7.Sliwa K, Mocumbi AO. Forgotten cardiovascular diseases in Africa. Clin Res Cardiol 2010;99:65–74 [DOI] [PubMed] [Google Scholar]
- 8.Sliwa K, Carrington M, Mayosi BM, et al. Incidence and characteristics of newly diagnosed rheumatic heart disease in urban African adults: insights from the heart of Soweto study. Eur Heart J 2010;31:719–27 [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation 2008;118:2360–7 [DOI] [PubMed] [Google Scholar]
- 10.Sani MU, Karaye KM, Borodo MM. Prevalence and pattern of rheumatic heart disease in the Nigerian savannah: an echocardiographic study. Cardiovasc J Afr 2007;18:295–9 [PMC free article] [PubMed] [Google Scholar]
- 11.Karaye KM, Saidu H, Bala MS, et al. Prevalence, clinical characteristics and outcome of pulmonary hypertension among admitted heart failure patients. Ann Afr Med 2013;12:197–204 [DOI] [PubMed] [Google Scholar]
- 12.Okello E, Wanzhu Z, Musoke C, et al. Cardiovascular complications in newly diagnosed rheumatic heart disease patients at Mulago Hospital, Uganda. Cardiovasc J Afr 2013;24:80–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olusegun-Joseph DA, Ajuluchukwu JNA, Okany CC, et al. Echocardiographic patterns in treatment-naïve HIV-positive patients in Lagos, south-west Nigeria. Cardiovasc J Afr 2012;23:e1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr 2012;23:90–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RF, Kaski JP, Hakim J, et al. Cardiac disease in adolescents with delayed diagnosis of vertically acquired HIV infection. Clin Infect Dis 2013;56:576–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aliyu ZY, Gordeuk V, Sachdev V, et al. Prevalence and risk factors for pulmonary artery systolic hypertension among sickle cell disease patients in Nigeria. Am J Hematol 2008;83:485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mokhtar GM, Tantawy AAG, Adly AAM, et al. Clinicopathological and radiological study of Egyptian β-thalassemia intermedia and β-thalassemia major patients: relation to complications and response to therapy. Hemoglobin 2011;35:382–405 [DOI] [PubMed] [Google Scholar]
- 18.Ahmed AEH, Ibrahim AS, Elshafie SM. Pulmonary hypertension in patients with treated pulmonary tuberculosis: analysis of 14 consecutive cases. Clin Med Insights Circ Respir Pulm Med 2011;5:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. World Health Organization : The World Health Report 2013: research for universal health coverage. Euro Surveill 2013;18:2055923968879 [Google Scholar]
- 20.Chu KM, Jayaraman S, Kyamanywa P, et al. Building research capacity in Africa: equity and global health collaborations. PLoS Med 2014;11:e1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noormahomed EV, Mocumbi AO, Preziosi M, et al. Strengthening research capacity through the medical education partnership initiative: the Mozambique experience. Hum Resour Health 2013;11:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009;30:2493–537 [DOI] [PubMed] [Google Scholar]
- 23.Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43–54 [DOI] [PubMed] [Google Scholar]
- 24.WHO. WHO International Classification of Diseases (ICD). WHO, 1994 [Google Scholar]
- 25.Henry WL, DeMaria A, Gramiak R, et al. Report of the American Society of Echocardiography Committee on nomenclature and standards in two-dimensional echocardiography. Circulation 1980;62:212–17 [DOI] [PubMed] [Google Scholar]
- 26.Jaffe CC, Weltin G. Echocardiography of the right side of the heart. Cardiol Clin 1992;10:41–57 [PubMed] [Google Scholar]
- 27.Sciomer S, Magrì D, Badagliacca R. Non-invasive assessment of pulmonary hypertension: Doppler-echocardiography. Pulm Pharmacol Ther 2007;20:135–40 [DOI] [PubMed] [Google Scholar]
- 28.Beigel R, Cercek B, Luo H, et al. Noninvasive evaluation of right atrial pressure. J Am Soc Echocardiogr 2013;26:1033–42 [DOI] [PubMed] [Google Scholar]
- 29.Quiñones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr 2002;15:167–84 [DOI] [PubMed] [Google Scholar]
- 30.Schachna L, Wigley FM, Chang B, et al. Age and risk of pulmonary arterial hypertension in scleroderma. Chest 2003;124:2098–104 [DOI] [PubMed] [Google Scholar]
- 31.Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–41 [DOI] [PubMed] [Google Scholar]
- 32.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr 2009;10:165–93 [DOI] [PubMed] [Google Scholar]
- 33.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J 2011;37:665–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013;62:D117–26 [DOI] [PubMed] [Google Scholar]
- 35.Penning S, Robinson KD, Major CA, et al. A comparison of echocardiography and pulmonary artery catheterization for evaluation of pulmonary artery pressures in pregnant patients with suspected pulmonary hypertension. Am J Obstet Gynecol 2001;184:1568–70 [DOI] [PubMed] [Google Scholar]
- 36.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009;179:615–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanzarini L, Fontana A, Campana C, et al. Two simple echo-Doppler measurements can accurately identify pulmonary hypertension in the large majority of patients with chronic heart failure. J Heart Lung Transplant 2005;24:745–54 [DOI] [PubMed] [Google Scholar]
- 38.Laaban JP, Diebold B, Zelinski R, et al. Noninvasive estimation of systolic pulmonary artery pressure using Doppler echocardiography in patients with chronic obstructive pulmonary disease. Chest 1989;96:1258–62 [DOI] [PubMed] [Google Scholar]
- 39.Matsuyama W, Ohkubo R, Michizono K, et al. Usefulness of transcutaneous Doppler jugular venous echo to predict pulmonary hypertension in COPD patients. Eur Respir J 2001;17:1128–31 [DOI] [PubMed] [Google Scholar]
- 40.Farber HW, Foreman AJ, Miller DP, et al. REVEAL Registry: correlation of right heart catheterization and echocardiography in patients with pulmonary arterial hypertension. Congest Heart Fail 2011;17:56–64 [DOI] [PubMed] [Google Scholar]
- 41.Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart 2011;97:612–22 [DOI] [PubMed] [Google Scholar]
- 42.Damy T, Goode KM, Kallvikbacka-Bennett A, et al. Determinants and prognostic value of pulmonary arterial pressure in patients with chronic heart failure. Eur Heart J 2010;31:2280–90 [DOI] [PubMed] [Google Scholar]
- 43.Prineas RJ, Crow RS, Blackburn H. The Minnesota code manual of electrocardiographic findings: standards and procedures for measurement and classification. Boston, Massachusetts: John Wright, 1982 [Google Scholar]
- 44.Elm von E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.