Abstract
Objective. This study aimed to determine whether BMI increases knee pain as measured from self-reported surveys even when controlling for OA severity as measured by osteophytes and joint space narrowing visible on X-rays.
Methods. Data available through the Osteoarthritis Initiative (OAI) were analysed, which included a sample of 4769 individuals, to answer the above question regarding OA, excess weight and pain. OA severity was assessed through baseline X-rays on right knees that were scored on a composite quasi-Kellgren and Lawrence grade. Weight was assessed through BMI. Pain was assessed through self-reports of the WOMAC pain subset as well as a 30-day pain severity question based on a 0–10 scale. Data were analysed using SPSS and analyses of covariance (ANCOVAs) were run to examine models adjusted for age, smoking, prior injury, pain medication and Heberden’s nodes. Critical alpha levels were set at 0.05.
Results. The results reported here confirm that knee pain does increase with OA severity. However, ANCOVA multiple regressions with controls reveal that even when taking into account OA severity, individuals with higher BMIs experience greater pain than individuals with lower BMIs.
Conclusion. Weight loss may reduce knee OA pain even if the osteological symptoms are not treated.
Keywords: knee osteoarthritis, pain, body mass index
Introduction
OA is a common cause of pain and disability in adults. Knee OA, which affects about one-quarter of adults, is one of the most disabling types of OA [1, 2]. OA changes include space narrowing between the connecting bones due to a loss of cartilage, cartilage hardening and thickening, tears or lesions in cartilaginous tissues and the formation of osteophytes [3]. OA aetiology is multifactorial; genes [4], age [1], activity [3], anatomical variation [5] and body weight [1, 6, 7] are among the commonly listed causes of OA.
Obesity is seen as the primary modifiable trait to prevent or reduce the effects of knee OA [6, 7]. An experimental study revealed that elevated leptin levels, which is a hormone found in adipose tissue, are related to OA and correlated with BMI [8]. Even after controlling for a variety of confounding factors, including sex, socio-economic status, smoking, diet, education and alcohol consumption, increased weight increases the risk of knee OA [9–11]. A clinical review article reported that 69% of knee replacements in middle-aged females can be attributed to obesity [10] and it has been estimated that if overweight and obese individuals reduced their weight to reach normal BMIs about 50% of knee OA cases would be eliminated [9].
Many studies reveal a correlation with excess weight and knee OA measured with the use of radiographs and MRI, but these measurements do not necessarily correspond to pain [2, 12]. Over time there has been an increase in knee replacements and in pain, but evidence of an increase in osteological OA severity is unclear [2]. Thus some researchers have assessed the effects of excess weight on OA by analysing self-reported surveys; studies using pain surveys reveal correlations with increased BMI and pain [6, 13]. Yet, the clinical literature has unresolved questions regarding pain tolerance in obese individuals [14]. Many studies have linked excess weight to an increase in pain or an increase in OA severity, but the aim in this study is to use data from the National Institutes of Health (NIH) Osteoarthritis Initiative (OAI) to determine whether excess weight results in more pain without more severe knee OA. The hypothesis is that individuals with higher BMIs will have greater knee OA pain even when controlling for the physical manifestations of OA severity.
Methods
Data used in the preparation of this article were obtained from the OAI database, which is available for public access at http://www.oai.ucsf.edu/. The OAI enrolled 4769 males and females between 45 and 79 years old at the onset of the 4-year study period. Data were collected at four urban clinical sites. OAI excluded individuals with RA or inflammatory arthritis. Individuals were also excluded if they had both knees replaced. The OAI study was Health Insurance Portability and Accountability Act (HIPAA) and institutional review board (IRB) compliant and all subjects included provided informed consent. The sample was divided into three subcohorts: control (those with no knee OA and no risk factors for knee OA; n = 122), incidence (those with no symptoms of knee OA, but with risk factors for knee OA; n = 3284) and progression (those with symptomatic knee OA; n = 1390). However, in this study the sample was treated as one population since some individuals considered in the incidence subcohort may, at the end of the OAI data collection, have symptomatic knee OA. Complete sample information can be viewed at http://www.oai.ucsf.edu/datarelease/docs/StudyDesignProtocol.pdf.
Database release version 8.2.1 was utilized. OA severity was assessed through right-side baseline X-rays that were scored on a composite quasi-Kellgren and Lawrence (KL) grade. The right side was utilized because there were no missing data on right knee OA severity, but data from 17 cases were missing on the left side. The quasi-KL OA grade, which is given for the whole joint, is based on a 0–4 scale. Only individuals with a quasi-KL OA grade ≥ 1 were utilized, which resulted in the removal of all control subcohorts. The quasi-KL OA scale is comprised of evidence of joint space narrowing and osteophytes and it is widely used in clinical research [2, 15].
Weight was assessed through BMI. BMI is calculated with the formula: weight (in kg)/[height (in m)]2. BMI is divided into the following categories: underweight, < 18.5; normal, 18.5–24.9; overweight, 25.0–29.9; obese, > 30.0. BMI is the variable most frequently used to assess the effects of excess weight on knee OA [9, 11, 13].
Pain was assessed through the WOMAC pain subset. The WOMAC pain subset is based on a 0–5 rating scale for each component, with a maximum score of 20. Only individuals with a WOMAC pain score of ≥ 1 were included in statistical tests where WOMAC pain was the pain variable utilized. Multiple studies examining pain use the WOMAC scale [6, 13]. The WOMAC pain scale asks about the severity of pain in the last 7 days when the patient engaged in the following activities: walking, going up or down stairs, laying or sitting and standing. More information about the pain scale can be found at the http://www.womac.org. Since the WOMAC scale is based on a 7-day cycle, a 30-day pain severity question based on a 0–10 scale was also utilized.
Covariates that were accounted for include age, whether the patient currently smokes, prior injury that resulted in the inability to walk for more than 1 week and Heberden’s nodes; these are common covariates in clinical research [6, 9, 13, 15]. Additional covariates included preventive pain treatments consisting of glucosamine, chondroitin sulphate, cortisone injections and hyaluronic acid injections. The frequency, during the past 6 months, of glucosamine or chondroitin sulphate was scored on a 4-point scale from no use to nearly every day or everyday use. For injections, the variable was scored as in the previous 6 months the patient either received an injection or did not receive an injection. Medicines to stop pain, such as NSAIDs and cyclooxygenase 2 (COX-2) selective inhibitors, were not controlled for since patients use these medicines when pain has already begun. Although sex is an obvious covariate listed in many studies, in this study sex was examined as an interaction factor. This allowed for testing hypotheses that the associations of pain, excess weight and OA severity are similar between males and females.
Using SPSS software (IBM, Armonk, NY, USA), analyses of covariances (ANCOVAs) were run to examine models adjusted for age, smoking, prior injury, pain treatments and Heberden’s nodes. Critical alpha levels were set at 0.05. The question that was tested was does an increase in BMI increase pain when knee joint OA severity is accounted for?
Results
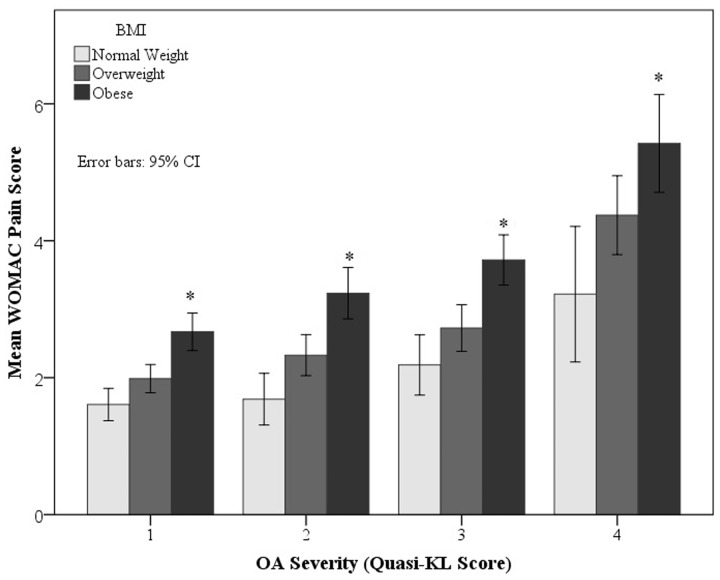
Table 1 displays the ANCOVA pain score results with covariate controls. WOMAC pain (see Fig. 1) and 30-day pain scores increase with an increase in BMI. Females experienced greater pain than males. No interactions between the main factors were statistically significant. OA severity correlated with both WOMAC and 30-day pain scores.
Table 1.
Two-factor (BMI and sex) ANCOVA results for WOMAC and 30-day pain scores
| Sum of squares | df | F | |||
|---|---|---|---|---|---|
| WOMAC pain score | Covariate | ||||
| OA severity | 745.839 | 1 | 75.062** | ||
| Main effects | (Combined) | 463.685 | 4 | 11.666** | |
| BMI | 345.803 | 3 | 11.601** | ||
| Sex | 117.882 | 1 | 11.864** | ||
| Interactions | BMI × sex | 19.243 | 3 | 0.646 | |
| Model | 2361.821 | 16 | 14.856** | ||
| Residual | 21 194.203 | 2133 | |||
| Total | 23 556.024 | 2149 | |||
| 30-day pain score | Covariate | ||||
| OA severity | 616.065 | 1 | 88.305** | ||
| Main effects | (Combined) | 218.155 | 4 | 7.817** | |
| BMI | 165.395 | 3 | 7.902** | ||
| Sex | 52.760 | 1 | 7.562* | ||
| Interactions | BMI × sex | 16.795 | 3 | 0.802 | |
| Model | 1619.378 | 16 | 14.507** | ||
| Residual | 14 846.077 | 2128 | |||
| Total | 16 465.455 | 2144 | |||
Covariates include age, injury, smoking, Heberden’s nodes and OA severity. OA severity and pain results are displayed. Other covariates not listed include age, injury, glucosamine/chondroitin sulphate use, cortisone or hyaluronic acid injections, Heberden’s nodes and smoking. ANCOVA: analysis of covariance; df: degrees of freedom; F: *P < 0.01, **P < 0.001.
Fig. 1.
BMI differences in mean WOMAC pain score (y-axis) by right knee OA severity (x-axis)
Discussion
OA is not reversible and it progresses through time, but understanding the impact of obesity on OA and pain can provide medical doctors, nurses and their patients with information on how to reduce the effects of the disease. OA pain corresponds with OA severity as measured by the presence of osteophytes and joint space narrowing. These results corroborate studies that suggest that knee pain is correlated with osteological symptoms [16]. Lethbridge-Çejku et al. [16], for example, using the Baltimore Longitudinal Study, found that people who reported pain were more likely to have X-ray evidence of OA than those without pain. Other studies, however, have not found a positive correlation between pain and osteological symptoms. Nguyen et al. [2], for example, found that increases in knee pain do not necessarily correlate with increases in radiographic OA. And Hannan et al. [12] examined a sample of ∼ 7000 individuals and found that ∼ 1000 reported knee pain, but only 15% of these individuals had X-ray evidence of OA and only 47% of the individuals with osteological symptoms experienced pain. Duncan et al. [17] suggest that some studies that do not show that osteological traits correspond with pain have X-rays taken from too few angles and that utilizing more images will reveal osteological evidence of OA. Although this study does reveal a correlation with pain and osteological changes, other factors also impact a patient’s perception of pain.
Excess weight affects OA severity and pain. With regard to OA severity and BMI, researchers have found that obesity is the main modifiable trait in helping reduce knee OA effects [6]. Review papers have suggested that more than half of all knee replacements in middle-aged females can be attributed to obesity [10]. Also, lifelong weight problems can be used to predict knee OA [11]. Excess weight increases the risk of knee OA even after controlling for smoking, diet, alcohol, Heberden’s nodes and socio-economic status [9]. In regard to pain, many studies have found a correlation between increased BMI and pain [6, 13]. For example, Lee et al. [6] ascertained that there is a correlation with BMI and questions regarding pain within the last 7 days. Laberge et al. [13] used > 100 individuals between 45 and 55 years of age to examine BMI, knee OA and pain as measured through the WOMAC score; the results showed that an increase in BMI led to a greater risk of knee pain.
All of the studies mentioned above focus either on the increase in OA severity or risk as it relates to BMI or on the increase in OA pain as it relates to BMI. In the current study, the two hypotheses are brought together. When controlling for a variety of additional factors (such as smoking, injury, age, preventative pain treatments and Heberden’s nodes) beyond OA severity, pain was still positively correlated with BMI.
Mechanical stress, such as excess weight on weight-bearing joints, can result in the induction of inflammatory factors [18]. Beyond mechanical stresses, leptins have been implicated as the source of the OA–obesity correlation [8]. Leptin, which resides in adipose cells, modulates with the inflammatory state and chronic low levels of inflammation are correlated with obesity [19]. Leptins may increase OA severity, but they may also increase OA pain without an increase in OA severity. Clinical researchers have yet to resolve questions regarding tolerance of pain in the obese and whether obesity in and of itself intensifies pain [14].
Sex differences could help researchers understand the impact of biochemicals. Females tend to have a higher ratio of body fat to muscle tissue compared with males. Some researchers argue that females are more affected by excess weight than males [7], while other researchers have found the opposite [15]. When examining the effects of obesity and pain on OA, Peltonen et al. [7] used a sample of > 6000 individuals over a 6-year period and they ascertained that females were more positively affected than males by weight loss surgery. In the current study, females experienced greater pain when OA severity was controlled for than did males. Females are also more likely than males to have increased susceptibility to autoimmune diseases and experience greater pain from inflammation [20].
In conclusion, self-reported pain increases with an increase in BMI even when controlling for OA severity. Even though OA severity may not be reversed and the osteological traits will likely increase, a lower BMI should reduce pain resulting from knee OA. Whether the decrease in pain is a result of changes in biochemicals or a reduction in mechanical stresses placed on the knee is difficult to ascertain. Research has also revealed that physical activities reduce pain caused by OA, and excess body weight is often tied to reduced activity levels [6]. To be active, however, weight loss may need to precede the physical therapy. Doctors should work with patients to explore effective weight loss techniques. Future studies may wish to include controls for additional pain drugs and fat metabolism medications since these drugs may decrease the perception of pain.
Rheumatology key messages.
OA pain increases with an increase in BMI even when controlling for OA severity.
Although OA severity cannot be reversed and will increase, a lower BMI should reduce pain in knee OA patients.
Patients should be encouraged to lose weight to manage knee OA pain.
Acknowledgements
The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261 and N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals, GlaxoSmithKline and Pfizer. Private-sector funding for the OAI is managed by the Foundation for the NIH. This manuscript was prepared using an OAI public use data set and does not necessarily reflect the opinions or views of the OAI investigators, the NIH or the private funding partners.
Disclosure statement: The author has declared no conflicts of interest.
References
- 1.Losina E, Walensky RP, Reichmann WM, et al. Impact of obesity and knee osteoarthritis on morbidity and mortality in older Americans. Ann Intern Med. 2011;154:217–26. doi: 10.1059/0003-4819-154-4-201102150-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen U, Yuqing Z, Yanyan Z, et al. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilak F. Biomechanical factors in osteoarthritis. Best Pract Res Clin Rheumatol. 2011;25:815–23. doi: 10.1016/j.berh.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector TD, MacGregor AJ. Risk factors for osteoarthritis: genetics. Osteoarthritis Cartilage. 2004;12(Suppl A):S39–44. doi: 10.1016/j.joca.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Hunter DJ, Niu J, Zhang Y, et al. Knee height, knee pain, and knee osteoarthritis: the Beijing Osteoarthritis Study. Arthritis Rheum. 2005;52:1418–23. doi: 10.1002/art.21017. [DOI] [PubMed] [Google Scholar]
- 6.Lee J, Song J, Hootman JM, et al. Obesity and other modifiable factors for physical inactivity measured by accelerometer in adults with knee osteoarthritis: data from the Osteoarthritis Initiative (OAI) Arthritis Care Res. 2013;65:53–61. doi: 10.1002/acr.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peltonen M, Lindroos A, Torgerson J. Musculoskeletal pain in the obese: a comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003;104:549. doi: 10.1016/S0304-3959(03)00091-5. [DOI] [PubMed] [Google Scholar]
- 8.Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–29. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- 9.Coggon D, Reading I, Croft P, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25:622. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 10.D’Arcy Y. Pain and obesity. Nurs Manage. 2012;43:21–6. doi: 10.1097/01.NUMA.0000411905.59061.5e. [DOI] [PubMed] [Google Scholar]
- 11.MacFarlane G, de Silva V, Jones G. The relationship between body mass index across the life course and knee pain in adulthood: results from the 1958 birth cohort study. Rheumatology. 2011;50:2251–6. doi: 10.1093/rheumatology/ker276. [DOI] [PubMed] [Google Scholar]
- 12.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 13.Laberge M, Baum T, Link T, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects-data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41:633–41. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janke E, Collins A, Kozak A. Overview of the relationship between pain and obesity: What do we know? Where do we go next? J Rehabil Res Dev. 2007;44:245–61. doi: 10.1682/jrrd.2006.06.0060. [DOI] [PubMed] [Google Scholar]
- 15.Holmberg S, Thelin A, Thelin N. Knee osteoarthritis and body mass index: a population-based case-control study. Scand J Rheumatol. 2005;34:59–64. doi: 10.1080/03009740510017922. [DOI] [PubMed] [Google Scholar]
- 16.Lethbridge-Çejku M, Scott WW, Reichle R, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Rheum. 1995;8:182–8. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 17.Duncan RC, Hay EM, Saklatvala J, Croft PR. Prevalence of radiographic osteoarthritis—it all depends on your point of view. Rheumatology. 2006;45:757–60. doi: 10.1093/rheumatology/kei270. [DOI] [PubMed] [Google Scholar]
- 18.Berenbaum F, Eymard F, Houard X. Osteoarthritis, inflammation and obesity. Curr Opin Rheumatol. 2013;25:114–8. doi: 10.1097/BOR.0b013e32835a9414. [DOI] [PubMed] [Google Scholar]
- 19.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaumond I, Arsenault P, Marchand S. The role of sex hormones on formalin-induced nociceptive responses. Brain Res. 2002;958:139–45. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]