Abstract
Although few recent studies have reported efficacy and safety data among patients with multiple sclerosis (MS) switching between immunotherapies, data on the mechanism of rebound activity postwithdrawal of fingolimod in patients with MS is scarce. A 36-year-old woman developed severe reactivation of her disease within 7 weeks of fingolimod's withdrawal despite the absence of breakthrough disease during the 8-week natalizumab washout period previously. The clinical presentation and radiological features were described indicating the diagnostic challenge given the potential risk of developing progressive multifocal leucoencephalopathy. The severe reactivation postwithdrawal of fingolimod could be due to the immune reconstitution inflammatory syndrome (IRIS) given the abrupt rise in lymphocyte count. Patients who discontinued fingolimod might be at risk of developing IRIS resulting in disease reactivation in the washout period.
Background
Patients with multiple sclerosis (MS) may be switched between different disease modifying therapies (DMTs) based on several factors such as breakthrough disease, adverse events or risk stratification of certain safety concerns. There is no consensus on washout periods between different DMTs. Most practice recommendations were based on retrospective data analysis. Immunological data suggested that potent DMTs such as natalizumab continue to exert the expected biological effects after their cessation, hence a notion of drug-holiday” was proposed to decrease the long-term risk of serious safety concerns such as progressive multifocal leucoencephalopathy (PML).1 2 However, it was soon learnt that the risk of disease recurrence or rebound activity after the cessation of potent DMTs such as natalizumab could pose a critical management controversy.3–5 Several treatment strategies were advocated, which included bridging therapy with monthly pulsed intravenous methylprednisolone6 7 or de-escalation to less potent DMTs such as β-interferons (IFN) or glatiramer acetate (GA).8 9 A randomised, partially placebocontrolled study to evaluate the effect on MS disease activity of a 24-week interruption in natalizumab treatment showed that disease activity began after 12 weeks and peaked at around 16 weeks after discontinuation of natalizumab in 167 of 175 patients regardless of whether on a drug holiday or if bridging or de-escalation therapy to IFN or GA was instituted.10 Fingolimod appeared as a promising switching therapy after its approval in 2010, especially in patients with highly active relapsing-remitting MS. Yet, a controversy concerning the appropriate washout period between fingolimod and natalizumab arose, given the potential risk of rebound disease activity once the immunological effects of natalizumab cease. Immune reconstitution inflammatory syndrome (IRIS) describes the paradoxical deterioration in clinical status following immune system recovery, and it has been increasingly seen in patients following the removal of the immune suppressive or immune-modulatory drugs.11 One such condition, which has recently received wide attention, is in the context of treatment of individuals with MS.12–14 We report a case of a patient with aggressive MS who was controlled on natalizumab and later switched to fingolimod due to John Cunningham (JC) virus seropositivity where she developed severe disease reactivation on discontinuation of fingolimod due to possible IRIS.
Case presentation
A 36-year-old woman, who was diagnosed with MS in 1998, continued to have breakthrough disease manifested by relapses despite being on IFN β-1a subcutaneously. She was escalated to natalizumab in February 2011. She remained in remission with an Expanded Disability Status Scale of 3.0. On completion of 24 infusions of natalizumab in February 2013 and due to JC seropositive status with a titre of 1.230, she elected to switch to fingolimod after a washout period of 2 months. A follow-up of MRI of the brain and cervical spine while on natalizumab showed no evidence of disease activity. She started to develop lymphopaenia in the first 6 months postfingolimod institution necessitating the interruption of the dosing regimen for 1 month and a subsequent gradual resumption of fingolimod with every other day dosing. After reinstitution of daily dose of fingolimod in November 2013, lymphocyte count was monitored closely on a monthly basis. However, she developed severe lymphopaenia of 0.1×109/L in February 2014 and fingolimod was discontinued. At the same time, a follow-up MRI of the brain revealed no evidence of disease activity (figure 1). The patient was counselled on the available treatment options and it was decided to resume natalizumab after the normalisation of lymphocyte count. She started to have slight imbalance and cognitive decline 7 weeks postfingolimod discontinuation. Natalizumab was started as the lymphocyte count was 1.2×109/L. Three days later, she was admitted to the hospital after developing dysarthria and right-sided pyramidal weakness, which rapidly progressed to drowsiness and coma within few days. She was unresponsive to verbal and pain stimuli. Her pupillary and corneal reflexes were preserved but her oculocephalic reflexes were suppressed. She had localising pain on the left upper and lower extremities and her deep tendon reflexes were asymmetrically brisker on the right side with bilateral upgoing plantars.
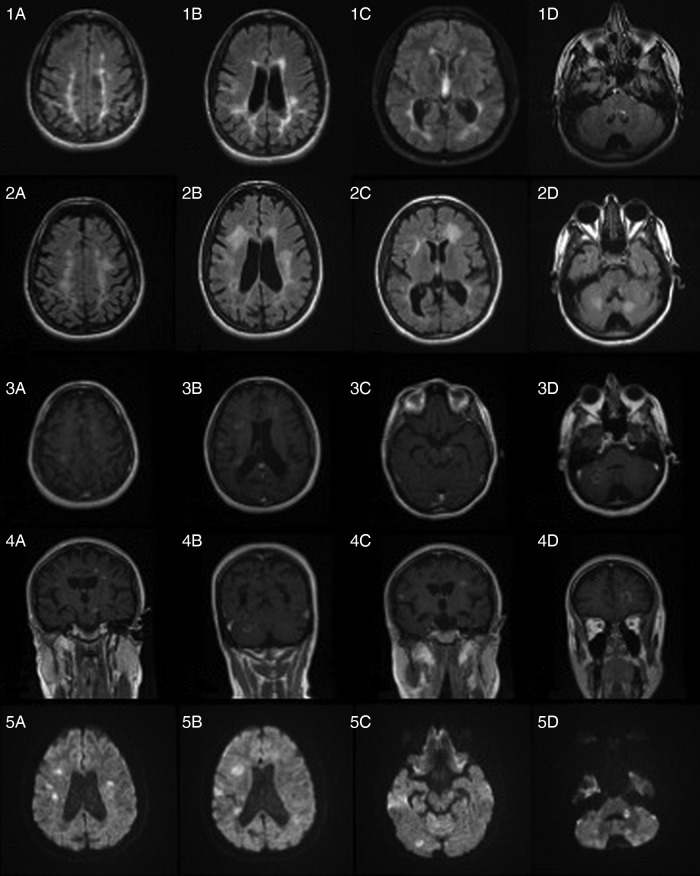
Figure 1.
MRI—1(A–D) baseline axial fluid-attenuated inversion recovery (FLAIR) at the time of fingolimod's withdrawal; 2(A–D) axial FLAIR images 8 weeks after fingolimod's withdrawal; 3(A–D) axial T1 with gadolinium; 4(A–D) coronal T1 with gadolinium and 5(A–D) axial diffusion-weighted images.
Investigations
The initial septic work up was negative including blood and urine cultures. MRI of the brain with gadolinium was performed on day 2 of admission, which revealed significant increase in T2/fluid-attenuated inversion recovery lesions in subcortical white matter, juxtacortical areas, brainstem and cerebellum when compared with the previous MRI (figure 1), along with at least 20 gad-enhancing lesions (figure 1). A lumbar puncture was performed the next day. Cerebrospinal fluid parameters revealed lymphocytic pleocytosis of 5 with normal protein and glucose. JC virus DNA was negative.
Differential diagnosis
Given the unusual clinical presentation and MRI findings, a diagnosis of PML was suspected. The differential diagnosis was reactivation of MS (relapse).
Treatment
Plasma exchange was initiated and six sessions were completed over 12 days in order to rapidly remove natalizumab since PML was the initial working diagnosis for her clinical deterioration. Subsequently, a course of intravenous methylprednisone 1 g once daily for 5 days was instituted and followed by oral prednisone (1 mg/kg) when IRIS was suspected.
Outcome and follow-up
The patient improved in terms of temporal orientation as she was responding to two-step commands, vocalising a few words and performing purposeful movements. She remained hemiplaegic on the right side and continued to be dependent on her daily activities. She was transferred to a rehabilitation centre.
Discussion
There are no established guidelines on the proper washout period between potent immunotherapies such as natalizumab and fingolimod. Two recent studies examined the risk of short-term relapse in patients switching from natalizumab to fingolimod.15 16 A French prospective cohort recommended a 3-month wash out period,15 while data derived from a large prospective international registry advised a maximum 2-month treatment gap for switches to fingolimod to decrease the hazard of relapse.16 The return to pretreatment disease activity should be differentiated from rebound phenomenon. Reactivation of MS can be worrisome and may lead to significant disabilities in patients who were initially escalated to second-line therapies due to their breakthrough disease.5 Miravalle et al12 studied a cohort of 32 patients who stopped natalizumab after 1 year under a planned suspension protocol where 38% of the patients with relapsing MS had at least moderately severe relapses within a mean of 4 months and they were worse than the relapses in the prenatalizumab period. With the accumulation of postmarketing data, several cases of rebound disease activity were reported after the discontinuation of fingolimod.17–19 The mechanism of rebound phenomenon is controversial and may differ depending on the stage of the disease, duration of prior remission and, most importantly, the drug's mechanism of action and the way the immune system is being targeted. In an Experimental Autoimmune Encephalomyelitis model, Yoshida et al20 found that a relapse postfingolimod's discontinuation was associated with infiltration of a clonal selection of myelin oligodendrocyte glycoprotein-specific T lymphocytes in secondary lymphoid tissue.
Ours is the first case reported in the literature to have had a severe rebound disease activity within 7 weeks of fingolimod's discontinuation despite the absence of any breakthrough disease activity during the natalizumab washout period. This highlights several observations. First, the differences in the mode of actions of the two drugs may play important roles in the time and mode of rebound activity. Second, despite the absence of breakthrough disease in the first washout period, rebound activity may appear with a second washout period. Third, the clinical presentation and radiological appearance of rebound phenomenon might be challenging to physicians in terms of diagnosis and symptomatic treatment.
We believe that our case had features suggestive of IRIS, given the abrupt rise in the lymphocyte count postfingolimod's withdrawal, which was associated with concurrent rapid clinical and radiological deterioration. The degree of inflammation postwithdrawal was indicative of a rapid influx of certain immune cells directing the inflammatory response. Although the term ‘IRIS’ was first introduced to describe a phenomenon of clinical deterioration despite successful immunological recovery in HIV-infected patients following treatment with antiretroviral therapy, it has been increasingly implicated in patients with rebound activity postnatalizumab interruption.11 12 IRIS is likely the consequence of lymphocyte redistribution to the target tissues following the recovery of immune function, cell numbers and prior defects in regulatory function.21 22 It may be challenging to differentiate IRIS from usual disease activity especially in patients who had received natalizumab, as PML could be in the differential diagnosis.14 This challenge translates into a therapeutic dilemma as to whether to rapidly remove the offending drug or to initiate the treatment of IRIS. Corticosteroids in the form of intravenous methylprednisolone for 3–5 days followed by an oral taper for 6–8 days remain the recommended treatment for acute IRIS based on retrospective studies.11 23 Although corticosteroids are not the most ideal drug for controlling IRIS because of the lack of specificity, they remain the most effective class of drugs in patients with severe inflammation.11 In our opinion, it might be practical to reduce the washout period to 4–6 weeks when switching from fingolimod to another DMTs to minimise disease reactivation and to avoid the acute stage of IRIS. Future studies are needed to examine the risk of disease reactivation in the setting of IRIS postwithdrawal of potent immunotherapies while consensus recommendations are urgently required to guide the treating physicians on the appropriate washout periods.
Learning points.
Reactivation of multiple sclerosis may occur during the washout period of potent immune-modulatory drugs, which may lead to severe rebound of previously halted disease activity.
Progressive multifocal leucoencephalopathy is a potential diagnostic challenge in patients who were prescribed natalizumab and experienced deterioration of their neurological condition or in those suspected to have relapses.
Discontinuation of fingolimod may lead to abrupt rise of lymphocyte count within 4–8 weeks, which could be implicated in fingolimod-associated IRIS (immune reconstitution inflammatory syndrome).
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Havla J, Kleiter I, Kumpfel T. Bridging, switching or drug holidays—how to treat a patient who stops natalizumab? Ther Clin Risk Manag 2013;9:361–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743–7 [DOI] [PubMed] [Google Scholar]
- 3.Killestein J, Vennegoor A, Strijbis EM, et al. Natalizumab drug holiday in multiple sclerosis: poorly tolerated. Ann Neurol 2010;68:392–5 [DOI] [PubMed] [Google Scholar]
- 4.Stuve O, Cravens PD, Frohman EM, et al. Immunologic, clinical, and radiologic status 14 months after cessation of natalizumab therapy. Neurology 2009;72:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Connor PW, Goodman A, Kappos L, et al. Disease activity return during natalizumab treatment interruption in patients with multiple sclerosis. Neurology 2011;76:1858–65 [DOI] [PubMed] [Google Scholar]
- 6.Borriello G, Prosperini L, Mancinelli C, et al. Pulse monthly steroids during an elective interruption of natalizumab: a post-marketing study. Eur J Neurol 2012;19:783–7 [DOI] [PubMed] [Google Scholar]
- 7.Magraner MJ, Coret F, Navarre A, et al. Pulsed steroids followed by glatiramer acetate to prevent inflammatory activity after cessation of natalizumab therapy: a prospective, 6-month observational study. J Neurol 2011;258:1805–11 [DOI] [PubMed] [Google Scholar]
- 8.Gobbi C, Meier DS, Cotton F, et al. Interferon beta 1b following natalizumab discontinuation: one year, randomized, prospective, pilot trial. BMC Neurol 2013;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Havla J, Gerdes LA, Meinl I, et al. De-escalation from natalizumab in multiple sclerosis: recurrence of disease activity despite switching to glatiramer acetate. J Neurol 2011;258:1665–9 [DOI] [PubMed] [Google Scholar]
- 10.Fox R, Kappos L, Cree B, et al., eds. Effects of a 24-week natalizumab treatment interruption on clinical and radiologic parameters of multiple sclerosis disease activity: the RESTORE study. 5th Joint Triennial Congress of the European and Americas Committees for Treatment an d Research in Multiple Sclerosis, 2011;19–22 October, Amsterdam, The Netherlands [Google Scholar]
- 11.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol 2011;24:284–90 [DOI] [PubMed] [Google Scholar]
- 12.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 2011;68:186–91 [DOI] [PubMed] [Google Scholar]
- 13.Clifford DB, De Luca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010;9:438–46 [DOI] [PubMed] [Google Scholar]
- 14.Tan IL, McArthur JC, Clifford DB, et al. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77:1061–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen M, Maillart E, Tourbah A, et al. Switching from natalizumab to fingolimod in multiple sclerosis: a French prospective study. JAMA Neurol 2014;71:436–41 [DOI] [PubMed] [Google Scholar]
- 16.Jokubaitis VG, Li V, Kalincik T, et al. Fingolimod after natalizumab and the risk of short-term relapse. Neurology 2014;82:1204–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Havla JB, Pellkofer HL, Meinl I, et al. Rebound of disease activity after withdrawal of fingolimod (FTY720) treatment. Arch Neurol 2012;69:262–4 [DOI] [PubMed] [Google Scholar]
- 18.Hakiki B, Portaccio E, Giannini M, et al. Withdrawal of fingolimod treatment for relapsing-remitting multiple sclerosis: report of six cases. Mult Scler 2012;18:1636–9 [DOI] [PubMed] [Google Scholar]
- 19.Ghezzi A, Rocca MA, Baroncini D, et al. Disease reactivation after fingolimod discontinuation in two multiple sclerosis patients. J Neurol 2013;260:327–9 [DOI] [PubMed] [Google Scholar]
- 20.Yoshida Y, Tsuji T, Fujita T, et al. Relapse of experimental autoimmune encephalomyelitis after discontinuation of FTY720 (fingolimod) treatment, but not after combination of FTY720 and pathogenic autoantigen. Biol Pharm Bull 2011;34:933–6 [DOI] [PubMed] [Google Scholar]
- 21.Chalkley JJ, Berger JR. Progressive multifocal leukoencephalopathy in multiple sclerosis. Curr Neurol Neurosci Rep 2013;13:408. [DOI] [PubMed] [Google Scholar]
- 22.Baldwin KJ, Hogg JP. Progressive multifocal leukoencephalopathy in patients with multiple sclerosis. Curr Opin Neurol 2013;26:318–23 [DOI] [PubMed] [Google Scholar]
- 23.McCarthy M, Nath A. Neurologic consequences of the immune reconstitution inflammatory syndrome (IRIS). Curr Neurol Neurosci Rep 2010;10:467–75 [DOI] [PMC free article] [PubMed] [Google Scholar]