Abstract
Loeys-Dietz syndrome is a recently described connective tissue disorder with a natural history of extreme vessel tortuosity and aggressive arterial aneurysm formation and rupture. This is the case of a 23-year-old woman with a large, dysplastic cavernous aneurysm who had successful endovascular treatment by flow diversion with the Pipeline embolisation device. Ten-month follow-up demonstrated complete aneurysm occlusion and curative reconstruction of the parent vessel without evidence of vessel injury or dissection. Endovascular treatment with flow-diverting devices is a valid treatment option and can be performed safely and effectively in this complex patient population.
Background
Loeys-Dietz syndrome (LDS) is a connective tissue disorder caused by mutations in the transforming growth factor β (TGFβ) receptors (TGFBR1, TGFBR2) and classically characterised by a triad of arterial tortuosity with predisposition for aneurysms and dissection, hypertelorism, and bifid uvula and/or cleft palate.1 Although LDS shares phenotypic traits with other connective tissue disorders (eg, vascular Ehlers-Danlos syndrome and Marfan's syndrome), the natural history is markedly different.2 Aneurysms in LDS, are particularly aggressive and prone to rupture or dissect at diameters smaller than predicted to cause such pathology in other connective tissue disorders.1–3 The tendency for extracranial, early, aggressive arterial aneurysms combined with proven low rates of intraoperative and perioperative mortality in patients with LDS have led to recommendations for serial imaging for early diagnosis and rapid intervention for aneurysm treatment.3
The incidence and significance of intracranial aneurysms are not well known in LDS. In the original paper describing the syndrome, two patients had cerebral aneurysms.1 In a subsequent paper, 10% (9 of 90) of patients with LDS in their cohort had arterial aneurysms of the head or neck.2 These aneurysms are clinically significant, because 7% of deaths in that cohort were from cerebral bleeding. Even less well studied is treatment of cerebral aneurysms in patients with LDS. The literature describes only two cases of cerebral aneurysms in patients with LDS treated by microsurgical clipping4 5 and only one case using endovascular treatment6 highlighting the dearth of data regarding treatment options and natural history. We report the first successful case of a cerebral aneurysm treatment in a patient with LDS using flow diversion via the Pipeline embolisation device (PED; Covidien Vascular Therapies, Mansfield, Massachusetts, USA).
Case presentation
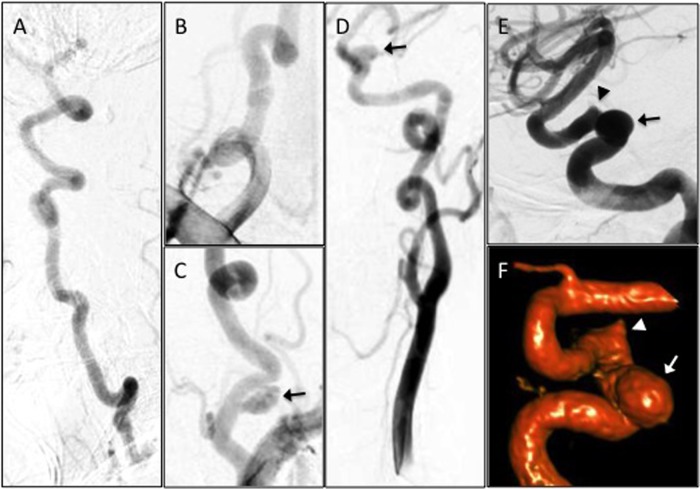
The patient is a 23-year-old woman with a history of ascending aortic aneurysm status post valve-sparing aortic root replacement at age 13 and rapidly progressive scoliosis status post-corrective spinal fusion at age 15. By age 21, she was noted on follow-up to have raphe in her uvula, easy bruising, soft skin texture and abnormal scarring. Genetic testing revealed mutation of the TGFBR2 gene (specifically, 1337 A>G causing aspartic acid to glycine substitution at amino acid 446) indicating LDS, and clinical findings were consistent with LDS type II. Screening CT angiography revealed a left cavernous internal carotid artery (ICA) aneurysm. Catheter cerebral angiography showed increased tortuosity of the bilateral ICAs, vertebral arteries (VAs) and a 10 mm pseudoaneurysm of the proximal left VA (figure 1A–D). An 8 mm×10 mm wide-necked proximal cavernous left ICA aneurysm and a 3 mm aneurysmal irregularity distal to this were also visualised (figure 1D–F). Initially, we chose to treat the intracranial ICA aneurysms and to monitor the vertebral aneurysm. For curative reconstruction of the entire cavernous segment, we recommended endovascular treatment of the ICA aneurysms using the PED. This treatment is classified as on-label use of the PED by the Food and Drug Administration (FDA). Pre-procedure, she was fully functional without disability and had a Modified Rankin scale (mRS) of 0.
Figure 1.
Angiography demonstrating marked tortuosity and aneurysms of the vertebral (VA) and carotid arteries. Digital subtraction angiography (DSA) of the right VA (A, lateral view), right VA origin (B), left VA origin (C) and left carotid artery (D, anteroposterior view; E, lateral view; F, three-dimensional reconstruction). There is a 10 mm pseudoaneurysm along the left V1 segment (C, arrow). There is an 8 mm×10 mm wide-necked proximal cavernous left internal carotid artery (ICA) aneurysm (D–F, arrow) and a 3 mm aneurysmal irregularity of the posterior genu of the cavernous left ICA (E and F, arrowhead).
Treatment
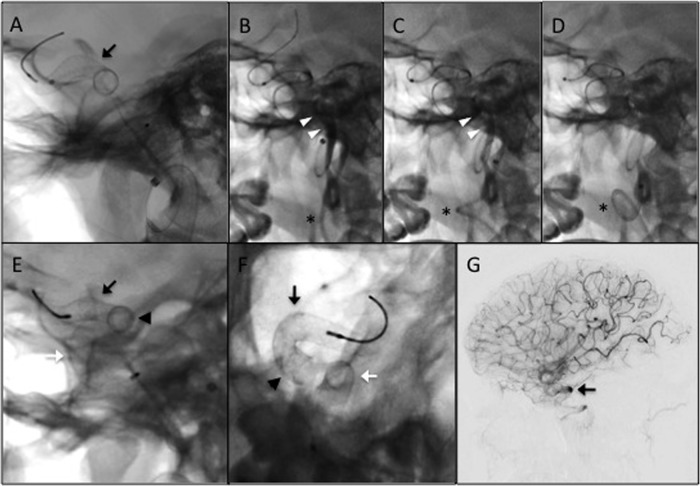
PED embolisation was performed using a tri-axial system as previously described.7 8 A 6 Fr Flexor Shuttle Sheath (Cook Medical Inc, Bloomington, Indiana, USA) was positioned in the proximal cervical left ICA. A 5 Fr Navien (Covidien Vascular Therapies, Mansfield, Massachusetts, USA) was positioned in the distal cervical ICA. Nicardipine was added to the Navien flush bag (1 mg/1000 mL heparinised saline) as vasospasm prophylaxis. A Marksman (Covidien Vascular Therapies, Mansfield, Massachusetts, USA) was introduced coaxially through the Navien and positioned initially in the ophthalmic segment of the ICA. A 5 mm×18 mm PED was then deployed across the aneurysm neck (figure 2A). Control angiography demonstrated incomplete neck coverage, so the decision was made to place a second PED. Following recapture of the PED delivery wire, the Marksman was re-advanced to a more distal position. During this process, the proximal cervical ICA loop straightened and contrast stasis was observed proximally (figure 2B, C). Shortly afterwards, the patient became asystolic. The wire was quickly withdrawn and the catheter was reloaded, restoring the natural looped configuration of the cervical carotid and anterograde flow (figure 2D). After <15 s, normal sinus rhythm resumed without further incident. The event was attributed to manipulation of the carotid bulb. A second 5 mm×18 mm PED was then telescoped without incident (figure 2E, F). Control angiography demonstrated complete neck coverage and contrast stasis within the aneurysm (figure 2G).
Figure 2.
Deployment of the Pipeline embolisation devices (PEDs). (A) Native fluoroscopy image demonstrating the first deployed PED (black arrow, also in E–F), sized 5 mm×18 mm. (B–D) Following deployment of the first PED, the Marskman microcatheter was readvanced over a Synchro-2 standard microwire and slack pulled from the system. These actions caused straightening of the lower cervical internal carotid artery (ICA) loop (astrisk) and contrast stasis (white arrowheads) distal to the guide catheter. The patient subsequently went asystolic, likely secondary to manipulation of the carotid bulb. The wire was quickly withdrawn and the microcatheter was reloaded, restoring the lower ICA loop and normal flow in the vessel (disappearance of contrast). Within 15 s, normal systolic cardiac rhythm restarted. Control angiography demonstrated that the first PED did not fully cover the aneurysm neck (not shown). A second PED, also sized 5 mm×18 mm, was subsequently deployed to provide full neck coverage. (E and F) Native fluoroscopy images (E, lateral; F, anteroposterior) following deployment of the second PED (white arrow). The arrowhead in (E) and (F) represents the region of overlap between the two telescoping PEDs. (G) Control digital subtraction angiography immediately postdeployment of the second PED demonstrating contrast stasis in the aneurysm (black arrow).
Outcome and follow-up
Follow-up cerebral angiography at 10 months demonstrated complete aneurysm occlusion and parent vessel reconstruction, without dissection or in-stent stenosis (figure 3). Clinically, she remained asymptomatic (except for baseline headaches) and was fully functional with an mRS of 0.
Figure 3.
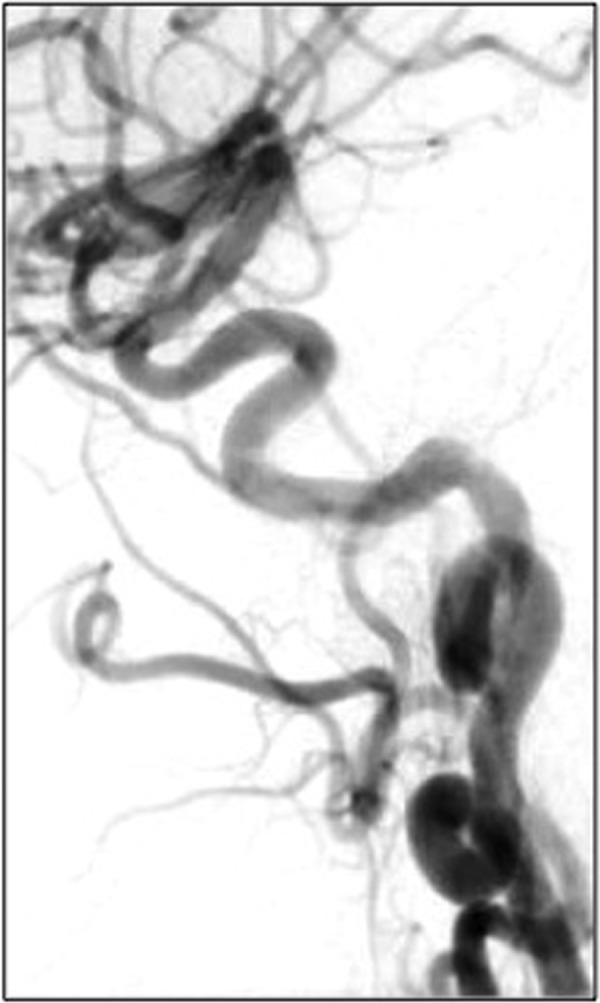
Ten-month follow-up. Digital subtraction angiography of the left common carotid artery 10 months post-Pipeline embolisation. There is parent vessel remodelling with no contrast opacification of the aneurysm. No dissection or in-stent stenosis is visualised.
Discussion
There is a paucity of literature on cerebral aneurysms in patients with LDS because the syndrome is recently discovered, rare and the incidence of cerebral aneurysms is unknown. Reports of cerebral aneurysm treatment by craniotomy (two cases) and by endovascular treatments (two cases) in patients with LDS is limited.4–6 Our report adds to this fund of knowledge, and includes the new option of flow diversion for patients with LDS.
Hesitancy to manipulate vessels because of fragility and propensity for dissection or catastrophic injury is a common theme in reports of cerebral aneurysm treatment in patients with LDS. For microsurgical clipping, adenosine was used rather than temporary clipping to minimise vessel manipulation.4 5 For endovascular treatment, vessel manipulation was minimised by excluding balloons and limiting aneurysm reaccess.6 We sought to limit vessel manipulation; however, PED cases are fundamentally and technically very different than stent-coilings. One difference is the support system. Levitt and colleagues used a bi-axial system with a 6 Fr Envoy.6 The Envoy position was not mentioned, but presumably it was proximal cervical ICA. For PED, distal support with an intermediate catheter is generally required. In this case, a 5 Fr Navien was tracked around two complete corkscrew loops in the cervical ICA to achieve the distal access. Our techniques using this catheter have been previously published.9 10 Management of catheter slack was critically important here, as reduction of the proximal cervical loop caused an episode of asystole. This issue could occur in any patient with extreme cervical tortuosity; however, the young age and vascular changes from LDS may have made this patient more susceptible to adverse effects of carotid manipulation. Although patients with LDS are prone to cardiac events, this particular event was likely non-cardiac in origin. The onset and resolution of the asystole were directly linked to catheter manipulation, and the catheter was not in contact with the heart. Furthermore, she had screening echocardiography every few years with the most recent demonstrating normal systolic function, normal chamber size and good valve function.
Another important difference is the stent properties. Self-expanding nitinol stents (eg, Neuroform) used by Levitt et al6 open without manipulation or postprocessing. The PED, however, can require considerable manipulation. The manipulation, such as ‘wagging’ the device, is of potential concern in patients with LDS because of suspected vessel fragility; however, it was performed without vessel injury in our case. This was likely facilitated by the distal intracranial support catheter, which improved the ability to manipulate the device and limited focused pressure on the vessel walls by the microcatheter.
Vessel reactivity and vasospasm were also a concern in this patient given during her young age and LDS. Hughes and colleagues noted extremely reactive vessels during arachnoid dissection for microsurgical clipping, necessitating multiple uses of papaverine to counteract vasospasm.5 We opted to use nicardipine in our guide catheter flush bag to prophylactically reduce vasospasm during the procedure, and no significant vasospasm was encountered.
Furthermore, follow-up at 10 months demonstrated complete aneurysm occlusion and normal parent vessel appearance. This demonstrates that PED treatment follows similar timelines in this patient population.
Learning points.
Pipeline embolisation has been successfully utilised to treat intracranial aneurysms in a patient with Loeys-Dietz syndrome.
Curative reconstruction of the parent vessel was achieved in a similar timeline as patients without connective tissue disorders.
A distal intracranial support catheter was used safely without vessel injury or dissection.
Endovascular treatment is a valid option for treatment of intracranial aneurysms in patients with Loeys-Dietz syndrome.
Footnotes
Contributors: GPC, SRZ and ALC were involved in patient care, manuscript preparation, figures, overall study design and final manuscript review; and L-ML was involved in patient care, figures, overall study design and final manuscript review.
Competing interests: GPC and ALC are consultants for Covidien. ALC is a proctor for the Pipeline embolisation device.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Loeys BL, Chen J, Neptune ER, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 2005;37:275–81 [DOI] [PubMed] [Google Scholar]
- 2.Loeys BL, Schwarze U, Holm T, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med 2006;355:788–98 [DOI] [PubMed] [Google Scholar]
- 3.Johnson PT, Chen JK, Loeys BL, et al. Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol 2007;189:W29–35 [DOI] [PubMed] [Google Scholar]
- 4.Rahme RJ, Adel JG, Bendok BR, et al. Association of intracranial aneurysm and Loeys-Dietz syndrome: case illustration, management, and literature review. Neurosurgery 2011;69:E488–92; discussion E492-483 [DOI] [PubMed] [Google Scholar]
- 5.Hughes BD, Powers CJ, Zomorodi AR. Clipping of a cerebral aneurysm in a patient with Loeys-Dietz syndrome: case report. Neurosurgery 2011;69:E746–55; discussion E755 [DOI] [PubMed] [Google Scholar]
- 6.Levitt MR, Morton RP, Mai JC, et al. Endovascular treatment of intracranial aneurysms in Loeys-Dietz syndrome. J Neurointerv Surg 2012;4:e37. [DOI] [PubMed] [Google Scholar]
- 7.Colby GP, Lin LM, Gomez JF, et al. Immediate procedural outcomes in 35 consecutive Pipeline embolization cases: a single-center, single-user experience. J Neurointerv Surg 2013;5:237–46 [DOI] [PubMed] [Google Scholar]
- 8.Lin LM, Colby GP, Kim JE, et al. Immediate and follow-up results for 44 consecutive cases of small (<10 mm) internal carotid artery aneurysms treated with the Pipeline embolization device. Surg Neurol Int 2013;4:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colby GP, Lin LM, Huang J, et al. Utilization of the Navien distal intracranial catheter in 78 cases of anterior circulation aneurysm treatment with the Pipeline embolization device. J Neurointerv Surg 2013;5(Suppl 3):iii16–21 [DOI] [PubMed] [Google Scholar]
- 10.Lin LM, Colby GP, Huang J, et al. Ultra-distal large-bore intracranial access using the hyperflexible Navien distal intracranial catheter for the treatment of cerebrovascular pathologies: a technical note. J Neurointerv Surg 2014;6:301.– [DOI] [PubMed] [Google Scholar]