Abstract
The accessibility and usage of body building supplements is on the rise with stronger internet marketing strategies by the industry. The dangers posed by the ingredients in them are underestimated. A healthy young man came to the emergency room with palpitations and feeling unwell. Initial history and clinical examination were non-contributory to find the cause. ECG showed atrial fibrillation. A detailed history for any over the counter or herbal medicine use confirmed that he was taking supplements to bulk muscle. One of the components in these supplements is yohimbine; the onset of symptoms coincided with the ingestion of this product and the patient is symptom free after stopping it. This report highlights the dangers to the public of consuming over the counter products with unknown ingredients and the consequential detrimental impact on health.
Background
Muscle strengthening supplements from over the counter (OTC) and internet are increasingly being consumed. The components in these substances can include anabolic steroids, carnitine, caffeine, creatine, dimethyl amylamine, glutamine, nitric oxide, prohormones, yohimbine and reserpine. These are claimed to burn fat, increase muscle bulk and strength. However, they can present a variety of acute and chronic health hazards that can have dire consequences on the body. Food and Drug Administration (FDA) has warned the public of the dangers posed by these products.1 Complications such as infertility, stunted growth, dyslipidaemias and stroke; serious kidney, liver and coronary artery disease; and pulmonary embolism are well described.
Case presentation
A 22-year-old healthy miner presented to the emergency room with palpitations and feeling unwell. He initially thought that his drink had been spiked, leading to his symptoms. He denied drug abuse. He noticed palpitations of 3-week duration. His medical and family history were insignificant. Physical examination was significant for tattoo marks and rapid irregularly irregular pulse.
Investigations
ECG confirmed atrial fibrillation (figure 1A, B). Urine toxicology screen was negative for amphetamines, cocaine and tetrahydrocannabinol. Troponin, thyroid function tests and a metabolic panel were normal. Echocardiogram revealed mild mitral regurgitation with good systolic function. Because of his abnormal liver function tests, he underwent ultrasonography followed by CT imaging, which confirmed a vascular malformation.
Figure 1.
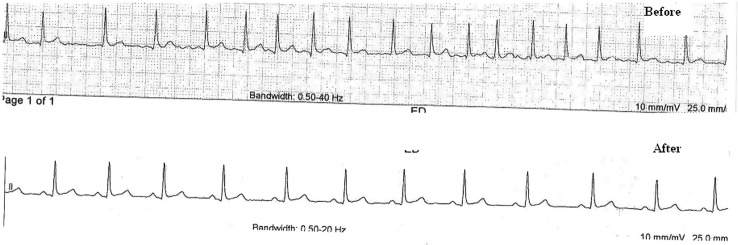
ECG before and after treatment.
Differential diagnosis
The common causes of atrial fibrillation are valvular heart disease, thyrotoxicosis, coronary heart disease, cardiomyopathies, heart failure, hypertension and drugs.
Treatment
Once our patient's urine screening was negative for amphetamines, he received metoprolol orally. This had no impact on the heart rate. Indeed the rate increased to 160/min. Amiodarone 300 mg bolus intravenous injection reverted the rhythm to sinus.
Outcome and follow-up
As our patient's findings were unexpected we questioned him on the use of any OTC medications or herbal medicines. Further detailed history revealed that he had started consuming the body build up supplement oxyelite recently and has been experiencing some thumping of the heart now and then. The usage had a temporal relationship with his symptoms. He remains symptom free at 4 months after stopping its ingestion and is under follow-up for his liver function tests.
Discussion
Our patient was consuming oxyelite containing unspecified qualities of herbs including Bauhinia purpurea, Pausinystalia johimbe (yohimbine), norcoclaurine HCL, Cirsium oligophyllum (whole plant extract), aegeline, 1,3-dimethylamylamine HCl, glucuronolactone and l-carnitine. Yohimbine is a crystalline indole plant alkaloid naturally occurring in Pausinystalia yohimbe (yohimbe, Indian snakeroot, niando). The half-life of yohimbine is 1 h and the active metabolite 11-hydroxyyohimbine has a half-life of 6 h.2 Yohimbine is added to many muscle bulking supplements as it has some lipid mobilising effects. It is used to treat erectile dysfunction. It is possible that oxyelite may contain other unknown substances having cardiovascular effects. However, yohimbine is the known culprit in our patient. Yohimbine has α2 antagonist action at the presynaptic and postsynaptic receptors. β-Blockers have not only not helped but also possibly made his tachycardia worse. Yohimbine increases the release of norepinephrine in the peripheral noradrenergic terminal.3 It also increases sympathetic outflow from the brain.4 Yohimbine can cause abnormal liver functions. Although our patient had a mild valvular disease, in this background a total α2 blockade induced this arrhythmia.
Fatalities are reported with consumption of high concentration yohimbine.5 The onset of action can be quick or delayed up to 3 weeks. In an earlier case reported from the UK, a patient with insulin-dependent diabetes with underlying cardiac disease manifested atrial fibrillation when yohimbine was prescribed for erectile dysfunction.6 We are not aware of reports of atrial fibrillation secondary to the use of body building supplements. OTC supplements can be causal to atypical disease manifestations. Oxyelite Pro products were linked to non-infectious liver failure attributed to adulteration.7 FDA ordered recall of some of these products in the USA.8 The reporting of the adverse events to drugs is streamlined. However, those attributable to dietary supplements are reported on a voluntary basis to the FDA in the USA. This can be done both by the general public and the clinical practitioners. These principles vary widely in different countries. Overall the industry is less regulated. Ensuring public safety is an ethical principle and forms part of duty of care. The inadequacies in monitoring of nutritional supplements and potential health hazards of legal and illegal ingredients is the focus of a latest perspective article.9 Our report highlights the perils of consuming OTC food supplements with unknown toxic ingredients leading to complex clinical conundrums.
Learning points.
Yohimbine as an ingredient in over the counter (OTC) supplements should be searched for and discouraged from use. Physicians should be aware of the side effects profiles.
Yohimbine causes atrial fibrillation.
OTC drugs are often not reported by patients during the routine medication history. Physicians should start modifying this section to medication, OTC and supplemental product use.
There is a need to review the regulation for marketing supplemental agents.
Acknowledgments
This case was presented as a poster at the Royal Australasian College of Physicians Congress in Auckland 2014.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.http://www.fda.gov/food/dietarysupplements/qadietarysupplements/ucm346576.htm (accessed 18 Nov 2013).
- 2.Sturgill MG, Grasing KW, Rosen RC, et al. Yohimbine elimination in normal volunteers is characterized by both one- and two-compartment behavior. J Cardiovasc Pharmacol 1997;29:697–703 [DOI] [PubMed] [Google Scholar]
- 3.Berlan M, Galitzky J, Riviere D, et al. Plasma catecholamine levels and lipid mobilization induced by yohimbine in obese and non-obese women. Int J Obes 1991;15:305–15 [PubMed] [Google Scholar]
- 4.Grossman E, Rea RF, Hoffman A, et al. Yohimbine increases sympathetic nerve activity and norepinephrine spillover in normal volunteers. Am J Physiol 1991;260:R142–7 [DOI] [PubMed] [Google Scholar]
- 5.Anderson C, Anderson D, Harre N et al. Two fatal case reports of acute yohimbine intoxication. J Anal Toxicol 2013;37:611–14 [DOI] [PubMed] [Google Scholar]
- 6.Varkey S. Overdose of yohimbine. BMJ 1992;304:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.http://www.fda.gov/food/recallsoutbreaksemergencies/outbreaks/ucm370849.htm (accessed 9 Sep 2014).
- 8.http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm374395. htm (accessed 30 Dec 2013).
- 9.Cohen PA. Hazards of hindsight—monitoring the safety of nutritional supplements. New Engl J Med 2014;370:1277–80 [DOI] [PubMed] [Google Scholar]


