Abstract
Eosinophilic cystitis (EC) is a rare disease. We describe three cases, where presentations of the disease are similar. To highlight probable causes of the disease, symptoms, clinical findings and treatment modalities, we reviewed 56 cases over a 10-year period. The most common symptoms were frequency, dysuria, urgency, pain and haematuria. Common clinical findings were presence of bladder mass, peripheral eosinophilia and thickened bladder wall. A variety of medical treatments were used, most frequently steroids, antibiotics and antihistamines. Recurrence occurred in patients on tapering or discontinuing prednisone, among other reasons. There is no consensus about the treatment of EC, but In light of our findings in this review, the treatment of choice in our department will be tapered prednisone over 6–8 weeks in combination with antihistamine.
Background
Eosinophilic cystitis (EC) is a rare bladder disease first described by Edwin Brown in 1960.1 Histological findings include transmural inflammation of the bladder, predominantly with eosinophil. In the chronic stage, fibrosis and muscle necrosis may also be seen which may lead to contracted bladder.2 3
The cause and pathogenesis of the disease is unclear, and not fully understood. It is speculated that an antigen–antibody complex is formed on antigen exposure in the bladder. This IgE-mediated response leads to degranulation of mast cells, thereby attracting eosinophil and causing an inflammatory response with tissue damage.4 5 Many aetiologies and associations to other diseases have been proposed.3 5 These include different medications, vesical injury, chronic vesical irritation, subsequent to vesical surgery, parasitosis, allergy to food and drugs, urinary tract infection (UTI), urothelial carcinoma, autoimmune disorders and eosinophilic enteritis.5 6
EC is described in all age groups,2 although to a lesser degree in the paediatric population.4 The condition usually causes irritative bladder symptoms, suprapubic pain and haematuria.3 In some cases the findings may simulate UTI or on radiological imaging and cystoscopy, mimic a malignant lesion.6 7
It is important to focus on this rare disease, as the variable symptomatic and clinical presentation of EC may lead to delayed diagnosis and treatment. In turn, delayed or insufficient treatment of EC can lead to increased discomfort for the patient due to the potential chronicity of the condition, as well as recurrence of symptoms.
Case presentation
Case 1
A 14-year-old boy with a medical history of type 1 diabetes, asthmatic bronchitis and allergies, was referred to paediatric outpatient evaluation due to 2 weeks persistent microscopic haematuria, dysuria, frequency and one event of painful terminal gross haematuria. Urine cultures were performed twice during the 2-week period, both negative. Physical examination was unremarkable. Urinalysis revealed pyuria and haematuria. Repeated urine culture was negative.
The patient was referred to our department. CT urography and blood analysis were normal. A flexible cystoscopy revealed hyperaemia of the whole bladder mucosa with no signs of bladder mass. Fluorescence cystoscopy with biopsies was performed 3 weeks later, and findings at this time were discreet. Postoperatively the patient was treated for suspected UTI due to recurrence of symptoms, but urine culture was negative. Histopathology was consistent with EC, showing severe subepithelial inflammatory changes with predominance of eosinophil (figure 1). The patient was evaluated at a specialist department. At this time, 2 months after biopsies were taken, the patient was asymptomatic. Owing to the patient's diabetes, and him being asymptomatic, no treatment was initiated. The patient remains asymptomatic 5 months after the biopsies revealed EC, and is scheduled for follow-up in 1 year.
Figure 1.
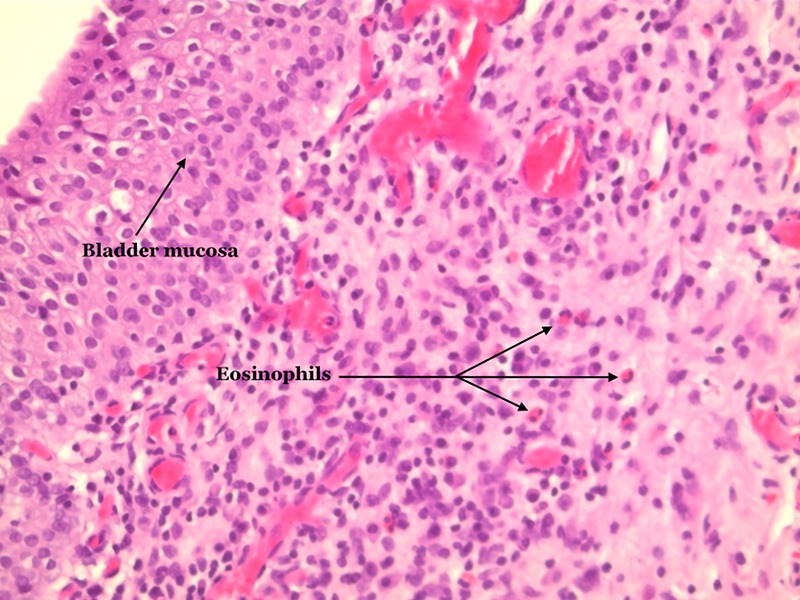
Bladder biopsy with inflammation in the lamina propria. Several eosinophilic granulocytes, with red granules in the cytoplasm and two nuclear segments (H&E ×20).
Case 2
A 78-year-old man was referred to our department due to gross haematuria, frequency and dysuria throughout 2 months. His medical history included hypertension, nephropathy and coronary bypass. CT scan revealed thickened bladder wall (figure 2). Urinalysis showed pyuria, haematuria and proteinuria and the patient was treated for suspected UTI. Subsequent urine culture was negative. Cystoscopy revealed oedematous, hyperaemic bladder mucosa suspicious of carcinoma in situ. Biopsies showed severely inflamed bladder mucosa and benign lymphoid follicles and eosinophilia infiltration (figure 3). The patient was treated with prednisone 37.5 mg per day for 10 days. Haematuria subsided and frequency at daytime improved, although the patient still reported frequent urination during the night. Urine culture was still negative.
Figure 2.
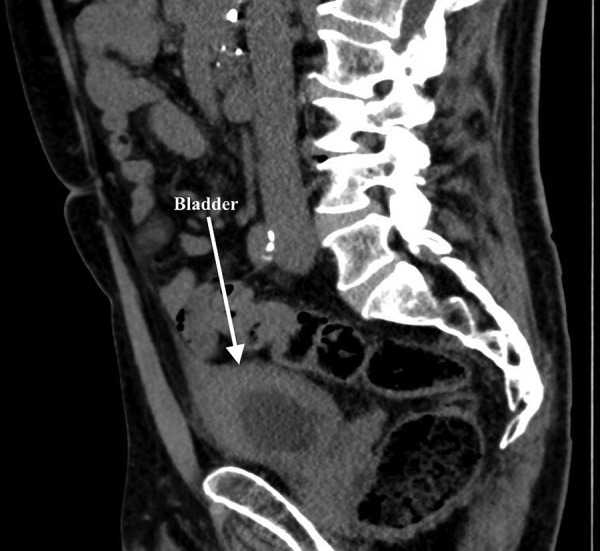
CT scan with sagittal section, an arrow shows thickened bladder wall.
Figure 3.
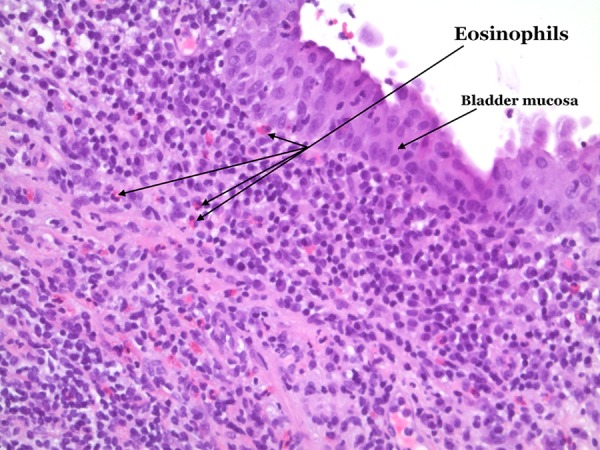
Bladder biopsy; inflammation with eosinophilic granulocytes in the lamina propria (H&E ×20).
Two and a half months later, the patient experienced gross haematuria. Urinalysis was still positive for erythrocytes, white cell count and protein. Treatment with prednisone for 10 days was initiated.
Three months later, the patient experienced symptom recurrence again. CT revealed sustained thickening of the bladder wall, and at cystoscopy, biopsies were taken from an erythematous plaque. Histopathology was consistent with EC. The patient's symptoms resolved without medical treatment and the patient is scheduled for follow-up in 6 months.
Case 3
A 83-year-old man was admitted to our department due to gross haematuria and urinary retention. His medical history included hypertension, atrial fibrillation and benign prostatic hypertrophy. Urinalysis revealed pyuria, haematuria and positive nitrite. He was treated with antibiotics and insertion of indwelling bladder catheter. CT urography showed benign, bilateral kidney cysts. Cystoscopy revealed vulnerable, erythematous bladder mucosa and a bladder mass. The bladder mass was resected and biopsies from bladder mucosa were obtained. Histological findings were acute and chronic inflammation, granulation tissue, eosinophilia and fibrosis. The patient's symptoms resolved after surgery. Twenty-three months later the patient had recurrence of gross haematuria and cystoscopy findings were normal apart from some bleeding from the prostate. Treatment with prednisone was not considered.
Investigations
Case 1
CT urography: normal
Blood analysis: normal.
Urinalysis: pyuria, haematuria, negative culture.
Cystoscopy: hyperaemia of bladder mucosa.
Case 2
CT scan: thickened bladder wall.
Blood analysis: normal.
Urinalysis: pyuria, proteinuria, haematuria, negative culture.
Cystoscopy: oedematous, hyperaemic bladder mucosa.
Case 3
CT urography: benign kidney cysts.
Blood analysis: marginally elevated creatinine (1.22 mg/dL) and slight anaemia (haemoglobin 7.8 mmol/L). Creatinine levels normalised after surgery, and haemoglobin reached normal levels 1 month postsurgery.
Urinalysis: haematuria. Five months later: pyuria, haematuria and positive nitrite, positive urine culture (at this time the patient had indwelling catheter).
Cystoscopy: erythema, bladder mass.
Differential diagnosis
Interstitial cystitis
Carcinoma in situ (CIS)
Haemorrhagic cystitis
Bladder cancer
Lower urinary track symptoms (LUTS)
Treatment
Case 1: Owing to the patient's diabetes and asymptomatic feature of EC, prednisone treatment was not initiated.
Case 2: Two courses of prednisone 37.5 mg for 10 days.
Case 3: Resection of bladder mass.
Outcome and follow-up
Case 1: Symptoms recurred after surgery, but subsided once again, spontaneously. The patient remains asymptomatic 5 months after bladder biopsies and a 1-year follow-up is scheduled.
Case 2: The patient was treated with prednisone 37.5 mg/day after surgery. At recurrence 3 months later, a new course of prednisolone initiated. At second recurrence bladder lesions were biopsied, and the patient's symptoms resolved with no further treatment. Follow-up is scheduled within 6 months.
Case 3: The patient became asymptomatic after resection of bladder mass. At recurrence of haematuria, there was no evidence of EC relapse.
Discussion
We performed a literature search in PubMed from 2003 to 2013 using the search word ‘eosinophilic cystitis’, ‘eosinophilic AND bladder’. The search generated 62 items. Of these, 30 were not of relevance on the topic or were not English. The remaining 32 articles included 56 cases of EC.
The most common symptoms and clinical findings of EC are listed in tables 1 and 2 respectively.
Table 1.
Common symptoms in EC
Table 2.
Clinical findings in EC
| Finding | N |
|---|---|
| Haematuria (gross, microscopic)2 4 7 9–21 23 24 27 32–35 | 28 (50%) |
| Bladder mass/velvety lesions2 4 7 9 10 13 15 16 18 20 23 28 29 33 35 36 | 27 (48%) |
| Peripheral eosinophilia4 7 9 11 12 15 16 18 20 22 25–27 31 33 34 | 21 (38%) |
| Thickened bladder wall2 4 7 9 10 13 15 16 18 20 23 28 29 33 35 36 | 21 (38%) |
| Haemorrhagic lesions/erythema/ulcers/erythematous plaques8 10 14 19 20 24 27 32 34 36 | 16 (29%) |
| Oedema/inflammation of the bladder mucosa2 4 9 11 12 14 17–20 25 27 34 | 15 (27%) |
| Hydronephrosis/hydroureteronephrosis2 4 11 15 16 29 32 33 | 11 (20%) |
| Pyuria4 9 11 12 16 18 23 28 34 | 10 (18%) |
| Positive urine culture4 11 13 16 18 20 32 | 9 (16%) |
| Reduced bladder capacity/compliance4 25 28 | 3 (5%) |
| Vesicoureteral reflux4 17 29 | 3 (5%) |
| Inflammation of the ureter/pelvis/ureteral orifice17 31 | 2 (4%) |
| Paracytosis8 32 | 2 (4%) |
| Stenosis of the ureteral orifice29 | 1 (2%) |
| Bladder neck contracture13 | 1 (2%) |
| Abdominal distention (free fluid in abdomen)30 | 1 (2%) |
In the majority of cases, there was no obvious cause for EC. Some cases of EC were most likely caused by medication (penicillin,25 Coumadin,13 BCG,15 levetiracetam,4 dimethyl sulfoxide and Mitomycin-C2 13). The most common causative and associative factors to EC are listed in table 3. Less common possible causes of EC were mononucleosis,20 recurrent UTI,20 32 chronic granulomatous disease,23 BK polyoma virus infection,13 blood cord transfusion,14 bladder stones,2 13 previous pelvic trauma,19 nephrogenic adenoma,29 parasitic infection8 32 and hypereosinophilic syndrome.7 12 27
Table 3.
Possible causes and associations of EC to other diseases
| Causes/associations | N |
|---|---|
| History of—or family history of asthma/allergy/atopy, family history and/or positive skintest2 4 10 11 13 18 20 22 25 31 35 | 15 (27%) |
| Bacterial infection4 11 13 16 18 20 32 | 9 (16%) |
| Medically induced2 4 13 15 25 31 | 7 (13%) |
EC, eosinophilic cystitis
Owing to the rarity of EC, treatment is not standardised and treatment modalities as well as course of treatment varied throughout cases (table 4). Steroids were most commonly used, but 21% of patients treated with steroids either had no relief of symptoms or symptoms recurred during or after treatment. Of these patients, three underwent surgical treatment,17 20 29 one patient received a longer treatment with steroids at recurrence,24 and one was started on ciclosporin combined with antihistamine.23 Finally, a parasitic infection was detected in one patient who was then treated accordingly.8 The most common combination of medications used, were antihistamines with steroids.2 10 11 16 23 27 31 33 Twelve patients (21%) underwent surgery as the only treatment.2 13 30 35 36
Table 4.
Treatments for EC
| Treatment | N |
|---|---|
| Steroids2 4 7–12 14–19 22–24 26 27 29 31 33 34 | 29 (52%) |
| Antibiotics (treatment or prophylactic)4 9 11–13 16 18 20 26 28 32 | 14 (25%) |
| Antihistamine2 10 11 16 20 23 27 28 31–33 | 14 (25%) |
| Surgery (excluding bladder biopsies/resection of bladder lesions)2 13 17 19–21 | 8 (14%) |
| NSAID7 10 20 33 | 5 (9%) |
| No treatment13 25 | 4 (7%) |
| Antiparasitic medication8 28 32 | 3 (5%) |
| Montelucast8 18 | 2 (4%) |
| Infravesical sodium pentosan polysulfate17 31 | 2 (4%) |
| Spasmolytic10 13 | 2 (4%) |
| Infravesical steroid17 32 | 2 (4%) |
| α-receptor blocker25 27 | 2 (4%) |
| TUR-B15 16 | 2 (4%) |
| Gabapentine31 | 1 (2%) |
| Nephrostomy15 | 1 (2%) |
| Ciclosporin23 | 1 (2%) |
| Sodium chromoglycate7 | 1 (2%) |
| Suplatast tosilate (cytokine inhibitor)9 | 1 (2%) |
NSAID, non-steroidal anti-inflammatory drug.
Sixteen per cent of patients had recurrence of symptoms either during or after primary treatment. In two patients, recurrence of symptoms occurred while tapering prednisone.7 23 Two patients had relapse of symptoms after discontinuing prednisone,24 27 and steroid treatment was reinitiated. Symptoms recurred in one patient after ended prednisone treatment while tapering montelucast,18 and in another while tapering antihistamine.28 One patient, where resection of EC lesions was performed without further medical treatment, had recurrence of symptoms 1 year postoperatively and was treated with prednisone causing symptoms to resolve.2 Two patients had debilitating cases of EC, where no medical treatment led to persistent resolution of symptoms, which prompted surgical intervention with cystoureterectomy17 and partial cystectomy.20
Of the cases with symptom recurrence, the most obvious cause was insufficient treatment with steroids (early treatment interruption or tapering). In light of this, as well as our experience with recurrence after short-term treatment with prednisone (case 2), the treatment of choice at our institution, will be removal of the suspected causative factor to the disease combined with long-term tapered prednisone (6–8 weeks) and antihistamine. To ensure timely treatment modification in cases of EC treatment failure and recurrence, we have now set up a scheduled follow-up regimen for patients diagnosed with EC in our department. In cases where patients become symptom-free after resection of EC lesions, medical treatment is not initiated, and patients are reassessed after 1 year. In cases where medical treatment is initiated, treatment evaluation is performed after 2 weeks. If symptoms are improving the patients are evaluated again after 4 weeks and at treatment completion where flexible cystoscopy is performed to ensure that lesions have fully regressed. If symptoms have not improved, or are worsening at first follow-up, flexible cystoscopy is performed. In cases where lesions are found, prednisone dosage is altered and re-evaluation of treatment efficacy is planned after 1–2 weeks. Hereafter, individual future evaluation is planned depending on symptoms and clinical findings.
At recurrence, or in cases where systemic steroids are relatively contraindicated (case 1), bladder instillation with steroids may become a treatment option. In refractory cases, surgery may be the only curative treatment.
Learning points.
Eosinophilic cystitis (EC) is a rare disease with transmural infiltration of eosinophil.
Diagnosis of EC is histopathological.
Patients with EC often have symptoms and clinical findings common to other urological disorders, such as urinary tract infection, malignancy and lower urinary symptoms.
No standardised treatment exists, but common, effective treatments include steroids, antihistamines, and surgery—often in combination.
Acknowledgments
The authors thank Anette Pedersen Pilt, PhD, Department of Pathology, Roskilde Hospital, Kogevej for preparing histological pictures.
Footnotes
Contributors: KSSM and NHA gave substantial contributions to the conception or design of the work. NHA and CD were involved in the drafting of the work or revising it critically for important intellectual content. NHA gave final approval of the version to be published.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brown EW. Eosinophilic granuloma of the bladder. J Urol 1960;83:665–8 [DOI] [PubMed] [Google Scholar]
- 2.Kumar S, Sharma V, Ganesamoni R, et al. Eosinophilic cystitis mimicking tuberculosis: an analysis of five cases with review of literature. Urol Ann 2013;5:50–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Ouden D. Diagnosis and management of eosinophilic cystitis: a pooled analysis of 135 cases. Eur Urol 2000;37:386–94 [DOI] [PubMed] [Google Scholar]
- 4.Sparks S, Kaplan A, DeCambre M, et al. Eosinophilic cystitis in the pediatric population: a case series and review of the literature. J Pediatr Urol 2013;9(6 Pt A):738–44 [DOI] [PubMed] [Google Scholar]
- 5.Teegavarapu PS, Sahai A, Chandra A, et al. Eosinophilic cystitis and its management. Int J Clin Pract 2005;59:356–60 [DOI] [PubMed] [Google Scholar]
- 6.Itano NM, Malek RS. Eosinophilic cystitis in adults. J Urol 2001;165:805–7 [PubMed] [Google Scholar]
- 7.Mallat F, Hmida W, Mestiri S, et al. Eosinophilic cystitis with eosinophilic cholecystitis: a rare association. Case Rep Urol 2013;2013:146020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerruto MA, D'Elia C, Artibani W. A case of eosinophilic cystitis in patients with abdominal pain, dysuria, genital skin hyperemia and slight toxocariasis. Arch Ital Urol Androl 2013;85:99–100 [DOI] [PubMed] [Google Scholar]
- 9.Yoshino T, Moriyama H. Case of eosinophilic cystitis treated with suplatast tosilate as maintenance therapy. Case Rep Urol 2012;2012:354219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caso J, Qin D, Sexton WJ. Eosinophilic cystitis following immediate post-resection intravesical instillation of mitomycin-C. Can J Urol 2010;17:5223–5 [PubMed] [Google Scholar]
- 11.Galutira PJ, Canonigo BB, Cabansag MR, et al. Presenting manifestations of eosinophilic cystitis in two Filipino children 2. Int Urol Nephrol 2010;42:557–63 [DOI] [PubMed] [Google Scholar]
- 12.Kim MS, Park H, Park CS, et al. Eosinophilic cystitis associated with eosinophilic enterocolitis: case reports and review of the literature. Br J Radiol 2010;83:e122–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Popescu OE, Landas SK, Haas GP. The spectrum of eosinophilic cystitis in males: case series and literature review. Arch Pathol Lab Med 2009;133:289–94 [DOI] [PubMed] [Google Scholar]
- 14.Tasaka T, Matsuhashi Y, Ohnishi H, et al. Eosinophilic cystitis following cord blood transplantation: a form of acute GVHD. A variant of hemorrhagic cystitis after hematopoietic SCT or drug-induced? 26. Bone Marrow Transplant 2008;42:495–6 [DOI] [PubMed] [Google Scholar]
- 15.Hidoussi A, Slama A, Jaidane M, et al. Eosinophilic cystitis induced by bacillus Calmette-Guerin (BCG) intravesical instillation. Urology 2007;70:591.e9–10 [DOI] [PubMed] [Google Scholar]
- 16.Salman M, Al-Ansari AA, Talib RA, et al. Eosinophilic cystitis simulating invasive bladder cancer: a real diagnostic challenge. Int Urol Nephrol 2006;38:545–8 [DOI] [PubMed] [Google Scholar]
- 17.Philip J, Ali FS, Zakhour HD, et al. Severe eosinophilic cystitis in a woman with anorexia nervosa. Int J Urol 2006;13:1132–3 [DOI] [PubMed] [Google Scholar]
- 18.Sterrett S, Morton J, Perry D, et al. Eosinophilic cystitis: successful long-term treatment with montelukast sodium. Urology 2006;67:423. [DOI] [PubMed] [Google Scholar]
- 19.Martino G, Torcasio A, Iavarone C, et al. Eosinophilic cystitis associated with urethral stricture disease from pelvic trauma. Case report and literature review. G Chir 2005;26:425–9 [PubMed] [Google Scholar]
- 20.Thompson RH, Dicks D, Kramer SA. Clinical manifestations and functional outcomes in children with eosinophilic cystitis 40. J Urol 2005;174:2347–9 [DOI] [PubMed] [Google Scholar]
- 21.Guerra LA, Pike J, Filler G, et al. Pseudo-tumoral eosinophilic cystitis in a 3 year-old girl. Can J Urol 2005;12:2846–8 [PubMed] [Google Scholar]
- 22.Amin MA, Hamid MA, Saba S. Hypereosinophilic syndrome presenting with eosinophilic colitis, enteritis and cystitis. Chin J Dig Dis 2005;6:206–8 [DOI] [PubMed] [Google Scholar]
- 23.Barese CN, Podesta M, Litvak E, et al. Recurrent eosinophilic cystitis in a child with chronic granulomatous disease. J Pediatr Hematol Oncol 2004;26:209–12 [DOI] [PubMed] [Google Scholar]
- 24.Thaxton CS, Eggener SE, Schaeffer AJ. Plasma cell infiltration of the urinary bladder 36. Urology 2004;64:156–7 [DOI] [PubMed] [Google Scholar]
- 25.Tsakiri A, Balslev I, Klarskov P. Eosinophilic cystitis induced by penicillin 44. Int Urol Nephrol 2004;36:159–61 [DOI] [PubMed] [Google Scholar]
- 26.Thomas JC, Ross JH. Eosinophilic cystitis in a child presenting with a bladder mass 47. J Urol 2004;171:1654–5 [DOI] [PubMed] [Google Scholar]
- 27.Kojima K, Maeda J, Mikami S, et al. Eosinophilic cystitis presented as a manifestation of hypereosinophilic syndrome: a case report and review of the literature. Nephron Extra 2013;3:30–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abilov A, Ozcan R, Polat E, et al. Rare cause of dysuria: eosinophilic cystitis 1. J Pediatr Urol 2013;9:e6–8 [DOI] [PubMed] [Google Scholar]
- 29.Rossi E, Pavanello P, Marzola A, et al. Eosinophilic cystitis and nephrogenic adenoma of the bladder: a rare association of 2 unusual findings in childhood. J Pediatr Surg 2011;46:e31–4 [DOI] [PubMed] [Google Scholar]
- 30.Hwang EC, Kwon DD, Kim CJ, et al. Eosinophilic cystitis causing spontaneous rupture of the urinary bladder in a child. Int J Urol 2006;13:449–50 [DOI] [PubMed] [Google Scholar]
- 31.Abramov Y, Goldberg RP, McGuire M, et al. Eosinophilic cystitis after bladder instillation with dimethyl sulfoxide. Urology 2004;63:1182–3 [DOI] [PubMed] [Google Scholar]
- 32.Zaman SR, Vermeulen TL, Parry J. Eosinophilic cystitis: treatment with intravesical steroids and oral antihistamines 4. BMJ Case Rep 2013;2013:pii: bcr2013009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HH, Yen TH, Huang CC, et al. Blood eosinophilia, corticoadrenal insufficiency and eosinophilic cystitis. Urol Int 2008;80:219–21 [DOI] [PubMed] [Google Scholar]
- 34.Tamai K, Koyama T, Saida S, et al. MR imaging findings of eosinophilic cystitis in an 8-year-old girl. Pediatr Radiol 2007;37:836–9 [DOI] [PubMed] [Google Scholar]
- 35.Al-Omar O, Fisher MB, Sarle R, et al. Eosinophilic cystitis in a neonate. J Urol 2005;173:576. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Das SK, Trivedi S, et al. Genito-urinary polyps: summary of the 10-year experiences of a single institute. Int Urol Nephrol 2008;40:901–7 [DOI] [PubMed] [Google Scholar]


