Abstract
Sialidosis is a lysosomal storage disease caused by deficit of neuraminidase. It is an autosomal recessive disease, heterogeneous in its onset, presentation and prognosis. We report a case of a male patient with molecular and enzymatic confirmation of the diagnosis. Symptoms began at age 26 with reduced visual acuity, bilateral cherry-red spots and later myoclonus. A brother, now deceased, had the same confirmed disease. We describe the symptoms and clinical findings of the patient, as well review the current knowledge on the topic. With this report, we highlight the importance of a clinical history integrating all the patient’s symptoms in order to achieve the diagnosis. In the presence of a cherry-red spot, a comprehensive study is mandatory. Despite being a rare disease, sialidosis carries a significant burden for its patients and its diagnosis should always be considered in the appropriate setting.
Background
Sialidosis is a rare lysosomal storage disorder1 caused by an isolated deficiency of the enzyme α-N-acetyl neuraminidase, resulting in an accumulation of its substrates. This disease has a wide range of clinical manifestations. It is a rare disease, but its diagnosis must always be considered in the presence of a cherry-red spot. A thorough clinical, biochemical and genetic approach may confirm the diagnosis and allow proper treatment, improving the patient’s quality of life.
Case presentation
A 53-year-old man, with non-consanguineous parents, presented to our hospital with a history of progressive decrease of visual acuity since the age of 26. At 36, he developed generalised myoclonus and ataxic gait.
He showed low visual acuity, ataxic gait, dysarthria and difficulty in writing. No significant medical or pharmacological history was known.
Ophthalmological evaluation revealed low visual acuity (best corrected visual acuity: 20/80 on the right eye and 20/40 on the left eye), bilateral horizontal nystagmus, bilateral cortical and posterior subcapsular cataracts and regular intraocular pressure. Funduscopic examination revealed bilateral macular cherry-red spots. Cup to disk ratio was 0.3 bilaterally.
Optical coherence tomography (OCT) revealed normal results, however, the presence of nystagmus hindered an appropriate retinal imaging. OCT of the nerve fibre layer revealed atrophy of nerve fibres, especially in the upper and lower quadrants. Perimetry tests presented with a defective temporal field in the right eye and an arcuate scotoma in the left eye, but these results provided a low level of confidence influenced by the presence of nystagmus. Multifocal electroretinography was consistent with the presence of maculopathy with cone dysfunction. EEG revealed an epileptic component of myoclonus. Brain MRI revealed cerebellar and cerebral cortical atrophy.
Skin biopsy with cultured fibroblasts and leucocytes (in 1995) showed null neuraminidase activity levels and normal β-galactosidase levels (in cultured fibroblasts 354, normal 166–2037; in cultured leucocytes 110, normal 73–585). This study was performed at a specialised institute. The chromatographic profile of oligosaccharides as considered compatible with the disease. The molecular study, conducted in 2009, identified presumably causal mutations (composite heterozygous mutations c.700G>A (p.D234N) exon 4, c.1021C>T(p.R341X) exon 5). Although none of these mutations was present in previous cohorts, the observed base change leads to the production of a truncated protein, and so was presumed as causal by the laboratory. The same mutation was later found in other patients with sialidosis type I, as described in more recent papers,2 3 thus reinforcing our previous diagnosis.
This patient is also followed in Neurology and in Physical Medicine and Rehabilitation, which provides a multidisciplinary approach and improves his quality of life.
One brother was also studied for sialidosis. He was not followed in our hospital so clinical data available are scarce. Skin biopsy with cultured fibroblasts and leucocytes also showed null neuraminidase activity levels (in 1995), with an abnormal pattern of urinary oligosaccharides (chromatographic profile of oligosaccharides with combined sialic acid compounds). He died of an unrelated cause.
Differential diagnosis
The cherry-red spot sign has also been described in Sandhoff disease, galactosialidosis, GM1 gangliosidosis, GM2 gangliosidosis, Goldberg syndrome, metachromatic leucodystrophy, Niemann-Pick disease types A, B, C and D, Farber lipogranulomatosis, multiple sulfatase deficiency, Gaucher disease, poisoning (dapsone) and Wolman disease.4 It often follows central retinal artery occlusion, which shows a pale retina as a result of reduced blood flow.
The characteristic appearance in sialidosis results from deposition of material in the ganglionic cells of the retina at the macula, where these cells are several layers thick. As ganglion cells are absent in the fovea, this area retains a relative transparency and contrasts with the surrounding opaque retina. This appearance is seen only in Caucasians. Some authors use the term ‘perifoveal white patch’ to describe it.5 Over time, the ganglion cells die and the spot becomes less evident.6
Treatment
Currently, there is no available therapy for sialidosis. Treatment consists of symptomatic measures involving a multidisciplinary team aimed at improving the patient's quality of life.
Our patient is being treated with antiepileptic and antispasmodic medication, which provides poor control of his symptoms.
Recent studies suggest a therapeutic role for a combination of a specific immunosuppressant and a proteosomal inhibitor aimed at enhancing mutant enzyme activity.7 Some of the NEU1 mutations associated with type I sialidosis may respond to PPCA-chaperone-mediated gene therapy with an increased enzymatic activity.8
Outcome and follow-up
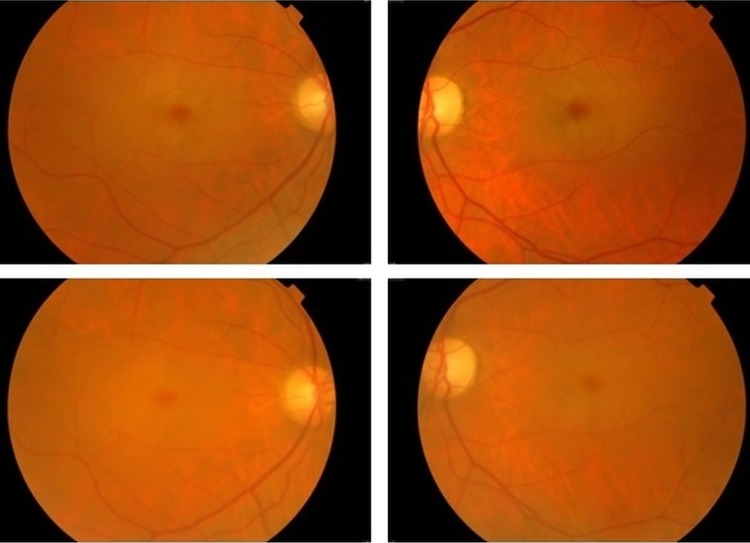
Best corrected visual acuity continued to decline and was 20/400 for the right eye and 20/200 for the left eye on the last medical observation (with an interval of 6 years from the first observation). Myoclonus and ataxic gait also showed no resolution. Funduscopic examination found that the bilateral cherry-red spots remained, yet were less noticeable (figure 1). The results of complementary studies (OCT, perimetry) were overlapping. The patient continues therapy with antiepileptic and antispasmodic medication.
Figure 1.
Color fundus photography discloses bilateral macular cherry-red spots but less prominently in 2014 (images below) compared with 2008 (images above).
Discussion
The lysosomal enzyme maps to chromosome 6p21 and various enzyme-inactivating mutations have been identified.9 The deficiency of α-N-acetyl neuraminidase (caused by mutations in the NEU1 gene) interrupts the normal catabolic pathway, resulting in cellular accumulation of substrates ordinarily degraded by that enzyme.10 This accumulation causes changes in cellular architecture, with emerging vacuolisation.11
Sialidosis is categorised into two clinical phenotypes, distinguished by the presence or absence of dysmorphic and somatic features. Patients with sialidosis type I (the less severe type)12 develop visual deficits, myoclonic epilepsy and ataxia in the second decade of life. Cherry-red spots are present in all patients. The type II sialidosis can be subdivided into two forms: congenital and infantile. The congenital type may be manifested by hydrops fetalis, hepatomegaly and premature death. The infantile form evolves with Hurler-like facies, osseous involvement and early developmental delay.1 The cherry-red spot is present in less than 75% of cases.
With respect to the ophthalmological findings, we can observe progressive decrease of visual acuity, cherry-red spots, nystagmus, corneal clouding, cataracts and optic atrophy. Sialidosis also presents with neurological symptoms (myoclonus, hypotonia, development delay), coarse facial features and skeletal and abdominal findings.1
The diagnosis is based on identification of a mutation in the NEU1 gene or of a deficient neuraminidase activity in leucocytes and cultured fibroblasts, assessed through skin biopsy.1 Assay of abnormal urinary oligosaccharides may suggest sialidosis. Brain MRI can show brain atrophy.13
Prognosis depends on the type of disease. In type I disease, life expectancy is unchanged despite the profound impact in the patient's quality of life. In type II, life expectancy is very low, with patients rarely surviving past the second decade of life.14
With this clinical report, we highlight the importance of a good clinical history integrating all the patient’s symptoms, in order to achieve a correct diagnosis. Ophthalmological observation is relevant and helpful. In the presence of a cherry-red spot sign, several differential diagnoses should be considered. An extensive laboratory and genetic study must be undertaken in order to rule in or out possible diagnoses. Despite sialidosis being a rare disease, it is a likely diagnosis in the appropriate setting, and trained clinicians should have the knowledge and clinical judgment to identify it.
Learning points.
Sialidosis is a rare disease, but a likely diagnosis in the presence of neurological findings accompanying a cherry-red spot sign.
An extended complete complementary study is necessary in the presence of a cherry red spot sign.
As sialidosis carries a significant burden to their patients, implementation of symptomatic measures can improve patient's quality of life.
Footnotes
Contributors: IS contributed to acquisition of the data, analysis and interpretation of the data, drafting of the manuscript, and gave final approval; MdLC and JF contributed to the critical revision of the manuscript and gave final approval; RS contributed to drafting and critical revision of the manuscript, and gave final approval.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vieira de Rezende Pinto WB, Sgobbi de Souza PV, Pedroso JL, et al. Variable phenotype and severity of sialidosis expressed in two siblings presenting with ataxia and macular cherry-red spots. J Clin Neurosci 2013;20:1327–8 [DOI] [PubMed] [Google Scholar]
- 2.Coutinho MF, Lacerda L, Macedo-Ribeiro S, et al. Lysosomal multienzymatic complex-related diseases: a genetic study among Portuguese patients. Clin Genet 2011;81:379–93 [DOI] [PubMed] [Google Scholar]
- 3.Fernández-Marmiesse A, Morey M, Pineda M, et al. Assessment of a targeted resequencing assay as a support tool in the diagnosis of lysosomal storage disorders. Orphanet J Rare Dis 2014;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leavitt JA, Kotagal S. The “cherry red” spot. Pediatr Neurol 2007; 37:74–5 [DOI] [PubMed] [Google Scholar]
- 5.Ospina LH, Lyons CJ, McCormick AQ. “Cherry-red spot” or “Perifoveal white patch”? Can J Ophthalmol 2005;40:609–10 [DOI] [PubMed] [Google Scholar]
- 6.NaKaya-Onishi M, Suzuki A, Okamoto N, et al. Observations on time course changes of retinal appearance in a patient with Tay Sachs disease. Br J Ophthalmol 2000;84:1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Leary EM, Igdoura SA. The therapeutic potential of pharmacological chaperones and proteosomal inhibitors, Celastrol and MG132 in the treatment of sialidosis. Mol Genet Metab 2012;107:173–85 [DOI] [PubMed] [Google Scholar]
- 8.Bonten EJ, Yogalingam G, Hu H, et al. Chaperone-mediated gene therapy with recombinant AAV-PPCA in a new mouse model of type I sialidosis. Biochim Biophys Acta 2013;1832:1784–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyrantepe V, Poupetova H, Froissart R, et al. Molecular pathology of NEU1 gene in sialidosis. Hum Mutat 2003;22:343–52 [DOI] [PubMed] [Google Scholar]
- 10.Chen CM, Lai SC, Chen IC, et al. First report of two Taiwanese siblings with sialidosis type I: a 10-year follow-up study. J Neurol Sci 2006;247:65–9 [DOI] [PubMed] [Google Scholar]
- 11.Uchihara T, Ohashi K, Kitagawa M, et al. Sialidosis type I carrying V217M/G243R mutations in lysosomal sialidase: an autopsy study demonstrating terminal sialic acid in lysosomal lamellar inclusions and cerebellar dysplasia. Acta Neuropathol 2010;119:135–45 [DOI] [PubMed] [Google Scholar]
- 12.Bonten EJ, Annunziata I, d'Azzo A. Lysosomal multienzyme complex: pros and cons of working together. Cell Mol Life Sci 2014;71:2017–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekijima Y, Nakamura K, Kishida D, et al. Clinical and serial MRI findings of a sialidosis type I patient with a novel missense mutation in the NEU1 gene. Intern Med 2013;52:119–24 [DOI] [PubMed] [Google Scholar]
- 14.Caciotti A, Di Rocco M, Filocamo M, et al. Type II sialidosis: review of the clinical spectrum and identification of a new splicing defect with chitotriosidase assessment in two patients. J Neurol 2009;256:1911–15 [DOI] [PubMed] [Google Scholar]