Abstract
The critical role of retinoids (vitamin A and its derivatives) for vision, reproduction, and survival has been well established. Vitamin A is produced from dietary carotenoids such as β-carotene by centric cleavage via the enzyme BCO1. The biochemical and molecular identification of a second structurally related β-carotene metabolizing enzyme, BCO2, has led to a prolonged debate about its relevance in vitamin A biology. While BCO1 cleaves provitamin A carotenoids, BCO2 is more promiscuous and also metabolizes nonprovitamin A carotenoids such as zeaxanthin into long-chain apo-carotenoids. Herein we demonstrate, in cell lines, that human BCO2 is associated with the inner mitochondrial membrane. Different human BCO2 isoforms possess cleavable N-terminal leader sequences critical for mitochondrial import. Subfractionation of murine hepatic mitochondria confirmed the localization of BCO2 to the inner mitochondrial membrane. Studies in BCO2-knockout mice revealed that zeaxanthin accumulates in the inner mitochondrial membrane; in contrast, β-carotene is retained predominantly in the cytoplasm. Thus, we provide evidence for a compartmentalization of carotenoid metabolism that prevents competition between BCO1 and BCO2 for the provitamin and the production of noncanonical β-carotene metabolites.—Palczewski, G., Amengual, J., Hoppel, C. L., von Lintig, J. Evidence for compartmentalization of mammalian carotenoid metabolism.
Keywords: BCO1, BCO2, β-carotene, zeaxanthin, vitamin A
Carotenoids are dietary lipids that can be divided into two classes of chemical compounds: carotenes, which are pure hydrocarbons (e.g., β-carotene), and their oxygenated derivatives, the xanthophylls (e.g., zeaxanthin and lutein). In humans, both carotenes and xanthophylls affect a large variety of physiological functions, serving as blue light filters and antioxidants in lipophilic environments (1, 2). For instance, the central area of the retina, the macula lutea, accumulates high levels of zeaxanthin and lutein, which gives rise to the distinctive yellow coloring of this structure. This accumulation of carotenoids in the macula has been associated with improved visual acuity and protection of the retina against light damage (3, 4). The function of carotenoids is complemented by the action of their cleavage products, the apo-carotenoids (5). Most notable of these compounds are the retinoids (vitamin A and its derivatives), which includes retinol, retinaldehyde, and retinoic acid (6). These β-carotene metabolites serve critical functions in vision, cellular differentiation, immunity, and cellular homeostasis (7–9). Retinaldehyde is the chromophore of visual pigments in photoreceptor cells of the retina (10). These G-protein-coupled receptors mediate phototransduction, the process by which light is converted into a neuronal signal (11). The nonvisual functions of retinoids are attributed to retinoic acid (12), the ligand of retinoic acid receptors (13, 14). These transcription factors form a heterodimeric complex with retinoid X receptors. This receptor dimer, together with corepressors and activators, serves as a potent gene regulator that controls the expression of many target genes throughout the mammalian life cycle (9).
In recent years much progress has been made in identifying key components of carotenoid metabolism, including enzymes that catalyze carotenoid breakdown to apo-carotenoids (refs. 2, 8 and Fig. 1). In mammals, two carotenoid cleaving enzymes (CCEs) have been identified with distinct substrate specificity and region selectivity for cleavage (15). β-Carotene-15,15′-oxygenase (BCO1) exhibits a narrow substrate specificity and cleaves carotenes such as β-carotene at the C15,C15′ double bond (16, 17). Rare mutations in patients and studies in knockout mice indicate that BCO1 is critical for vitamin A production and homeostasis (18, 19). The second enzyme β-carotene-9′,10′-oxygenase (BCO2) displays broad substrate specificity and cleaves both carotenes and xanthophylls at the C9,C10 double bond (20–23). Genetic studies in cattle, sheep, and chicken implicate BCO2 as being critical for carotenoid homeostasis of various tissues (24–27). Studies in a BCO2-knockout mouse model confirm these findings and further suggest that BCO2 exerts a protective role against oxidative stress caused by xanthophyll accumulation in mitochondria (23, 28).
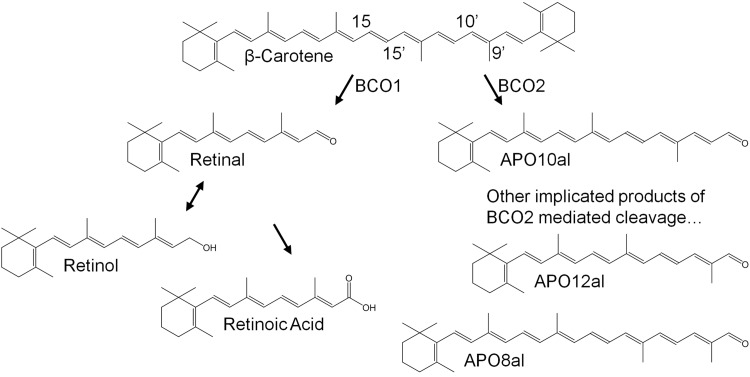
Figure 1.
Scheme for the proposed metabolism of β-carotene. β-Carotene is cleaved symmetrically by BCO1 in the cytoplasm to two molecules of retinal. Retinal can be converted to biologically active retinoic acid to serve as a gene regulator or to retinol for distribution throughout the body. BCO2, cleaves β,β-carotene asymmetrically at the 9′,10′ position generating β-apo-10′-carotenal (Apo10al) and β-ionone. β-apo-8′-carotenal(Apo8al) and β-apo-12′-carotenal(Apo12al) have also been implicated as products of BCO2 mediated cleavage.
How mammals maintain two distinctive CCEs with overlapping substrate specificity has long since been a matter of debate (29, 30). Aside from symmetric cleavage to retinaldehyde, β-carotene conversion to long-chain apo-carotenoids has been repeatedly reported in cell free protein extracts from mammals (refs. 31–37 and Fig. 1). These long-chain apo-carotenoids have been shown to exert specific biological activities in cell culture systems (38–41). Furthermore, these chemically related products can compete with canonical retinoids for downstream components of the vital retinoid signaling pathway (16, 39, 40). Considering that β-carotene is the major dietary source of vitamin A for humans, competition between two different enzymes for this nutrient would increase the prevalence of vitamin A deficiency. The putative problem of different metabolic fates and functions of apo-carotenoid metabolites is not only restricted to β-carotene. Common dietary carotenoids, including lycopene and β-cryptoxanthin, have also been shown to be metabolized by either type of CCE in the test tube (16, 20–23).
Studies in human volunteers reveal that absorbed β-carotene is converted mainly to retinoids (42, 43). Only one study found the production of nonretinoid apo-carotenoids from β-carotene, but in low quantities (44). Studies in mice also indicate that central cleavage by BCO1 is the major metabolic fate of β-carotene (16, 19). In addition, these studies provided evidence that BCO2 is critical for xanthophyll metabolism but does not significantly contribute to β-carotene turnover (16, 23). An explanation for the selectivity of β-carotene conversion may be found in the subcellular localization of BCO1 and BCO2. Studies in cell lines and mice have shown that human and murine BCO1 localizes to the cytoplasm as a soluble enzyme (17, 45). In contrast, murine BCO2 has been associated with mitochondria (23). The different subcellular localization of the two CCEs led us to hypothesize that carotenoid metabolism is compartmentalized. This compartmentalization would spatially separate the metabolism of β-carotene and xanthophylls, thereby avoiding competition between CCEs for common carotenoid substrates such as β-carotene and β-cryptoxanthin. We here employed mammalian cell lines and mouse models to approach this hypothesis.
MATERIALS AND METHODS
BCO2 plasmid construction for expression in Escherichia coli and HepG2
E. coli containing pReceiver-M02 that harbors the full-length open reading frame of human BCO2 was purchased from GeneCopoeia (GeneCopeia, Rockville, MD, USA) and was used a template for the following PCR reactions. To generate inserts suitable for tagless bacterial expression, the following forward primers for PCR were used; 5′-ATGTTTTTTCGAGTCTTTCTCC-3′, 5′-ATGGTGCACCGGCTCC-3′, 5′-ATGGGAAATACTCCTCAGAAAAAAGCCGTC-3′, 5′-ATGCTGCTGACCACAGTGGAAGAGG-3′, for the generation of the 579-aa (NP_114144.4), 556-aa (ACA05951.1), 545-aa (NP_001032367.2), and 522-aa isoforms of BCO2, as described in the Ensemble database (http://www.ensembl.org) and predicted with MitoProt software (http://ihg.gsf.de/ihg/mitoprot.html). The reverse primer for these reactions was 5′-TCAGATGGGTATGAAGGTACCATGG-3′ and included the common stop codon of the BCO2 isoforms. The amplified BCO2 isoforms were then cloned in frame into pTrcHis2 TOPO TA (Invitrogen, Grand Island, NY, USA) for bacterial expression and enzyme activity assay.
For expression of BCO2 in bacteria with a V5-tag, the following primers were used for PCR; 5′-ATGTTTTTTCGAGTCTTTCTCC-3′ and 5′-ATGCTGCTGACCACAGTGGAAGAGG-3′ for the generation of the 579- and 522-aa isoforms of BCO2, respectively. The reverse primer for these reactions was 5′-GATGGGTATGAAGGTACCATGG-3′. The amplified BCO2 isoforms were then cloned in frame into pBAD TOPO TA (Invitrogen) and translationally fused to a V5-tag on the C terminus.
For expression of BCO2 in the human hepatoma cell line HepG2 (American Type Culture Collection, Manassas, VA, USA) the following primers were used to incorporate a Kozak sequence ([G/A]NNATGG) to aid eukaryotic expression; 5′-GAAATGTTTTTTCGAGTCTTTCTCC-3, 5-GTGATGGTGCACCGGCTCC-3′, 5′-GACATGGGAAATACTCCTCAGAAAAAAGCCGTC-3′, 5′-GCAATGCTGCTGACCACAGTGGAAGAGG-3′ for the generation of the 579-, 556-, 545-, and 522-aa isoforms of BCO2, respectively. The reverse primer for these reactions was 5′-GATGGGTATGAAGGTACCATGG-3′. The amplified BCO2 isoforms were then cloned in frame into pcDNA 3.1/V5-His TOPO TA (Invitrogen) and translationally fused to the C-terminal V5-tag for expression in HepG2.
All PCRs were carried out with the Expanded High Fidelity PCR system (Roche, Indianapolis, IN, USA). All plasmid constructs were verified by sequence analysis (Genomics Core Sequencing Facility, Case Western Reserve University, Cleveland, OH, USA).
BCO2 enzymatic activity, high-performance liquid chromatography (HPLC), and mass spectrometry (MS)
XL1-Blue strain of E. coli carrying the genes for carotenoid synthesis (46) were transformed with different pTrcHis2 TOPO TA plasmid constructs. Cells were grown in lysogeny broth medium at 37°C to an optical density of one absorbance unit (AU) at 600 nm. Temperature was then reduced to 27°C for 1 h to allow for the accumulation of β-carotene. Following carotenoid accumulation, ascorbic acid, ferrous sulfate, and isopropyl β-d-1-thiogalactopyranoside were added to a final concentration of 5, 20, and 500 μM, respectively. Cells were then maintained at 27°C for 15 h. Cells were harvested by centrifugation at 4000 g for 10 min at 4°C. Carotenoids were extracted from the bacterial pellet under dim red light (<600 nm) with hydroxylamine, methanol, acetone, diethyl ether, and hexane as described previously (47). Briefly, the cell pellets were homogenized in 2 M hydroxylamine (pH 6.8), methanol, and acetone. The homogenate was then incubated for 30 min at 21°C to allow hydroxylamine to react with aldehydes. Then, lipids were extracted from the homogenate with hexane and diethyl ether. Extracted lipids were then separated by HPLC performed on a 1100 Agilent HPLC series equipped with a diode array detector and normal-phase Zorbax silica column (5 μm 4.6×150 mm; Agilent, Santa Clara, CA, USA). Chromatographic separation was achieved with isocratic flow of 20% ethyl acetate in hexane at a flow rate of 1.4 ml/min. The eluate was directed into a LXQ linear ion trap mass spectrometer (Thermo Scientific, Waltham, MA, USA) through atmospheric pressure chemical ionization (APCI) source working in the positive mode. To ensure optimal sensitivity, the instrument was tuned with β-carotene as well as apo-carotenoids. Identification of BCO2 cleavage products was based on retention time, mass, MS/MS fingerprint, and spectral characteristics to that of a known standard of 10′-β-apocarotenal (BASF, Ludwigshafen, Germany).
Cell lines, cell culture, and transient transfection
HepG2 cells (American Type Culture Collection) were maintained in DMEM with 10% FBS and 10 U/mL penicillin-streptomycin antibiotics, at 37°C with 5% CO2. To transfect HepG2 cells, the cells were grown to 50–70% confluence and then transfected with purified plasmid DNA using X-tremeGENE HP (Roche) transfection reagent. At 24 h post-transfection, cells were either used for in situ immunocytochemistry (as described below) or used for protein isolation. To isolate total protein, cells were scrapped then centrifuged at 600 g for 5 min at 4°C. Cell pellets were dissolved in M-PER (Thermo Scientific) and incubated on ice for 10 min. The solution was then centrifuged again at 10,000 g for 10 min at 4°C. The supernatant was stored at −80°C for further analysis.
Confocal microscopy
Transfected HepG2 cells grown on polylysine-treated glass coverslips were fixed in freshly prepared solution of 4% paraformaldehyde in PBS overnight at 4°C. Cells were washed and permeabilized with 0.1% Triton X-100 (Roche) in PBS (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, and 1.4 mM KH2PO4, pH 7.3; PBS-T) and blocked with 10% BSA and 5% goat serum (Sigma) in PBS-T (blocking buffer). Cells were then incubated overnight at 4°C in blocking buffer containing a mouse anti-V5 serum (to detect BCO2; Invitrogen) and rabbit anti-COX IV serum (Cell Signaling, Boston, MA USA) diluted 1:200. Following, cells were washed and incubated at room temperature with anti-mouse and anti-rabbit secondary antibody conjugated to Alexa 594 and Alexa 488 (Life Technologies, Grand Island, NY, USA), respectively, diluted 1:400 in blocking buffer. DAPI was used to stain nuclei. Confocal images were acquired with a Zeiss LSM 510 META laser scanning confocal microscope (Carl Zeiss MicroImaging, Jena, Germany) by using a multiline argon laser (excitation 488 and 594 nm) or a 405 diode laser (excitation 405) with a 63X C-Apochromat, NA 1.2-O objective.
Animals, husbandry, and experimental diets
Animal procedures and experiments were approved by the Case Western Reserve University Animal Care Committee and conformed to recommendations of both the American Veterinary Medical Association Panel on Euthanasia and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. In all experiments, mice were maintained at 24°C in a 12-h light–dark cycle and had free access to food and water. The generation of Bco1−/− and Bco1−/−;Bco2−/− (ko/ko) mice has been described elsewhere (16, 19, 23). Bco1−/−, ko/ko, and wild-type (WT) control mice with a C57/BL6;129Sv mixed genetic background were used for the described experiments. During breeding and weaning periods (up to 5 wk of age), all mice were maintained on breeder chow containing ∼29,000 IU vitamin A/kg diet (Prolab RMH 3000; LabDiet, St. Louis, MO, USA).
Mitochondrial isolation and fractionation
For localization of carotenoids, 4 wk old Bco1−/− and ko/ko mice (n=4) were fed a vitamin A-deficient pelleted diet based on the AIN-93G formulation, containing 0.05 mg/g zeaxanthin (zeaxanthin diet) or 0.05 mg/g β-carotene (β-carotene diet), for 4 wk. The diet was prepared by Research Diets, Inc. (New Brunswick, NJ, USA) by cold extrusion (to protect carotenoids from heat) and incorporated a water-soluble formulation of beadlets (DSM Ltd., Sisseln, Switzerland). After 4 wk of dietary intervention, mice were anesthetized by intraperitoneal injection of a mixture containing ketamine (80 mg/kg body weight) and xylazine (20 mg/kg body weight) in 10 mM sodium phosphate (pH 7.2), with 100 mM NaCl; blood was collected directly from the heart after cutting the right atrium. Mice were perfused with 10 ml of PBS and euthanized by cervical dislocation before tissue collection. Perfused liver tissue was extirpated from euthanized mice. Tissue was blotted dry and weighed, followed by washing in ice-cold mannitol, sucrose, and MOPS (MSM) buffer (220 mM mannitol, 70 mM sucrose, and 10 mM MOPS, pH 7.4). The tissue was then homogenized in the presence of MSM buffer with 2 mM EDTA.
This homogenate was then centrifuged at 350 g for 10 min at 4°C. The supernatant was then subjected to centrifugation at 7000 g for 10 min. The resulting supernatant was discarded; the pellet was resuspended in MSM buffer and again centrifuged at 7000 g for 10 min. The pellet was again resuspended in MSM buffer and layered on top of 30% Percoll MSM solution and subjected to ultracentrifuged at 150,000 g for 60 min at 4°C. The mitochondrial-rich layer was harvested and washed 3× as described before with centrifugation at 7000 g for 10 min in MSM buffer at 4°C (48).
Mitochondrial integrity was then ensured by activity assay as well as by electron microscopy. For submitochondrial fractionation, we used a previously described method (49). Briefly, isolated mitochondria were diluted with MSM buffer to a concentration of 60 mg mitochondrial protein/ml. Digitonin was added to a final concentration of 0.12 mg/mg protein. This solution was stirred on ice for 2 min and then diluted with MSM buffer. The solution was then centrifuged at 12,000 g for 10 min. The supernatant, which contained the outer membrane and inter membrane space, was saved and set aside. The pellet, which contained the mitoplast fraction, was then washed with MSM buffer. The pellet was resuspended in MSM buffer and then sonicated in an ice bath for 30 s (4 s on, 10 s off cycles). The sonicated material, as well as the previously saved supernatant, was then ultracentrifuged at 150,000 g at 4°C.
The ultracentrifuged pellet from the mitoplast fraction contained the inner membrane, and the pellet from the digitonin treated supernatant contained the outer membrane. Fractions were then verified by enzymatic activity assay (measuring activity of citrate synthase and succinate dehydrogenase) and by immunoblot (against carnitine palmitoyltransferase 1, citrate synthase, and complex III). Carotenoid extraction from total mitochondria and submitochondrial fractions was performed as described previously above.
Succinate dehydrogenase and citrate synthase activity
Protein from freshly prepared mouse hepatic mitochondria was quantified via Bradford assay. Samples were diluted with 5% cholate, 25 mM KPi, and 2 mM EDTA (pH 7.4) in water to a final concentration of 1 mg protein/ml. Prior to assaying activity, the cholate-treated protein sample was further diluted to 0.1 mg/ml of protein using 25 mM KPi and 2 mM EDTA (pH 7.4) in water.
To assay succinate dehydrogenase, 0.1 ml of cholate extract was added to a cuvette containing 1.2 mM KCN, 0.5 mM phenazine ethosulphate, 1 mM 2,6 dichlorophenol-indophenol, 112.5 mM Tris, and 1.2% (w/v) BSA in water at pH 7.8. The assay was preincubated for 5 min at 37°C. The reaction was started by adding succinate to a final concentration of 20 mM, and absorbance was measured for 10 min at 600 nm using a Hewlett-Packard diode array spectrophotometer (Hewlett-Packard, Palo Alto, CA, USA; ref. 48).
To assay citrate synthase activity, 0.02 ml protein solution was added to a cuvette containing 100 μM 5,5′-dithiobis(2-nitrobenzoic acid) (pH 8.1), prepared in 1.0 M Tris buffer and 336 μM acetyl coenzyme A (pH 8.1), dissolved in water to a total volume of 1 ml. The solution was preincubated for 5 min at 37°C. The reaction was started by adding oxalacetate in Tris buffer (pH 8.1) at a final concentration of 500 μM and absorbance measured for 10 min at 600 nm using a diode array spectrophotometer (Hewlett-Packard). Rates were calculated my measuring rate of change of absorbance per minute divided by corresponding extinction coefficients. Extinction coefficients used were 21 for succinate dehydrogenase and 13.6 for citrate synthesis, per milligram of protein (50).
Immuno-electron microscopy analysis
Transfected monkey COS7 cells were lightly fixed for 5 min at 4°C with 3% formaldehyde and 20 mM ethylacetimidate in HEPES-buffered saline (30 mM HEPES, 151 mM NaCl, 4.7 mM KCl, 1.2 mM MgCl2, and 7.8 mM glucose, pH 7.3) at room temperature. Samples were washed and further fixed for 45 min in 3% w/v formaldehyde containing 0.25% w/v glutaraldehyde in HEPES buffered saline at room temperature, then dehydrated in ethanol and embedded in LR White resin (Polysciences, Inc., Warrington, PA, USA). Thin sections were blocked with PBS containing 1% w/v BSA and 0.01% v/v Tween 20 (PBT). Grids were then incubated with anti-V5 mouse monoclonal antibody (Invitrogen) at 1:5 and 1:25 dilution in PBT for 12 h at 4°C. Negative controls included normal mouse serum and PBT replaced as the primary antibody. After washing, grids were incubated for 1 h in 5 nm gold-conjugated goat anti-mouse IgG (BBInternational; Ted Pella Inc., Redding, CA, USA) diluted 1:30 in PBT, rinsed with PBS, and fixed with glutaraldehyde to stabilize the gold particles. Samples were stained with uranyl acetate and lead citrate, and then examined in a Jeol 1200EX electron microscope (Jeol, Tokyo, Japan).
Mouse liver electron microscopy analysis
Bco2−/− female mice (4 wk old) were maintained on a vitamin A-deficient diet supplemented with or without lutein (50 mg/kg) prepared by Research Diets, for 10 wk. Mice were euthanized, their livers perfused and extirpated. Tissue was fixed in 4% paraformaldehyde in PBS overnight at 4°C and sectioned for electron microscopy. Samples were stained with uranyl acetate and lead citrate and then examined in a Jeol 1200EX electron microscope.
RESULTS
Human BCO2 contains a mitochondrial localization sequence on the N terminus
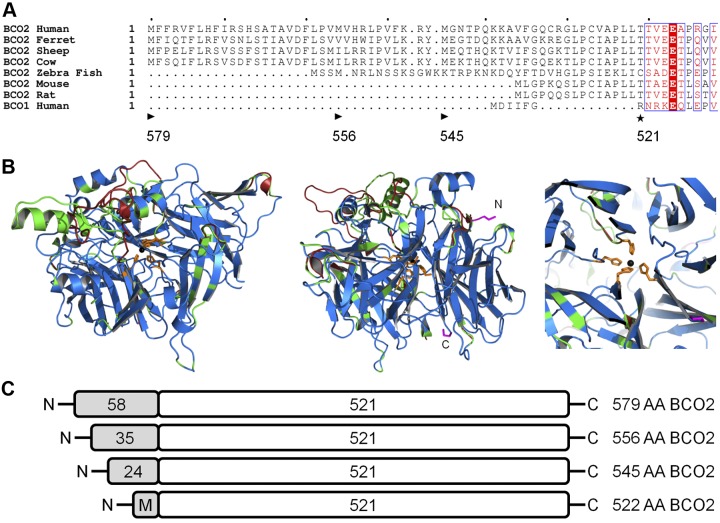
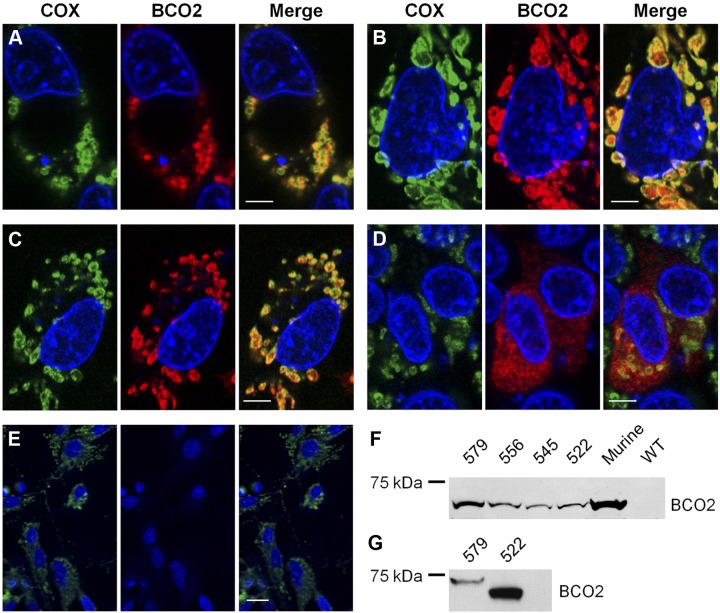
Human BCO2 is expressed in several tissues, including the liver and the retinal pigment epithelium of the eyes (51), but little is known about the enzyme function and subcellular localization. The Ensemble database reports 19 splice isoforms for human BCO2 encoded on chromosome 11. Among predicted protein isoforms, the 579-aa (NP_114144.4), 556-aa (ACA05951.1), and 545-aa (NP_001032367.2) isoforms showed overall sequence identity to the biochemically well-characterized 532-aa murine BCO2 (22, 23) but display an N-terminal extension (Fig. 2A, Supplemental Fig. S1, and ref. 52). Molecular modeling using the retinal pigment epithelium-specific 65 kDa protein (RPE65) as a template predicted that human BCO2 and murine BCO2 adopted the same overall structure (Fig. 2B and ref. 53, 54). The observation that murine BCO2 is a mitochondrial enzyme (23) led us to speculate that the N-terminal portion of the human isoforms encodes a mitochondrial import sequence (55). Hence, we analyzed the human BCO2 protein sequence computationally. MitoProt software predicted (P>0.989) a 58-aa leader sequence responsible for mitochondrial import of the 579-aa BCO2 (Fig. 2A and ref. 56). Part of this leader sequence is also present in the other isoforms of BCO2 (named here 556 BCO2 and 545 BCO2 based on their total amino acid count; Fig. 2). To investigate the role of the N terminus for the subcellular localization of BCO2, we expressed these different isoforms in the human hepatoma cell line HepG2 as C-terminal V5 tagged proteins (Fig. 2C). In addition, we constructed a plasmid for the expression of an isoform lacking the N-terminal leader sequence (522-aa) also tagged with a C-terminal V5 tag (Fig. 2C). Immunostaining and confocal imaging revealed that the 579-, 556-, and 545-aa BCO2 isoforms distributed in a similar pattern to COX IV, a mitochondrial marker protein (Fig. 3A–C). In contrast to this colocalization, the 522 BCO2 isoform, with the truncated N-terminal sequence, had a diffuse pattern of distribution that did not coincide with staining for the mitochondrial marker (Fig. 3D). Untransformed cells and cells not treated with primary antibodies were used as negative controls (Fig. 3E). To provide further evidence for a cleavable N-terminal leader sequence, protein from the transfected HEPG2 was isolated, separated by SDS-PAGE, and examined by immunoblotting. Isolated protein from the transfected HEPG2 cells had an indistinguishable size as compared to the 522-aa isoform that lacks the mitochondrial leader sequence. In addition, the different human isoforms displayed the same apparent size to the murine BCO2 that lacks the N-terminal leader sequence present in the human isoforms (Fig. 2A, B). In contrast, the 579 and 522 BCO2 isoforms expressed in E. coli displayed a significant size difference when separated on SDS-PAGE and examined by immunoblot (Fig. 3G). These findings indicated that the N-terminal leader sequence of human BCO2 is proteolytically removed during import into mitochondria in HEPG2 cells (55).
Figure 2.
Scheme for different human BCO2 variants. Three isoforms of human BCO2 have been previously identified that have overall sequence identity but differ at the length of their N termini. A) Alignment of the N-terminal sequence of BCO2 from several species, including the 579-aa (NP_114144.4), 556-aa (ACA05951.1), and 545-aa (NP_001032367.2) isoforms of human BCO2, as compared to human BCO1. Computational analysis predicts (P>0.989) a mitochondrial targeting sequence on the N terminus of the longest isoform. Arrowhead indicates the starting location of the different human isoforms; black asterisk indicates the putative proteolytic cleavage sites. Letters with a red background indicate sequence identity, red letters designate sequence similarity, and a period denotes a lack of homologous sequence. B) Overlay cartoon view of predicted human (modeled residues 54–578; green), mouse (modeled residues 10–531; red), and spatially aligned (blue) BCO2 structures using the SWISS-MODEL program based on template 3FSN model of related protein RPE65 (2.14 Å). Iron (black), chelating histidines (orange) and terminal residues (pink) are shown in detail. Helices and sheets point toward the C terminus. Left, the tunnel for RPE65 that leads to the active site iron is conserved in modeled murine and human BCO2 structures. Center, the two termini of BCO2 are visible on the surface. Right, the central chelating histidines are shown in detail. No difference in relative position was predicted for the active site histidines. C) Scheme of the different human BCO2 isoforms that were employed in this study. Note that the mitochondrial targeting sequence is lacking in the 522-aa BCO2 isoform, and a new translational start site was introduced by mutagenesis for this study.
Figure 3.
Human BCO2 localization is dependent on an N-terminal mitochondrial targeting sequence. A–D) HepG2 cells were transfected with expression plasmids encoding the 579-aa (A), 556-aa (B), 545-aa (C), and 522-aa (D) isoforms of human BCO2. E) As a control, untransfected HepG2 cells were used. Immunostaining was performed with anti-V5 antibody for BCO2 (red) and anti-COX IV antibody (green). DAPI was used to stain the nuclei. Merged images show a colocalization of BCO2 and COX IV for the 579-, 556-, and 545-aa BCO2 isoforms. Cells expressing the 522-aa isoform do not exhibit such staining. Untransfected control cells display no anti-V5 antibody staining. Scale bars = 5 μm (A–D); 25 μm (E). F, G) Immunoblots for BCO2 from transfected HepG2 cells (F) and transformed E. coli (G). As a control, HepG2 cells that express murine BCO2 and untransfected HepG2 (WT) were used. Total protein (20 μg) was separated by SDS-PAGE. Recombinant BCO2 was stained with anti-V5 antibody.
Human BCO2 isoforms cleaves β-carotene at the 9′,10′ position
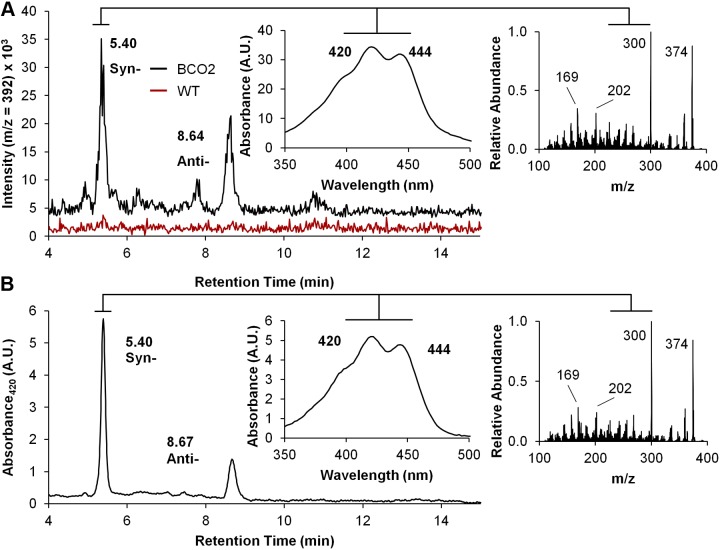
To demonstrate that the N-terminal leader sequence is not required for BCO2 enzyme activity, we transformed E. coli strain that can synthesize β-carotene with the different construct containing the various human BCO2 isoforms (including the truncated 522 BCO2 isoform). This test system was established in our laboratory for the initial biochemical characterization of murine BCO2 (20). On expression of different BCO2 proteins, bacteria were harvested by centrifugation and lipophilic compounds were extracted in the presence of hydroxylamine to protect the aldehyde groups of the apocarotenal cleavage products. Analysis of lipid extracts from cells expressing the different BCO2 isoforms was then carried out by LC-MS/MS. These analyses revealed the presence of two mass peaks with an m/z of 392.3 (expected m/z for β-10′-apo-carotenaloxime) and identical spectral characteristics, retention times, and tandem MS/MS diffraction patterns to that of a standard of β-10′-apo-carotenaloxime (Fig. 4). The two observed mass peaks corresponded to the syn- and anti-conformation of the oximes of this compound. The peaks were present in extracts from E. coli strains transformed with the distinct BCO2 expression plasmids but were absent in extracts from nontransformed β-carotene-producing bacterial cells (Fig. 4A and Supplemental Fig. S2). These findings led us to the conclusion that all four recombinant isoforms of BCO2 were enzymatically active, including the 522 BCO2 isoform that lacked the N-terminal leader sequence.
Figure 4.
Recombinant human BCO2 converts β-carotene to β-apo-10′-carotenal. Recombinant human 579-aa BCO2 isoform was expressed in β-carotene producing E. coli. Lipids were extracted and subjected to LC-MS analyses. Putative apocarotenal cleavage products were converted to the corresponding oximes (syn and anti) by hydroxylamine. A) LC-MS diagram at m/z 392 of lipid extracts isolated from cells expressing BCO2 (black trace) as compared to cells that lack BCO2 expression (red trace). B) LC-MS diagram of a β-apo-10′-carotenal standard was extracted under the same conditions to verify the retention time, absorbance spectra, and mass. Insets on the right show MS/MS fragmentation patterns of 392 m/z peak of the E. coli extract and the β-apo-10′-carotenaloxime standard, respectively.
Since murine BCO2 has been reported to convert xanthophylls (23), we also expressed the 522-aa human BCO2 isoform and murine BCO2 in a zeaxanthin-producing E. coli strain (57). Surprisingly, HPLC analysis of extracts from these bacterial cells revealed that the β-carotene derivative, β-10′-apo-carotenaloxime, was produced. In addition, compounds with spectral characteristics of 10′-apo-lycopenaloximes became detectable (Supplemental Fig. S3A, B and ref. 20). Thus, the biosynthetic precursors of zeaxanthin were already converted by BCO2 when expressed in this E. coli strain. Interestingly, the same picture emerged when we expressed BCO1. Though this enzyme cannot metabolize zeaxanthin (17), it converted lycopene to acyclo-retinoids and β-carotene to retinoids when expressed in zeaxanthin producing bacteria (Supplemental Fig. S3C). Hence, we concluded that the zeaxanthin-producing E. coli strain did not provide a robust test system to characterize xanthophyll cleavage activities of mammalian CCEs.
BCO2 localizes to the inner membrane of the mitochondria
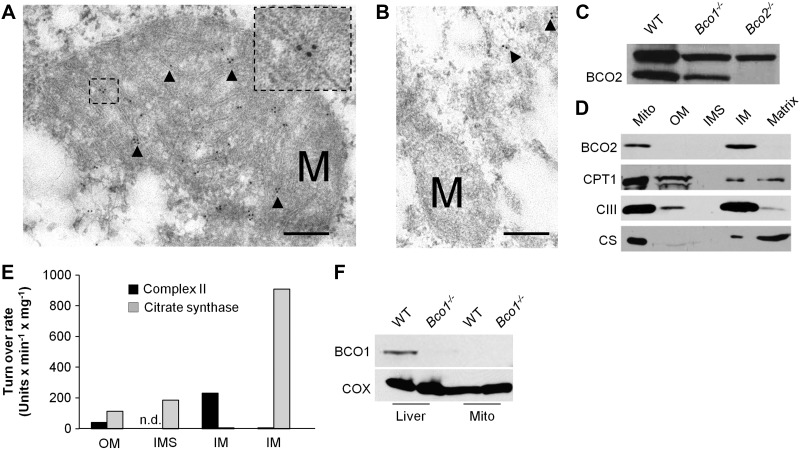
We next questioned where human BCO2 exactly resides in the mitochondria. To achieve the required resolution, we employed immunogold staining and electron microscopy. To that end, COS7 cells were transfected with the 545 BCO2 isoform. In addition, we employed the 522 BCO2 isoform that lacks the N-terminal mitochondrial leader sequence. After fixation and staining procedures, cells were subjected to transmission electron microscopy for imaging. The COS7 cells transfected with the 545 BCO2 transcripts had gold particles primarily within the mitochondria and in close proximity to the cristae that are formed by the inner mitochondrial membrane (Fig. 5A). The 522 BCO2 isoform displayed an altered cellular localization; gold particles were only observed outside of mitochondria in unrecognizable patterns (Fig. 5B).
Figure 5.
BCO2 localizes to the inner membrane of the mitochondria. A, B) COS7 cells were transfected with the 545-aa (A) and the 522-aa (B) BCO2 isoforms. Immunogold labeling was performed with anti-V5 antibody and imaged with transmission electron microscopy. Immunogold labeling for the human 545-aa isoform was found at the inner membrane of mitochondria. Cells transfected with the 522-aa BCO2 isoform had no observable gold particles within the mitochondria. Arrows indicate gold particles; M, mitochondria. Scale bars = 200 μm. Inset: zoom of the marked area. Note the gold particles associate with the inner mitochondrial membrane. Several images were taken, representative samples are shown. C) Immunoblot analysis for BCO2 of whole liver protein extracts of WT, Bco1−/−, and Bco2 mice confirmed the specificity of the BCO2 antiserum. Note that the antibody shows a nonspecific cross-reaction with a protein of ∼80 kDa in size. D) Hepatic mitochondria were isolated and fractionated. Protein extracts from total mitochondria (Mito), outer membrane (OM), inter membrane space (IMS), inner membrane (IM), and matrix were subjected to immunoblot analysis for BCO2, carnitine palmitoyltransferase 1 (CPT1) and complex III (CIII). E) Test for enzymatic activity of citrate synthase (matrix) and complex II (succinate dehydrogenase, inner membrane) of different mitochondrial fractions. n.d., not detectable. F) Immunoblot analysis for BCO1 with protein extracts of whole liver and isolated mitochondria of WT and Bco1−/− mice. Analysis in Bco1−/− mice confirmed the specificity of the BCO1 antiserum.
To complement this result from cell lines and to provide evidence for a general inner mitochondrial membrane localization of mammalian BCO2s, we isolated mitochondria from mouse liver. The structural integrity of the isolated mitochondria was routinely confirmed by electron microscopy and mitochondrial oxidative phosphorylation rates. The subsequent fractionation into different mitochondrial compartments was achieved with the use of the detergent digitonin, sonication, and ultracentrifugation. Protein extracts from intact mitochondria and the mitochondrial fractions were then separated by SDS-PAGE and subjected to immunoblot analysis for BCO2 using antiserum produced against purified full-length murine BCO2 (23). The specificity of the antiserum for murine BCO2 was verified in liver protein extracts of mice. This antiserum specifically detected a ∼60 kDa protein in extracts of WT and Bco1−/− mice, whereas the band was absent in Bco2−/− mice (Fig. 5C). With this antiserum, a strong band was observed in protein extracts of whole mitochondria and in the inner membrane fraction of mitochondria (Fig. 5D). No band was observed in the outer membrane fraction, intermembrane space, or the mitochondria matrix fractions. To verify the identity of the different mitochondrial fractions, we performed immunoblotting for marker proteins and tested for activity of key enzymes. These analyses revealed that digitonin treatment, differential centrifugation, sonication, and the separation by ultracentrifugation yielded highly enriched mitochondria fractions. As shown in Fig. 5D, the identity of the outer membrane fraction was verified by staining for CPT1, the matrix fraction by citrate synthase, and the inner membrane fraction by Complex III. Activity for citrate synthase and Complex II was observed primarily in the matrix and inner membrane, respectively (Fig. 5E). Thus, we concluded that recombinant human BCO2 in cell lines as well as native murine BCO2 in liver localizes to the inner membrane of mitochondria.
We also determined the subcellular localization of the vitamin A forming BCO1 by comparing its expression in isolated mitochondrial and whole liver protein extracts. When samples were probed with rabbit antiserum raised against BCO1 (19), we detected BCO1 in whole liver protein extracts from WT mice but not in BCO1−/− mice (Fig. 5F). In protein extracts from isolated mitochondria no staining for BCO1 was observed. (Fig. 5F).
β-Carotene accumulates in the inter membrane space in BCO1-deficient mice, while zeaxanthin accumulates in the inner membrane in BCO2-deficient mice
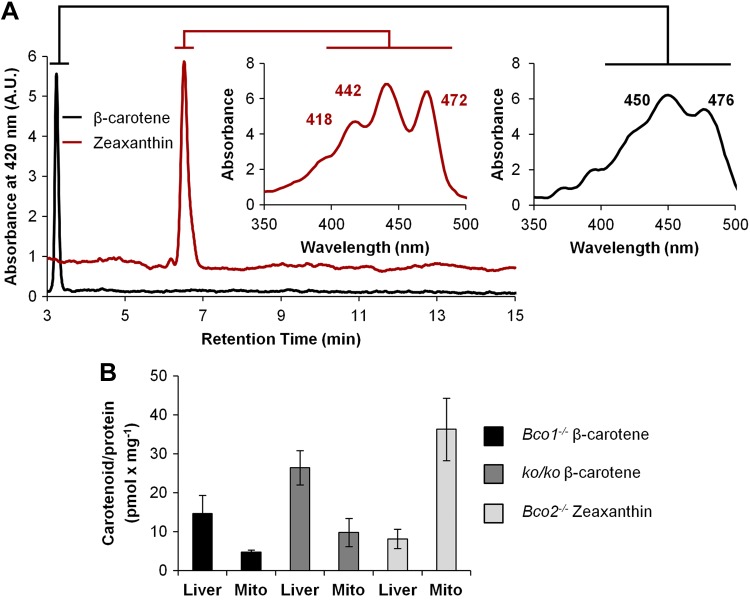
Our analyses revealed that BCO2 resides in the inner membrane of the mitochondria. Previously, we and others have localized human and murine BCO1 to the cytosol of cells (17, 45). The distinct localization of the two CCEs (Fig. 5) suggested a compartmentalization of carotenoid metabolism. Thus, we next examined whether the subcellular distribution of carotenoid substrates may reflect the cellular localization of the two CCEs. To test this, we employed mice that are deficient for specific CCEs and accumulate carotenoids in the liver, as WT mice readily convert ingested carotenoids by BCO1 and BCO2 (19, 23). Mice deficient for BCO1 (Bco1−/− mice) and BCO2 (Bco2−/− mice) and mice deficient for both BCO1 and BCO2 (ko/ko) were fed a defined diet that was supplemented with β-carotene or zeaxanthin (each 50 mg/kg). After 4 wk, mice were euthanized, and livers were dissected. Carotenoids were extracted from whole livers and from isolated hepatic mitochondria. HPLC analyses showed that β-carotene levels were higher in total liver extracts than in isolated mitochondria when compared on a carotenoid per protein basis (Fig. 6). This state changed when we determined zeaxanthin accumulation in mice. This xanthophyll accumulated in oxidized form and the levels of these compounds were higher in hepatic mitochondria as compared to whole liver extracts (Fig. 6). This finding indicated that zeaxanthin, but not β-carotene, became enriched in mitochondria.
Figure 6.
β-Carotene and zeaxanthin accumulation in hepatic mitochondria of mice. Five-week-old Bco1−/−, Bco2−/−, and ko/ko mice (n=4 each) were subjected to a 4 wk feeding with controlled diets containing β-carotene or zeaxanthin, respectively. Lipids were extracted from liver and quantified by HPLC analysis. A) Representative HPLC diagrams at 420 nm of lipid extracts of mice fed diets supplemented with β-carotene (black trace) or zeaxanthin (red trace). Insets: spectral characteristics of accumulated carotenoids. Note that zeaxanthin accumulates in the form of its oxidized 3-dehyro-derivative, as indicated by retention time and spectral characteristics. B) Carotenoid levels per protein in extracts of total liver and isolated mitochondria. Data represent means ± sd of 4 mice/data point.
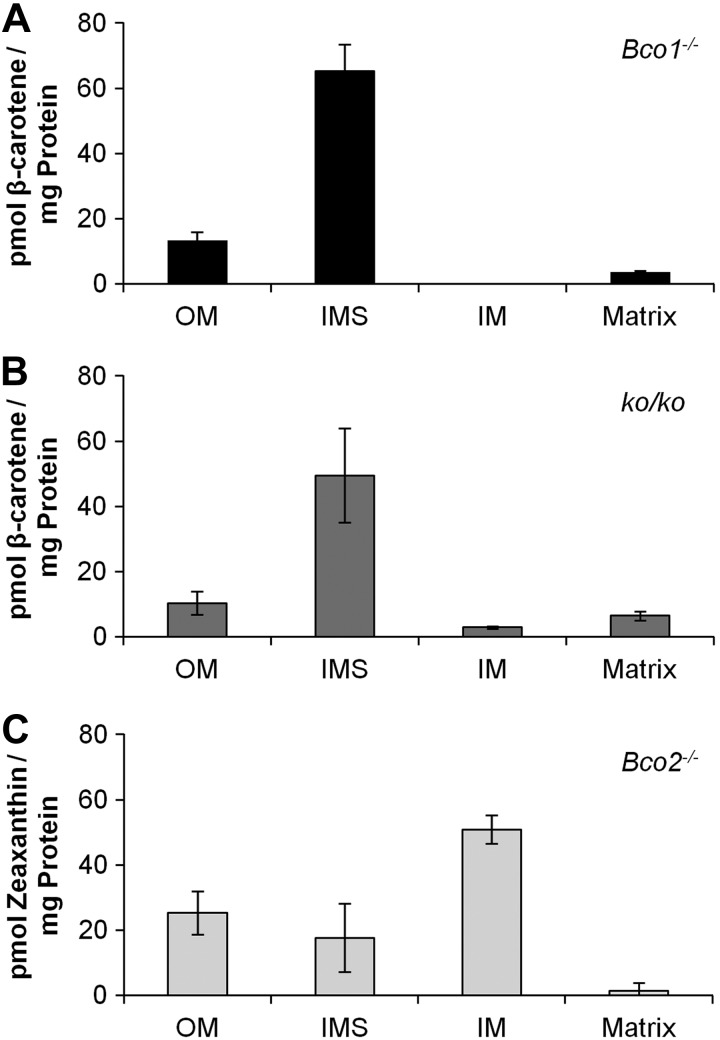
We next fractionated mitochondria as described above. We then subjected the different mitochondrial fractions to HPLC analyses for the quantification of carotenoids. For Bco1−/− mice, this analysis revealed β-carotene levels were highest in the intramembrane space, again calculated on a carotenoid per protein basis (Fig. 7A). In the mitochondrial matrix, very small amounts of β-carotene were detectable, and in the inner membrane, fraction β-carotene levels were below detection levels of the HPLC system. Analysis of ko/ko mice revealed essentially the same picture with one notable difference, trace amounts of β-carotene were present in the inner membrane (Fig. 7B). We previously reported the occurrence of small amounts of β-apo-10′carotenol in livers of Bco1−/− and WT mice (16). Hence, this accumulation in inner mitochondrial membrane of the double mutant is in agreement with the finding that trace amounts of β-carotene can be converted by BCO2 in Bco1−/− and the WT mice (16).
Figure 7.
Zeaxanthin, but not β-carotene, accumulates in the inner mitochondrial membrane. A, B) Five-week-old Bco1−/− (A) and ko/ko (B) mice (n=4 each) were subjected to a 4 wk feeding with β-carotene (50 mg/kg). C) Five-week-old Bco2−/− mice (n=4) were subjected to a 4 wk feeding with zeaxanthin (50 mg/kg). Hepatic mitochondria were isolated and fractionated into outer membrane (OM), inter membrane space (IMS), inner membrane (IM), and matrix. Protein amount was determined and lipids were extracted for HPLC analyses. Values represent means ± sd of 4 mice/mitochondrial fraction.
Subfractionation of mitochondria from zeaxanthin fed Bco2−/− mice and HPLC analyses revealed that zeaxanthin accumulated in oxidized form in all mitochondrial membranes (Fig. 7C). The highest levels of these compounds were found in the inner membrane fraction. Thus, the spatial accumulation of xanthophyll in double-mutant mice coincided with the localization of BCO2 in the WT situation. Previously, we reported that accumulation of xanthophylls can cause oxidative stress in mitochondria (23). To rule out that the accumulation of oxidized carotenoids in the inner membrane is related to morphological changes in mitochondrial or cell morphology, we performed liver histology with mice deficient for BCO2. These mice were subjected to xanthophyll feeding or control diet feeding. This analysis revealed that cell morphology was comparable among different diets, and no change in structure, size, or number of mitochondria was observed (Supplemental Fig. S3).
DISCUSSION
Both centric and eccentric cleavage of β-carotene has been observed in cell-free protein extracts of mammals. This observation has led to a long-lasting debate about the pathway for vitamin A production in mammals. Later, the respective β-carotene cleavage enzymes were molecularly identified to be encoded in mammalian genomes. Though eccentric cleavage of β-carotene occurs in cell-free tissue homogenates, this reaction is not favored in the in vivo situation. Only one report describes, besides centric, an eccentric conversion of some of the administered β-carotene in a human volunteer (44). The large majority of publications describe centric conversion to retinoids only (42, 44, 58). Centric conversion and retinoid production is also the major metabolic fate of β-carotene in rodents (19). β-Carotene even accumulates in BCO1 deficiency, and only trace amounts of β-apo-carotenoids are produced by BCO2 (16, 59). Herein, we show that compartmentalization of carotenoid metabolism prevents competition between BCO1 and BCO2 for β-carotene and the production of noncanonical β-carotene metabolites.
BCO1 and BCO2 localize to different subcellular compartments
The vitamin A-forming enzyme BCO1 localizes to the cytoplasm as a soluble protein, as shown in human cell lines and mouse tissues (17, 18, 45). Consistently, the vitamin A-forming enzyme has been described as soluble in tissue homogenates (60, 61). In contrast, human and murine BCO2 are mitochondrial proteins as shown here. This proposal is in agreement with the finding that human BCO2 is part of the cardiac mitochondrial proteome (62). Human BCO2 exists as several splice variants that differ at the N terminus from murine BCO2. We provide evidence in HepG2 cells that the N terminus of the human isoforms encodes a mitochondrial import sequence. Since cell-based protein expression systems may run the risk of protein mislocalization, we removed the N-terminal leader sequence, which resulted in a truncated 522 BCO2 isoform. This isoform no longer localizes to mitochondria in the HepG2 cell system, thus indicating that the N-terminal leader sequence is mandatory for mitochondrial import. As nuclear encoded mitochondrial proteins reach their destination, they are predominantly processed by mitochondrial-specific peptidase that remove the N-terminal import sequence (55). The evidence provided here suggests that BCO2 follows this model of mitochondrial localization. On expression in HepG2 cells, the 579-, 556-, 545-, and 522-aa isoforms show no apparent size difference in migration on SDS-PAGE. Notably, the leader sequence of human BCO2 contains an R-10 mitochondrial cleaving site motif (R)X(↓)(F/L/I)XX(T/S/G)XXXX(↓). This motif is present in all three human BCO2 isoforms (Fig. 2A). Hence, this 2-step peptidase motif is a likely candidate for cleavage of the different isoforms and explains the comparable size of different isoforms on expression in HepG2 cells. This finding also is of particular interest as future attempts at biochemically characterizing human BCO2 may want to focus on the 522 BCO2 isoform, as it may represent the physiologically relevant enzyme. This assumption is supported by the finding that the 522-aa isoform similar to the other isoforms converted β-carotene to β-10′-apocarotenal in the E. coli test system, thus clearly demonstrating that this N-terminal sequence is not critical for enzyme activity. Furthermore, sequence comparison revealed that long N-terminal leader sequences exist in other mammalian BCO2s, including the ferret and bovine enzymes (Fig. 1A and Supplemental Fig. S1). Ferret BCO2 has been biochemically characterized (22), and bovine and sheep BCO2 are required for the control of carotenoid tissue levels (24, 27). Previously, the ferret enzyme has been localized to the cytosol on expression in cell culture using immunofluorescence microscopy (21). Of note, the characterized ferret BCO2 isoform was a 540-aa variant and was cloned by PCR approach. This isoform lacks the N-terminal leader sequence of other ferret BCO2 isoforms found in the database, e.g., the 575-aa (XP_004791526.1) and 586-aa (XP_004791525.1) BCO2 isoforms. In addition, the applied microscopy method for the localization of the 540-aa ferret BCO2 may not provide high enough resolution to distinguish between mitochondrial and cytoplasmic localization. Thus, we propose that a mitochondrial localization is common to mammalian BCO2s.
Within mitochondria, BCO2 is associated with the inner membrane. We provide evidence for this localization by fractionation of isolated murine hepatic mitochondria, as well as by immunogold staining and electron microscopy in HepG2 cells. These approaches employ different primary antibodies and examine the localization of BCO2 in vivo and in cell culture. Examination of the BCO2 amino acid sequence does not suggest the existence of a transmembrane region, thus excluding BCO2 as an integral membrane protein of the inner mitochondrial membrane (Fig. 2B). Association of BCO2 with the membrane may be a result of secondary modification of specific residues or the presence of a hydrophobic patch that has been described for monotopic membrane proteins. This mechanism of interaction between protein and membrane has been recently demonstrated for the structurally related RPE65 protein (53, 54, 63).
Xanthophylls and β-carotene accumulate in different subcellular compartments
The localization of BCO1 and BCO2 in different subcellular compartments is mirrored in the distribution of β-carotene and zeaxanthin in the liver of Bco1- and Bco2-knockout mice. Zeaxanthin is enriched in oxidized form in hepatic mitochondria, whereas β-carotene is enriched in the cytoplasm. In fractionated mitochondria β-carotene exists primarily in the intramembrane space. This compartment is not physically separated from the cytosol and small molecules can diffuse between these compartments. Though β-carotene accumulates to similar levels in ko/ko and Bco1−/− mice, a notable difference was found between these genotypes. Double-mutant mice display trace amounts β-carotene in the inner membrane of mitochondria that is absent in Bco1−/− mice. This finding is in agreement with previous findings that report the production of small amounts of β-apo-carotenoids in Bco1−/− mice (16, 59). In contrast to β-carotene, zeaxanthin, in the form of its oxidized derivatives, is enriched in the inner membrane of mitochondria. This accumulation in the inner membrane of mitochondria also may explain our previous observations that Bco2−/− mouse cells and cell lines not expressing BCO2 have decreased mitochondrial respiration rates and increased oxidative stress on zeaxanthin supplementation (23, 28).
The accumulations of β-carotene in cytoplasm and zeaxanthin in the inner membrane of mitochondria coincide with the subcellular localization of their respective cleavage enzymes. The observed cellular distribution of carotenes and xanthophylls likely involves specific transport mechanism, which includes binding proteins. Isoprenoid compounds such cholesterol also are shuttled by specific binding proteins among different subcellular compartments (64). Xanthophyll-binding proteins have been identified previously in the retina of the primate eyes (65, 66). Related binding proteins may help to retain β-carotene in a soluble form in the cytoplasm and to shuttle zeaxanthin to mitochondria. Future research is required to elucidate the molecular players that help shuttle carotenoids between different cellular compartments for metabolism by either CCE.
In summary, we have shown both in cell culture and mouse models that the eccentric carotenoid cleavage enzyme BCO2 resides in the inner mitochondrial membrane. In knockout mouse models for BCO1 and BCO2, we demonstrate that carotenoid metabolism is compartmentalized. This compartmentalization elegantly prevents a competition between BCO1 and BCO2 for the common substrate β-carotene and the production of β-apo-carotenoids that can hamper the vital retinoid signaling cascade. These findings provide an explanation for the observation that eccentric enzymatic cleavage of β-carotene can occur in the test tube but is largely absent in living organisms.
Supplementary Material
Acknowledgments
The authors thank Dr. Marcin Golczak [Case Western Reserve University (CWRU)] for help with LC-MS analysis of carotenoids and apo-carotenoids. The authors thank Dr. Janos Kerner (CWRU) and Dr. Mariana Rosca (CWRU) for help with methods in mitochondrial preparation. The authors thank Hiral Patel (CWRU) for assaying mitochondrial fractions for purity. The authors are grateful to Dr. Philip Kiser (CWRU) for helping with modeling of carotenoid oxygenases. The authors are grateful to Dr. Hisashi Fujioka and the Case Electron Microscopy Core Facility for assistance with immunogold staining and imaging. The authors are grateful to Dr. Hansgeorg Ernst (BASF, Ludwigshafen, Germany) for the gift of apo-carotenoid standards. The authors thank Maryanne Pendergast and the Neurosciences Imaging Center for assistance with confocal microscopy.
This work was supported by U.S. National Institute of Health grants EY019641 and EY02551 and the visual science training program T32-EY07157.
Footnotes
- BCO1
- β-carotene-15,15′-oxygenase
- BCO2
- β-carotene-9′,10′-oxygenase
- CCE
- carotenoid cleaving enzyme
- HPLC
- high-performance liquid chromatography
- MS
- mass spectrometry
- MSM
- mannitol, sucrose, and MOPS
- RPE65
- retinal pigment epithelium-specific 65 kDa protein
- WT
- wild type
REFERENCES
- 1. Krinsky N. I., Johnson E. J. (2005) Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26, 459–516 [DOI] [PubMed] [Google Scholar]
- 2. Von Lintig J. (2010) Colors with functions: elucidating the biochemical and molecular basis of carotenoid metabolism. Annu. Rev. Nutr. 30, 35–56 [DOI] [PubMed] [Google Scholar]
- 3. SanGiovanni J. P., Neuringer M. (2012) The putative role of lutein and zeaxanthin as protective agents against age-related macular degeneration: promise of molecular genetics for guiding mechanistic and translational research in the field. Am. J. Clin. Nutr. 96, 1223S–1233S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabour-Pickett S., Nolan J. M., Loughman J., Beatty S. (2012) A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol. Nutr. Food Res. 56, 270–286 [DOI] [PubMed] [Google Scholar]
- 5. Moise A. R., von Lintig J., Palczewski K. (2005) Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids. Trends Plant Sci. 10, 178–186 [DOI] [PubMed] [Google Scholar]
- 6. D'Ambrosio D. N., Clugston R. D., Blaner W. S. (2011) Vitamin A metabolism: an update. Nutrients 3, 63–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hall J. A., Grainger J. R., Spencer S. P., Belkaid Y. (2011) The role of retinoic acid in tolerance and immunity. Immunity 35, 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Lintig J., Kiser P. D., Golczak M., Palczewski K. (2010) The biochemical and structural basis for trans-to-cis isomerization of retinoids in the chemistry of vision. Trends Biochem. Sci. 35, 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rhinn M., Dolle P. (2012) Retinoic acid signalling during development. Development 139, 843–858 [DOI] [PubMed] [Google Scholar]
- 10. Von Lintig J. (2012) Metabolism of carotenoids and retinoids related to vision. J. Biol. Chem. 287, 1627–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palczewski K. (2006) G protein-coupled receptor rhodopsin. Ann. Rev. Biochem. 75, 743–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duester G. (2008) Retinoic acid synthesis and signaling during early organogenesis. Cell 134, 921–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Petkovich M., Brand N. J., Krust A., Chambon P. (1987) A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature 330, 444–450 [DOI] [PubMed] [Google Scholar]
- 14. Giguere V., Ong E. S., Segui P., Evans R. M. (1987) Identification of a receptor for the morphogen retinoic acid. Nature 330, 624–629 [DOI] [PubMed] [Google Scholar]
- 15. von Lintig J., Wyss A. (2001) Molecular analysis of vitamin A formation: cloning and characterization of beta-carotene 15,15′-dioxygenases. Arch. Biochem. Biophys. 385, 47–52 [DOI] [PubMed] [Google Scholar]
- 16. Amengual J., Widjaja-Adhi M. A., Rodriguez-Santiago S., Hessel S., Golczak M., Palczewski K., von Lintig J. (2013) Two carotenoid oxygenases contribute to mammalian provitamin A metabolism. J. Biol. Chem. 288, 34081–34096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindqvist A., Andersson S. (2002) Biochemical properties of purified recombinant human beta-carotene 15,15′-monooxygenase. J. Biol. Chem. 277, 23942–23948 [DOI] [PubMed] [Google Scholar]
- 18. Lindqvist A., Sharvill J., Sharvill D. E., Andersson S. (2007) Loss-of-function mutation in carotenoid 15,15′-monooxygenase identified in a patient with hypercarotenemia and hypovitaminosis A. J. Nutr. 137, 2346–2350 [DOI] [PubMed] [Google Scholar]
- 19. Hessel S., Eichinger A., Isken A., Amengual J., Hunzelmann S., Hoeller U., Elste V., Hunziker W., Goralczyk R., Oberhauser V., von Lintig J., Wyss A. (2007) CMO1 deficiency abolishes vitamin A production from beta-carotene and alters lipid metabolism in mice. J. Biol. Chem. 282, 33553–33561 [DOI] [PubMed] [Google Scholar]
- 20. Kiefer C., Hessel S., Lampert J. M., Vogt K., Lederer M. O., Breithaupt D. E., von Lintig J. (2001) Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J. Biol. Chem. 276, 14110–14116 [DOI] [PubMed] [Google Scholar]
- 21. Hu K. Q., Liu C., Ernst H., Krinsky N. I., Russell R. M., Wang X. D. (2006) The biochemical characterization of ferret carotene-9′,10′-monooxygenase catalyzing cleavage of carotenoids in vitro and in vivo. J. Biol. Chem. 281, 19327–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mein J. R., Dolnikowski G. G., Ernst H., Russell R. M., Wang X. D. (2010) Enzymatic formation of apo-carotenoids from the xanthophyll carotenoids lutein, zeaxanthin and beta-cryptoxanthin by ferret carotene-9′,10′-monooxygenase. Arch. Biochem. Biophys. 506, 109–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amengual J., Lobo G. P., Golczak M., Li H. N., Klimova T., Hoppel C. L., Wyss A., Palczewski K., von Lintig J. (2011) A mitochondrial enzyme degrades carotenoids and protects against oxidative stress. FASEB J. 25, 948–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berry S. D., Davis S. R., Beattie E. M., Thomas N. L., Burrett A. K., Ward H. E., Stanfield A. M., Biswas M., Ankersmit-Udy A. E., Oxley P. E., Barnett J. L., Pearson J. F., van der Does Y., Macgibbon A. H., Spelman R. J., Lehnert K., Snell R. G. (2009) Mutation in bovine beta-carotene oxygenase 2 affects milk color. Genetics 182, 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tian R., Pitchford W. S., Morris C. A., Cullen N. G., Bottema C. D. (2009) Genetic variation in the beta, beta-carotene-9′, 10′-dioxygenase gene and association with fat colour in bovine adipose tissue and milk. Anim. Genet. 41, 253–259 [DOI] [PubMed] [Google Scholar]
- 26. Eriksson J., Larson G., Gunnarsson U., Bed'hom B., Tixier-Boichard M., Stromstedt L., Wright D., Jungerius A., Vereijken A., Randi E., Jensen P., Andersson L. (2008) Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 4, e1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vage D. I., Boman I. A. (2010) A nonsense mutation in the beta-carotene oxygenase 2 (BCO2) gene is tightly associated with accumulation of carotenoids in adipose tissue in sheep (Ovis aries). BMC Genetics 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lobo G. P., Isken A., Hoff S., Babino D., von Lintig J. (2012) BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway. Development 139, 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wolf G. (2001) The enzymatic cleavage of beta-carotene: end of a controversy. Nutr. Rev. 59, 116–118 [DOI] [PubMed] [Google Scholar]
- 30. Krinsky N., Russell R. M. (2001) Regarding the conversion of beta-carotene to vitamin A. Nutr. Rev. 59, 309. [DOI] [PubMed] [Google Scholar]
- 31. Hansen S., Maret W. (1988) Retinal is not formed in vitro by enzymatic central cleavage of beta-carotene. Biochemistry 27, 200–206 [DOI] [PubMed] [Google Scholar]
- 32. Tang G. W., Wang X. D., Russell R. M., Krinsky N. I. (1991) Characterization of beta-apo-13-carotenone and beta-apo-14′-carotenal as enzymatic products of the excentric cleavage of beta-carotene. Biochemistry 30, 9829–9834 [DOI] [PubMed] [Google Scholar]
- 33. Tibaduiza E. C., Fleet J. C., Russell R. M., Krinsky N. I. (2002) Excentric cleavage products of beta-carotene inhibit estrogen receptor positive and negative breast tumor cell growth in vitro and inhibit activator protein-1-mediated transcriptional activation. J. Nutr. 132, 1368–1375 [DOI] [PubMed] [Google Scholar]
- 34. Wang X. D., Krinsky N. I., Tang G. W., Russell R. M. (1992) Retinoic acid can be produced from excentric cleavage of beta-carotene in human intestinal mucosa. Arch. Biochem. Biophys. 293, 298–304 [DOI] [PubMed] [Google Scholar]
- 35. Wang X. D., Tang G. W., Fox J. G., Krinsky N. I., Russell R. M. (1991) Enzymatic conversion of beta-carotene into beta-apo-carotenals and retinoids by human, monkey, ferret, and rat tissues. Arch. Biochem. Biophys. 285, 8–16 [DOI] [PubMed] [Google Scholar]
- 36. Sharma R. V., Mathur S. N., Ganguly J. (1976) Studies on the relative biopotencies and intestinal absorption of different apo-beta-carotenoids in rats and chickens. Biochem. J. 158, 377–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glover J. (1960) The conversion of beta-carotene into vitamin A. Vit. Hormones 18, 371–386 [DOI] [PubMed] [Google Scholar]
- 38. Eroglu A., Harrison E. H. (2013) Carotenoid metabolism in mammals including man: formation, occurrence & function of apocarotenoids. J. Lipid Res. 54, 1719–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Eroglu A., Hruszkewycz D. P., Curley R. W., Jr., Harrison E. H. (2010) The eccentric cleavage product of beta-carotene, beta-apo-13-carotenone, functions as an antagonist of RXRalpha. Arch. Biochem. Biophys. 504, 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eroglu A., Hruszkewycz D. P., dela Sena C., Narayanasamy S., Riedl K. M., Kopec R. E., Schwartz S. J., Curley R. W., Jr., Harrison E. H. (2012) Naturally occurring eccentric cleavage products of provitamin A beta-carotene function as antagonists of retinoic acid receptors. J. Biol. Chem. 287, 15886–15895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ziouzenkova O., Orasanu G., Sukhova G., Lau E., Berger J. P., Tang G., Krinsky N. I., Dolnikowski G. G., Plutzky J. (2007) Asymmetric cleavage of beta-carotene yields a transcriptional repressor of retinoid X receptor and peroxisome proliferator-activated receptor responses. Mol. Endocrinol. 21, 77–88 [DOI] [PubMed] [Google Scholar]
- 42. Burri B. J., Clifford A. J. (2004) Carotenoid and retinoid metabolism: insights from isotope studies. Arch. Biochem. Biophys. 430, 110–119 [DOI] [PubMed] [Google Scholar]
- 43. Reboul E., Borel P. (2011) Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog. Lipid Res. 50, 388–402 [DOI] [PubMed] [Google Scholar]
- 44. Ho C. C., de Moura F. F., Kim S. H., Clifford A. J. (2007) Excentral cleavage of beta-carotene in vivo in a healthy man. Am. J. Clin. Nutr. 85, 770–777 [DOI] [PubMed] [Google Scholar]
- 45. Kowatz T., Babino D., Kiser P., Palczewski K., von Lintig J. (2013) Characterization of human beta,beta-carotene-15,15′-monooxygenase (BCMO1) as a soluble monomeric enzyme. Arch. Biochem. Biophys. 539, 214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Misawa N., Nakagawa M., Kobayashi K., Yamano S., Izawa Y., Nakamura K., Harashima K. (1990) Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J. Bacteriol. 172, 6704–6712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. von Lintig J., Vogt K. (2000) Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving beta-carotene to retinal. J. Biol. Chem. 275, 11915–11920 [DOI] [PubMed] [Google Scholar]
- 48. Hoppel C., DiMarco J. P., Tandler B. (1979) Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J. Biol. Chem. 254, 4164–4170 [PubMed] [Google Scholar]
- 49. Hoppel C. L., Tomec R. J. (1972) Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J. Biol. Chem. 247, 832–841 [PubMed] [Google Scholar]
- 50. Faloona G. R., Srere P. A. (1969) Escherichia coli citrate synthase. Purification and the effect of potassium on some properties. Biochemistry 8, 4497–4503 [DOI] [PubMed] [Google Scholar]
- 51. Lindqvist A., He Y. G., Andersson S. (2005) Cell type-specific expression of beta-carotene 9′,10′-monooxygenase in human tissues. J. Histochem. Cytochem. 53, 1403–1412 [DOI] [PubMed] [Google Scholar]
- 52. Gouet P., Robert X., Courcelle E. (2003) ESPript/ENDscript: Extracting and rendering sequence and 3D information from atomic structures of proteins. Nucleic Acids Res. 31, 3320–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kiser P. D., Farquhar E. R., Shi W., Sui X., Chance M. R., Palczewski K. (2012) Structure of RPE65 isomerase in a lipidic matrix reveals roles for phospholipids and iron in catalysis. Proc. Natl. Acad. Sci. U. S. A. 109, E2747–E2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kiser P. D., Golczak M., Lodowski D. T., Chance M. R., Palczewski K. (2009) Crystal structure of native RPE65, the retinoid isomerase of the visual cycle. Proc. Natl. Acad. Sci. 106, 17325–17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gakh O., Cavadini P., Isaya G. (2002) Mitochondrial processing peptidases. Biochim. Biophys. Acta 1592, 63–77 [DOI] [PubMed] [Google Scholar]
- 56. Claros M. G., Vincens P. (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur. J. Biochem. 241, 779–786 [DOI] [PubMed] [Google Scholar]
- 57. Bouvier F., Suire C., Mutterer J., Camara B. (2003) Oxidative remodeling of chromoplast carotenoids: identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell 15, 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Borel P., Grolier P., Mekki N., Boirie Y., Rochette Y., Le Roy B., Alexandre-Gouabau M. C., Lairon D., Azais-Braesco V. (1998) Low and high responders to pharmacological doses of beta-carotene: proportion in the population, mechanisms involved and consequences on beta-carotene metabolism. J. Lipid Res. 39, 2250–2260 [PubMed] [Google Scholar]
- 59. Amengual J., Gouranton E., van Helden Y. G., Hessel S., Ribot J., Kramer E., Kiec-Wilk B., Razny U., Lietz G., Wyss A., Dembinska-Kiec A., Palou A., Keijer J., Landrier J. F., Bonet M. L., von Lintig J. (2011) Beta-carotene reduces body adiposity of mice via BCMO1. PloS One 6, e20644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Olson J. A., Hayaishi O. (1965) The enzymatic cleavage of beta-carotene into vitamin A by soluble enzymes of rat liver and intestine. Proc. Natl. Acad. Sci. U. S. A. 54, 1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goodman D. S., Huang H. S. (1965) Biosynthesis of vitamin A with rat intestinal enzymes. Science 149, 879–880 [DOI] [PubMed] [Google Scholar]
- 62. Taylor S. W., Fahy E., Zhang B., Glenn G. M., Warnock D. E., Wiley S., Murphy A. N., Gaucher S. P., Capaldi R. A., Gibson B. W., Ghosh S. S. (2003) Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 21, 281–286 [DOI] [PubMed] [Google Scholar]
- 63. Golczak M., Kiser P. D., Lodowski D. T., Maeda A., Palczewski K. (2010) Importance of membrane structural integrity for RPE65 retinoid isomerization activity. J. Biol. Chem. 285, 9667–9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arakane F., Sugawara T., Nishino H., Liu Z., Holt J. A., Pain D., Stocco D. M., Miller W. L., Strauss J. F., 3rd (1996) Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc. Natl. Acad. Sci. U. S. A. 93, 13731–13736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bhosale P., Larson A. J., Frederick J. M., Southwick K., Thulin C. D., Bernstein P. S. (2004) Identification and characterization of a Pi isoform of glutathione S-transferase (GSTP1) as a zeaxanthin-binding protein in the macula of the human eye. J. Biol. Chem. 279, 49447–49454 [DOI] [PubMed] [Google Scholar]
- 66. Li B., Vachali P., Frederick J. M., Bernstein P. S. (2011) Identification of StARD3 as a lutein-binding protein in the macula of the primate retina. Biochemistry 50, 2541–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.