Abstract
Mitochondria, the major source of cellular energy in the form of ATP, respond to changes in substrate availability and bioenergetic demands by employing rapid, short-term, metabolic adaptation mechanisms, such as phosphorylation-dependent protein regulation. In mammalian cells, an intramitochondrial CO2-adenylyl cyclase (AC)-cyclic AMP (cAMP)-protein kinase A (PKA) pathway regulates aerobic energy production. One target of this pathway involves phosphorylation of cytochrome c oxidase (COX) subunit 4-isoform 1 (COX4i1), which modulates COX allosteric regulation by ATP. However, the role of the CO2-sAC-cAMP-PKA signalosome in regulating COX activity and mitochondrial metabolism and its evolutionary conservation remain to be fully established. We show that in Saccharomyces cerevisiae, normoxic COX activity measured in the presence of ATP is 55% lower than in the presence of ADP. Moreover, the adenylyl cyclase Cyr1 activity is present in mitochondria, and it contributes to the ATP-mediated regulation of COX through the normoxic subunit Cox5a, homologue of human COX4i1, in a bicarbonate-sensitive manner. Furthermore, we have identified 2 phosphorylation targets in Cox5a (T65 and S43) that modulate its allosteric regulation by ATP. These residues are not conserved in the Cox5b-containing hypoxic enzyme, which is not regulated by ATP. We conclude that across evolution, a CO2-sAC-cAMP-PKA axis regulates normoxic COX activity.—Hess, K. C., Liu, J., Manfredi, G., Mühlschlegel, F. A., Buck, J., Levin, L. R., Barrientos, A. A mitochondrial CO2-adenylyl cyclase-cAMP signalosome controls yeast normoxic cytochrome c oxidase activity.
Keywords: Cyr1, mitochondrial energy metabolism, normoxic Cox5a, hypoxic Cox5b
As the major source of cellular energy in the form of ATP and a hub of intermediate metabolism, mitochondria play fundamental roles in cell signaling and regulation (1–3). They are able to respond to changes in substrate availability and bioenergetic demands by taking advantage of rapid, short-term, metabolic adaptation mechanisms. Over the past decade, numerous studies in eukaryotic cells from yeast to mammals have linked post-translational modifications of mitochondrial enzymes to the regulation of energy metabolism (4–6). Phosphorylation-dependent regulation of mitochondrial proteins plays important roles in mitochondrial and cellular stress responses (5, 6). Recently, the role of the ubiquitous cyclic AMP (cAMP)/protein kinase A (PKA) pathway in the phosphorylation of mitochondrial proteins to regulate mitochondrial metabolism has begun to emerge (3).
In mammalian cells, two independent cAMP domains signaling in mitochondria have been identified, one associated with the cytosolic-mitochondrial interface (7–9), and a second contained in the mitochondrial matrix (7, 10, 11). In regard to the source of cAMP in the mitochondrial matrix, it has been proposed that cAMP is generated in the cytosol and translocated into the mitochondrial matrix (12). However, we (13–15) and others (7, 10) have demonstrated that matrix cAMP is only generated locally by resident soluble adenylyl cyclase (sAC). Matrix-generated cAMP regulates oxidative phosphorylation (oxphos) and ATP generation (7, 10, 14, 15) via phosphorylation of numerous proteins, including subunits of the mitochondrial respiratory chain complex IV, cytochrome c oxidase (COX; ref. 14, 15). S58 in COX subunit 4 isoform 1 (COX4i1) is one of the targets of phosphorylation (11, 14). This modification regulates COX allosteric inhibition by matrix ATP: when COX4i1 is dephosphorylated, COX activity is inhibited by ATP (11). ATP inhibition may play a role in the transition to an energy storage state by facilitating accumulation of unused substrates, such as fat and glycogen.
Carbonic anhydrases (CAs) are ubiquitous enzymes that equilibrate CO2 levels with bicarbonate and a proton, and 2 isoforms of type V CA have been shown to reside inside mammalian mitochondria (16, 17). The coincident localization of sAC, CAs, and the tricarboxylic acid (TCA) cycle suggests that the CO2 produced by the TCA cycle is maintained in equilibrium with bicarbonate via mitochondrial CA and sensed via sAC. We demonstrated that sAC-cAMP regulation of oxphos was sensitive to physiologically relevant intramitochondrial bicarbonate concentrations, metabolically generated CO2, and CA inhibition (11). Thus, the intramitochondrial sAC-PKA pathway senses metabolic activity, providing a mechanism linking respiratory activities to nutritional availability. As nutrients become available to the cell, local CO2 levels increase, and matrix bicarbonate concentration rises, stimulating the mitochondrial sAC-cAMP pathway. This leads to increased oxphos activity, while simultaneously limiting reactive oxygen species (ROS) production. Recently, Di Benedetto et al. (10) demonstrated that the matrix-localized sAC-cAMP cascade is also regulated by uptake of cytosolic Ca2+ into mitochondria, suggesting this cascade mediates hormone-induced regulation of oxphos activity. Finally, we identified an isoform of the 3′,5′-cyclic-nucleotide phosphodiesterase 2A (PDE2A), which hydrolyzes cAMP into 5′AMP and thereby inactivates PKA, as a component of this intramitochondrial, sAC-defined domain. The unique NH2 terminus of isoform 2 of PDE2A targets the enzyme to the mitochondrial matrix, where it opposes the action of sAC in regulating oxphos (13).
The role of the CO2-sAC-cAMP-PKA signalosome in the regulation of COX activity and mitochondrial metabolism and its evolutionary conservation remain to be fully established. We have chosen to explore the existence of this pathway in the yeast Saccharomyces cerevisiae. In addition to the usual reasons for using yeast as a model system (its ease of genetic manipulation, well-annotated genome, short generation time, and simplicity, yet with the same fundamental biology as humans), S. cerevisiae is a unicellular eukaryote well suited for studying metabolism because it is a facultative aerobe/anaerobe, and anaerobic vs. aerobic respiration can be experimentally distinguished by growth in medium containing respiratory or fermentable carbon sources. Most of our knowledge on eukaryotic COX biogenesis derives from original studies in yeast (18, 19). In addition, S. cerevisiae posses a Ras/cAMP/PKA pathway well characterized as a nutrient-sensitive signaling cascade that regulates carbohydrate metabolism, proliferation, stress resistance, life span, and sporulation (20, 21). S. cerevisiae contains a single adenylyl cyclase, Cyr1, which synthesizes cAMP from ATP (22, 23). The activity of Cyr1 is stimulated by the small GTP-binding proteins Ras1 and Ras2, as well as the glucose-sensing G-protein-coupled receptor Gpr1 and the Gα-like protein Gpa2 (21). As in mammals, the PKA enzyme in yeast is a heterotetramer consisting of 2 regulatory and 2 catalytic subunits. On binding of 2 molecules of cAMP to each regulatory subunit, the catalytic subunits are able to phosphorylate their targets. Unlike mammals, however, yeast possess a single gene for the PKA regulatory subunit, BCY1 (24), and 3 genes that code for the catalytic subunits of PKA: TPK1, TPK2, and TPK3 (25). Bcy1 and the catalytic subunits of PKA have been detected in yeast mitochondria (26–29).
Like mammalian sAC, the CYR1 adenylyl cyclases from Candida albicans and Cryptococcus neoformans are directly regulated by bicarbonate (30–32), and the same appears to be true for S. cerevisiae Cyr1 (33). Mammals contain 16 different CAs, including 2 targeted to the mitochondria. All yeasts thus far analyzed contain ≥1 CA, several of which are known to localize to the mitochondria (34). The subcellular distribution of the only known CA of S. cerevisiae, Nce103, has not yet been characterized; however, it has been shown to supply bicarbonate ions for the carboxylation reactions of pyruvate carboxylase, acetyl-CoA carboxylase, and carbamoyl phosphate synthetase, functions attributed to the mammalian mitochondrial CA isoforms (35). Although it is not clear whether Nce103 is expressed inside and/or on the surface of mitochondria, it presumably can sense the CO2 generated from the Krebs cycle. The production of cAMP is counteracted by the 3′,5′-cyclic-nucleotide PDEs Pde1 and Pde2, which hydrolyze cAMP into 5′AMP and thereby inactivate PKA (36). Notably, the activity of Pde1 has been detected in the cytoplasm, nucleus, and mitochondria (37).
Thus, we hypothesize that a full CO2-sAC-cAMP-PKA signalosome could exist in yeast mitochondria and regulate target proteins by phosphorylation, thereby influencing cell growth, metabolism, and fundamental cellular functions (20, 21). Here, we show that adenylyl cyclase activity is present in yeast mitochondria and that normoxic COX is one of the targets of the mitochondrial cAMP pathway, while the hypoxic enzyme is not. Our results demonstrate that the intramitochondrial CO2-sAC-cAMP-PKA signalosome is highly conserved from lower eukaryotes to humans and acts as a major regulatory mechanism for metabolic adaptation not only in mammals but also in yeast.
MATERIALS AND METHODS
Strains and growth conditions
The genotypes and sources of the S. cerevisiae strains used in this study are listed in Supplemental Table SI. The following media were used routinely to grow yeast: YPD (2% glucose, 1% yeast extract, and 2% peptone), WO-Gal (2% galactose and 0.67% W/O nitrogen base), and YPEG (2% ethanol, 2% glycerol, 1% yeast extract, and 2% peptone).
Construction of strains expressing only one of the two COX subunit 5 (Cox5) isoforms (aW303Δcox5aΔcox5b/YIP351-COX5a and aW303Δcox5aΔcox5b/YIP351-ProCOX5a-COX5b)
An aW303Δcox5aΔcox5b strain was created by replacement of the full COX5b gene with a KANMX4X cassette from BY4741Δcox5b obtained from Open Biosystems/Thermo Scientific (Huntsville, AL, USA). The KANMX4X cassette and flanking sequences of the COX5b gene were PCR-amplified using primers: 5′-GGATCCGACACAAAGATTGGGTATGAGC-3′ and 5′-CTGCAGTGGATTGATGTAAGCAGTGGTAT-3′. The amplicon was transformed into an aW303Δcox5a strain to create aW303Δcox5aΔcox5b strain. The aW303Δcox5aΔcox5bYIP351-COX5a and aW303Δcox5aΔcox5b/YIP351-ProCOX5a-COX5b were created by transform aW303Δcox5aΔcox5b strain with linearized plasmid containing COX5a gene or COX5b gene under the promoter of COX5a. The plasmid containing ProCOX5a-COX5b-2HA was constructed by ligating both the 1 kb promoter sequence of COX5a, and COX5b-2HA into the SacI and PstI restriction sites of YIplac351 plasmid. The 1 kb portion of COX5a promoter was amplified using primers 5′-GAGCTCGTTGCCAAAGAAATACCGAAC-3′ and 5′-GACACGGGATCCTGTAGATGCGTTCTTAGTTG-3′. COX5b-2HA was amplified using primers 5′-GACACGGGATCCAGCATGTATAACAAACACTG-3′ and 5′-CGATGTCTGCAGTTAAGCGTAGTCTGGGACGTCGTATGGGTAAGCGTAGTCTGGGACGTCGTATGGGTATTTAGATTGAACTTGAGAATAACCTCC-3′. The ProCOX5a-COX5b plasmid was constructed by creating a stop codon before the C-terminal HA tag sequence, using primers 5′-CTCAAGTTCAATCTAAATAACCATACGACGTCCCAGACTACGC-3′ and 5′-GCGTAGTCTGGGACGTCGTATGGTTATTTAGATTGAACTTGAG-3′.
Adenylyl cyclase assays
Mitochondria with intact outer membrane were isolated from yeast cells as reported previously (38). To obtain highly purified mitochondria virtually free of plasma membrane, endoplasmic reticulum, and other organelles, we used a linear Nicodenz gradient, as described previously (38). Highly purified mitochondria were sonicated in 20 mM Tris-HCl (pH 7.4), 0.5 mM EDTA, and 5 mM DTT in the presence of proteinase inhibitors. Protein (50 μg) was incubated for 30 min at 30°C with 10 mM Mg2+-ATP in the presence of indicated concentrations of sodium bicarbonate or cyclase inhibitors (KH7 and MDL-12330A). Assays were quenched with equal volume of 0.2 N HCl, and cAMP was determined using the Correlate-EIA cAMP assay (Assay Designs, Inc., Ann Arbor, MI, USA).
Yeast PDE assays
Mitochondrial fractions were prepared and then sonicated in 20 mM Tris-HCl (pH 7.4) and 0.5 mM EDTA in the presence of proteinase inhibitors. Protein (50 μg) was incubated for 30 min at 30°C with 10 mM MgCl2 in the presence or absence of 40 mM sodium bicarbonate, with exogenous cAMP but no ATP added. cAMP was determined using the Correlate-EIA cAMP assay (Assay Designs).
Respiratory growth assays
Yeast were grown in liquid cultures for 20 h at 30°C while shaking at 250 rpm. The starting number of yeast was reduced such that at the completion of the experiment, the cells did not reach stationary phase. Half the samples were grown in YPD medium (glucose containing), and the other half were grown in ethanol/glycerol-containing medium. The initial cell concentration was 105 cells/ml for cells grown in YPD and 106 cells/ml for those grown in ethanol/ glycerol medium (since cells grow less rapidly under these conditions). Before the incubation, both KH7 and MDL-12330A were titrated into duplicate tubes corresponding to each growth condition. The final DMSO (solvent for both drugs) concentration was 0.4%, including vehicle control tubes. At h 20, absorbance (OD600) of the cultures was measured, as an indicator of cell number. In another experiment, a growth curve was established by measuring the OD600 of the culture every 2 h. Values were reported as percentage of the DMSO controls.
Mitochondrial function assays
In vivo endogenous cell respiration was measured polarographically in log-phase cultures as reported previously (39). For some experiments, the cultures were preincubated with sAC inhibitors or the vector (0.2% DMSO). Rates of oxygen consumption were standardized to cell number. The compounds (alone) were tested on the polarograph apparatus as a control for unspecific oxygen readings, and determined to have no effects.
For all the in organello experiments, we used mitochondria with intact outer membranes that were isolated from yeast cells as reported previously (38). For in organello oxygen consumption measurements, 40 μg of mitochondria with intact outer membranes was injected into a polarographic chamber (Hansatech Instruments, King's Lynn, UK) containing 1 ml of respiration medium (20 mM HEPES, pH 7.4; 2 mM MgCl2; 1 mM EDTA; 0.6 mM sorbitol; and freshly added 0.1% BSA) in the presence or absence of sAC inhibitors (0.2% DMSO final concentration). We measured state 1, state 2 following addition of 1 mM NADH, state 3 following addition of 80 mM ADP, and maximal uncoupled respiration by addition of 12 μM carbonyl cyanide m-chlorophenylhydrazone (CCCP). Finally, 700 μM potassium cyanide (KCN) was added to block oxphos via COX inhibition. The values reported are KCN-sensitive oxygen consumption rates (36, 39). Cytochrome c oxidase was measured spectrophotometrically (Shimadzu Corp., Kyoto, Japan) as reported previously (36, 39) following the oxidation of reduced cytochrome c at 550 nm.
Assay of allosteric regulation of COX activity
Mitochondria with intact outer membranes were resuspended in a buffer containing 0.6 M sorbitol and 20 mM HEPES, pH 7.4 (SH buffer). The intactness of the outer membrane is essential to maintain active the exogenous ATP regeneration system described below, since pyruvate kinase is allosterically inhibited by ATP (40), and to prevent perturbations of intramitochondria signaling cascades. The concentration of heme a + a3 in mitochondria was calculated by quantitatively extracting all cytochromes in the presence of sodium deoxycholate, obtaining the differential reduced vs. oxidized mitochondrial total cytochrome spectra, measuring the heme a/a3 peak area (maxima at 603 nm), and obtaining its concentration by using an extinction coefficient (ε=605–630) of 24.0. In wild-type W303 mitochondria, the concentration calculated was 6.0 ± 0.54 nmol heme a/a3 per milligram mitochondrial protein. In all the assays, we used 30 μg of mitochondria in 100 μl reactions, thus containing ∼ 60 nM heme a/a3.
Following a modified version of the method of Arnold and Kadenbach (40), mitochondria at 60 nM heme a/a3 were incubated for 20 min at 25°C in the presence of either 5 mM ADP or 5 mM ATP plus an ATP-regenerating system, consisting of 10 mM phosphenolpyruvate (Sigma, St. Louis, MO, USA), 5 mM MgSO4, and 20 U/ml pyruvate kinase (Roche, Indianapolis, IN, USA) in final volume of 100 μl. In some of the experimental settings, the reactions additionally included 150 μM of the sAC inhibitors KH7 or MDL or corresponding volumes of the vector; or 40 mM NaHCO3. Subsequently, COX activity was measured spectrophotometrically at 25°C as reported previously (39), using 395 μl of the incubated mitochondrial suspension mixed with 395 μl of 50 mM KPO4 buffer with 1% Tween 20 and 25 μl of 0.1 M ADP or ATP and with various concentrations of cytochrome c (0.25–100 μM) for titration experiments or 60 μM for all other experiments.
Miscellaneous procedures
Standard procedures were used for the preparation and ligation of DNA fragments, and for transformation and recovery of plasmid DNA from Escherichia coli (41). Yeast were transformed as described previously (42). Protein concentration was measured with the Folin phenol reagent (43). Proteins were separated by SDS-PAGE in the buffer system of Laemmli (44), and membranes with immobilized proteins were treated with antibodies against the appropriate proteins, followed by a second reaction with anti-mouse or anti-rabbit IgG conjugated to horseradish peroxidase (Sigma). The Super Signal chemiluminescent substrate kit (Pierce, Rockford, IL, USA) was used for the final detection. Phosphorylation sites within the N-terminal Cox5a sequence were predicted by bioinformatics analysis using the NetPhos 2.0 server (45), which produces neural network predictions for serine, threonine, and tyrosine phosphorylation sites in eukaryotic proteins.
Statistical analyses
At least 2 independent experiments with 3 replicas were performed for each analysis. Data are presented as means ± sd of absolute values or percentage of control. The values obtained for wild-type and drug-treated strains for the different parameters studied were compared by Student's t test. Values of P < 0.05 were considered significant.
RESULTS
Adenylyl cyclase activity of S. cerevisiae Cyr1 is present in mitochondria
CYR1 is an essential gene that encodes the single adenylyl cyclase present in the yeast S. cerevisiae. Cyr1 activation was thought to be dependent on its localization to the plasma membrane (22), a peripheral association that requires the Ras GTPase-activating protein Ira1 (23). However, because mammalian sAC has been demonstrated inside mitochondria (7, 10, 11, 15), we asked whether Cyr1 might also reside in mitochondria. Two high-throughput studies analyzing the mitochondrial proteome identified Cyr1 as a mitochondrial component (46, 47), and recent subcellular localization analyses of Ras signaling complex proteins by both fluorescent tagging and biochemical cell membrane fractionation on sucrose gradients showed that Cyr1 is present in the mitochondrial fraction (48). Therefore, we explored whether adenylyl cyclase activity is present in S. cerevisiae mitochondria.
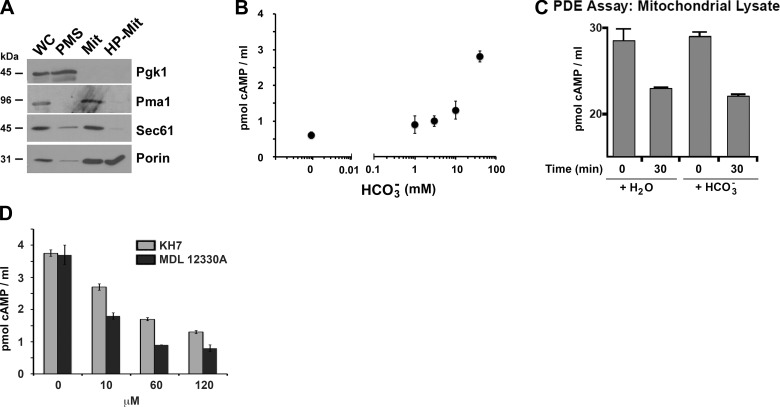
Highly purified S. cerevisiae mitochondrial fractions isolated from wild-type W303 cells grown in respiratory medium (Fig. 1A) contained adenylyl cyclase activity, which was potently stimulated by bicarbonate (Fig. 1B). In these experiments, we were unable to pharmacologically inhibit PDE activity; therefore, the HCO3−-dependent increase in cAMP measured in mitochondria (Fig. 1C) could be due to a constant level of adenylyl cyclase activity coupled with HCO3− inhibiting endogenous PDEs. We directly tested whether endogenous mitochondrial PDE activity was affected by HCO3− and observed a similar reduction of exogenously added cAMP over time in the presence or absence of HCO3− (Fig. 1C). Thus, HCO3− does not affect yeast mitochondrial PDE enzymatic activity, suggesting that the observed elevation of cAMP in HCO3−-treated mitochondria was due to stimulation of the endogenous adenylyl cyclase (i.e., Cyr1).
Figure 1.
Cyr1 and PDE activities are present in mitochondria from S. cerevisiae. A) Whole cell (WC) fractionation into postmitochondrial supernatant (PMS), which mostly contains soluble cytoplasmic proteins, and mitochondria-enriched fraction (Mit), was performed as explained in Materials and Methods. Highly purified mitochondria (HP-Mit) were obtained by a Nicodenz gradient fractionation of the crude mitochondrial fraction. Pgk1 (3-phosphoglycerate kinase) is a cytoplasmic marker; Pma1 is a plasma membrane H+-ATPase; and Sec61 is a subunit of the endoplasmic reticulum protein translocation channel. B) Adenylyl cyclase activity, measured in purified yeast mitochondrial fractions over 30 min in the presence of 10 mM Mg2+-ATP, 100 mM Tris-HCl (pH 7.5), and the indicated concentration of bicarbonate. C) PDE activity in mitochondrial lysates, estimated by measuring the depletion of 28 pmol/ml of exogenously added cAMP, in the presence or absence of sodium bicarbonate. D) Adenylyl cyclase activity, measured in purified yeast mitochondrial fractions over 30 min in the presence of 10 mM Mg2+-ATP, 100 mM Tris-HCl (pH 7.5), 40 mM bicarbonate, and the indicated concentrations of KH7 and MDL-12330A .
To gain temporal and reversible control over yeast adenylyl cyclase activity, we explored whether a number of commercially available pharmacological inhibitors of mammalian adenylyl cyclases inhibited the adenylyl cyclase activity in yeast mitochondria. MDL-12330A, an inhibitor of both mammalian transmembrane adenylyl cyclases and sACs (49) previously shown to inhibit the Cyr1 of C. albicans (50), and KH7, a selective inhibitor of mammalian mitochondrial sAC (49, 51), dose dependently inhibited S. cerevisiae mitochondrial adenylyl cyclase activity (Fig. 1D).
The ability of Cyr1 to respond to HCO3− suggests that the cyclase is capable of sensing CO2 generated by the Krebs cycle. This raises the hypothesis that Cyr1 could serve a functional role in yeast mitochondria, analogous to that of sAC in mammals.
Cyr1 is a positive regulator of oxphos
If Cyr1 serves a respiratory regulatory function in yeast analogous to that of sAC in mammalian cells, then inhibiting the cyclase in vivo would be expected to reduce oxphos activity in wild-type W303 yeast cells.
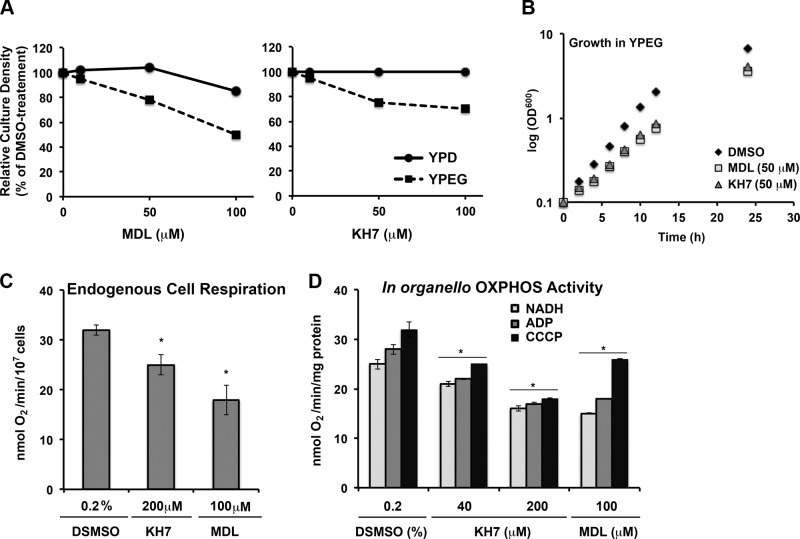
To examine this possibility, we first tested whether AC inhibitors would preferentially affect growth in medium containing respiratory (ethanol/glycerol-YPEG) vs. fermentable (glucose-YPD) carbon sources. Pharmacological inhibition of the cyclase attenuated cell growth to a greater extent in yeast grown in YPEG than it did in yeast grown in YPD, consistent with a prorespiratory function of Cyr1 (Fig. 2A). KH7 at both 50 and 100 μM was sufficient to decrease the growth of yeast in YPEG medium, while it had virtually no effect in YPD. MDL-12330A caused a similar respiratory phenotype. High MDL concentrations (100 μM) had, however, a slight negative effect on cell growth in YPD, which could reflect nonspecific toxicity (Fig. 2A). A growth curve confirmed that 50 μM KH7 or MDL did not affect cell growth in YPD medium (not shown), but both extended the growth rate in YPEG (Fig. 2B). Thus, respiratory growth seems to be more sensitive to adenylyl cyclase inhibition than growth in the presence of glucose, a fermentable carbon source.
Figure 2.
In vivo Cyr1 Inhibition causes a respiratory phenotype. A) Wild-type W303 yeast were incubated for 20 h in medium containing either glucose (YPD) or ethanol/glycerol (YPEG) in the presence of the indicated concentrations of MDL-12330A or KH7. Culture density was measured as OD600, and data are reported as a percentage of DMSO control. B) Growth curve of wild-type W303 yeast in YPEG medium in the presence of 50 μM of MDL-12330A or KH7. C) KCN-sensitive endogenous cell respiration of wild-type W303 yeast measured polarographically in the presence of 50 μM of MDL-12330A or KH7. D) NADH-driven oxidation in coupled (+ADP) and uncoupled (+CCCP) mitochondria in the presence of the indicated concentrations of MDL-12330A or KH7. Bars represent means ± sd. *P < 0.05.
Consistently with the growth assay results, cyanide-sensitive endogenous cell respiration of wild-type yeast was significantly attenuated by KH7 and MDL (Fig. 2C). Although KH7 has been shown to uncouple mammalian mitochondria, treatment of isolated mitochondria with either KH7 or MDL caused a significant decline in NADH oxidation in both coupled (+ADP) and uncoupled (+CCCP) conditions (Fig. 2D).
Normoxic yeast COX is allosterically regulated by ATP
In mammalian mitochondria, CO2/bicarbonate-regulated sAC responds to metabolically generated CO2 to modulate COX activity by facilitating the phosphorylation of subunit COX4i1 (11, 15).
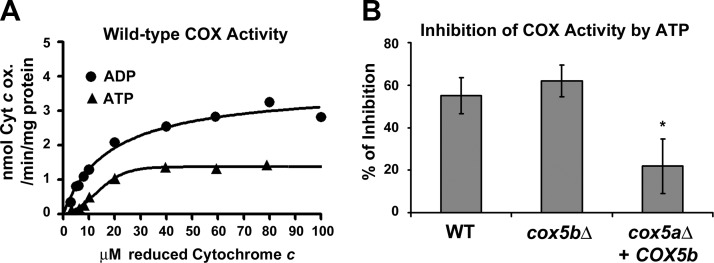
To examine whether yeast COX function is modulated in a Cyr1-dependent manner, we started by establishing the experimental conditions (mitochondria with intact outer membranes kept in an isotonic buffer in the presence of an ATP-regenerating system) to demonstrate that yeast COX is allosterically regulated by ATP (Fig. 3A) as previously reported (52). COX activity measured in the presence of ATP was ∼55% lower than the activity measured in the presence of ADP (Fig. 3A, B).
Figure 3.
Percentage of COX inhibition by ATP in mitochondria from strains expressing COX5a is greater than in strains expressing COX5b. A) Cytochrome c titration and measurement of COX activity in the presence of excess ATP (5 mM) or ADP (5 mM) following a previously reported procedure (73) using highly enriched fractions containing mitochondria with intact outer membranes isolated from wild-type W303 cells. Data represent an average of 2 independent experiments. For each value, the differences between the 2 replicas where within 5%. B) Percentage of inhibition of COX activity measured using 60 μM reduced cytochrome c as the substrate in the presence of 5 mM ATP compared with the COX activity measured in the presence of 5 mM ADP. Bars represent means ± sd of 3 independent experiments. *P < 0.05.
COX plays a role in both short-term (allosteric regulation by ATP binding) and long-term regulation (adaptation to oxygen concentrations) of the mitochondrial respiratory chain. Both regulatory mechanisms involve subunit 5 (Cox5) in yeast, the homologue of mammalian COX4 subunit. Cox5/COX4 exists in 2 interchangeable isoforms encoded by the nuclear genes COX5a/COX4-1 and COX5b/COX4-2. Their expression is inversely regulated transcriptionally by oxygen concentration and oxidative stress (53–55). In mammals, COX4i2 is expressed in lung and placenta, highly oxygenated tissues, where oxidative stress could be potentially high. Cox5/COX4 isoforms have differential effects on COX enzymatic activity. The hypoxic isoforms modify an internal step in electron transport between heme a and the binuclear reaction center in COX subunit 1, which occurs 3 to 4 times faster than in the normoxic COX isoenzyme, thus altering the enzyme maximal turnover number (TNmax) but not its Km (56). As a result, by regulating the proportion of each isoform assembled into the holoenzyme, the catalytic efficiency of COX is modulated adjusting the mitochondrial respiratory chain electron transfer rate to adapt to changes in the environmental oxygen tension (53).
Under hypoxia and oxidative stress, yeast switch from oxidative phosphorylation to glycolysis. We hypothesized that under these conditions, the cell would no longer need to regulate oxphos through ATP-mediated allosteric regulation. We tested whether hypoxic COX undergoes allosteric regulation by constructing mutant yeast strains expressing only one Cox5 isoform, either Cox5a or Cox5b, under the control of the normoxic COX5a promoter, and estimated the percentage of COX inhibition by ATP. We observed that Cox5b-containing COX was significantly less inhibited by ATP (∼20% of inhibition) than Cox5a-containing COX (Fig. 3B).
Mitochondrial sAC pathway modulates the allosteric regulation of COX activity by ATP
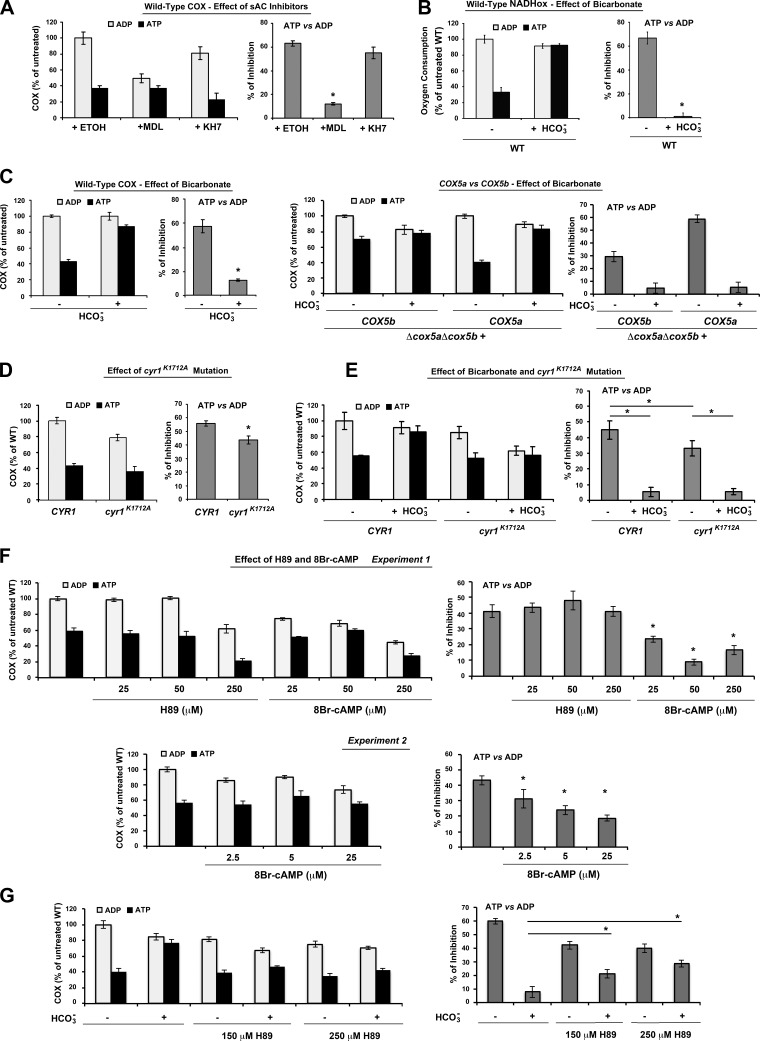
To test whether COX regulation by ATP was at least partially dependent on the mitochondria-localized yeast cyclase, we repeated the COX activity assays in isolated W303 mitochondria in the presence of either ATP or ADP and the inhibitors MDL or KH7. Both inhibitors significantly lowered COX activity in the presence of ADP (Fig. 4A, left panel), although only MDL significantly reduced the ATP inhibition rate by ∼50% (Fig. 4A, right panel). Further supporting the involvement of Cyr1, the ATP-mediated regulation of mitochondrial respiration, measured as NADH oxidation and COX activity, was dramatically sensitive to bicarbonate in wild-type mitochondria (Fig. 4B, C). Notably, while bicarbonate abrogated the inhibition of COX activity by ATP in cells expressing exclusively Cox5a, it did not alter the already abrogated ATP-mediated COX inhibition in mitochondria exclusively expressing Cox5b (Fig. 4C, right panel).
Figure 4.
The CO2-sAC-cAMP-PKA signalosome regulates the allosteric regulation of COX activity by ATP. A) Graphs represent total COX activity (left panel) and percentage of inhibition of COX activity by ATP (right panel) in the presence of sAC inhibitors (150 μM KH7 or MDL) or just the vehicle (DMSO). Mitochondria with intact outer membranes were incubated with 5 mM ADP or ATP with ATP regeneration system with or without the inhibitors for 20 min before assessment of COX activity. KH7 and MDL were dissolved in ethanol with 10% DMSO. Final concentration of DMSO in the COX measurement was 0.0165%. B) NADH oxidation, measured polarographically, using mitochondria preincubated in the presence of either ADP or ATP. C) Effect of sodium bicarbonate (40 mM) on COX activity in wild-type cells (right panel) and in cells expressing exclusively COX5A or COX5b under the control of the COX5a promoter (left panel). D) Effect of a Cyr1K1712A mutation on total COX activity (left panel) and percentage of inhibition of COX activity by ATP (right panel). E) As in C, but activity was measured in the presence or absence of 40 mM sodium bicarbonate. F) Effect of H89 (PKA inhibitor) and the cAMP analog 8-bromoadenosine 3′,5′-cyclic monophosphate (8Br-cAMP) on total COX activity (left panel) and percentage of inhibition of COX activity by ATP (right panel). Two independent experiments are presented. G) Effect of sodium bicarbonate (40 mM) on COX activity in wild type cells treated with H89. Bars represent means ± sd of 3 independent experiments. *P < 0.05.
To exclude the possibility that the effect of the sAC inhibitors on COX allosteric regulation may have come from off-target effects of the compounds, we measured COX activity and allosteric inhibition in the diploid yeast strain cyr1-K1712A carrying a mutation in Cyr1 (33). The cyr1K1712A variant affects a lysine residue within the catalytic center involved in substrate binding and severely decreases cAMP production. Yeast expressing cyr1K1712A accumulate significant amount of glycogen compared with wild-type yeast, a signature of reduced cAMP/PKA signaling (33). We showed that the CYR1 and cyr1K1712A strains have similar steady-state levels of Cox5 and Cox1 (Supplemental Fig. S1), the latter a catalytic COX subunit whose levels directly correlate to levels of assembled COX holoenzyme in the yeast S. cerevisiae (56). However, consistent with the inhibitor studies, the cyr1K1712A mutant had lower COX activity in the presence of ADP and retained a significantly lower ATP inhibition rate compared with wild-type (Fig. 4D). The cyr1 mutant had lower COX activity compared with the wild type in the presence of bicarbonate and ADP. However, in the presence of bicarbonate and ATP, both the mutant and corresponding wild-type strains were insensitive to ATP-mediated COX allosteric regulation (Fig. 4E).
We pharmacologically probed the signaling pathway described here by testing the effect of the cAMP analog 8-bromoadenosine 3′,5′-cyclic monophosphate (8Br-cAMP) and a PKA inhibitor, H89, on total COX activity and percentage of inhibition of COX activity by ATP. Addition of 8Br-cAMP significantly lowered the percentage of inhibition of COX activity by ATP (Fig. 4F), mimicking the effect of bicarbonate-induced adenylyl cyclase stimulation, while H89 abrogates the effect of bicarbonate on the activity of COX (Fig. 4G). We noticed that H89-treated wild-type mitochondria have lower COX activity in the presence of ADP. The lower ADP activity with no change in ATP activity compared with non-H89 treated cells could be explained if in these conditions COX is inhibited due to constitutive binding of ATP to COX5a, thus preventing further ATP binding. In summary, our results indicate that while in untreated cells bicarbonate blocks ATP inhibition of COX by promoting phosphorylation of the ATP-binding pocket, H89 inhibits PKA abrogating the effect of bicarbonate.
Taken together, the data presented in this section demonstrate that the mitochondrial CO2-sAC-cAMP-PKA pathway modulates the allosteric regulation of yeast COX activity by ATP.
T65 and S43 in yeast COX5a mediate the allosteric regulation of COX activity by ATP
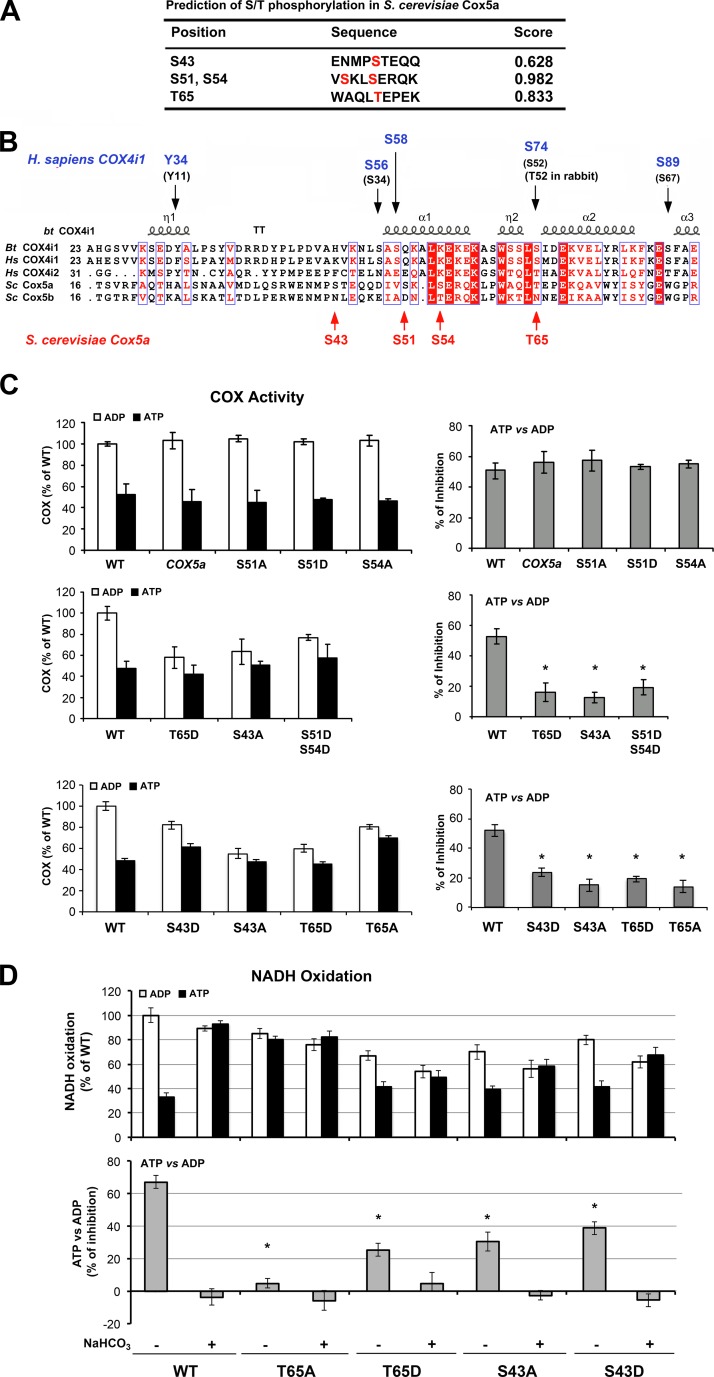
Next, we aimed to identify the residues within the ATP binding domain of Cox5a that could be responsible for the ATP-dependent allosteric regulation in yeast mitochondria. As mentioned earlier, phosphorylation of S58 on mammalian COX4i1 abolishes ATP binding and thus prevents the allosteric regulation of COX by ATP (14). Consistently, a nonphosphorylatable S58A variant of COX4i1 resulted in loss of allosteric regulation (14). Mammalian COX4 and yeast Cox5 have remarkably similar structures and functions. The crystal structure determined for bovine COX4i1 (58) and the predicted 3-dimensional structure of yeast Cox5a are superimposable, thus allowing a similar predicted spatial relationship between these proteins and the catalytic core subunits 1 and 2 (59). We have created structural models of the ATP binding pocket in yeast Cox5a by superimposition of the Cox5a and COX4i1 structures (Supplemental Fig. S2A). The model indicates that relevant residues (see below) such as Cox5a-T65 and COX4i1-S74 superimpose on each other, while the serine pairs Cox5a-[S51, S54] and COX4i1-[S56, S58] do not precisely superimpose (Supplemental Fig. S2A, B). In yeast, the closest predicted phosphorylation sites to mammalian COX4i1-S58 are Cox5a-S51 and -S54 (Fig. 5A, B). Unlike the mammalian enzyme, mutation of either of these predicted phosphorylation sites on the matrix domain of Cox5a to nonphosphorylatable S51A and S54A, or phosphomimetic S51D or S54D Cox5a, did not affect COX function nor abolish the inhibition of COX activity by ATP (Fig. 5C). However, a double-mutant S51D-S54D Cox5a decreased COX activity and the allosteric inhibition of COX in the presence of ATP (Fig. 5C). In silico analysis of possible phosphorylation sites in Cox5a predicted that both S43 and T65 near the ATP binding site could be phosphorylated (Fig. 5A, B). Indeed, strains expressing T65D or S43A Cox5a mutants had lowered COX activity and abolished the inhibition of COX by ATP (Fig. 5C). Notably, T65A or S43D Cox5a substitutions also largely abolished ATP inhibition; however, the COX activities in strains expressing T65A or S43D Cox5a mutants, while lower than wild type, were higher than the COX activities in the T65D or S43A mutants. Notably, none of these mutations did affect the steady-state levels of Cox5 or Cox1 (Supplemental Fig. S1).
Figure 5.
Mutations in predicted threonine and serine phosphorylation sites within the yeast Cox5a ATP binding pocket abolish allosteric regulation of COX activity by ATP. A) Predicted PKA substrate residues (predicted phosphorylation sites) within the N-terminal Cox5a sequence, where the ATP binding pocket is located, by bioinformatics analysis using Netphos 2.0. B) Multiple alignments of COX4/Cox5 show the conservation from yeast to mammals of key residues in the normoxic and hypoxic isoforms. Above the alignment, residues known to be phosphorylated in mammalian COX4i1 are identified in purple, following the residue number of the precursor protein, or in black in parenthesis, following the residue number of the mature protein. Below the alignment, residues predicted to be phosphorylated in yeast Cox5a (following the residue number of the precursor protein) are identified in red. Bt, Bos taurus; Hs, Homo sapiens; Sc, Saccharomyces cerevisiae. C) Total COX activity (left panel) and percentage of inhibition of COX activity by ATP (right panel) in mitochondria isolated from wild-type cells, or cells expressing exclusively wild-type Cox5a or the indicated Cox5a variants (S51A, S51D, S54A, T65D, T65A, S43D, S43A, or the double mutant S51D-S54D). Bars represent means ± sd of 3 independent experiments. *P < 0.05. D) Effect of NaHCO3 in NADH oxidation, measured polarographically, using mitochondria preincubated in the presence of either ADP or ATP. Bars represent means ± sd of 3 independent experiments. *P < 0.05.
To further inspect the role of the T65 and S43 residues in COX allosteric regulation by ATP in a mitochondrial CO2-dependent manner, we measured the effect of bicarbonate in the NADH oxidation rate of isolated mitochondria preincubated in the presence of ATP or ADP. In wild-type yeast, bicarbonate treatment reduced ATP-dependent inhibiton of COX and of NADH oxidation by ∼60% (Figs. 4B and 5D). Presumably, the inhibition was mediated via phosphorylation of Cox5a preventing ATP binding. In the T65 mutant strains, the change to nonphosphorylatable T65A fully prevented ATP-dependent inhibition of NADH oxidation, while the change to phosphomimetic T65D allowed ∼25% of inhibition, a significant proportion, considering the negative effect the mutation has on maximum COX activity. Concerning the S43 mutant strains, both changes S43A and S43D allowed ∼35–40% of NADH oxidation inhibition by ATP. In the 4 T65 and S43 mutant strains, bicarbonate treatment abolished ATP inhibition. These results suggest that, although both residues might play a role in mediating COX allosteric regulation by ATP, T65 is clearly a target of the mitochondrial CO2-sAC-cAMP-PKA signalosome.
DISCUSSION
In the yeast S. cerevisiae, the Ras/cAMP/PKA pathway is a nutrient-sensitive signaling cascade that regulates carbohydrate metabolism, proliferation, stress resistance, and sporulation (20, 21). In mammals, it was recently discovered that cAMP is generated inside mitochondria in response to CO2/bicarbonate by sAC. Completely contained within mitochondria, sAC together with mitochondrial forms of PDE (mtPDE) and PKA (mtPKA), constitute a cAMP functional domain that regulates the production of ATP and ROS. The precise nature of the mtPKA remains, however, to be fully understood. The intramitochondrial CO2-sAC-cAMP-PKA cascade regulates the phosphorylation pattern and activity of cytochrome c oxidase (COX) in response to the changing metabolic needs of the cell (13–15). Here, we demonstrate that the CO2-sAC-cAMP-PKA signalosome is an ancient pathway conserved in the yeast S. cerevisiae. Several lines of evidence support this conclusion: the single adenylyl cyclase, Cyr1, resides in the mitochondria and is regulated by bicarbonate; the regulatory and catalytic subunits of yeast PKA localize to mitochondria (28, 29), and Pde1 is also expressed in S. cerevisiae mitochondria; Cyr1 is an positive regulator of oxphos; yeast normoxic COX, which contains the Cox5a isoform, is allosterically regulated by ATP, which is in turn regulated by the mitochondrial sAC pathway; and the effect of ATP on COX activity is significantly abrogated in a concentration-dependent manner by either a cAMP analog or by a PKA inhibitor. Furthermore, we have demonstrated that the ATP-mediated regulation is absent in the Cox5b-containing hypoxic COX isozyme as it is in the mammalian COX4i2-containing isozyme, which suggests that the regulatory function evolved along with the adaptation of eukaryotes to an oxygenated atmosphere.
COX is a Cu-heme terminal oxidase in the mitochondria respiratory chain, which exerts important regulatory roles on the overall respiratory rate. Both yeast and mammalian COX are multimeric complexes of dual genetic origin. The enzymatic core of the enzyme is formed by 3 mitochondrion-encoded subunits, COX1, COX2, and COX3, which are conserved in the prokaryotic enzyme. During the evolution of eukaryotic COX, several additional subunits encoded in the nuclear genome were progressively incorporated. One of the earliest nuclear addition events involved the emergence of subunit Cox5 (yeast)/COX4 (mammals), which exist in 2 isoforms whose expression is inversely regulated transcriptionally by oxygen tension and oxidative stress (55, 60–62). This subunit is highly conserved across phyla and provides a substrate for regulation of COX function and eukaryotic respiratory metabolism (63). Although among all of the nucleus-encoded subunits that are conserved across taxonomy, yeast Cox5 and human COX4 shared the lowest sequence conservation (63, 64), yet they perform similar regulatory functions in response to hypoxia or oxidative stress. In addition, the matrix domains of the normoxic isoforms Cox5a and COX4i1, which contain the putative ATP binding site, share relative high amino acid identity and similarity, which could support the conservation of its regulatory function.
Despite of the similarity between the mammalian and yeast cAMP-mediated allosteric regulation of COX, the amino acid residues involved in the process are different in mammalian COX4i1 and yeast Cox5a. The residue involved in the regulation of COX4i1, S58 (14) is only conserved among mammals and not in yeast Cox5a. In yeast, the S43 residue located adjacent to the first α-helix in the matrix domain of Cox5a is sensitive to phosphomimetic mutations and controls ATP binding. An electrostatic potential map showed that the matrix domain of Cox5a is more electropositive than in mammalian COX4i1 (Supplemental Fig. S2C). Thus, ATP may dock differently into the ATP binding pocket of yeast COX. and different amino acids may be involved in the electrostatic interaction with ATP. On the other hand, the residue T65, located within a small helix stretch adjacent the possible purine binding pocket (14), is conserved from yeast to higher eukaryotes. Previous phosphoproteomic analysis of COX from rabbit heart identified residue T52 (T74 in the rabbit precursor protein) as being phosphorylated after ischemic-reperfusion damage (65). However, functional analyses of this phosphorylation event have not yet been performed. Here we have demonstrated by mutagenesis studies that phosphorylation of this residue in yeast Cox5a results in lowered COX activity and abolition of ATP-mediated COX regulation. The S43 and T65 mutations do not change the overall conformation of the Cox5a matrix domain (Supplemental Fig. S2D), suggesting that phosphorylation of these residues could be physiologically relevant. Our results indicate that multiple amino acids near the ATP binding pocket may be involved in allosteric regulation and that those may vary among species and Cox5/COX4 isoforms. It is possible that during ischemic reperfusion, phosphorylation of the T65 residue and subsequent attenuation of COX activity could provide an adaptive mechanism to minimize ROS production through the respiratory chain. Notably, with the exception of S54, none of the predicted phosphorylation sites in Cox5a is conserved in Cox5b (Fig. 5B). Specifically, Cox5b has asparagines in the Cox5a positions S43 or T65, which may impede ATP binding. Similarly, the mammalian COX4i1 residue S58 is absent from COX4i2, suggesting that the COX4i2-containing isozyme cannot be regulated by ATP (14) as shown in mouse cortical neurons overexpressing COX4i2 (66).
The Ras/AC pathway in S. cerevisiae regulates the balance between the cell's investments in either growth or antistress defenses (reviewed in ref. 67). While abrogation of the pathway (i.e., knockout) is lethal, since yeast cannot progress through the cell cycle, reduced activity of the pathway slows growth and increases storage of nutrients, such as glycogen and trehalose; it also enhances stress resistance and causes constitutive expression of heat-shock and antioxidant genes. As reported for ras2 mutants (68), here we found that low doses of cyclase inhibitors did not affect growth in glucose, but had an effect on the growth of yeast in respiratory media, indicating respiration is more sensitive to lowered cAMP levels.
Nutrient-sensing pathways, such as the Ras/cAMP/PKA and the Sch9/TOR pathways, have a major impact on life span (69). As with cyr1, loss-of-function mutations in sch9 have dramatic positive effects on development of resistance to several kinds of stress and enhanced accumulation of reserve nutrients, and both cyr1 and sch9 mutants dramatically extended chronological longevity, defined as survival in stationary phase (69, 70). Equivalent nutrient-sensing pathways govern longevity in worms, flies, and mammals (reviewed in ref. 71). We have recently reported that mitochondrial respiratory thresholds regulate yeast chronological life span and mediate its extension by caloric restriction (72, 73). Therefore, future studies exploiting the power of S. cerevisiae to study the mitochondrial CO2-sAC-cAMP-PKA signalosome are likely to shed light on mechanisms for adaptation to stress and the regulation of life span in yeast and mammals.
In summary, the discovery of a mitochondrial CO2-sAC-cAMP-PKA signalosome, conserved from the yeast S. cerevisiae to human cells, modulating the allosteric inhibition of COX by ATP, underlies a universal mechanism for metabolic regulation in eukaryotes exposed to environmental fluctuations in temperature, oxygen, and nutrient availability.
Supplementary Material
Acknowledgments
The authors thank Dr. Flavia Fontanesi and Dr. Iliana Soto for critically reading the manuscript. The authors thank Dr. Christof Taxis (Philipps Universität Marburg, Marburg, Germany) for providing the cyr1 yeast mutant strain.
This study was supported by the Muscular Dystrophy Association (to A.B. and G.M.) and by U.S. National Institutes of Health (NIH) grants GM-071775 (to A.B.); GM-088999A (to G.M.); and GM-062328 and HD-059913 (to J.B. and L.R.L.). J.B. and L.R.L. own equity interest in CEP Biotech (Tamarac, FL, USA), which has licensed commercialization of a panel of monoclonal antibodies directed against sAC.
The other authors declare no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 8Br-cAMP
- 8-bromoadenosine 3′,5′-cyclic monophosphate
- CA
- carbonic anhydrase
- cAMP
- cyclic AMP
- CCCP
- carbonyl cyanide m-chlorophenyl hydrazone
- COX
- cytochrome c oxidase
- COX4i1
- cytochrome c oxidase subunit 4 isoform 1
- Cox5a
- cytochrome c oxidase subunit 5 isoform a
- Cox5b
- cytochrome c oxidase subunit 5 isoform b
- oxphos
- oxidative phosphorylation
- PDE
- phosphodiesterase
- PDE2A
- phosphodiesterase 2A
- PKA
- protein kinase A
- ROS
- reactive oxygen species
- sAC
- soluble adenylyl cyclase
REFERENCES
- 1. McBride H. M., Neuspiel M., Wasiak S. (2006) Mitochondria: more than just a powerhouse. Curr. Biol. 16, R551–560 [DOI] [PubMed] [Google Scholar]
- 2. Tait S. W., Green D. R. (2012) Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Valsecchi F., Ramos-Espiritu L. S., Buck J., Levin L. R., Manfredi G. (2013) cAMP and mitochondria. Physiology (Bethesda) 28, 199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Henriksen P., Wagner S. A., Weinert B. T., Sharma S., Bacinskaja G., Rehman M., Juffer A. H., Walther T. C., Lisby M., Choudhary C. (2012) Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol. Cell. Proteomics 11, 1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grimsrud P. A., Carson J. J., Hebert A. S., Hubler S. L., Niemi N. M., Bailey D. J., Jochem A., Stapleton D. S., Keller M. P., Westphall M. S., Yandell B. S., Attie A. D., Coon J. J., Pagliarini D. J. (2012) A quantitative map of the liver mitochondrial phosphoproteome reveals posttranslational control of ketogenesis. Cell Metab. 16, 672–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reinders J., Wagner K., Zahedi R. P., Stojanovski D., Eyrich B., van der Laan M., Rehling P., Sickmann A., Pfanner N., Meisinger C. (2007) Profiling phosphoproteins of yeast mitochondria reveals a role of phosphorylation in assembly of the ATP synthase. Mol. Cell. Proteomics 6, 1896–1906 [DOI] [PubMed] [Google Scholar]
- 7. Lefkimmiatis K., Leronni D., Hofer A. M. (2013) The inner and outer compartments of mitochondria are sites of distinct cAMP/PKA signaling dynamics. J. Cell Biol. 202, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar S., Kostin S., Flacke J. P., Reusch H. P., Ladilov Y. (2009) Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J. Biol. Chem. 284, 14760–14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appukuttan A., Kasseckert S. A., Micoogullari M., Flacke J. P., Kumar S., Woste A., Abdallah Y., Pott L., Reusch H. P., Ladilov Y. (2012) Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovasc. Res. 93, 340–349 [DOI] [PubMed] [Google Scholar]
- 10. Di Benedetto G., Scalzotto E., Mongillo M., Pozzan T. (2013) Mitochondrial Ca(2)(+) uptake induces cyclic AMP generation in the matrix and modulates organelle ATP levels. Cell. Metab. 17, 965–975 [DOI] [PubMed] [Google Scholar]
- 11. Acin-Perez R., Salazar E., Kamenetsky M., Buck J., Levin L. R., Manfredi G. (2009) Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell. Metab. 9, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DiPilato L. M., Cheng X., Zhang J. (2004) Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. U. S. A. 101, 16513–16518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acin-Perez R., Russwurm M., Gunnewig K., Gertz M., Zoidl G., Ramos L., Buck J., Levin L. R., Rassow J., Manfredi G., Steegborn C. (2011) A phosphodiesterase 2A isoform localized to mitochondria regulates respiration. J. Biol. Chem. 286, 30423–30432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Acin-Perez R., Gatti D. L., Bai Y., Manfredi G. (2011) Protein phosphorylation and prevention of cytochrome oxidase inhibition by ATP: coupled mechanisms of energy metabolism regulation. Cell Metab. 13, 712–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acin-Perez R., Salazar E., Brosel S., Yang H., Schon E. A., Manfredi G. (2009) Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. EMBO Mol. Med. 1, 392–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodgson S. J., Forster R. E., 2nd, Storey B. T., Mela L. (1980) Mitochondrial carbonic anhydrase. Proc. Natl. Acad. Sci. U. S. A. 77, 5562–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henry R. P. (1996) Multiple roles of carbonic anhydrase in cellular transport and metabolism. Annu. Rev. Physiol. 58, 523–538 [DOI] [PubMed] [Google Scholar]
- 18. Soto I. C., Fontanesi F., Liu J., Barrientos A. (2012) Biogenesis and assembly of eukaryotic cytochrome c oxidase catalytic core. Biochim. Biophys. Acta 1817, 883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zee J. M., Glerum D. M. (2006) Defects in cytochrome oxidase assembly in humans: lessons from yeast. Biochem. Cell Biol. 84, 859–869 [DOI] [PubMed] [Google Scholar]
- 20. Santangelo G. M. (2006) Glucose signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 70, 253–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zaman S., Lippman S. I., Zhao X., Broach J. R. (2008) How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42, 27–81 [DOI] [PubMed] [Google Scholar]
- 22. Mitts M. R., Grant D. B., Heideman W. (1990) Adenylate cyclase in Saccharomyces cerevisiae is a peripheral membrane protein. Mol. Cell. Biol. 10, 3873–3883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mitts M. R., Bradshaw-Rouse J., Heideman W. (1991) Interactions between adenylate cyclase and the yeast GTPase-activating protein IRA1. Mol. Cell. Biol. 11, 4591–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsumoto K., Uno I., Oshima Y., Ishikawa T. (1982) Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U. S. A. 79, 2355–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toda T., Cameron S., Sass P., Zoller M., Wigler M. (1987) Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell 50, 277–287 [DOI] [PubMed] [Google Scholar]
- 26. Muller G., Bandlow W. (1989) An amphitropic cAMP-binding protein in yeast mitochondria. 2. Phospholipid nature of the membrane anchor. Biochemistry 28, 9968–9973 [DOI] [PubMed] [Google Scholar]
- 27. Rodel G., Muller G., Bandlow W. (1985) Cyclic AMP receptor protein from yeast mitochondria: submitochondrial localization and preliminary characterization. J. Bacteriol. 161, 7–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McEntee C. M., Cantwell R., Rahman M. U., Hudson A. P. (1993) Transcription of the yeast mitochondrial genome requires cyclic AMP. Mol. Gen. Genet. 241, 213–224 [DOI] [PubMed] [Google Scholar]
- 29. Rahman M. U., Hudson A. P. (1995) Substrates for yeast mitochondrial cAMP-dependent protein kinase activity. Biochem. Biophys. Res. Commun. 214, 188–194 [DOI] [PubMed] [Google Scholar]
- 30. Hall R. A., De Sordi L., Maccallum D. M., Topal H., Eaton R., Bloor J. W., Robinson G. K., Levin L. R., Buck J., Wang Y., Gow N. A., Steegborn C., Muhlschlegel F. A. (2010) CO(2) acts as a signalling molecule in populations of the fungal pathogen Candida albicans. PLoS Pathog. 6, e1001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klengel T., Liang W. J., Chaloupka J., Ruoff C., Schroppel K., Naglik J. R., Eckert S. E., Mogensen E. G., Haynes K., Tuite M. F., Levin L. R., Buck J., Muhlschlegel F. A. (2005) Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 15, 2021–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mogensen E. G., Janbon G., Chaloupka J., Steegborn C., Fu M. S., Moyrand F., Klengel T., Pearson D. S., Geeves M. A., Buck J., Levin L. R., Muhlschlegel F. A. (2006) Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot. Cell 5, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jungbluth M., Mosch H. U., Taxis C. (2012) Acetate regulation of spore formation is under the control of the Ras/cyclic AMP/protein kinase A pathway and carbon dioxide in Saccharomyces cerevisiae. Eukaryot. Cell 11, 1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Elleuche S., Poggeler S. (2010) Carbonic anhydrases in fungi. Microbiology 156, 23–29 [DOI] [PubMed] [Google Scholar]
- 35. Supuran C. T. (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 [DOI] [PubMed] [Google Scholar]
- 36. Barrientos A., Fontanesi F., Diaz F. (2009) Evaluation of the mitochondrial respiratory chain and oxidative phosphorylation system using polarography and spectrophotometric enzyme assays. Curr. Protoc. Hum. Genet. 19, Unit 19.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sander M., Ramotar D. (1997) Partial purification of Pde1 from Saccharomyces cerevisiae: enzymatic redundancy for the repair of 3′-terminal DNA lesions and abasic sites in yeast. Biochemistry 36, 6100–6106 [DOI] [PubMed] [Google Scholar]
- 38. Glick B. S., Pon L. A. (1995) Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 260, 213–223 [DOI] [PubMed] [Google Scholar]
- 39. Barrientos A., Korr D., Tzagoloff A. (2002) Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh's syndrome. EMBO J. 21, 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arnold S., Kadenbach B. (1997) Cell respiration is controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase. Eur. J. Biochem. 249, 350–354 [DOI] [PubMed] [Google Scholar]
- 41. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA [Google Scholar]
- 42. Schiestl R. H., Gietz R. D. (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339–346 [DOI] [PubMed] [Google Scholar]
- 43. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 44. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 45. Blom N., Gammeltoft S., Brunak S. (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294, 1351–1362 [DOI] [PubMed] [Google Scholar]
- 46. Sickmann A., Reinders J., Wagner Y., Joppich C., Zahedi R., Meyer H. E., Schonfisch B., Perschil I., Chacinska A., Guiard B., Rehling P., Pfanner N., Meisinger C. (2003) The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. U. S. A. 100, 13207–13212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Reinders J., Zahedi R. P., Pfanner N., Meisinger C., Sickmann A. (2006) Toward the complete yeast mitochondrial proteome: multidimensional separation techniques for mitochondrial proteomics. J. Proteome Res. 5, 1543–1554 [DOI] [PubMed] [Google Scholar]
- 48. Belotti F., Tisi R., Paiardi C., Rigamonti M., Groppi S., Martegani E. (2012) Localization of Ras signaling complex in budding yeast. Biochim. Biophys. Acta 1823, 1208–1216 [DOI] [PubMed] [Google Scholar]
- 49. Bitterman J. L., Ramos-Espiritu L., Diaz A., Levin L. R., Buck J. (2013) Pharmacological distinction between soluble and transmembrane adenylyl cyclases. J. Pharmacol. Exp. Ther. 347, 589–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jain P., Akula I., Edlind T. (2003) Cyclic AMP signaling pathway modulates susceptibility of candida species and Saccharomyces cerevisiae to antifungal azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 47, 3195–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hess K. C., Jones B. H., Marquez B., Chen Y., Ord T. S., Kamenetsky M., Miyamoto C., Zippin J. H., Kopf G. S., Suarez S. S., Levin L. R., Williams C. J., Buck J., Moss S. B. (2005) The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Follmann K., Arnold S., Ferguson-Miller S., Kadenbach B. (1998) Cytochrome c oxidase from eucaryotes but not from procaryotes is allosterically inhibited by ATP. Biochem. Mol. Biol. Int. 45, 1047–1055 [DOI] [PubMed] [Google Scholar]
- 53. Hodge M. R., Kim G., Singh K., Cumsky M. G. (1989) Inverse regulation of the yeast COX5 genes by oxygen and heme. Mol. Cell. Biol. 9, 1958–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poyton R. O. (1999) Models for oxygen sensing in yeast: implications for oxygen-regulated gene expression in higher eucaryotes. Respir. Physiol. 115, 119–133 [DOI] [PubMed] [Google Scholar]
- 55. Liu J., Barrientos A. (2012) Transcriptional regulation of yeast oxphos hypoxic genes by oxidative stress. Antioxid. Redox Signal. 19, 1916–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Allen L. A., Zhao X. J., Caughey W., Poyton R. O. (1995) Isoforms of yeast cytochrome c oxidase subunit V affect the binuclear reaction center and alter the kinetics of interaction with the isoforms of yeast cytochrome c. J. Biol. Chem. 270, 110–118 [DOI] [PubMed] [Google Scholar]
- 57. Barrientos A., Zambrano A., Tzagoloff A. (2004) Mss51p and Cox14p jointly regulate mitochondrial Cox1p expression in Saccharomyces cerevisiae. EMBO J. 23, 3472–3482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsukihara T., Aoyama H., Yamashita E., Tomizaki T., Yamaguchi H., Shinzawa-Itoh K., Nakashima R., Yaono R., Yoshikawa S. (1996) The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science 272, 1136–1144 [DOI] [PubMed] [Google Scholar]
- 59. Burke P. V., Poyton R. O. (1998) Structure/function of oxygen-regulated isoforms in cytochrome c oxidase. J. Exp. Biol. 201, 1163–1175 [DOI] [PubMed] [Google Scholar]
- 60. Huttemann M., Kadenbach B., Grossman L. I. (2001) Mammalian subunit IV isoforms of cytochrome c oxidase. Gene 267, 111–123 [DOI] [PubMed] [Google Scholar]
- 61. Fukuda R., Zhang H., Kim J. W., Shimoda L., Dang C. V., Semenza G. L. (2007) HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 [DOI] [PubMed] [Google Scholar]
- 62. Castello P. R., Woo D. K., Ball K., Wojcik J., Liu L., Poyton R. O. (2008) Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc. Natl. Acad. Sci. U. S. A. 105, 8203–8208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pierron D., Wildman D. E., Huttemann M., Markondapatnaikuni G. C., Aras S., Grossman L. I. (2012) Cytochrome c oxidase: evolution of control via nuclear subunit addition. Biochim. Biophys. Acta 1817, 590–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Das J., Miller S. T., Stern D. L. (2004) Comparison of diverse protein sequences of the nuclear-encoded subunits of cytochrome c oxidase suggests conservation of structure underlies evolving functional sites. Mol. Biol. Evol. 21, 1572–1582 [DOI] [PubMed] [Google Scholar]
- 65. Fang J. K., Prabu S. K., Sepuri N. B., Raza H., Anandatheerthavarada H. K., Galati D., Spear J., Avadhani N. G. (2007) Site specific phosphorylation of cytochrome c oxidase subunits I, IVi1 and Vb in rabbit hearts subjected to ischemia/reperfusion. FEBS Lett. 581, 1302–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Horvat S., Beyer C., Arnold S. (2006) Effect of hypoxia on the transcription pattern of subunit isoforms and the kinetics of cytochrome c oxidase in cortical astrocytes and cerebellar neurons. J. Neurochem. 99, 937–951 [DOI] [PubMed] [Google Scholar]
- 67. Thevelein J. M. (1994) Signal transduction in yeast. Yeast 10, 1753–1790 [DOI] [PubMed] [Google Scholar]
- 68. Fraenkel D. G. (1985) On ras gene function in yeast. Proc. Natl. Acad. Sci. U. S. A. 82, 4740–4744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fabrizio P., Pozza F., Pletcher S. D., Gendron C. M., Longo V. D. (2001) Regulation of longevity and stress resistance by Sch9 in yeast. Science 292, 288–290 [DOI] [PubMed] [Google Scholar]
- 70. Longo V. D., Shadel G. S., Kaeberlein M., Kennedy B. (2012) Replicative and chronological aging in Saccharomyces cerevisiae. Cell 16, 18–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Longo V. D., Finch C. E. (2003) Evolutionary medicine: from dwarf model systems to healthy centenarians? Science 299, 1342–1346 [DOI] [PubMed] [Google Scholar]
- 72. Ocampo A., Liu J., Schroeder E. A., Shadel G. S., Barrientos A. (2012) Mitochondrial respiratory thresholds regulate yeast chronological life span and its extension by caloric restriction. Cell Metab. 16, 55–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Barrientos A. (2012) Complementary roles of mitochondrial respiration and ROS signaling on cellular aging and longevity. Aging (Albany, NY) 4, 578–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bender E., Kadenbach B. (2000) The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 466, 130–134 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.