Abstract
Up-regulation of placental soluble fms-like tyrosine kinase 1 (sFlt1) contributes to the pathogenesis of preeclampsia. To evaluate novel upstream pathways that regulate placental sFlt1 production, we screened a library of natural compounds (n=502) in human placental cell lines. Here, we report 3 compounds in the cardiac glycoside family, ouabain, gitoxigenin, and digitoxin, that inhibit placental sFlt1 production at nanomolar concentrations in vitro. We further characterized ouabain and demonstrated that it inhibits sFlt1 mRNA and protein expression in human placental cytotrophoblasts and explant cultures in a dose- and time-dependent manner. Ouabain down-regulated sFlt1 production by inhibiting hypoxia-inducible factor 1 (HIF-1α) protein expression in the placenta. Furthermore, we found that phosphorylation of heat-shock protein 27 (HSP27) was necessary for ouabain to inhibit HIF-1α translation. In a rat model of pregnancy-induced hypertension, ouabain reduced mean arterial pressure and enhanced placental HSP27 phosphorylation without any adverse effects on pups. Further studies are needed to explore the usefulness of targeting HIF-1α/HSP27 pathway in preeclampsia.—Rana, S., Rajakumar, A., Geahchan, C., Salahuddin, S., Cerdeira, A. S., Burke, S. D., George, E. M., Granger, J. P., Karumanchi, S. A. Ouabain inhibits placental sFlt1 production by repressing HSP27-dependent HIF-1α pathway.
Keywords: preeclampsia, hypertension, angiogenesis
Preeclampsia is a common medical complication of pregnancy that is characterized by new onset hypertension and often associated with vascular damage in target organs, such as in the glomerulus of the kidney (1, 2). It is associated with increased maternal and fetal morbidity and mortality, and, to date, delivery is the only treatment. Preeclampsia is also a major cause of iatrogenic prematurity when the disease presents early (3). Therefore, any strategy for treatment that can safely prolong pregnancy will have a significant effect on reduction in preterm births secondary to preeclampsia.
Although the etiology of preeclampsia is unknown, epidemiological and experimental studies suggest that imbalances in angiogenic factors and inflammation may be central to its pathogenesis (2, 4). While there are several abnormalities in angiogenic factors, a key antiangiogenic protein that has been extensively studied is the soluble version of the vascular endothelial growth factor (VEGF) receptor known as soluble fms-like tyrosine kinase 1 (sFlt1), a potent circulating antiangiogenic molecule that is produced in abundant quantities within the placenta (5). Circulating sFlt1 levels are increased prior to onset of preeclampsia, are altered in most women with severe preeclampsia, and correlate with adverse maternal and neonatal outcomes related to preeclampsia (6, 7). Overexpression of sFlt1 in rodents is sufficient to produce hypertension, proteinuria, and glomerular endotheliosis, the classic hallmarks of preeclampsia (5).
Placental production of sFlt1 may be regulated through angiotensin II signaling (8), hypoxia (9), and altered heme oxygenase expression (10). However, it is unclear which of these pathways are important in preeclampsia. Exploration using small molecules to dissect signaling pathways and disease biology has attracted a lot of interest with the availability of novel chemical libraries and high-throughput screening methodologies. The primary purpose of this study was to identify novel therapeutic targets upstream of sFlt1, which may lead to new strategies to prevent or treat preeclampsia. We screened a chemical library of natural compounds, and report here that ouabain, a cardiac glycoside, down-regulates sFlt1 production and that this inhibition was mediated via its effects on the hypoxia-inducible factor 1α (HIF-1α)/heat-shock protein 27 (HSP27) pathway.
MATERIALS AND METHODS
Chemical and drug library
Commercially available natural product library (cat. no. BML-2865) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). Ouabain (ouabain octahydrate; cat. no. O3125), dimethyl sulfoxide (DMSO; cat. no. 276855), and 5-(5-ethyl-2-hydroxy-4-methoxyphenyl)-4-(4-methoxyphenyl) isoxazole (KRIBB3; cat. no. K4514) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dimethyl oxaloylglycine (DMOG; cat. no. D1070) was purchased from Frontier Scientific (Logan, UT, USA).
Human studies
Placentas were collected from normal individuals and patients with preeclampsia using an institutional review board-approved protocol at the Beth Israel Deaconess Medical Center (Boston, MA, USA). Patients with preeclampsia in our study had new-onset hypertension (≥140/90 after 20 wk of gestation in a woman with previously normal blood pressure) and proteinuria (protein/creatinine ratio ≥0.3) and when evaluated using the updated ACOG criteria were classified as preeclampsia (1). Patients with history of diabetes, chronic hypertension, renal disease, or multiple gestations were excluded in this study.
Cytotrophoblast culture and high-throughput screening
Cytotrophoblasts were isolated from normal and preeclamptic placentas, as described elsewhere (11). Cells were isolated and frozen in liquid nitrogen in batches. For screening experiments, cells were thawed and plated in a 96-well flat-bottom plate (Microtest 96; Becton Dickinson, Franklin Lakes, NJ, USA) in medium 199 with 5% fetal bovine serum (FBS) and 1% penicillin and streptomycin overnight. Medium was removed in the morning, and fresh medium was added with either a compound from the drug library or vehicle (DMSO). Plates placed in a cell culture incubator with 5% CO2–95% room air (21% O2) for 72 h. At the end of the experiment, cell culture supernatant was collected and stored at −80°C for future analysis.
Placental villous explant cultures
Placental villous explant preparation and culture were carried out according to published protocols (12). Several villous biopsies (2 cm3) were excised from the maternal surface midway between the chorionic and basal plates, within 30 min of delivery, and decidual layers were carefully removed. The villous tissue collected was cut into 0.5-cm3 pieces and was thoroughly rinsed with phosphate-buffered saline (PBS) to remove maternal blood. Explants were placed in a 24-well flat-bottom plate (Falcon multiwell tissue culture plate; Becton Dickinson) in 1 ml of medium 199 with 5% FBS, 1% glutamine, and 1% penicillin and streptomycin at 37°C for 72 h on an orbital shaker (60 rpm; Belly Dancer; Stovall Life Science, Greensboro, NC, USA) under standard tissue culture conditions of 5% CO2–95% room air (21% O2) in a cell culture incubator with varying concentrations of ouabain. At the end of the incubation period, the explants were removed, blotted with sterile cotton gauze to remove any excess medium, and flash-frozen in liquid nitrogen and stored at −80°C. The incubation medium was stored in aliquots at −80°C. Experiments were carried out in duplicate on explants from normal individuals and patients with preeclampsia.
Enzyme-linked immunosorbent assay (ELISA)
sFlt1 in culture medium was measured by ELISA using the human VEGF receptor 1 (VEGFR1) Quantikine kit (DVR 100B; R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions, which identifies the soluble VEGFR1 (also referred to as sFlt1) in biological fluids. Sensitivity of the assay was 5 pg/ml, with an intra-assay coefficient of variation of 2.6–3.8% and an interassay coefficient of variation of 7.0–8.1%. Mouse-soluble VEGFR1 immunoassay (MVR 100B; R&D Systems) was used to measure plasma concentration of sFlt1 in mouse plasma following the manufacturer's instructions. Sensitivity of the assay was 9.8 pg/ml, with an intra-assay coefficient of variation of 3.2–7.4% and an interassay coefficient of variation of 6.3–8.4%. Cytotoxicity was analyzed using a cytotoxicity detection kit (LDH; Roche Diagnostics, Indianapolis, IN, USA).
Heparin-agarose chromatography
Soluble Flt1 protein in conditioned medium from explant cultures was concentrated by heparin-agarose affinity chromatography using published protocols (13). Briefly, 250 μl of conditioned medium (diluted to 1 ml with PBS) was incubated with heparin-agarose beads (Sigma-Aldrich) at 4°C for 1.5 h, then centrifuged, and the pellet washed three times with PBS buffer. After the final wash, the heparin-agarose beads were resuspended in minimal volume of 1× Laemmli's solution and processed for Western blot analysis as described below. Equal volumes of the conditioned medium from control and ouabain-treated explants were used to standardize loading for Western blot analysis.
Western blot analysis
Proteins were separated on SDS containing 8% polyacrylamide gels for sFlt1 and HIF expression studies and 12% gels for HSP27 studies. Western blot analysis was conducted using published procedures with minor modifications (14). Mouse monoclonal anti-human Flt1 (V4262; Sigma-Aldrich) was used to detect sFlt1 protein isoforms and used at 1:1000 dilution (2.2 μg/ml) in Tris-buffered saline (TBS) containing 0.05% Tween-20. The presence of sFlt1 immunoreactivity on Western blots (sFlt1-13 and -14 isoforms) was confirmed by a second commercial anti-human Flt1 antibody (AF 321; R&D Systems). The anti-HIF-1α monoclonal antibody (610958; Transduction Laboratories, San Jose, CA, USA) and a rabbit polyclonal anti-HIF-2α antibody (NB 100-122B2; Novus Biologicals, Littleton, CO, USA) were utilized for Western blot analysis. HIF-1α antibody was used at 1:200 dilution (1.25 μg/ml) in TBS containing 0.05% Tween-20, and HIF-2α antibody was used at 1:1000 dilution (2.2 μg/ml). Rabbit anti-human/mouse HSP27 antibody (AF1580; R&D Systems) was used to detect HSP27 and rabbit anti-phospho-HSP27 (S78/S82) antibody (AF2314; R&D Systems) was used to detect phosphorylated HSP27 (pHSP27) in human studies. Both of these antibodies were diluted 1:1000 (2.2 μg/ml) in TBS containing 0.05% Tween 20. The mouse monoclonal anti-β-actin antibody (A 5441; Sigma-Aldrich) was diluted 1:1000, yielding a final concentration of 2.2 μg/ml. Rabbit anti-human/mouse/rat HSP27 antibody (AF1580; R&D Systems) was used to detect HSP27 and rabbit anti-phospho-HSP27 (S78/S82) antibody (AF2314, human; MAB23141, rat; R&D Systems) was used to detect pHSP27 in rat studies. Total protein (50 μg) was electrophoresed for detection of sFlt1, HIF-1α, and HIF-2α expression, and 15 μg for HSP27 and pHSP27 expression studies. Membranes were stripped and reprobed for β-actin for loading control.
RNA extraction and real-time quantitative polymerase chain reaction (PCR)
Total RNA was isolated from placental tissue as described previously (14). TaqMan real-time quantitative PCR was performed for the quantitation of Flt1, sFlt1-13, sFlt1-14, HIF-1α, and HIF-2α messenger RNAs (mRNAs) between controls and treated explants, from normal individuals and patients with preeclampsia. Supplemental Table S1 contains primer sequence details. For each real-time PCR reaction, 1 μl cDNA (cDNA diluted 1:10, generated from 1 μg RNA), 1 μl gene expression assay, and 10 μl of TaqMan gene expression master mix (Applied Biosystems, Foster City, CA, USA) were combined with water in a well on the reaction plate for a total volume of 20 μl. Each reaction was run in duplicate with endogenous control on the same reaction plate. This eliminated any differences in input cDNA variation and allowed the data to be read as a relative quantity. The real-time PCR reactions were read and analyzed using the 7900HT Sequence Detection System (Applied Biosystems).
Coimmunoprecipitation studies
Villous explants were homogenized using an Omni homogenizer (Omni International, Kennesaw, GA, USA) in a buffer containing 1% Brij 99 [20 mM HEPES, 0.15 M NaCl, 50 mM ethylenediaminetetraacetic acid (EDTA), and 1% Brij99] and centrifuged for 10 min at 10,500 g; protein estimation was done. For coimmunoprecipitation studies, 1 mg of protein was mixed with 30 μl of protein G (Protein G Plus-Agarose; SC-2002; Santa Cruz Biotechnologies, Santa Cruz, CA, USA) and incubated on a rocker at 4°C to preclear nonspecific binding to protein G. After centrifugation at 800 g for 2 min, the supernatant was collected and incubated with 1 μg of HSP27 antibody (SC-13132; Santa Cruz Biotechnology) overnight. Protein G magnabeads (30 μl) were added to the samples and further incubated for 30 min. Pellets and supernatants were separated using the magnetic particle concentrator. After 3 washes, 40 μl of 1× Laemmli buffer was added to the pellets, and the samples were heated at 65°C and centrifuged at 800 g for 2 min. The supernatants were collected and subjected to Western blot analysis for detection of HIF-1α.
Animal studies
The reduced uterine perfusion pressure (RUPP) model of placental ischemia and pregnancy-induced hypertension has been previously described (15). Timed pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were received on d 10 or 11 of gestation. This protocol was approved by the University of Mississippi Medical Center Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Rats were maintained on a 12-h light-dark cycle, at 23°C constant temperature, and were provided food and water ad libitum. On gestational d 14, all animals were anesthetized and subjected to aortic and bilateral ovarian artery constriction, as described previously (16). Briefly, animals were anesthetized by controlled 3% isoflurane (Webster, Devens, MA, USA), and a midline abdominal incision was made. After externalization of both uterine horns, one single 0.203-mm silver surgical clip was placed on the lower abdominal aorta above the iliac bifurcation. One 0.100-mm silver surgical clip was placed on both the left and right ovarian arteries that supply the uterus to prevent compensatory flow. Rats absorbing all pups as a result of the procedure were excluded from the study. Animals were given either ouabain dissolved in PBS (20 μg/kg/d) or vehicle (PBS) intraperitoneally from gestational d 12 to 19. On d 18 of gestation, under a short-acting anesthetic (2% isoflurane) delivered by an anesthesia apparatus, a catheter of V-3 tubing (SCI, Lake Hayasu City, AZ, USA) was inserted into the carotid artery, tunneled to the back of the neck, and exteriorized for direct monitoring of blood pressure. On d 19 of gestation, pregnant rats were placed in individual restraining cages, and blood pressure measurements were recorded continuously for two 20-min periods after 30 min of stabilization using a pressure transducer (Cobe II Transducer CDX; Sema, Birmingham, AL, USA), as described previously (17). At the time of euthanizing the animals, the placenta was flash-frozen, all fetuses were weighed, blood was collected in EDTA tubes and processed by centrifuge at 1200 g for 8 min, and plasma was collected and stored at −80°C for further analysis.
For experiments using mice, animal protocols were approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Female CD-1 mice (10–12 wk old, 20–25 g initial body weight) were purchased from Charles River Laboratories International (Wilmington, MA, USA). Pregnant mice were obtained on gestational d 9 of pregnancy and housed at the Animal Research Facility under controlled conditions as recommended. Ouabain was injected intraperitoneally at a dose of 20 μg/kg/d or vehicle (PBS) from gestational d 12 to 17. Ultrasound examination was done on gestational d 12 and 17. Umbilical artery parameters, fetal heart rate, and uterine artery measurements were evaluated using a Vevo 2100 (VisualSonics, Toronto, ON, Canada), as described previously (18). Animals were anesthetized using 3% isoflurane in medical air via nose cone anesthesia and euthanized by cardiac puncture under anesthesia, according to institutional protocols. Fetuses and placentas were weighed, and placental tissue was flash-frozen. Blood samples were processed, and plasma was stored at −80°C.
Statistical analyses
All data are expressed as means ± sem. Statistical analyses were performed using GraphPad software (GraphPad, La Jolla, CA, USA). Student's t test was used to analyze comparisons between control and experimental groups. Comparisons between multiple groups were made by analysis of variance (ANOVA) with Dunn's post hoc test. Specific statistical tests used are reported in the figure captions. Values of P < 0.05 were considered significant.
RESULTS
Screening for inhibitors of sFlt1 protein
To screen for compounds that inhibit sFlt1 production, we isolated human cytotrophoblasts from normal human placentas, and 100,000 cells were plated under standard cell culture conditions and then exposed to either drug or vehicle (DMSO). The cell culture supernatant was then collected after 72 h, and sFlt1 protein was measured and evaluated for inhibition as a percentage of protein expression in untreated cells. We screened 502 natural compounds and focused on drugs that inhibited sFlt1 production with evidence of no cytotoxicity. The cells were monitored for visual cytotoxicity on light microscopy and also by measurement of LDH levels in the supernatant. Supplemental Table S2 shows 38 compounds that inhibited sFlt1 at nanomolar concentrations. Interestingly, several members of cardiac glycosides (ouabain, gitoxigenin, and digitoxin) were found to inhibit sFlt1 concentration. Of the several cardiac glycosides that inhibited sFlt1 (Supplemental Table S2), we focused on ouabain, as this compound has been most extensively studied in the literature, and a role for endogenous ouabain during rat pregnancy has been reported (19).
Ouabain inhibits sFlt1 production in a dose- and time-dependent fashion
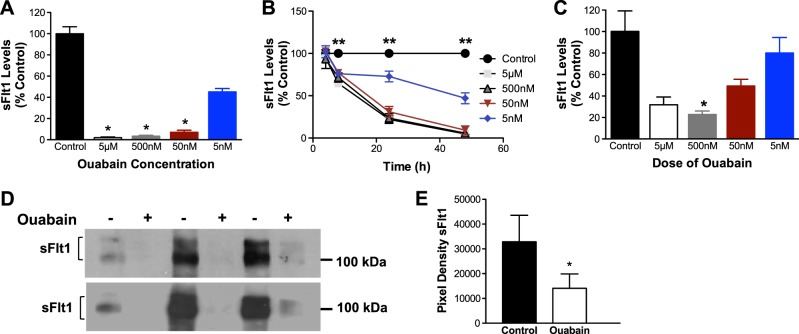
We incubated primary human cytotrophoblasts from normal pregnancy with varying concentrations of ouabain and measured sFlt1 protein by ELISA. In Fig. 1A, B, we demonstrate that ouabain treatment inhibited sFlt1 production from cytotrophoblasts in a dose- and time-dependent fashion. Similar to human cytotrophoblasts, we also noted that ouabain inhibited sFlt1 protein in human placental villous cultures by ELISA (Fig. 1C) and Western blot analysis of supernatant-enriched using heparin agarose chromatography (Fig. 1D, E).
Figure 1.
Ouabain inhibits sFlt1 production in primary cytotrophoblast cells and villous explants from normal pregnancy. A, B) Ouabain inhibits sFlt1 production in a dose-dependent (A; n=6) and time-dependent manner (B; n=4) in culture supernatants of cytotrophoblasts from normal pregnancy. Data are expressed as a percentage of the sFlt1 (pg/ml) levels in controls; average amount of sFlt1 produced by normal cytotrophoblast (control) was 1974 ± 1076 pg/ml (A). *P < 0.05 vs. control; 1-way ANOVA with Dunn's multiple-comparison test. **P < 0.05 vs. control; 2-way ANOVA, repeated measures for the analysis of data. C) Similar dose-dependent reduction of sFlt1 is observed in culture supernatant of villous explants (n=3) from normal pregnancy. Average amount of sFlt1 produced by normal placental villous explant (control) was 771 ± 95 pg/ml. D) Effects of ouabain (500 nM) on sFlt1 protein levels by Western blots in culture supernatant of villous explants (n=3) enriched by heparin-agarose chromatography (top panel using Sigma-Aldrich antibody; bottom panel using R&D antibody). E) Quantification of the Western blots for Sigma-Aldrich antibody. *P < 0.05 vs. control; Student's t test.
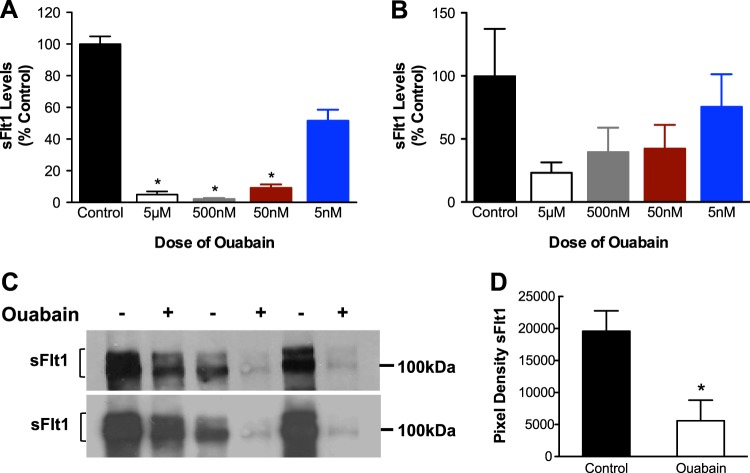
We then assessed whether ouabain would also regulate sFlt1 production from preeclamptic tissue. Not surprisingly, ouabain inhibited sFlt1 protein expression in preeclamptic cytotrophoblasts, with significant results at ≥50 nM concentrations (Fig. 2A). Similar trends were noted in ELISA studies of preeclamptic villous cultures, although this was not significant (Fig. 2B). Western blot analysis of preeclamptic villous supernatant, enriched using heparin-agarose chromatography, confirmed that 500 nM ouabain strongly inhibited sFlt1 protein expression (Fig. 2C, D).
Figure 2.
Ouabain inhibits sFlt1 production in preeclamptic cytotrophoblasts and villous explants. A, B) Ouabain inhibits sFlt1 production in a dose-dependent manner in cytotrophoblasts (A; n=6) and in villous explants (B; n=3) from preeclamptic pregnancies. Data are expressed as a percentage of the sFlt1 (pg/ml) levels in controls. Average amount of sFlt1 produced by preeclamptic cytotrophoblasts (control) was 1556 ± 717 pg/ml (A); average amount of sFlt1 produced by preeclamptic placental villous explant (control) was 2106 ± 547 pg/ml (B). *P < 0.05 vs. control, 1-way ANOVA with Dunn's multiple-comparison test. C) Effects of ouabain (500 nM) on sFlt1 protein levels in culture supernatant of preeclamptic explants (n=3) enriched by heparin-agarose chromatography top panel using Sigma-Aldrich antibody; bottom panel using R&D antibody). D) Quantification of the Western blots for Sigma-Aldrich antibody. *P < 0.05 vs. control; Student's t test.
Ouabain inhibits sFlt1 and HIF protein
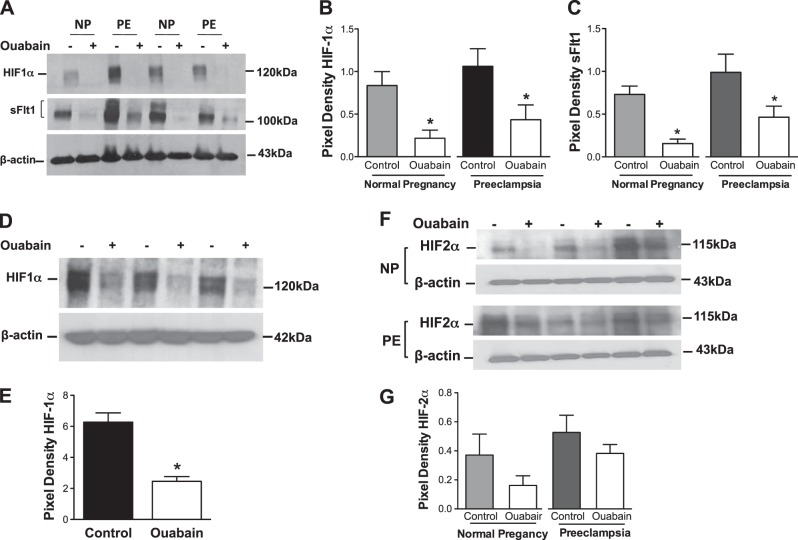
As prior work had suggested that digoxin (a cardiac glycoside family member) inhibits HIF expression and suppresses tumor growth (20) and cell culture studies had suggested that HIF-1α is a major regulator of sFlt1 production in placental tissue (21), we hypothesized that ouabain was inhibiting sFlt1 production by repressing HIF-1α levels. Western blot analysis of the placental explants exposed to ouabain showed 3- or 4-fold reduction in HIF-1α and sFlt1 protein expression (Fig. 3A–C). Interestingly, we noted that nuclear expression of HIF-1α was also attenuated by ouabain exposure (Fig. 3D, E). Moreover, ouabain therapy modestly inhibited HIF-2α expression in both normal and preeclamptic villous explants, although this was not statistically significant (Fig. 3F, G). Collectively, these data suggested that in human placental tissue, ouabain inhibited both HIF and sFlt1 protein expression coordinately. Although sFlt1 and HIF1 proteins are up-regulated in preeclamptic placentas at delivery (5, 22), interestingly, we did not find elevated sFlt1 and HIF-1α protein levels in preeclamptic cytotrophoblasts when compared to control cytotrophoblasts that were cultured for 72 h. These data are consistent with reports that there is reversal of gene dysregulation in cultured cytotrophoblasts from patients with preeclampsia, suggesting that in vivo conditions are critical to maintain the preeclamptic state in the placental trophoblasts (23).
Figure 3.
Ouabain treatment leads to concurrent decrease in HIF-1α and sFlt1 levels. A) Reduced HIF-1α and sFlt1 protein levels in explants from normal and preeclamptic placentas (n=6/group) treated with 500 nM ouabain. B, C) Quantification of Western blots for HIF-1α (B) and sFlt1 (C). D) Nuclear HIF-1α of explants (n=3) treated with 500 nM of ouabain is significantly down-regulated compared to controls. E) Quantification of Western blot. F) HIF-2α levels in NP (n=3) and PE (n=3) explants treated with 500 nM of ouabain show a significant decrease in HIF2-α compared to controls. NP, normal pregnancy; PE, preeclampsia. G) Quantification of Western blots. *P < 0.05 vs. control; Student's t test.
Ouabain inhibits sFlt1 mRNA but not HIF mRNA
To evaluate whether ouabain regulates sFlt1 protein at a transcriptional level that is mediated by HIF, we measured mRNA expression of sFlt1 in ouabain-exposed villous cultures. Similar to protein expression, ouabain inhibited mRNA expression of both isoforms sFlt1 (sFlt1-13 and sFlt1-14) and Flt1 in normal and preeclamptic placental explants (Fig. 4A). These data suggested that ouabain was not specifically inhibiting splicing of sFlt1, but rather acting by blocking transcription of both Flt1 and sFlt1 gene products. In contrast to sFlt1 expression, we noted that ouabain did not inhibit mRNA for either HIF-1α and HIF-2α in normal or preeclamptic villous explants (Fig. 4B).
Figure 4.
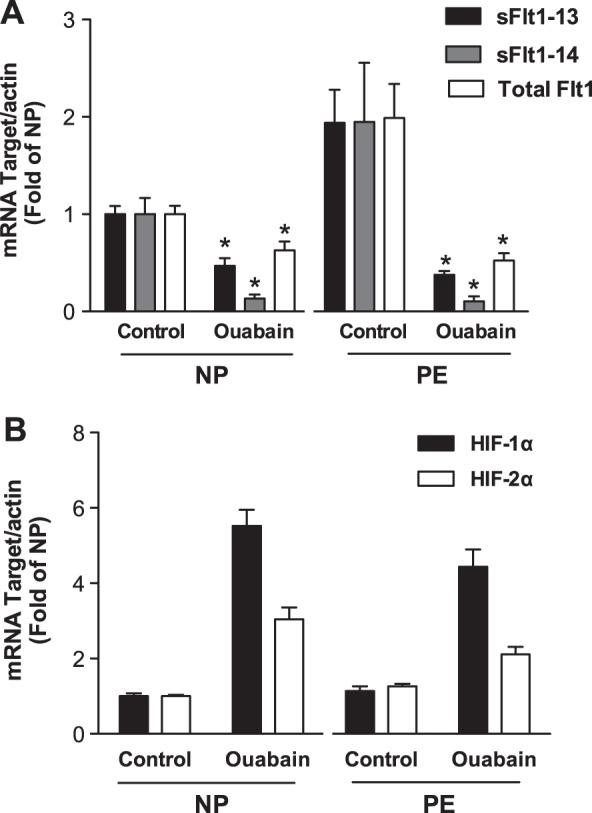
mRNA expression studies for sFlt1 and HIF-1α. mRNA levels were normalized to actin and presented as ratios relative to the normal explants. A) Quantitative real-time PCR showing reduction in mRNA for total Flt1, sFlt1-13, and sFlt1-14 isoforms in normal (n=6) and preeclamptic (n=6) villous explants exposed to 500 nM ouabain. B) Quantitative PCR shows an increase in HIF-1α and HIF-2α mRNA in normal (n=6) and preeclamptic (n=6) explants exposed to 500 nM ouabain. NP, normal pregnancy; PE, preeclampsia. *P < 0.05 vs. control; Student's t test.
Molecular mechanisms for HIF inhibition
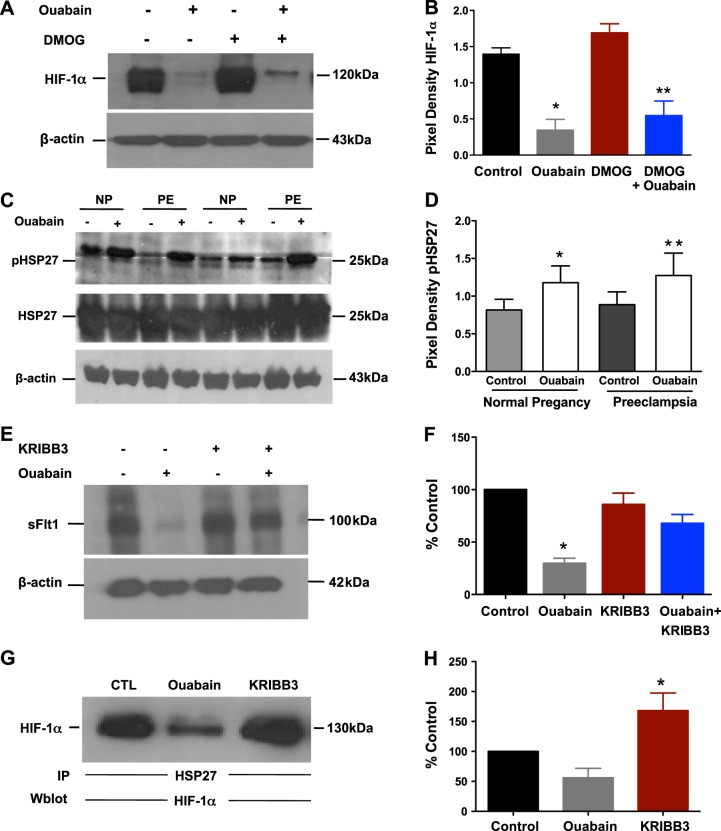
To further explore the mechanism of HIF protein inhibition by ouabain, we studied the effect of ouabain in the presence and absence of DMOG, an inhibitor of prolyl hydroxylase and asparaginyl hydroxylase factor inhibiting HIF-1 (FIH-1). DMOG has been previously noted to up-regulate HIF-1α (24). When we tested the effects of DMOG in a human cytotrophoblast cell line, we noted that HIF-1α protein levels increased as expected. But incubated together with ouabain, DMOG was unable to reverse the effects of ouabain, implying that ouabain was inhibiting HIF-1α protein independent of the conventional PHD-VHL-proteasome pathway (Fig. 5A, B). Since HSPs have been noted to regulate HIF translation through its chaperone function, we then explored the role of HSPs, and specifically HSP27, as it is abundantly expressed in placental tissue (25). We observed increased phosphorylation of HSP27 after exposure to ouabain in both normal and preeclamptic explants, with no changes in the total HSP27 levels (Fig. 5C, D). The addition of KRIBB3 (a potent inhibitor of phosphorylation of HSP27) resulted in a mild increase in sFlt1 (nonsignificant). However, the addition of ouabain and KRIBB3 together rescued the inhibition of sFlt1 levels by ouabain (Fig. 5E, F). Moreover, coimmunoprecipitation studies confirmed that HSP27 and HIF-1α interacted and that ouabain treatment blocked this interaction. The addition of KRIBB3 restored the HSP27/HIF-1α interaction (Fig. 5G, H). These experiments indicate that inhibition of HIF1-α by ouabain may be mediated through phosphorylation of HSP27, and inhibition of this phosphorylation using KRIBB3 attenuated this effect.
Figure 5.
Mechanisms of HIF inhibition. A) Western blot analysis of HIF-1α in normal explants (n=4) exposed to ouabain (500 nM) ± DMOG (100 μM). B) Quantification of Western blot. *P < 0.05 vs. control; **P < 0.05 vs. DMOG; Student's t test. C) Western blot analysis of explants for HSP27 and pHSP27 in control ± ouabain (n=6 NP and 6 PE). D) Quantification of pHP27 as a ratio of pHSP27/HSP27 for both groups. *P < 0.05 vs. control in normal pregnancy; **P < 0.05 vs. control in preeclampsia; Student's t test. E) Western blot analysis of sFlt1 in normal explants (n=3) exposed to ouabain (100 nM) ± KRIBB3 (500 nM). F) Quantification of Western blot. Data are presented as a percentage of the sFlt1 expression in control. *P < 0.05 vs. control; Student's t test. G) In coimmunoprecipitation studies, HSP27 was immunoprecipitated (IP) and then analyzed for HIF1-α in Western blot analysis. Normal explants (n=4, ouabain 100 nM and KRIBB3 500 nM). Data are presented as a percentage of the sFlt1 expression in control. H) Quantification of Western blot. *P < 0.05 vs. ouabain; Student's t test.
Ouabain reverses signs of preeclampsia in RUPP model
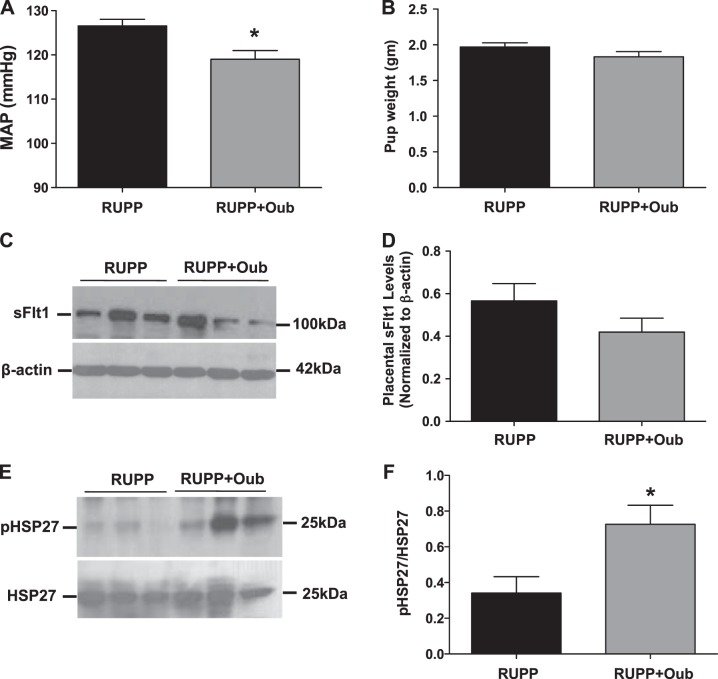
We then evaluated the effect of ouabain therapy on systemic blood pressure and fetal weights in the reduced uterine perfusion pressure model (RUPP), which is characterized by pregnancy-induced hypertension in response to placental ischemia in pregnant rats (15). Prior studies in this model had revealed that sFlt1 was elevated during uterine ischemia and that the hypertension can be rescued by treatment with VEGF-121 (26). In this model, we noted that treatment with ouabain (20 μg/kg/d) from gestational d 12 to 19 reduced the mean arterial pressure (Fig. 6A) with no difference in pup weights (Fig. 6B). Interestingly, while there was a modest decrease in placental sFlt1 expression by ouabain therapy in the RUPP animals, this was not significant (Fig. 6C, D). Notably, placental phosphorylated HSP27 was significantly increased in ouabain-treated RUPP animals (Fig. 6E, F).
Figure 6.
Characteristics of RUPP animals injected with ouabain. A) Mean arterial pressure (MAP) in RUPP (n=9) and in RUPP treated with ouabain (Oub; n=12). *P < 0.05 vs. RUPP; Student's t test. B) Pup weight between RUPP (n=9) compared to RUPP treated with ouabain (n=12). C, D) Representative (3 animals/group) Western blot of sFlt1 expression in placenta (C) and summary data (D) from RUPP (n=11) and RUPP rats treated with ouabain (n=8). E, F) Representative (3 animals/group) Western blot of pHSP27 and HSP27 expression in placenta (E) and summary data (F) from RUPP (n=11) and RUPP rats treated with ouabain (n=8). *P < 0.05 vs. RUPP; Student's t test.
Ouabain therapy does not alter uteroplacental hemodynamics during normal mouse pregnancy
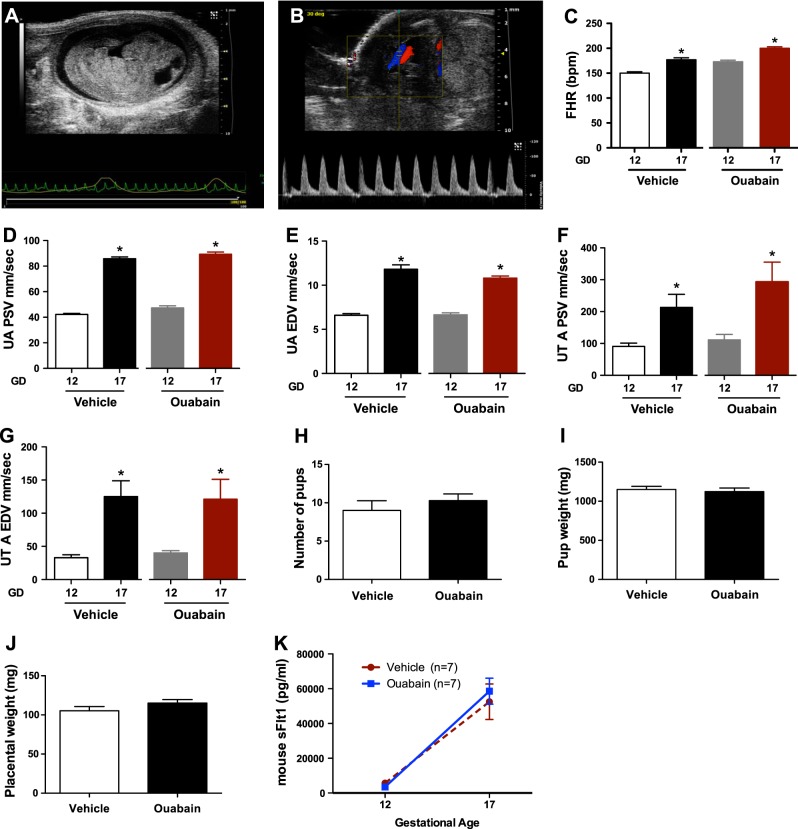
To evaluate whether ouabain had direct effects on the maternal and fetal health during normal gestation, we evaluated pregnant CD1 mice treated with 20 μg/kg/d of ouabain from gestation d 12 to 17. These mice displayed no change in blood flow velocity in umbilical and uterine arteries, as measured by Doppler studies. There was normal physiological increase in fetal heart rate, umbilical artery peak systolic and end diastolic velocity, and uterine artery peak systolic and end diastolic velocity in both vehicle- and ouabain-treated mice (Fig. 7A–G). Similarly, there was no change in number of pups, pup weight, placental weight (Fig. 7H–J) or serum sFlt1 levels with ouabain therapy (Fig. 7K). These data suggest that, at least at 20 μg/kg/d dose, ouabain did not induce any adverse outcomes in normal pregnant mice.
Figure 7.
Uteroplacental hemodynamics in normal pregnancy with ouabain therapy. A) Sagittal view of the mouse fetus at gestational d 12. B) The placental cord insertion is first identified by color Doppler. A waveform is obtained showing the peak systolic velocity (PSV) and end-diastolic velocity (EDV). C) Change in the fetal heart rate (FHR) at gestational d 12 and gestational d 17 among vehicle-injected (n=7) and ouabain-injected (n=7) mice. *P < 0.05 vs. gestational d 12; Student's t test. D, E) Change in the umbilical artery (UA) PSV (D) and EDV (E) at gestational d 12 and gestational d 17 among vehicle-injected (n=7) and ouabain-injected (n=7) mice. *P < 0.05 vs. gestational d 12; Student's t test. F, G) Change in the uterine artery (UT A) PSV (F) and EDV (G) at gestational d 12 and gestational d 17 among vehicle-injected (n=7) and ouabain-injected (n=7) mice. *P < 0.05 vs. gestational d 12; Student's t test. H–J) Number of pups (H), pup weight (I), and placental weight (J) among vehicle-injected and ouabain-injected mice. K) Plasma sFlt1 levels at gestational d 12 and gestational d 17 in vehicle-injected (n=7) and ouabain-injected (n=7) mice.
DISCUSSION
Using an unbiased screen of a library of natural compounds, we report here that cardiac glycosides, such as ouabain, were potent inhibitors of sFlt1 protein and mRNA synthesis. We also noted that ouabain inhibits sFlt1 expression through the HIF-1α pathway and that this effect was mediated through ouabain-induced HSP27 phosphorylation. Furthermore, in an animal model of pregnancy-induced hypertension, ouabain therapy improved hypertension and restored placental pHSP27 levels without any adverse consequences to the pups. In addition, ouabain had no negative effect on uteroplacental hemodynamics in normal pregnant mice, as assessed by Doppler studies of uterine and umbilical vessels. These data suggest that the biological effect of ouabain occurs primarily in the setting of placental ischemia, where HIF-1α protein is up-regulated. Taken together with prior work linking placental ischemia to preeclampsia, our data suggest that targeting HIF-1α/HSP27 pathway may be a novel approach to ameliorate signs and symptoms of preeclampsia in humans.
Ouabain is a cardiac glycoside derived from plants of the genera Digitalis, Strophanthus, and others, which have been prescribed for decades to treat congestive heart failure and arrhythmias (27). In cell culture studies, we demonstrated that ouabain was a potent inhibitor of sFlt1 production in a time- and dose-dependent fashion. We further demonstrated that sFlt1 repression by ouabain was mediated through HIF-1α inhibition. Interestingly, other cardiac glycosides, such as digoxin, have been shown to be potent HIF-1α inhibitors and have been used to ameliorate ischemic disorders, such as pulmonary hypertension (20) and retinal ischemia (28). In contrast to sFlt1 expression, we found that the levels of mRNA for HIF-1α and HIF-2α were not reduced by ouabain treatment. These data suggested that regulation of sFlt1 expression by ouabain may be occurring at a post-transcriptional level via impairment of HIF-1α protein levels. These data are consistent with prior work in pulmonary tissue in which digoxin was shown to inhibit HIF-1α protein, but not HIF-1α mRNA expression (20). Experiments with DMOG showed that inhibition of HIF-1α levels by ouabain were not related to the PHD-VHL-proteasome pathway. Notably, we observed that ouabain induced HSP27 phosphorylation, and this was necessary for repression of HIF-1α and sFlt1.
HSPs are a family of proteins expressed by all cells, and their expression is induced in response to a wide variety of unfavorable physiological and environmental conditions (29). One of the functions of HSP27 includes its role as a protein chaperone and protection against heat shock and oxidative stress (29). HSP27 can undergo phosphorylation at serine 15, 78, and 82 and can significantly alter its function (30, 31). In prior studies, other chaperone proteins, such as HSP90, have been shown to regulate HIF expression (20). However, the role of HSP27 phosphorylation in regulating HIF biology has not been described. In fact, expression of HSP27 and pHSP27 has been found to be higher in placental tissues derived from preeclamptic placentas compared to controls (32) and was shown to have differential spatial distribution within the placenta (25). These data suggest that perhaps increased pHSP27 observed in human preeclampsia may be an adaptive response to the placental ischemia.
Our in vivo studies in a uterine ischemia model revealed that short exposure to ouabain to pregnant RUPP rats resulted in partial rescue of the hypertension phenotype. Although there was a robust increase in rat placental pHSP27 levels following ouabain therapy, we only noted a modest decrease in placental sFlt1 expression. We speculate that selective sampling of placentas related to differential placental sFlt1 expression between litters may account for a lack of more robust effect, as noted by others (33). Preliminary safety studies in pregnant mice also did not reveal any major adverse consequences to the fetus, although this needs to be further explored. These data suggest that a short course of ouabain therapy may reduce signs and symptoms of preeclampsia, with no measurable effects on the fetal and placental size.
Our findings have many implications to our understanding of the pathogenesis of preeclampsia. Human studies have suggested that endogenous cardiac glycosides are elevated in patients with preeclampsia (34). While it has been argued that these endogenous cardiac glycosides contribute to preeclampsia, our data imply that these endogenous cardiac glycosides could be elevated as a compensatory response to elevated HIF-1α and sFlt1 noted in preeclampsia. Although we need more data to support our conclusions, it is likely that targeting endogenous cardiac glycosides may not ameliorate preeclampsia. It is not surprising that early human studies targeting the cardiac glycoside pathways have not had any major success (35, 36). Long-term ouabain therapy in animals has led to hypertension (37). Conversely, our studies suggest that, at least in the setting of uterine ischemia, ouabain, when given for a short period of time, lowers the blood pressure. Recent findings by Jacobs et al. (19), suggesting that there is resistance to ouabain-induced hypertensive effects during normal rodent pregnancy, support our hypothesis. In contrast to the placenta, HIF-1α is thought to promote angiogenesis in systemic vasculature (9); therefore, we do not know whether HIF inhibition systemically during pregnancy would not have any adverse consequences. Evaluation of other HIF antagonists unrelated to the cardiac glycoside family in cell culture and rodent models of preeclampsia may shed further light on the benefit of targeting the HIF pathway. It is also possible that the beneficial effects of ouabain in the placenta may be related to its effects on other pathways, such as blocking autophagy-mediated cell death or “autosis” that was recently described to occur in stressful situations (38). Further studies should be done to evaluate the effects of ouabain in other animal models of pregnancy-induced hypertension and to test whether ouabain therapy will lead to enhanced VEGF signaling in vascular beds affected by preeclampsia.
Reduction of sFlt1 by other mechanisms, such as statins (39), relaxin (40), and extracorporeal removal of sFlt1 by apheresis (41), have been shown to be beneficial in animal models and small pilot studies in humans. Our findings suggest that ouabain or other HIF-1α inhibitors may prove to be an effective treatment of preeclampsia by reducing the placental production of sFlt1. Further studies need to be done to confirm our findings and evaluate whether targeting the HIF-1α/HSP27 pathway will reduce sFlt1 levels in human pregnancies and attenuate signs of preeclampsia without inducing adverse consequences to the mother or the fetus.
Supplementary Material
Acknowledgments
The authors thank Dawn McCullough, R.N., for patient placental sample collection.
S.R. is supported by grant K08HD068398-01A1 (U.S. National Institutes of Health/National Institute of Child Health and Development). A.S.C is funded by the Gulbenkian Programme for Advanced Medical Education (Lisbon, Portugal). S.A.K is an investigator of the Howard Hughes Medical Institute. S.A.K. is a coinventor on multiple patents for preeclampsia markers and reports service as a consultant to Siemens Diagnostics (Munich, Germany) and has financial interest in Aggamin LLC (New York, NY, USA).
The other authors report no conflicts of interest.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- ANOVA
- analysis of variance
- DMOG
- dimethyl oxaloylglycine
- DMSO
- dimethyl sulfoxide
- EDTA
- ethylenediaminetetraacetic acid
- ELISA
- enzyme-linked immunosorbent assay
- FBS
- fetal bovine serum
- HIF-1α
- hypoxia-inducible factor 1α
- HIF-2α
- hypoxia inducible factor 2α
- HSP27
- heat-shock protein 27
- KRIBB3
- 5-(5-ethyl-2-hydroxy-4-methoxyphenyl)-4-(4-methoxyphenyl) isoxazole
- mRNA
- messenger RNA
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- pHSP27
- phosphorylated heat-shock protein 27
- RUPP
- reduced uterine perfusion pressure
- sFlt1
- soluble fms-like tyrosine kinase 1
- TBS
- Tris-buffered saline
- VEGF
- vascular endothelial growth factor
- VEGFR1
- vascular endothelial growth factor receptor 1
REFERENCES
- 1. American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy (2013) Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 122, 1122–1131 [DOI] [PubMed] [Google Scholar]
- 2. Powe C. E., Levine R. J., Karumanchi S. A. (2011) Preeclampsia, a disease of the maternal endothelium: the role of antiangiogenic factors and implications for later cardiovascular disease. Circulation 123, 2856–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Backes C. H., Markham K., Moorehead P., Cordero L., Nankervis C. A., Giannone P. J. (2011) Maternal preeclampsia and neonatal outcomes. J. Pregnancy 2011, 214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Redman C. W., Sargent I. L. (2005) Latest advances in understanding preeclampsia. Science 308, 1592–1594 [DOI] [PubMed] [Google Scholar]
- 5. Maynard S. E., Min J. Y., Merchan J., Lim K. H., Li J., Mondal S., Libermann T. A., Morgan J. P., Sellke F. W., Stillman I. E., Epstein F. H., Sukhatme V. P., Karumanchi S. A. (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine R. J., Maynard S. E., Qian C., Lim K. H., England L. J., Yu K. F., Schisterman E. F., Thadhani R., Sachs B. P., Epstein F. H., Sibai B. M., Sukhatme V. P., Karumanchi S. A. (2004) Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 350, 672–683 [DOI] [PubMed] [Google Scholar]
- 7. Rana S., Powe C. E., Salahuddin S., Verlohren S., Perschel F. H., Levine R. J., Lim K. H., Wenger J. B., Thadhani R., Karumanchi S. A. (2012) Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125, 911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhou C. C., Zhang Y., Irani R. A., Zhang H., Mi T., Popek E. J., Hicks M. J., Ramin S. M., Kellems R. E., Xia Y. (2008) Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat. Med. 14, 855–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karumanchi S. A., Bdolah Y. (2004) Hypoxia and sFlt-1 in preeclampsia: the “chicken-and-egg” question. Endocrinology 145, 4835–4837 [DOI] [PubMed] [Google Scholar]
- 10. Cudmore M., Ahmad S., Al-Ani B., Fujisawa T., Coxall H., Chudasama K., Devey L. R., Wigmore S. J., Abbas A., Hewett P. W., Ahmed A. (2007) Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation 115, 1789–1797 [DOI] [PubMed] [Google Scholar]
- 11. Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd (1986) Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology 118, 1567–1582 [DOI] [PubMed] [Google Scholar]
- 12. Rajakumar A., Doty K., Daftary A., Harger G., Conrad K. P. (2003) Impaired oxygen-dependent reduction of HIF-1α and -2α proteins in pre-eclamptic placentae. Placenta 24, 199–208 [DOI] [PubMed] [Google Scholar]
- 13. Rajakumar A., Powers R. W., Hubel C. A., Shibata E., von Versen-Hoynck F., Plymire D., Jeyabalan A. (2009) Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta 30, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rajakumar A., Michael H. M., Rajakumar P. A., Shibata E., Hubel C. A., Karumanchi S. A., Thadhani R., Wolf M., Harger G., Markovic N. (2005) Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26, 563–573 [DOI] [PubMed] [Google Scholar]
- 15. Granger J. P., LaMarca B. B., Cockrell K., Sedeek M., Balzi C., Chandler D., Bennett W. (2006) Reduced uterine perfusion pressure (RUPP) model for studying cardiovascular-renal dysfunction in response to placental ischemia. Methods Mol. Med. 122, 383–392 [DOI] [PubMed] [Google Scholar]
- 16. Alexander B. T., Kassab S. E., Miller M. T., Abram S. R., Reckelhoff J. F., Bennett W. A., Granger J. P. (2001) Reduced uterine perfusion pressure during pregnancy in the rat is associated with increases in arterial pressure and changes in renal nitric oxide. Hypertension 37, 1191–1195 [DOI] [PubMed] [Google Scholar]
- 17. Murphy S. R., LaMarca B. B., Parrish M., Cockrell K., Granger J. P. (2013) Control of soluble fms-like tyrosine-1 (sFlt-1) production response to placental ischemia/hypoxia: role of tumor necrosis factor-alpha. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R130–R135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khankin E. V., Hacker M. R., Zelop C. M., Karumanchi S. A., Rana S. (2012) Intravital high-frequency ultrasonography to evaluate cardiovascular and uteroplacental blood flow in mouse pregnancy. Pregnancy Hypertens. 2, 84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jacobs B. E., Liu Y., Pulina M. V., Golovina V. A., Hamlyn J. M. (2012) Normal pregnancy: mechanisms underlying the paradox of a ouabain-resistant state with elevated endogenous ouabain, suppressed arterial sodium calcium exchange, and low blood pressure. Am. J. Physiol. Heart Circ. Physiol. 302, H1317–H1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H., Qian D. Z., Tan Y. S., Lee K., Gao P., Ren Y. R., Rey S., Hammers H., Chang D., Pili R., Dang C. V., Liu J. O., Semenza G. L. (2008) Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth. Proc. Natl. Acad. Sci. U. S. A. 105, 19579–19586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagamatsu T., Fujii T., Kusumi M., Zou L., Yamashita T., Osuga Y., Momoeda M., Kozuma S., Taketani Y. (2004) Cytotrophoblasts up-regulate soluble fms-like tyrosine kinase-1 expression under reduced oxygen: an implication for the placental vascular development and the pathophysiology of preeclampsia. Endocrinology 145, 4838–4845 [DOI] [PubMed] [Google Scholar]
- 22. Rajakumar A., Whitelock K. A., Weissfeld L. A., Daftary A. R., Markovic N., Conrad K. P. (2001) Selective overexpression of the hypoxia-inducible transcription factor, HIF-2α, in placentas from women with preeclampsia. Biol. Reprod. 64, 499–506 [DOI] [PubMed] [Google Scholar]
- 23. Zhou Y., Gormley M. J., Hunkapiller N. M., Kapidzic M., Stolyarov Y., Feng V., Nishida M., Drake P. M., Bianco K., Wang F., McMaster M. T., Fisher S. J. (2013) Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J. Clin. Invest. 123, 2862–2872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 25. Abdulsid A., Lyall F. (2014) Heat shock protein 27 expression is spatially distributed in human placenta and selectively regulated during preeclampsia. J. Reprod. Immunol. 101–102, 89–95 [DOI] [PubMed] [Google Scholar]
- 26. Gilbert J. S., Verzwyvelt J., Colson D., Arany M., Karumanchi S. A., Granger J. P. (2010) Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placental ischemia-induced hypertension. Hypertension 55, 380–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mueller P. (1965) Ouabain effects on cardiac contraction, action potential, and cellular potassium. Circ. Res. 17, 46–56 [DOI] [PubMed] [Google Scholar]
- 28. Yoshida T., Zhang H., Iwase T., Shen J., Semenza G. L., Campochiaro P. A. (2010) Digoxin inhibits retinal ischemia-induced HIF-1α expression and ocular neovascularization. FASEB J. 24, 1759–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Acunzo J., Katsogiannou M., Rocchi P. (2012) Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int. J. Biochem. Cell Biol. 44, 1622–1631 [DOI] [PubMed] [Google Scholar]
- 30. Garrido C., Paul C., Seigneuric R., Kampinga H. H. (2012) The small heat shock proteins family: the long forgotten chaperones. Int. J. Biochem. Cell Biol. 44, 1588–1592 [DOI] [PubMed] [Google Scholar]
- 31. Oya-Ito T., Liu B. F., Nagaraj R. H. (2006) Effect of methylglyoxal modification and phosphorylation on the chaperone and anti-apoptotic properties of heat shock protein 27. J. Cell. Biochem. 99, 279–291 [DOI] [PubMed] [Google Scholar]
- 32. Shin J. K., Jeong Y. T., Jo H. C., Kang M. Y., Chang I. S., Baek J. C., Park J. K., Lee S. A., Lee J. H., Choi W. S., Paik W. Y. (2009) Increased interaction between heat shock protein 27 and mitogen-activated protein kinase (p38 and extracellular signal-regulated kinase) in pre-eclamptic placentas. J. Obstet. Gynaecol. Res. 35, 888–894 [DOI] [PubMed] [Google Scholar]
- 33. Surmon L., Bobek G., Chiu C. L., Young S., Makris A., Lind J. M., Hennessy A. (2014) The expression of placental soluble fms-like tyrosine kinase 1 in mouse placenta varies significantly across different litters from normal pregnant mice. [E-pub ahead of print] Hypertens. Pregnancy doi: 10.3109/10641955.2014.903963 [DOI] [PubMed] [Google Scholar]
- 34. Graves S. W., Lincoln K., Cook S. L., Seely E. W. (1995) Digitalis-like factor and digoxin-like immunoreactive factor in diabetic women with preeclampsia, transient hypertension of pregnancy, and normotensive pregnancy. Am. J. Hypertens. 8, 5–11 [DOI] [PubMed] [Google Scholar]
- 35. Adair C. D., Luper A., Rose J. C., Russell G., Veille J. C., Buckalew V. M. (2009) The hemodynamic effects of intravenous digoxin-binding fab immunoglobulin in severe preeclampsia: a double-blind, randomized, clinical trial. J. Perinatol. 29, 284–289 [DOI] [PubMed] [Google Scholar]
- 36. Adair C. D., Buckalew V. M., Graves S. W., Lam G. K., Johnson D. D., Saade G., Lewis D. F., Robinson C., Danoff T. M., Chauhan N., Hopoate-Sitake M., Porter K. B., Humphrey R. G., Trofatter K. F., Amon E., Ward S., Kennedy L., Mason L., Johnston J. A. (2010) Digoxin immune fab treatment for severe preeclampsia. Am. J. Perinatol. 27, 655–662 [DOI] [PubMed] [Google Scholar]
- 37. Yuan C. M., Manunta P., Hamlyn J. M., Chen S., Bohen E., Yeun J., Haddy F. J., Pamnani M. B. (1993) Long-term ouabain administration produces hypertension in rats. Hypertension 22, 178–187 [DOI] [PubMed] [Google Scholar]
- 38. Liu Y., Shoji-Kawata S., Sumpter R. M., Jr., Wei Y., Ginet V., Zhang L., Posner B., Tran K. A., Green D. R., Xavier R. J., Shaw S. Y., Clarke P. G., Puyal J., Levine B. (2013) Autosis is a Na+,K+-ATPase-regulated form of cell death triggered by autophagy-inducing peptides, starvation, and hypoxia-ischemia. Proc. Natl. Acad. Sci. U. S. A. 110, 20364–20371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Costantine M. M., Cleary K.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Obstetric–Fetal Pharmacology Research Units Network (2013) Pravastatin for the prevention of preeclampsia in high-risk pregnant women. Obstet. Gynecol. 121, 349–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Conrad K. P. (2011) Emerging role of relaxin in the maternal adaptations to normal pregnancy: implications for preeclampsia. Seminars Nephrol. 31, 15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thadhani R., Kisner T., Hagmann H., Bossung V., Noack S., Schaarschmidt W., Jank A., Kribs A., Cornely O. A., Kreyssig C., Hemphill L., Rigby A. C., Khedkar S., Lindner T. H., Mallmann P., Stepan H., Karumanchi S. A., Benzing T. (2011) Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124, 940–950 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.