Abstract
Mitochondrial dysfunction in adipose tissue occurs in obesity, type 2 diabetes, and some forms of lipodystrophy, but whether this dysfunction contributes to or is the result of these disorders is unknown. To investigate the physiological consequences of severe mitochondrial impairment in adipose tissue, we generated mice deficient in mitochondrial transcription factor A (TFAM) in adipocytes by using mice carrying adiponectin-Cre and TFAM floxed alleles. These adiponectin TFAM-knockout (adipo-TFAM-KO) mice had a 75–81% reduction in TFAM in the subcutaneous and intra-abdominal white adipose tissue (WAT) and interscapular brown adipose tissue (BAT), causing decreased expression and enzymatic activity of proteins in complexes I, III, and IV of the electron transport chain (ETC). This mitochondrial dysfunction led to adipocyte death and inflammation in WAT and a whitening of BAT. As a result, adipo-TFAM-KO mice were resistant to weight gain, but exhibited insulin resistance on both normal chow and high-fat diets. These lipodystrophic mice also developed hypertension, cardiac hypertrophy, and cardiac dysfunction. Thus, isolated mitochondrial dysfunction in adipose tissue can lead a syndrome of lipodystrophy with metabolic syndrome and cardiovascular complications.—Vernochet, C., Damilano, F., Mourier, A., Bezy, O., Mori, M. A., Smyth, G., Rosenzweig, A., Larsson, N.-G., Kahn, C. R. Adipose tissue mitochondrial dysfunction triggers a lipodystrophic syndrome with insulin resistance, hepatosteatosis, and cardiovascular complications.
Keywords: TFAM, WAT, BAT, diabetes, hypertension, cardiomegaly
Both excess fat (obesity) and lack of fat (lipodystrophy) are associated with increased risks of diabetes, metabolic syndrome, and cardiovascular disease (1–3). Mitochondrial dysfunction has been reported to cause adipose tissue dysfunction in both obesity and lipodystrophy (3). In humans, down-regulation of the expression of different components of the oxidative phosphorylation (oxphos) system in white adipose tissue (WAT) correlates with the level of obesity (3, 4). It is thought that mitochondrial dysfunction can lead to impaired substrate oxidation in adipose tissue, thereby contributing to the development of obesity. Mitochondrial dysfunction in adipose tissue has also been reported in the lipodystrophy observed in some HIV-1-infected patients undergoing antiretroviral treatment (5). An in vitro study of adipocytes has shown that exposure to certain nucleoside analogues and nucleotide reverse transcriptase inhibitor, such as stavudine and zidovudine, can lead to a depletion of mitochondrial DNA (mtDNA), decreased mitochondrial activity, reduced lipid content, and impaired adipocyte survival (6).
The 2 main types of adipose tissue in mammals can be distinguished by their mitochondrial content and function. Brown adipose tissue (BAT) has a high mitochondrial content that gives it a high capacity for both glucose and fatty acid β-oxidation. BAT mitochondria also specifically express uncoupling protein 1 (UCP1), which allows a leak of protons across the mitochondrial inner membrane, resulting in a high level of energy expenditure through thermogenesis (7). WAT contains fewer mitochondria, but mitochondrial function is still crucial for adipogenic differentiation (8) and is essential for maintenance of white adipocyte function, including normal adipokine secretion (9–11). One of the major controllers of mitochondrial mass and function is mitochondrial transcription factor A (TFAM). TFAM plays an important role in mitochondrial biology (12) and is crucial for regulation of mitochondria-encoded gene transcription, mtDNA stability, and mtDNA replication (13–15).
In the current study, we induced mitochondrial dysfunction specifically in adipose tissue by using Cre-lox–induced recombination in a mouse carrying floxed alleles of the TFAM gene and in mice carrying Cre recombinase driven by the strong and highly specific adipose tissue promoter adiponectin. The resultant mice had markedly reduced TFAM expression in BAT and WAT and developed a syndrome of lipodystrophic diabetes associated with hepatosteatosis and dyslipidemia. These adiponectin TFAM-knockout (adipo-TFAM-KO) mice also developed cardiovascular abnormalities, including hypertension, cardiac hypertrophy, and cardiac dysfunction. These data demonstrate the broad and systemic effect of adipose tissue mitochondrial dysfunction on whole-body physiology and indicate how the dysfunction may contribute to metabolic syndrome.
MATERIALS AND METHODS
Animals and diets
Adiponectin Cre transgenic (16) and TFAM floxed (TFAMf/f) mice have been described previously (12). Both are on mixed genetic backgrounds consisting of 129-FVB-B6 strains; thus, littermate controls on the same mixed background were used in all experiments. All the mice were housed in a mouse facility on a 12-h light-dark cycle in a 22°C temperature-controlled room. The animals were maintained on a standard chow diet (CD) containing 22% calories from fat, 23% from protein, and 55% from carbohydrates (Mouse Diet 9F 5020; PharmaServ, Framingham, MA, USA) or subjected to a high-fat diet (HFD) containing 60% calories from fat, 20% from protein, and 20% from carbohydrates (OpenSource Diet D12492; Research Diet, New Brunswick, NJ, USA) beginning at ∼8 wk of age as indicated. Calorie-restricted mice were obtained from the National Aging Institute Repository, as described previously (17). Animal care and study protocols were approved by the Animal Care Committee of Joslin Diabetes Center and were in accordance with the U.S. National Institutes of Health (NIH) guidelines.
Body composition and metabolic analysis
Body composition of 24-wk-old male mice, fed either a CD or HFD ad libitum (starting at 6 wk of age), was determined with dual-energy X-ray absorptiometry (DEXA) scanning (Lunar PIXImus2 densitometer; GE Medical Systems, Valhalla, NY, USA) following anesthesia with Avertin (tribromoethanol:tert-amyl alcohol, 0.015 ml/g by intraperitoneal injection). For metabolic cage analysis, 24-wk-old control (flox) and adipo-TFAM-KO mice were fed a CD. The mice were housed individually and evaluated for ambulatory activity with an OPTO-M3 sensor system [Comprehensive Laboratory Animal Monitoring System (CLAMS); Columbus Instruments, Columbus, OH, USA]. Indirect calorimetry was performed on the same mice with the open-circuit Oxymax system (Columbus Instruments). After a 48 h acclimation period, each mouse was studied for 48 h in the fed state and 24 h in the unfed state, for determination of O2 consumption and CO2 production.
Circulating factors and glucose tolerance test
Fed glucose was measured between 9 and 11 AM in tail vein blood samples (Ascensia Elite; Bayer Healthcare Wayne, NJ, USA). Insulin was measured with a rat insulin enzyme-linked immunosorbent assay (ELISA) with mouse standards. Interleukin 6 (IL6), tumor necrosis factor α (TNFα), leptin and adiponectin were measured with ELISA kits (Crystal Chem, Downers Grove, IL, USA). Angiotensinogen (AGTN) and angiotensin II Ang II) were measured by Clontech Laboratories (Mountain View CA, USA) and Cayman Chemical (Madison WI, USA), respectively. Intraperitoneal glucose (2 g/kg body weight) and insulin tolerance (1.25 U/kg) tests were performed in unrestrained, conscious mice from which food had been withheld for 16 and 4 h, respectively.
Analysis of gene expression and mtDNA by quantitative PCR
Total RNA was isolated with an RNeasy minikit (Qiagen, Valencia, CA, USA). Total RNA (3 μg) was reverse transcribed in a 100 μl volume with a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). A portion (5 μl) of the diluted (1:5) reverse transcription reaction was amplified with specific primers (300 nM each) in a 10 μl PCR reaction with SYBR Green PCR Master Mix (Applied Biosystems). Analysis of gene expression was performed in the ABI Prism 7900HT sequence detector (Applied Biosystems) with an initial denaturation at 95°C for 10 min, followed by 40 PCR cycles consisting of 95°C for 15 s, 60°C for 1 min, and 72°C for 1 min, and the SYBR green fluorescence emission was monitored after each cycle. For each gene, mRNA expression was calculated relative to the TATA-binding protein (TBP) expression. Amplification of specific transcripts was confirmed by the melting-curve profiles (cooling the sample to 68°C and heating slowly to 95°C with measurement of fluorescence) at the end of each PCR. Primer sequences have been published previously (18) except for the following: agtn, F 5′-cgagtgggagaggttctcaa-3′, R 5′ ctcgtagatggcgaacagga-3′; collagen type 1a (col1a), F 5′-gagcggagagtactggatcg-3′, R 5′-gcttcttttccttggggttc-3′; collagen type 3a1 (col3a1), F 5′-gcacagcagtccaacgtaga-3′, R 5′-tctccaaatgggatctctgg-3′; connective tissue growth factor (CTGF), F 5′-caaagcagctgcaaatacca-3′, R 5′-ggccaaatgtgtcttccagt-3′; brain natriuretic peptide (BNP), F 5′-cagctcttgaaggaccaagg-3′, R 5′-agacccaggcagagtcagaa-3′; brain natriuretic peptide (ANP), F 5′-cctaagcccttgtggtgtgt-3′, R 5′-cagagtgggagaggcaagac-3′; TNFa, F 5′-ccctcacactcagatcatcttct-3′; R 5′gctacgacgtgggcracag-3′; IL6, F 5′-gccttccctacttcacaag-3′, R 5′-aggtctgttgggagtggtatcc-3′; ATP2A, F 5′-tggtgatatagtggaaattgctg-3′, R 5′-gagttgtagacttgatggatgtcaa-3′; Na+/Ca2+-exchange protein 1 (NCX1), F 5′-gatcatccgattccctctactg-3′, R 5′-gtcagtggctgcttgtcatc-3′; and 18s, F 5′-cgtctgccctatcaactttcg-3′; R 5′-cttggatgtggtagccgtttc-3′.
Histology
Subcutaneous inguinal WAT (iWAT), intra-abdominal epididymal WAT (eWAT), and interscapular BAT from mice fed a CD for 24 wk (n=4/group) were fixed in 10% formalin and embedded in paraffin; 8 μm sections were cut and stained with hematoxylin and eosin. Five digital images (×20 for iWAT and eWAT and ×40 for BAT) from nonoverlapping fields were taken of each slide. Adipocyte diameters were calculated with ImageJ software (NIH, Bethesda, MD, USA).
Paraffin-embedded left ventricles (LVs) were cut in 5 μm sections and stained with Masson's trichrome, to determine collagen deposition. Frozen optimal cutting temperature (OCT) compound (Tissue-Tek OCT; Sakura Finetek, Torrance, CA, USA)–embedded LVs were cut in sections of 5 μm.
Mitochondrial isolation
Adipocytes were first isolated after collagenase digestion of minced pieces of iWAT, eWAT, and BAT of 10- to 12-wk-old male mice. Isolated adipocytes were then resuspended into 2 vol of ice-cold homogenization buffer (0.25 M sucrose, 20 mM HEPES, and 0.2 mM potassium EDTA, pH 7.4) and homogenized in a loose-fitting homogenizer. Homogenates were centrifuged at 600 g for 10 min at 4°C. The supernatant was centrifuged at 10,000 g for 10 min at 4°C. The pellets containing mitochondria were suspended in 2 vol of 0.25 M sucrose and again centrifuged at 10,000 g for 10 min at 4°C. Purified mitochondria were then used for Western blotting and analysis of electron transport chain (ETC) complex activity.
Mitochondrial ETC complex enzymatic activity
Mitochondrial proteins (15–50 μg) were diluted in phosphate buffer (50 mM KH2PO4, pH 7.4) followed by spectrophotometric analysis of isolated respiratory chain complex activities at 37°C with a UV-3600 spectrophotometer (Hitachi USA, Troy, MI, USA). Succinate dehydrogenase (SDH) activity was measured at 600 nm (E=21,000 M−1·cm−1) after the addition of 10 mM succinate, 35 μM dichlorphenolindophenol (DCPIP), and 1 mM KCN. NADH dehydrogenase activity was determined at 340 nm (E=6220 M−1·cm−1) after the addition of 0.25 mM NADH, 0.25 mM decylubiquinone and 1 mM KCN. Cytochrome c reductase activity was assessed at 540 nm (E=18,000 M−1·cm−1) in the presence of 1 mM cytochrome c, 20 mM succinate, and 1 mM KCN. Cytochrome c oxidase activity was measured by N,N,N′,N′-tetramethylbenzene-1,4-diamineascorbate (TMPD) standard assays. All chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA). Citrate synthase activity was measured at 412 nm (E=13,600 M−1·cm−1) after the addition of 0.1 mM acetyl-CoA, 0.5 mM oxaloacetate, and 0.1 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB).
Triglyceride quantification
Liver triglyceride content was assessed in 24-wk-old male mice fed a CD. Liver homogenates were prepared by homogenizing tissue (∼300 mg) in 2 ml of standard diluent in a Bullet blender (Next Advance, Averill Park, NY, USA). Samples were centrifuged at 1000 g for 10 min, and the supernatant was collected. Triglyceride quantification was performed on serial dilutions of liver extracts according to the manufacturer's instructions, with a colorimetric kit (Cayman Chemical). Absorbance was determined at 540 nm. All samples were determined in duplicate, and triglyceride values were expressed as milligrams triglycerides per gram tissue and normalized to the control (Lox, i.e., mice carrying the TFAM allele flanked with Lox sites but without Cre) value.
Western blot analysis
Proteins were extracted from isolated mitochondria from white and brown adipocytes and homogenized in 1× RIPA buffer containing 0.1% SDS. Protein (20 μg) was subjected to SDS-PAGE and transferred to polyvinylidene fluoride membranes, and the blots were probed with the following antibodies at the dilution indicated: anti-TFAM, 1:200 (sc-23588; Santa Cruz Biotechnology, Santa Cruz, CA, USA); anti-voltage-dependent anion channel (VDAC; shown as a loading control), 1:1000 (4866; Cell Signaling Technology, Danvers, MA, USA); and the MitoProfile Total Oxphos antibody cocktail, which includes antibodies against C-I-20 (NDUFB8), C-II-30 (FeS), C-III-Core2, CoI, and C-V-a, 1:250 (MS604; Mitosciences, Eugene, OR, USA). The appropriate secondary antibodies conjugated to horseradish peroxidase (HRP; Amersham, Piscataway, NJ, USA) were used at 1:5000 (except for the actin antibody, which was used at 1:10,000 dilution). Membranes were visualized by using SuperSignal West Pico substrate (Pierce Biotechnologies, Rockford, IL, USA) or Immobilon Western HRP Substrate (Millipore, Billerica, MA, USA). Quantification of Western blots was performed with ImageJ software.
LVs were dissected and frozen in liquid nitrogen for storage at −80°C. LV tissue was lysed in a 1% Triton X-100 buffer (120 mM NaCl; 50 mM Tris HCl, pH 8.0; and 1% Triton X-100) with addition of protease inhibitor cocktail (Complete Mini; Roche Diagnostics, Indianapolis, IN, USA), 10 mM sodium pyrophosphate, 5 mM NaF, 1% PMSF, and 1 mM sodium orthovanadate. Nitrocellulose membranes were incubated overnight with Abs against Na+/Ca2+ Exchanger 1 (R&D Systems, Minneapolis, MN), SERCA2 ATPase (Affinity Bioreagents, Golden, CO, USA), and GAPDH (Cell Signaling Technology).
Echography
Short-axis, 2D-guided, M-mode echocardiography was performed in conscious wild-type and KO mice with a Sonos 5500 echocardiograph (Philips, Eindhoven, The Netherlands). Echo measurement was obtained as the average of the analysis of ≥3 frames/animal. The fractional shortening percentage (FS%) was calculated as (LVIDd − LVIDs)/(LVIDd) × 100, where LVIDd and LVIDs are LV internal dimension at diastole and systole, respectively. Relative wall thickness (RWT) was calculated as [(IVSd + LVPWd)/2]/(LVIDd/2), where IVSd is interventricular septal thickness at end-diastole, and LVPWd is LV posterior wall at diastole (19, 20).
Statistics
All differences were analyzed by a Student's t test. Results were considered significant at P < 0.05.
RESULTS
TFAM levels in adipose tissue are regulated by HFD and caloric restriction (CR)
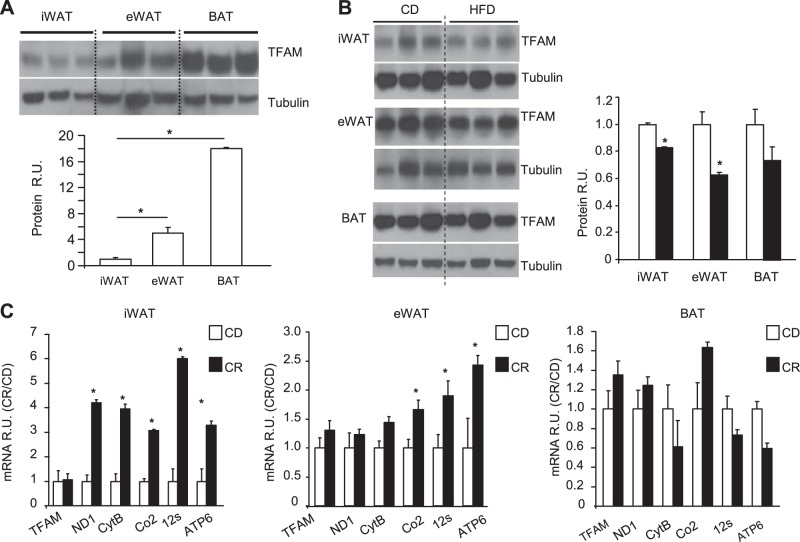
The mitochondrial transcription factor TFAM plays a dual role in maintenance of mtDNA function by stabilizing mtDNA and initiating transcription of the mtDNA, which encodes 13 proteins involved in mitochondrial oxphos (15, 21). To understand the role of TFAM in adipose tissue function, we analyzed the levels of TFAM protein in different adipose depots of the mouse and also determined how these levels changed in response to nutritional status. In mice fed a normal CD, Western blot analysis revealed that the level of TFAM was lowest in subcutaneous iWAT. The relative TFAM levels were 4.6-fold higher in eWAT and 18.1-fold higher in BAT, in comparison with iWAT (Fig. 1A). When mice were challenged with a 60% HFD for 12 wk (starting at 8 wk of age), TFAM protein decreased by 18% in iWAT (P<0.05) and 35% in eWAT (P<0.05), with a trend toward a decrease in BAT (Fig. 1B). CR, which has been shown to maintain mitochondrial function and is associated with increased longevity (22), did not have any effect on TFAM expression in WAT or BAT, but the targets of TFAM regulation, including several mtDNA-encoded genes (ND1, CytB, Co2, 12S, and ATP6), were significantly upregulated in iWAT, eWAT, and BAT (Fig. 1C).
Figure 1.
TFAM expression in WAT and BAT from mice fed HFD or subjected to CR. A) Top panel: TFAM expression was analyzed by Western blot analysis of iWAT, eWAT, and BAT. Bottom panel: quantitation of data (n=6 samples/tissue). *P < 0.05. B) Left panel; TFAM expression was analyzed by Western blot in iWAT, eWAT, and BAT from mice fed a CD or HFD at 16 wk of age. Data are representative of 6 samples/genotype. Right panel: quantitation of data. *P < 0.05. C) TFAM and mRNA levels of mitochondria-encoded genes in mice fed a CD or subjected to CR, using qPCR of iWAT, eWAT, and BAT from 16-wk-old male mice. Data are means ± sem of 6 samples/group. *P < 0.05.
Deletion of TFAM in adipose tissue decreases expression of both mitochondria- and nucleus-encoded mitochondrial proteins and leads to oxphos dysfunction
Mitochondria play an important role in both BAT and WAT (11, 23, 24). We had attempted in earlier work to determine the role of TFAM in adipose tissue by crossing aP2-Cre mice with TFAM floxed mice (18). The resulting mice had a moderate reduction in TFAM in iWAT and BAT, but with the aP2-Cre, there was no reduction in TFAM in eWAT. These mice also had some reduction in linear growth, suggesting some off-target effects of the aP2-Cre (16, 18). Therefore, to better understand the effect of TFAM deletion, specifically in fat, we created a new strain of mice with a stronger and more adipose-specific ablation of TFAM. Mice carrying the adiponectin promoter-Cre transgene were bred with mice carrying a floxed TFAM allele that contained LoxP sites surrounding exons 6 and 7 of the Tfam gene (12). The resultant adiponectin-Cre; TFAM f/f mice (termed adipo-TFAM-KO mice) were viable, born at the expected mendelian ratio, and of normal size.
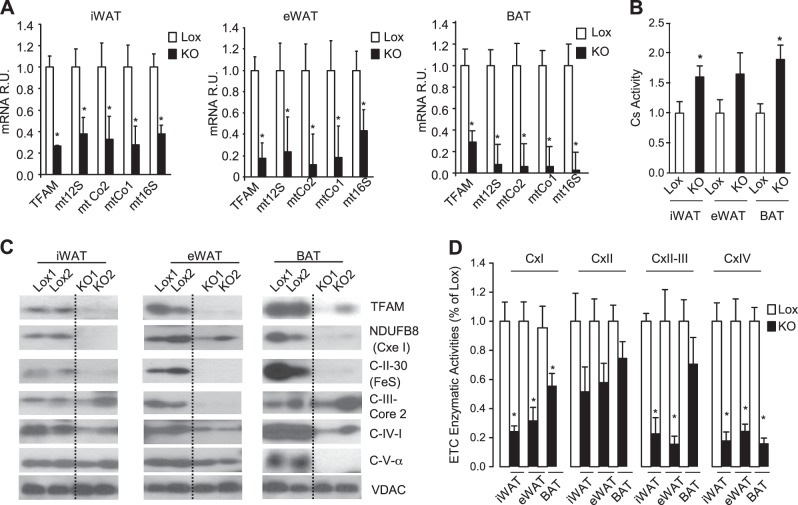
In contrast to the previous TFAM-KO mice, in the current line of mice, TFAM mRNA was decreased in the adipocyte fraction of all fat depots (68% in BAT, 75% in iWAT, and 81% in eWAT) but not in their stromal vascular fraction (SVF; ref. 16). This decrease was accompanied by a marked reduction in expression of mitochondria-encoded genes, averaging ∼65% in iWAT and up to 90% in BAT (Fig. 2A). In all fat depots, this effect was accompanied by an increase in citrate synthase activity, a surrogate marker of mitochondria mass, which reached significance in iWAT and BAT (Fig. 2B). The reduction in TFAM was further confirmed at the protein level by Western blot analysis of isolated mitochondria of the adipocyte fractions of WAT and BAT from control (Lox) and adipo-TFAM-KO mice (Fig. 2C). Interestingly, there was also a dramatic reduction in the levels of nucleus-encoded mitochondrial proteins, such as NDUFB8 (subunit of complex I), C-II-30 (subunit of complex II), and C-IV-I (MTCOI, subunit of complex IV), in the mitochondrial fractions from the TFAM-KO adipocytes. Complex III (C-III)-core 2 protein levels in the adipo-TFAM-KO mitochondria were also reduced in eWAT, but were similar to Lox levels in mitochondria of iWAT and BAT adipocytes (Fig. 2C). On the other hand, C-V-α protein content was strongly reduced in TFAM-KO BAT mitochondria, but was not different from that in the control in mitochondria from either WAT depot of TFAM-KO mice. These differential effects in different adipose depots suggest differences in mitochondrial protein turnover in the different adipocyte populations.
Figure 2.
TFAM KO efficiency in iWAT, eWAT, and BAT and effect on oxphos expression and function. A) TFAM and mRNA of mitochondria-encoded genes, according to qPCR, in isolated adipocytes from iWAT, eWAT, and BAT from 10- to 12-wk-old male control (Lox) and adipo-TFAM-KO (KO) mice. Data are means ± sem of 6 samples/group. *P < 0.05. B) Citrate synthase (Cs) activity was assessed from isolated mitochondria of iWAT, eWAT, and BAT adipocytes. Cells were isolated from 10- to 12-wk-old male Lox and KO mice. Data are means ± sem of 7 samples/group. *P < 0.05. C) Expression of TFAM and oxphos members was analyzed by Western blot from mitochondria isolated from iWAT, eWAT, and BAT adipocytes of 12- to 15-wk-old male KO and Lox mice. Data are representative of 4 samples/genotype. D) Isolated mitochondria respiratory chain enzyme activities were assessed in mitochondria isolated from white and brown adipocytes (n=7/genotype). Cx, complex. *P < 0.05.
Notably, the activities of complexes I and IV of the ETC in mitochondria from both eWAT and iWAT of adipo-TFAM-KO mice were decreased by 45–77% and by 75–84%, respectively, compared to the corresponding control levels (Fig. 2D). Activities of complexes II and III of iWAT and eWAT from the KO mice were also downregulated by 77 and 84%, respectively, whereas complex II and III activity in BAT from the KO mice was not significantly altered compared to that in the control. Complex II enzymatic activity was also moderately decreased in the various fat depots of adipo-TFAM-KO mice, but these changes did not reach statistical significance. Thus, adipo-TFAM-KO mice exhibit altered protein composition of ETC complexes and reduced ETC activity in subcutaneous iWAT, intra-abdominal eWAT, and interscapular BAT.
TFAM KO prevents age- and diet-induced weight gain but impairs glucose tolerance
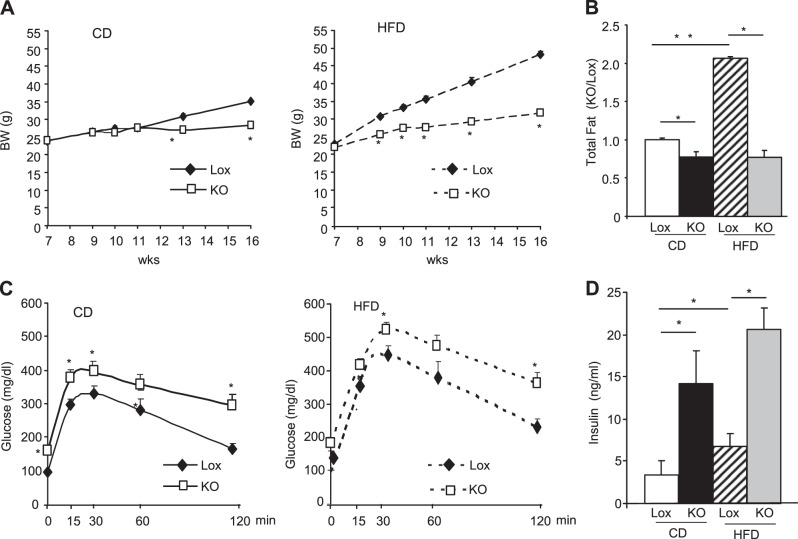
Adipose tissue mitochondrial dysfunction has been linked to metabolic diseases, including obesity and diabetes (11, 25). To examine the role of TFAM KO in adipose tissue on whole-body physiology, adipo-TFAM-KO mice were monitored both while on standard CD and after exposure to an HFD from age 8 to 16 wk. On the CD, the Lox and adipo-TFAM-KO mice displayed similar weights until 11–12 wk of age, then diverged such that by 16 wk of age, the KO mice weighed 23% less than the controls (Fig. 3A, right panel). This difference in weight gain was exaggerated on the HFD, with 34% lower body weight by 16 wk in the adipo-TFAM-KO mice (Fig. 3A, left panel). DEXA analysis revealed that this difference was due to decreases in fat mass of 28% while on the CD and 65% while on the HFD by 16 wk of age (Fig. 3B). This observation was associated with a marked decrease in the levels of both leptin and adiponectin (leptin, 30.6±5.7 ng/ml Lox vs. 11.2±1.2 ng/ml KO; adiponectin, 16.1±1.1 μg/ml Lox vs. 2.7±0.4 KO at 24 wk of age on the CD diet; Table 1).
Figure 3.
TFAM deletion in adipose tissues decreases fat mass and triggers insulin resistance. A) Body weight of male control (Lox) and adipo-TFAM-KO (KO) mice on CD (left panel) or HFD (right panel). Data are means ± sem of 6–8 animals/group. *P < 0.05. B) Total fat mass was assessed by DEXA analysis of male Lox and KO mice on a CD or HFD at 24 wk of age. Data are means ± sem, normalized to Lox data (n=8/genotype). *P < 0.05. C) Intraperitoneal glucose test on male Lox and KO male on CD (left panel) or HFD (right panel) at 24 wk of age. *P < 0.05. Data are means ± sem of 6–8 samples/genotype. *P < 0.05. D) Insulin serum levels of male Lox and KO mice at 24 wk of age. Data are means ± sem of 6–8 samples/genotype. *P < 0.05.
Table 1.
Circulating factors in male control (Lox) and adipo-TFAM-KO (KO) mice at 24 wk of age
| Factor | Lox | KO | P |
|---|---|---|---|
| Glucose (mg/dl) | 179.2 ± 6.8 | 242.2 ± 8.7 | 9.5E-05 |
| Insulin (pg/ml) | 776.8 ± 266.3 | 4361.7 ± 961.2 | 6.8E-03 |
| IL6 (pg/ml) | 4.3 ± 0.6 | 8.6 ± 1.7 | 4.2E-02 |
| TNFα (pg/ml) | 6.9 ± 0.2 | 9 ± 1.8 | 4.1E-05 |
| Leptin (ng/ml) | 30.6 ± 5.7 | 11.1 ± 1.2 | 0.017 |
| Adiponectin (mg/ml) | 16.1 ± 1.1 | 2.7 ± 0.4 | 0.04 |
| FFA (mEq/ml) | 1.2 ± 0.1 | 1.4 ± 0.1 | 0.04 |
| TG (mg/dl) | 43.6 ± 2.1 | 53.1 ± 3.4 | 0.04 |
| PAI-1 (ng/ml) | 2.8 ± 0.9 | 4.1 ± 0.4 | 0.01 |
| AGTN (μg/ml) | 4.9 ± 0.2 | 4.1 ± 0.2 | 0.30 |
Data are means ± sem of 6 samples/genotype.
P < 0.05.
Reduction in circulating adiponectin level is usually associated with a reduction in insulin sensitivity (26). Indeed, adipo-TFAM-KO mice showed impaired glucose tolerance on both CD (Fig. 3C, left panel) and HFD (Fig. 3C, right panel) at 24 wk of age. In addition, fasting glucose levels and insulin levels were higher in the adipo-TFAM-KO mice than in the controls (Fig. 3C, D and Table 1), consistent with insulin resistance.
Adipo-TFAM-KO mice have decreased energy expenditure and develop hepatosteatosis
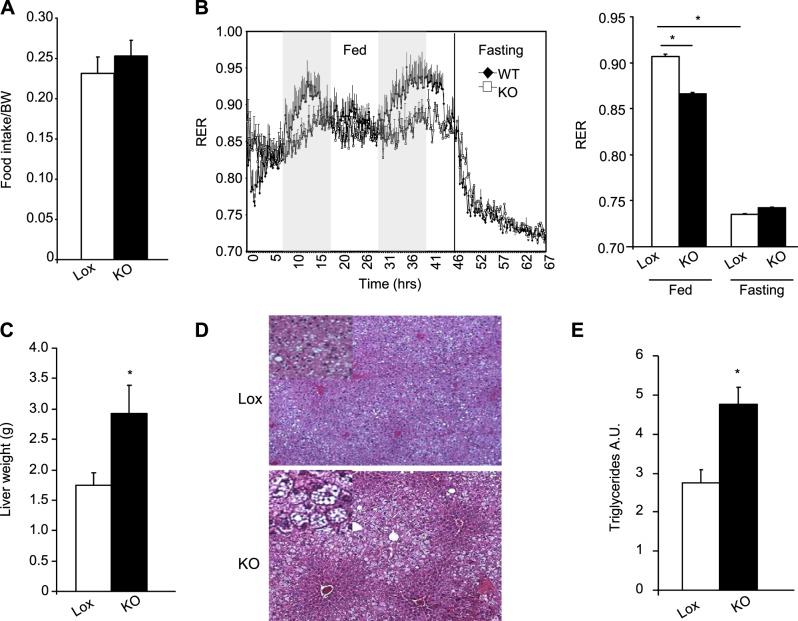
To better understand how adipose tissue–specific deletion of TFAM affects whole-body physiology, we subjected 24-wk-old Lox and KO mice fed the CD to indirect calorimetry analysis in CLAMS metabolic cages. Despite the reduction in serum leptin in the KO mice, the adipo-TFAM-KO mice had levels of food intake similar to those of the control mice when normalized to body weight (Fig. 4A). Indirect calorimetry revealed a slight decrease in oxygen consumption rate (4748±35 ml/kg/h Lox vs. 4148±34 ml/kg/h KO, data not shown) and a mild, but statistically significant, decrease in respiratory exchange ratio (RER) in the KO animals (0.91±0.002 Lox vs. 0.86±0.001 KO; P≤0.05) at night (i.e., when the animals were feeding, consistent with increased fat oxidation in the KO mice). This difference in RER between the 2 groups disappeared during food withdrawal (Fig. 3B). Both free fatty acid (FFA) and triglyceride levels were increased in the circulation of the adipo-TFAM-KO mice compared with those in the Lox mice, indicating a greater availability of these substrates for oxidation (Table 1). Also, consistent with the defect in fat storage, adipo-TFAM-KO mice developed severe hepatosteatosis, as shown by the doubling of liver weight (Fig. 4C) with gross lipid accumulation seen on routine histology (Fig. 4D) and increased triglyceride content in a biochemical assay (Fig. 4E).
Figure 4.
Adipo-TFAM-KO mice have decreased energy expenditure and develop hepatosteatosis. A) Food intake of male control (Lox) and adipo-TFAM-KO (KO) mice at 24 wk of age. Data are means ± sem of 6 samples/genotype. *P < 0.05. B) Left panel: fed and fasting RER values of male Lox and KO male mice at 24 wk of age. Right panel: quantification of data. Data are means ± sem of 6 samples/genotype.*P < 0.05. C) Liver weight of male Lox and KO mice at 24 wk of age. Data are means ± sem of 6–8 samples/genotype. *P < 0.05. D) Representative sections of livers from 24-wk-old male Lox and KO mice, stained with hematoxylin and eosin (×10). E) Triglyceride content of livers from 24-wk-old male Lox and KO mice, represented as arbitrary units (A.U.) per gram of liver weight vs. Lox. Data are means ± sem of 6–8 samples/genotype. *P < 0.05.
Fat TFAM deletion triggers adipocyte death and inflammation in WAT and whitening of the BAT
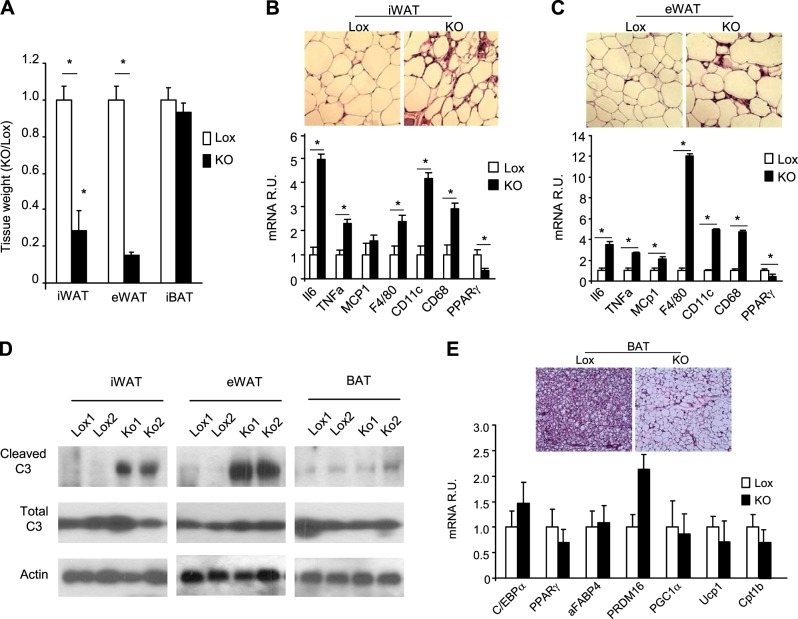
Consistent with the DEXA data, on euthanasia at 24 wk of age, the TFAM-KO mice had a marked decrease in WAT mass (87% reduction in eWAT and 71% reduction in iWAT), but no change in BAT mass. Interestingly, despite the marked reduction in WAT mass, histology revealed that adipocyte sizes in iWAT and eWAT were similar in the TFAM-KO and Lox controls (Fig. 5B, C, top panels, and Supplemental Fig. S1A). Histology also revealed an increased number of nonadipocyte cells and crownlike structures in both WAT depots, indicative of adipocyte death and tissue inflammation. Consistent with this, mRNA levels of inflammatory cytokines [IL6, TNF-α, and monocyte chemotactic protein 1 (MCP1)] and macrophage markers (F4/80, CD11c, and CD68) were increased in iWAT and eWAT of 24-wk-old adipo-TFAM KO (Fig. 5B, C, bottom panel). Peroxisome proliferator-activated receptor γ (PPARγ) expression, on the other hand, was reduced in the iWAT and eWAT of KO mice, most probably because of the invasion of nonadipocyte cells in the adipose tissue (Fig. 5B, C, bottom panel). Western blot analysis also revealed higher levels of cleaved caspase 3 in the iWAT and eWAT of the TFAM of KO mice, indicating a higher rate of apoptosis (Fig. 5D). Thus, the reduction in WAT mass in the TFAM-KO mice was due to increased apoptosis and a reduction in the number of adipocytes rather than in the size of the cells, and this change was associated with increased inflammation in the WAT.
Figure 5.
TFAM deletion leads to lipodystrophy, inflammation, and adipocyte death. A) Individual adipose tissue mass (iWAT, eWAT, and BAT) assessed on dissection of male control (Lox) and adipo-TFAM-KO (KO) mice at 24 wk of age. Data are means ± sem of 6 samples/genotype. *P < 0.05. B, C) Top panel: representative sections of iWAT (B) and eWAT (C) from 24-wk-old male Lox and KO mice, stained with hematoxylin and eosin (×20). Bottom panel: expression levels of the inflammation (IL6, MCP1, and TNFα) and macrophage (CD11c, CD68, and F4/80) markers and PPARγ mRNA were compared by qPCR of iWAT from 24-wk-old male Lox and KO mice. Data are means ± sem of 6 samples/genotype. *P < 0.05. D) Assessment of cleaved and total caspase 3 by Western blot analysis of 24-wk-old male Lox and KO mice. Data are means ± sem of 6 samples/genotype. *P < 0.05. E) Representative sections of BAT from 24-wk-old male Lox and KO mice, stained with hematoxylin and eosin.
In contrast to the WAT, BAT mass was similar in the TFAM-KO and control mice (Fig. 5A), and there was no up-regulation in cleaved caspase 3, according to Western blot analysis (Fig. 5D). Nonetheless, histological analysis revealed alterations in BAT with the presence of large, unilocular lipid droplets in the brown adipocytes of TFAM-KO mice, rather than the classic multilocular fat droplets seen in BAT of control animals (Fig. 5E, top panel). Despite this whitening of the BAT, the mRNA levels of the general adipocyte markers (C/EBPα, PPARγ, and aFABP4), mitochondrial markers (PGC1α and Cpt1b), and BAT markers (UCP1 and PRDM16) were similar or even increased in the adipo-TFAM-KO BAT compared to that of the control (Fig. 5E). These results suggest that large lipid droplets in the TFAM-KO brown adipocytes could be the result of various factors, such as impairment of mitochondrial ETC function, reduced fatty acid oxidation, and increased circulating fatty acids, rather than a conversion of brown to white adipocytes.
Adipo-TFAM-KO mice develop hypertension and cardiac dysfunction
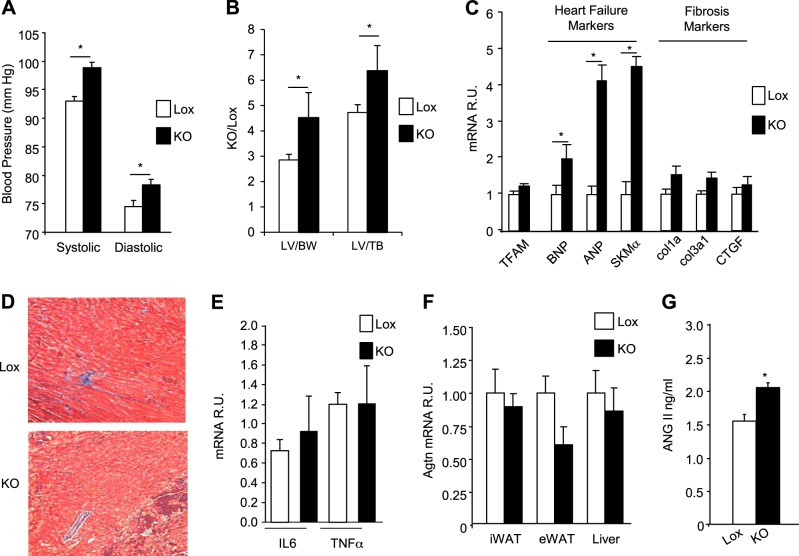
Patients with inherited or acquired lipodystrophies sometimes present with hypertension and cardiac abnormalities similar to hypertrophic cardiomyopathy (27–30). At 24 mo of age on a normal CD, the adipo-TFAM-KO mice exhibited significant increases in both systolic (98.7±0.9 vs. 93.2±0.8 mm Hg; P≤0.05) and diastolic (78.1±1 vs. 74.7±1.1 mm Hg; P≤0.05) blood pressure compared with that of the control animals (Fig. 6A). Furthermore, the adipo-TFAM-KO mice displayed LV hypertrophy, as indicated by increased LV weight when normalized to either body weight or tibial length (Fig. 6B). Echocardiograms revealed increased LVIDs and a consequent 16% reduction in FS% in the adipo-TFAM-KO mice compared with control Lox animals (Table 2), indicative of decreased systolic function.
Figure 6.
TFAM ablation in adipose tissue triggered hypertension and cardiac dysfunction. A) Systolic and diastolic blood pressure of male control (Lox) and adipo-TFAM-KO (KO) male mice at 24 wk of age. B) LV heart weight of male Lox and KO mice at 24 wk of age, normalized either to body weight (BW; mg/g) or tibialis (TB) length (mg/ml). Data are means ± sem of 6–8 samples/genotype. *P < 0.05. C) Expression levels of TFAM, fibrosis marker (Col1a, col3a1, and CTGF), and heart failure marker (BNP, ANP, and SKMα) mRNAs were compared by using qPCR in heart tissue of male Lox and KO mice at 24 wk of age. Data are means ± sem of 6 samples/genotype. *P < 0.05. D) Representative sections of heart tissue from 24-wk-old male Lox and KO mice, stained with Masson's trichrome. E) Expression levels of IL6 and TNF-α mRNA in heart of male Lox and KO mice at 24 wk of age, compared by qPCR. Data are means ± sem of 6 samples/genotype. *P < 0.05. F) AGTN mRNA level, assessed by qPCR, in iWAT, eWAT, and liver of male Lox and KO mice at 24 wk of age. Data are means ± sem of n = 6/genotype. *P < 0.05. G) Circulating level of Ang II in male Lox and KO mice at 24 wk of age. Data are means ± sem of n = 10/genotype. *P < 0.05.
Table 2.
Echocardiography of male control (Lox) and adipo-TFAM-KO (KO) mice at 24 wk of age
| Genotype | IVSd | LVIDd | LVPWd | IVSs | LVIDs | LVPWs | FS% | RWT | HR |
|---|---|---|---|---|---|---|---|---|---|
| Lox | 1.06 ± 0.04 | 3.05 ± 0.08 | 0.99 ± 0.04 | 1.67 ± 0.04 | 1.61 ± 0.08 | 1.53 ± 0.04 | 47.60 ± 1.42 | 0.68 ± 0.02 | 657.69 ± 12.15 |
| KO | 1.02 ± 0.04 | 3.19 ± 0.08 | 0.98 ± 0.04 | 1.66 ± 0.05 | 1.86* ± 0.07 | 1.49 ± 0.04 | 41.28* ± 1.22 | 0.63 ± 0.03 | 638.95 ± 17.17 |
Data are means ± se of 7–9 mice/genotype.
P < 0.05.
The contractile dysfunction observed in the adipo-TFAM-KO mice was associated with increased expression of the markers of heart failure, including BNP, ANP, and skeletal muscle-α (SKMα) actin, confirming pathological cardiac remodeling. However, there was no evidence of fibrosis, as indicated by normal expression levels of collagens Col1a1 and Col3a1 and CTGF (Fig. 6C) and the absence of fibrosis detected by Masson's trichrome staining (Fig. 6D). Likewise, there was no evidence of inflammation on histology, and expression levels of IL6 and TNFα were normal (Fig. 6E). TFAM mRNA levels were also similar in Lox and KO mice (Fig. 6C), confirming that the cardiac phenotype in adipo-TFAM-KO heart was not due to an off-target recombination of TFAM in the myocardium. Taken together, these data suggest that the cardiac hypertrophy and contractile dysfunction are secondary to the hypertension present in these mice, or reflect the cardiotoxic effects of circulating factors altered by the lipodystrophy, or both.
There are several potential links between the adipose tissue and control of blood pressure. For example, the renin–angiotensin system (RAS) is a major regulator of blood pressure and may also contribute to heart failure (31). In addition, adipose tissue is a source of AGTN, and PPARγ activators have been shown to repress the vascular RAS and Ang II receptor 1 expression (32). qPCR analysis revealed similar levels of Agtn expression in liver, iWAT, and eWAT of the control and KO mice (Fig. 6F). Consistent with this, circulating levels of AGTN were similar between controls and adipo-TFAM-KO mice (4.9 ±0.2 μg/ml Lox vs. 4.1±0.2 μg/ml KO, P=ns; Table 1). In contrast, circulating levels of Ang II were higher in the adipo-TFAM-KO mice than in the control animals by 41% (Fig. 6G), suggesting that increased conversion of AGTN to Ang II is a contributing factor to the hypertension observed in the adipo-TFAM-KO mice. Changes in cardiac SERCA2 (SLC8A1) and NCX1 have been described as potential mediators of Ang II-induced contractile dysfunction (33, 34), but no change in mRNA or protein levels was found for these proteins in the hearts of the adipo-TFAM-KO mice (Supplemental Fig. S1). Finally, circulating levels of adiponectin and PAI-1 and PAI-2 hormones, secreted by adipose tissue and known to protect cardiac function, were drastically down-regulated in the adipo-TFAM-KO mice (Table 1), suggesting that multiple hormones may contribute to cardiac dysfunction in these animals.
DISCUSSION
Mitochondrial dysfunction is a feature of normal aging and age-related diseases, including obesity, type 2 diabetes, and metabolic syndrome (35). It is also essential for normal tissue metabolism, and mitochondrial dysfunction has been shown to alter adipocyte differentiation and function (8, 10, 11). We have studied the role of mitochondria in adipose tissue by creating a mouse with a adipose-specific reduction of TFAM, the key mitochondrial transcription factor, in adipocytes by using TFAMlox/lox mice and mice carrying an aP2-Cre transgene (18). This approach, however, produced only a moderate reduction in TFAM in subcutaneous iWAT and BAT and failed to induce recombination in intra-abdominal (perigonadal) adipocytes, thus limiting the potential effect of the recombination on whole-body metabolism. These aP2-Cre TFAM-KO mice also demonstrated a defect in linear growth, suggesting some off-target recombination in other tissues, a feature known to occur with aP2 promoter transgenes (19). Therefore, to investigate the effects of mitochondrial dysfunction on adipose tissue biology, metabolism, and cardiovascular disease more specifically and precisely, we generated a mouse with deletion of TFAM in adipose tissue by using an adiponectin promoter-Cre, a transgene that we have shown to be highly effective and specific to adipose tissue (16).
The resulting adipo-TFAM-KO mice showed major reductions in TFAM mRNA and protein in subcutaneous and intra-abdominal WAT depots, as well as in BAT. These decreases were accompanied by parallel decreases in expression of many of the transcriptional targets of TFAM, including multiple proteins involved in the ETC. These changes were also associated with marked reductions at the protein level of several nucleus-encoded mitochondrial genes, probably as a result of changes in the stability of the mitochondrial complex. This change causes severe reductions in the activity of ETC complexes I, III, and IV.
Note that, even with similar decreases in TFAM level, the various adipose tissue depots demonstrated different responses to the reduction in TFAM. For instance, C-III-core 2 protein was decreased in eWAT, but not in iWAT or BAT, whereas C-V-α protein was only slightly reduced in iWAT and eWAT, but was completely absent in BAT of the TFAM KO mice. It is known that the mitochondrial protein repertoire shows tissue differences, even among adipose depots (36). Mitochondria from white adipocytes are less numerous, but contain a more diverse protein repertoire than mitochondria from most other tissues (36). Thus, the mitochondrial capacity to remodel their ETC components in response to TFAM deletion may be different from that of other tissues.
These alterations in mitochondrial ETC protein and activity in the adipo-TFAM-KO mice ultimately led to adipocyte death and adipose tissue degeneration. Hence, although the body weights of the adipo-TFAM-KO and control mice were similar until 12 wk of age, with further aging, the control mice continued to gain weight, whereas the adipo-TFAM-KO mice remained lean. Histological examination of adipose tissues revealed that those of the TFAM-KO mice were smaller because of a reduction in the number of adipocytes rather than in size. In addition, the fat pads of adipo-TFAM-KO mice show increased apoptosis, as evidenced by increased levels of cleaved caspase 3 and by macrophage infiltration, both histologically and by the presence of increased macrophage markers. Interestingly, TFAM KO led to significant adipocyte death in the white, but not in the brown, fat depot, again indicating adipose depot–specific responses to reduction in TFAM levels. This perturbation of WAT homeostasis triggered inflammation in WAT. TFAM KO mice also developed systemic inflammation with higher circulating levels of TNFα and IL6.
The adipo-TFAM-KO phenotype is thus strikingly different from the aP2-TFAM-KO mouse model due to TFAM KO efficiency and adipose tissue mitochondrial adaptation. In aP2-TFAM-KO mice, TFAM expression was decreased moderately in subcutaneous adipose tissue and BAT, but not in visceral adipose tissue. As a result, complex I and IV activity was downregulated, whereas complex II was upregulated, provoking a shift in substrate utilization and an increase in energy utilization. At a young age, whole-body energy expenditure of the aP2-TFAM-KO mice increased, and thus they were protected from body weight gain and from age- and diet-induced insulin resistance. In adipo-TFAM-KO mice, TFAM expression was drastically reduced in all adipose (subcutaneous, visceral, and brown) tissues, and activities of all the ETC complexes were significantly down-regulated. We hypothesized that this mitochondrial dysfunction plays a major role in adipocyte death in visceral and subcutaneous adipose tissue, impairing one of the tissue's main functions, lipid storage. The adipo-TFAM-KO mice share these features with other lipodystrophic models created by blocking the adipocyte differentiation process, such as A-ZIP/F-1 mice, nSREBP-1c mice, and PPARγ hyp/hyp mice (37–39). As a direct consequence of the lack of lipid storage capacity in these lipoatrophic mice, high levels of circulating fatty acids are found, leading to significant ectopic lipid accumulation (hepatosteatosis) and insulin resistance. These animal models also resemble human lipodystrophic syndromes, including genetic forms and the HIV lipodystrophy syndrome. Interestingly, antiretroviral therapy has also been shown to reduce mtDNA in WAT, which may contribute to the lipodystrophy in these patients (37). A reduction in mtDNA with moderate lipoatrophy and a decrease in the number of circulating adipokines has also been reported in thymidine kinase 2 (TK2)-deficient mice (38).
Another interesting feature of the adipo-TFAM-KO mice is the development of hypertension associated with cardiac hypertrophy and reduced cardiac contractility in the absence of fibrosis or inflammation. Similar features are present in some patients with inherited or acquired lipodystrophies (28). Lipoatrophic A-ZIP/F-1 mice develop arterial hypertension (39), but have not been reported to develop cardiac hypertrophy. In the adipo-TFAM KO mice, the hypertension was associated with increased circulating levels of Ang II, a positive modulator of blood pressure with adverse effects on cardiac contractility (40), whereas levels of adiponectin, a cardioprotective hormone secreted by adipose tissue (41), were reduced. It seems likely that the combination of these alterations culminates in the cardiac phenotypes observed.
In summary, a loss of TFAM in adipose tissue results in mitochondrial dysfunction in fat, leading to lipoatrophy, whole-body insulin resistance, hepatosteatosis, hypertension, and cardiac dysfunction. These findings demonstrate the important crosstalk between adipose tissue mitochondrial dysfunction and whole-body metabolism and cardiovascular abnormalities.
Supplementary Material
Acknowledgments
This work was supported by U.S. National Institutes of Health (NIH) grant R01-DK082659 (C.R.K.), a grant from the Eli Lilly Foundation, Joslin Diabetes Research Center (NIH grant P30-DK036836), the Mary K. Iacocca Professorship, and NIH grant R01-HL094677 (A.R.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- adipo-TFAM-KO
- adiponectin mitochondrial transcription factor A–knockout
- AGTN
- angiotensinogen
- Ang II
- angiotensin II
- ANP
- atrial natriuretic peptide
- BAT
- brown adipose tissue
- BNP
- brain natriuretic peptide
- CD
- chow diet
- CLAMS
- comprehensive lab animal monitoring system
- Col1a1
- collagen type 1a1
- Col3a1
- collagen type 3a1
- CR
- caloric restriction
- CTGF
- connective tissue growth factor
- DEXA
- dual-energy X-ray absorptiometry
- ELISA
- enzyme-linked immunosorbent assay
- ETC
- electron transport chain
- eWAT
- epididymal white adipose tissue
- FS%
- fractional shortening percentage
- HFD
- high-fat diet
- HRP
- horseradish peroxidase
- IL6
- interleukin 6
- IVSd
- interventricular septal thickness at end-diastole
- IVSs
- interventricular septal thickness at end-systole
- iWAT
- inguinal white adipose tissue
- KO
- knockout
- LV
- left ventricle
- LVIDd
- left ventricle internal dimension at diastole
- LVIDs
- left ventricle internal dimension at systole
- LVPWd
- left ventricle posterior wall at diastole
- LVPWs
- left ventricle posterior wall at systole
- MCP1
- monocyte chemotactic protein-1
- mtDNA
- mitochondrial DNA
- NCX1
- Na+/Ca2+-exchange protein 1
- oxphos
- oxidative phosphorylation
- PPARγ
- peroxisome proliferator-activated receptor γ
- RAS
- renin–angiotensin system
- RER
- respiratory exchange ratio
- RWT
- relative wall thickness
- SKMα
- skeletal muscle α
- TFAM
- mitochondrial transcription factor A
- TNFα
- tumor necrosis factor α
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue
REFERENCES
- 1. Garg A., Misra A. (2004) Lipodystrophies: rare disorders causing metabolic syndrome. Endocrinol. Metab. Clin. North Am. 33, 305–331 [DOI] [PubMed] [Google Scholar]
- 2. Haslam D. W., James W. P. (2005) Obesity. Lancet 366, 1197–1209 [DOI] [PubMed] [Google Scholar]
- 3. De Pauw A., Tejerina S., Raes M., Keijer J., Arnould T. (2009) Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations. Am. J. Pathol. 175, 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaaman M., Sparks L. M., van Harmelen V., Smith S. R., Sjolin E., Dahlman I., Arner P. (2007) Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 50, 2526–2533 [DOI] [PubMed] [Google Scholar]
- 5. Giralt M., Domingo P., Villarroya F. (2011) Adipose tissue biology and HIV-infection. Best Pract. Res. Clin. Endocrinol. Metab. 25, 487–499 [DOI] [PubMed] [Google Scholar]
- 6. Caron M., Auclair M., Lagathu C., Lombes A., Walker U. A., Kornprobst M., Capeau J. (2004) The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS 18, 2127–2136 [DOI] [PubMed] [Google Scholar]
- 7. Ricquier D. (2005) Respiration uncoupling and metabolism in the control of energy expenditure. Proc. Nutr. Soc. 64, 47–52 [DOI] [PubMed] [Google Scholar]
- 8. Villarroya J., Giralt M., Villarroya F. (2009) Mitochondrial DNA: an up-and-coming actor in white adipose tissue pathophysiology. Obesity 17, 1814–1820 [DOI] [PubMed] [Google Scholar]
- 9. Koh E. H., Park J. Y., Park H. S., Jeon M. J., Ryu J. W., Kim M., Kim S. Y., Kim M. S., Kim S. W., Park I. S., Youn J. H., Lee K. U. (2007) Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes 56, 2973–2981 [DOI] [PubMed] [Google Scholar]
- 10. Kusminski C. M., Scherer P. E. (2012) Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol. Metab. 23, 435–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patti M. E., Corvera S. (2010) The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 31, 364–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Larsson N. G., Wang J., Wilhelmsson H., Oldfors A., Rustin P., Lewandoski M., Barsh G. S., Clayton D. A. (1998) Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 [DOI] [PubMed] [Google Scholar]
- 13. Gaspari M., Larsson N. G., Gustafsson C. M. (2004) The transcription machinery in mammalian mitochondria. Biochim. Biophys. Acta 1659, 148–152 [DOI] [PubMed] [Google Scholar]
- 14. Campbell C. T., Kolesar J. E., Kaufman B. A. (2012) Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta 1819, 921–929 [DOI] [PubMed] [Google Scholar]
- 15. Asin-Cayuela J., Gustafsson C. M. (2007) Mitochondrial transcription and its regulation in mammalian cells. Trends Biochem. Sci. 32, 111–117 [DOI] [PubMed] [Google Scholar]
- 16. Lee K. Y., Russell S. J., Ussar S., Boucher J., Vernochet C., Mori M. A., Smyth G., Rourk M., Cederquist C., Rosen E. D., Kahn B. B., Kahn C. R. (2013) Lessons on conditional gene targeting in mouse adipose tissue. Diabetes 62, 864–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mori M. A., Raghavan P., Thomou T., Boucher J., Robida-Stubbs S., Macotela Y., Russell S. J., Kirkland J. L., Blackwell T. K., Kahn C. R. (2012) Role of microRNA processing in adipose tissue in stress defense and longevity. Cell Metab. 16, 336–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vernochet C., Mourier A., Bezy O., Macotela Y., Boucher J., Rardin M. J., An D., Lee K. Y., Ilkayeva O. R., Zingaretti C. M., Emanuelli B., Smyth G., Cinti S., Newgard C. B., Gibson B. W., Larsson N. G., Kahn C. R. (2012) Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metab. 16, 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scherrer-Crosbie M., Kurtz B. (2010) Ventricular remodeling and function: insights using murine echocardiography. J. Mol. Cell. Cardiol. 48, 512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rottman J. N., Ni G., Brown M. (2007) Echocardiographic evaluation of ventricular function in mice. Echocardiography 24, 83–89 [DOI] [PubMed] [Google Scholar]
- 21. Kang D., Kim S. H., Hamasaki N. (2007) Mitochondrial transcription factor A (TFAM): roles in maintenance of mtDNA and cellular functions. Mitochondrion 7, 39–44 [DOI] [PubMed] [Google Scholar]
- 22. Fontana L., Partridge L., Longo V. D. (2010) Extending healthy life span–from yeast to humans. Science 328, 321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahima R. S. (2006) Adipose tissue as an endocrine organ. Obesity 14(Suppl 5), 242S–249S [DOI] [PubMed] [Google Scholar]
- 24. Farmer S. R. (2008) Molecular determinants of brown adipocyte formation and function. Genes Dev. 22, 1269–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dela F., Helge J. W. (2013) Insulin resistance and mitochondrial function in skeletal muscle. Int. J. Biochem. Cell Biol. 45, 11–15 [DOI] [PubMed] [Google Scholar]
- 26. Ye R., Scherer P. E. (2013) Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2, 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nelson M. D., Victor R. G., Szczepaniak E. W., Simha V., Garg A., Szczepaniak L. S. (2013) Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am. J. Cardiol. 112, 1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lupsa B. C., Sachdev V., Lungu A. O., Rosing D. R., Gorden P. (2010) Cardiomyopathy in congenital and acquired generalized lipodystrophy: a clinical assessment. Medicine 89, 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Onen N. F., Overton E. T., Seyfried W., Stumm E. R., Snell M., Mondy K., Tebas P. (2010) Aging and HIV infection: a comparison between older HIV-infected persons and the general population. HIV Clinical Trials 11, 100–109 [DOI] [PubMed] [Google Scholar]
- 30. Ho J. E., Hsue P. Y. (2009) Cardiovascular manifestations of HIV infection. Heart 95, 1193–1202 [DOI] [PubMed] [Google Scholar]
- 31. Yvan-Charvet L., Quignard-Boulange A. (2011) Role of adipose tissue renin-angiotensin system in metabolic and inflammatory diseases associated with obesity. Kidney Int. 79, 162–168 [DOI] [PubMed] [Google Scholar]
- 32. Herichova I., Szantoova K. (2013) Renin-angiotensin system: upgrade of recent knowledge and perspectives. Endocr. Regul. 47, 39–52 [DOI] [PubMed] [Google Scholar]
- 33. Rivard K., Grandy S. A., Douillette A., Paradis P., Nemer M., Allen B. G., Fiset C. (2011) Overexpression of type 1 angiotensin II receptors impairs excitation-contraction coupling in the mouse heart. Am. J. Physiol. Heart Circ. Physiol. 301, H2018–H2027 [DOI] [PubMed] [Google Scholar]
- 34. Gusev K., Domenighetti A. A., Delbridge L. M., Pedrazzini T., Niggli E., Egger M. (2009) Angiotensin II-mediated adaptive and maladaptive remodeling of cardiomyocyte excitation-contraction coupling. Circ. Res. 105, 4250. [DOI] [PubMed] [Google Scholar]
- 35. Romano A. D., Greco E., Vendemiale G., Serviddio G. (2014) Bioenergetics and mitocondrial dysfunction in aging: recent insights for a therapeutical approach. Curr. Pharm. Des. 20, 2978–2992 [DOI] [PubMed] [Google Scholar]
- 36. Pagliarini D. J., Calvo S. E., Chang B., Sheth S. A., Vafai S. B., Ong S. E., Walford G. A., Sugiana C., Boneh A., Chen W. K., Hill D. E., Vidal M., Evans J. G., Thorburn D. R., Carr S. A., Mootha V. K. (2008) A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Apostolova N., Blas-Garcia A., Esplugues J. V. (2011) Mitochondrial interference by anti-HIV drugs: mechanisms beyond Pol-gamma inhibition. Trends Pharmacol. Sci. 32, 715–725 [DOI] [PubMed] [Google Scholar]
- 38. Villarroya J., Dorado B., Vila M. R., Garcia-Arumi E., Domingo P., Giralt M., Hirano M., Villarroya F. (2011) Thymidine kinase 2 deficiency-induced mitochondrial DNA depletion causes abnormal development of adipose tissues and adipokine levels in mice. PloS One 6, e29691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lamounier-Zepter V., Bornstein S. R., Kunes J., Zicha J., Krsek M., Ehrhart-Bornstein M., Ziegler C. G., Kiessling A., Funk R. H., Haluzik M. (2008) Adrenocortical changes and arterial hypertension in lipoatrophic A-ZIP/F-1 mice. Mol. Cell. Endocrinol. 280, 39–46 [DOI] [PubMed] [Google Scholar]
- 40. Massiera F., Bloch-Faure M., Ceiler D., Murakami K., Fukamizu A., Gasc J. M., Quignard-Boulange A., Negrel R., Ailhaud G., Seydoux J., Meneton P., Teboul M. (2001) Adipose angiotensinogen is involved in adipose tissue growth and blood pressure regulation. FASEB J. 15, 2727–2729 [DOI] [PubMed] [Google Scholar]
- 41. Shibata R., Murohara T., Ouchi N. (2012) Protective role of adiponectin in cardiovascular disease. Curr. Med. Chem. 19, 5459–5466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.