Abstract
The systemic vasculitides are multifocal diseases characterized by the presence of blood vessel inflammation in multiple organ systems. Their clinical presentation is variable extending from self-limited illness to critical complications including diffuse alveolar hemorrhage and glomerulonephritis. Alveolar hemorrhage is a life-threatening manifestation of pulmonary vasculitis that can rapidly progress into acute respiratory failure requiring ventilatory support. We present the case of a 74-year-old patient admitted to the Intensive Care Unit with severe hypoxic respiratory failure and diffuse alveolar infiltrates in chest imaging that was later diagnosed as antineutrophil cytoplasmic antibodies-associated vasculitis. The report highlights the importance of differentiate between alveolar hemorrhage and acute respiratory distress syndrome of other etiology because alveolar hemorrhage is reversible with prompt initiation of treatment.
Key words: Respiratory failure, vasculitis, antineutrophil cytoplasmic antibodies, alveolar infiltrates
Introduction
Vasculitides are disorders of inflammation and necrosis of the blood vessel wall.1 They can affect multiple organ systems with frequent respiratory and renal involvement. Their clinical manifestations are diverse, often mimicking the symptoms of much more common disorders. Thus, vasculitides remain an important diagnostic challenge to the clinicians especially in critical care setting.2 Early recognition and treatment is important as the presence of advanced disease at diagnosis limits the potential benefit of therapy. We report the case of a 74-year-old woman who was admitted in the Intensive Care Unit (ICU) with severe respiratory dysfunction and diffuse alveolar infiltrates in chest imaging and was finally diagnosed with antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis.
Case Report
A 74-year-old woman presented to the emergency department with a 2-month history of dyspnea on exertion that had become significantly worse during the last 48 h, with associated dry cough and chest pain. During the previous 12 months she was complaining of myalgias, malaise and anorexia. At that time, she was diagnosed with polymyalgia rheumatica and placed on low dose prednisolone (5 mg/day).
On examination she was tachypneic with central cyanosis and use of the inspiratory accessory muscles. Bilateral inspiratory crackles were noted on chest auscultation. Blood gas analysis on room air revealed severe hypoxemia with a pH of 7.48, partial pressure of oxygen 49 mmHg and partial pressure of carbon dioxide 31 mmHg with an oxygen saturation of 83%. Initial laboratory investigations revealed anemia with a hemoglobin level of 10.9 g/dL, leukocytosis (white blood cell count 11.5x109/L, 82% neutrophils) and a normal platelet count (213×109/L). The inflammatory marker levels were elevated; erythrocyte sedimentation rate at 80 mm/h and C-reactive protein at 3710 mg/dL. Kidney function testing was impaired with serum creatinine of 1.73 mg/dL. Chest x-ray demonstrated diffuse bilateral patchy opacities (Figure 1A) while sinus radiographs were normal.
Figure 1.
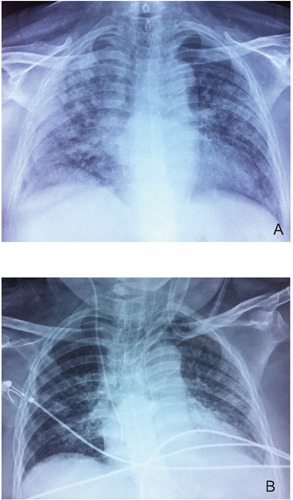
A) Chest x-ray on admission showing diffuse bilateral patchy opacities. B) Resolution of infiltrates after 2 weeks of treatment.
The patient was admitted in the Pulmonary Department with the diagnosis of severe lower respiratory tract infection and was commenced empirically on intravenous ceftriaxone and azithromycin. Due to worsening respiratory insufficiency, she was supported with noninvasive ventilation (BiPAP S/T, Philips Healthcare, Amsterdam, the Netherlands; inspiratory positive pressure 20 cm H2O, expiratory pressure 8 cm H2O). High-resolution computed tomography showed extensive ground-glass opacities and consolidation in right upper lobe with relative subpleural sparing (Figure 2) whereas abdomen computed tomography showed no pathological findings.
Figure 2.

A) and B) High-resolution computed tomography demonstrating extensive ground-glass opacities and consolidation in right upper lobe with relative subpleural sparing.
During the first days of hospitalization, the patient’s clinical condition deteriorated. On the fourth day, she was transferred to the ICU with rapidly worsening respiratory distress and commencing circulatory failure. On admission Acute Physiology and Chronic Health Evaluation II (APACHE II) score was calculated at 24. She was intubated and mechanical ventilation was initiated [pressure control ventilation with a pressure of 22 cm H2O, positive end-expiratory pressure 8 and PaO2/FiO2 125 on day one, fulfilling the criteria for acute respiratory distress syndrome (ARDS)] along with cardiovascular support with fluids and vasopressors. Laboratory tests in ICU demonstrated a significant decline of hemoglobin concentration (hemoglobin level at 7.9 g/dL) with marked leukocytosis (white blood cell count 18.5x109/L, 92% neutrophils) and worsening renal function (creatinine at 2.06 mg/dL). Urinalysis detected active urine sediment with dysmorphic red blood cells and red blood casts. A transthoracic echocardiogram performed on admission in the ICU was negative for valvular vegetations; left ventricular systolic function was normal.
After clinical stabilization the patient proceeded to fiberoptic bronchoscopy where no endobronchial lesions were detected. Bronchoalveolar lavage fluid was found hemorrhagic. Standard bacterial and mycobacterial cultures of lavage fluid were negative while no cytological evidence of malignancy was detected. Meanwhile the patient presented blood in the stool and colonoscopy was performed; gut biopsy showed mild edema; histopathologic changes of chronic colitis were found in the biopsy specimen. ANCA were positive for proteinase 3 (PR3)-cANCA with a titer of 1/80. Testing for antiglomerular basement membrane, antinuclear antibodies and extractable nuclear antigens were all negative. Serology for hepatitis B and C virus was negative. Rheumatoid factor was 10 IU/mL (<20) while complement levels were found within normal range. On the basis of ANCA positivity, the presence active urine sediment, together with acute renal failure, fecal blood and diffuse hemorrhagic alveolar infiltrates, the patient was diagnosed with ANCA-associated vasculitis presenting as pulmonary-renal syndrome.
The patient was managed aggressively with high-dose steroids (1 g intravenous methylprednisolone 1 g daily for 3 days followed by prednisone 1 mg/kg/day) in combination with intravenous pulses of cyclophosphamide (repeated at 2-week interval) and ten consecutive regimens of plasmapheresis. Over two-week treatment period, the patient developed remarkable clinical stabilization with improvement of arterial blood gases. Chest x-ray showed complete resolution of ARDS (Figure 1B). She was gradually weaned from mechanical ventilation and referred to the rehabilitation unit within 40 days after presentation. Unfortunately, she died of sepsis caused by nosocomial pneumonia before hospital discharge.
Discussion
Systemic vasculitides are characterized by histological evidence of blood vessel inflammation. They can occur in association with an underlying disease or exposure or as part of a primary vasculitides, which are entities of unknown cause.3 ANCA-associated vasculitis is necrotizing vasculitis, with few or no immune deposits, predominantly affecting small vessels and is characterized by the presence of circulating ANCA specific for myeloperoxidase or PR3-ANCA.4 The clinical presentation of vasculitides is highly variable extending from self-limited illness to critical or even life-threatening complications, including diffuse alveolar hemorrhage and glomerulonephritis. Presenting features may mimic those of polymyalgia rheumatica, a common disease of the elderly. In a single center study one in eight patients diagnosed with glomerulonephritis due to small vessel vasculitis had been treated for polymyalgia rheumatica for an average of one year prior to diagnosis.5
Diffuse alveolar hemorrhage, characterized by the widespread extravasation of red blood cells into the pulmonary alveolar spaces, represents the most common clinical manifestation of pulmonary vasculitis.6 Hemoptysis is regarded as the guiding clinical symptom of the syndrome. Even though the majority of patients experience a variable degree of hemoptysis, approximately one third of all patients with diffuse alveolar hemorrhage are lacking this symptom.7,8 As a result, the diagnosis of pulmonary vasculitis in the ICU may be delayed or be completely unrecognized. In a series of 37 patients with the syndrome requiring ICU support, diffuse alveolar hemorrhage was an unexpected diagnosis in most patients and the diagnosis had not been considered before bronchoalveolar lavage analysis.9 It is therefore essential that vasculitis is included in the differential diagnosis of unexplained, persistent pulmonary infiltrates. Both bronchoalveolar lavage fluid and iron stain are mandatory diagnostic means.
Since the introduction of combination immunosuppressive therapy, consisting of corticosteroids and cyclophosphamide, the prognosis of patients with vasculitis has been improved. While potentially lifesaving, the aforementioned treatments are not without significant risk. Notably, the prognosis of vasculitis patients in the intensive care setting remains poor.2,10,11 For example, for patients admitted to the ICU with suspected pulmonary vasculitis, a mortality of 25-50% has been reported.12 One of the main reasons for this finding might be the enhanced susceptibility to infections when under treatment in the ICU with high doses of immunosuppressive agents. Risk factors that have been associated with an unfavorable outcome of critical patients with connective tissue disease are higher APACHE II scores at ICU admission, longer stay in the ICU (>10 days)13 and the presence of respiratory failure.14 Unfortunately, all of the aforementioned factors were present in our patient and may account for her final adverse outcome, despite the initial prompt response to therapy.
Conclusions
Life-threatening acute respiratory failure requiring ventilatory support can be the first manifestation of pulmonary vasculitis. Alveolar hemorrhage should be considered in differential diagnosis of acute respiratory distress syndrome. Early diagnosis and prompt initiation of treatment is vital to prevent irreversible critical organ injury. However, clinicians should take into account the significant toxicities of immunosuppressive treatment on the ICU.
References
- 1.Lapraik C, Watts R, Bacon P, et al. BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology (Oxford) 2007;46:1615-6 [DOI] [PubMed] [Google Scholar]
- 2.Wilfong EM, Seo P. Vasculitis in the intensive care unit. Best Pract Res Clin Rheumatol 2013;27:95-106 [DOI] [PubMed] [Google Scholar]
- 3.Langford CA. Vasculitis. J Allergy Clin Immunol 2010;125:S216-25 [DOI] [PubMed] [Google Scholar]
- 4.Jennette JC, Falk RJ, Bacon PA, et al. 2012 revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum 2013;65:1-11 [DOI] [PubMed] [Google Scholar]
- 5.Little MA, Nazar L, Farrington K. Polymyalgia rheumatica preceding small-vessel vasculitis: changed spots or misdiagnosis? QJM 2004;97:289-92 [DOI] [PubMed] [Google Scholar]
- 6.Krause ML, Cartin-Ceba R, Specks U, Peikert T. Update on diffuse alveolar hemorrhage and pulmonary vasculitis. Immunol Allergy Clin North Am 2012;32: 587-600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Prost N, Parrot A, Picard C, et al. Diffuse alveolar haemorrhage: Factors associated with inhospital and long-term mortality. Eur Respir J 2010;35:1303-11 [DOI] [PubMed] [Google Scholar]
- 8.Diaz J, Calamia KT, Lee AS. Pulmonary vasculitis in the intensive care unit. J Intensive Care Med 2011;26:88-104 [DOI] [PubMed] [Google Scholar]
- 9.Rabe C, Appenrodt B, Hoff C, et al. Severe respiratory failure due to diffuse alveolar hemorrhage: clinical characteristics and outcome of intensive care. J Crit Care 2010;25:230-5 [DOI] [PubMed] [Google Scholar]
- 10.Cruz BA, Ramanoelina J, Mahr A, et al. Prognosis and outcome of 26 patients with systemic necrotizing vasculitis admitted to the intensive care unit. Rheumatology (Oxford) 2003;42:1183-8 [DOI] [PubMed] [Google Scholar]
- 11.Semple D, Keogh J, Forni L, Venn R. Clinical review: Vasculitis on the intensive care unit-part 1: diagnosis. Crit Care 2005;9:92-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith M, Brett S. The pulmonary physician in critical care illustrative case 3: pulmonary vasculitis. Thorax 2003;58:543-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkhardt O, Köhnlein T, Wrenger E, et al. Predicting outcome and survival in patients with Wegener’s granulomatosis treated on the intensive care unit. Scand J Rheumatol 2007;36:119-24 [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Yim JJ, Yang SC, et al. Outcome of patients with connective tissue disease requiring intensive care for respiratory failure. Rheumatol Int 2012;32:3353-8 [DOI] [PubMed] [Google Scholar]


