Abstract
Background.
The ability to dynamically control fingertip force vector magnitude and direction is critical for dexterous manipulation. We quantified the dynamic control of fingertip forces to examine how dexterous manipulation declines with age.
Methods.
The strength–dexterity (SD) test measures fingertip forces during compression of a slender spring prone to instability and buckling. The greatest sustained compression (designed to be under 3 N), and force dynamics therein, have been shown to be simple and quick measures of dynamic dexterous manipulation ability. We measured pinch strength and strength–dexterity test in a cross-sectional population of 98 people from 18 to 89 years of age.
Results.
Dexterous manipulation ability is poorer at older ages, beginning in middle age (p < .001), with greater decline past 65 years of age. Fingertip force dynamics during spring compression and stabilization show a deterioration of neuromuscular control with age. Importantly, this novel detection of decline in dynamic manipulation ability is not correlated with, and thus cannot be entirely explained by, the known decline in pinch strength. We also measured standardized tests of dexterity in participants older than 45, and discuss how the strength–dexterity test uniquely captures features of sensorimotor capabilities for dexterous manipulation in this adult population.
Conclusions.
Starting in middle age, changes in the functional interactions among sensory, motor, and neural capabilities result in measurably poorer dynamic dexterous manipulation. This deterioration of neuromuscular control motivates and enables future studies to understand the physiological bases for this functional decline so critical to activities of daily living and quality of life.
Key Words: Dexterous manipulation, Aging, Middle age, Pinch strength, Dynamical analysis.
The worsening of fine motor skills for manipulation, whereas not a health risk in the elderly people like loss of strength (1) and risk of falls (2), is nevertheless a critical and little understood contributor to the decline of quality of life, social participation, and work capabilities (3). Pick-and-place standardized tests of dexterity (4–6) reveal behavioral impairments in upper extremity and hand function after the age of 65 (7) and have been of some clinical utility. These methods, however, lack the specificity and resolution to quantify in detail the dynamic control of fingertip force vectors that is so critical to dexterous manipulation. Given this unmet need, we developed the strength–dexterity (SD) test to measure dynamic dexterous manipulation, which we define as the dynamic regulation of fingertip force vector magnitude and direction. It is based on the mechanical perspective of evaluating the dynamical ability of the fingertips to stabilize unstable objects (8–14). This simple test consists of measuring a person’s ability to compress slender springs with the fingertips. The physical characteristics of each spring define how stiff, compliant, stable, and prone to buckling it is. As such, the SD test has been shown to assess the interactions among sensory, motor, and neural processing capabilities in the behavioral context of dexterous manipulation (9,11,12,14). The SD test differs from available tests of static forces and pick-and-place tasks in important ways (see Discussion section). For example, the Nine Hole Peg test (4) measures the time it takes to pick up, insert into holes, and remove nine pegs. Given that it studies the use of the whole upper extremity to grasp and orient rigid objects, an individual can apply excessive forces or adapt their kinematic trajectories to achieve the same score, and the directional control of the fingertips forces is not measured in detail.
Using the SD test to quantify dynamic dexterous manipulation is also relevant to fine manipulation in activities of daily living. The dynamic control of fingertip force vector magnitude and direction is important functionally for manipulating fragile, deformable, or small objects in daily life. Basic research studies have shown that when older people are asked to produce static fingertip forces against a rigid surface, they tend to misdirect (15) the force vector and fail to precisely regulate its magnitude (16,17), especially at low magnitudes (17). This has been attributed to differential weakening of hand musculature or changes in the neural coupling among muscles (18). Such deficits in the control of static fingertip force vectors could compromise force and moment balance of the object being manipulated (19). However, these directional deficits are not observed or implicated consistently (20,21)—and even if present, it is unclear whether and how they would affect dynamic manipulation of hand-held objects. Here we extend those studies of static force regulation and pick-and-place tasks by quantifying the dynamical control of fingertip force vector magnitude and direction in middle-aged and older adults.
Methods
Ninety-eight adults (18–89 years of age) were assigned into three age groups with around 33 participants in each group: young, 18–34 (28.38 ± 3.69, 18 F, 2L), middle-aged, 45–65 (57.06 ± 6.58, 19 F, 2 L), and older adults, 66–89 (75.6 ± 7.15, 19 F, 3 L). Ethical approval was obtained from the University of Southern California institutional review board, and all participants consented to participate in this study. All the participants reported no medical conditions, pain, or surgery affecting their hands.
Instrumentation and Experimental Procedure to Quantify Dexterous Manipulation
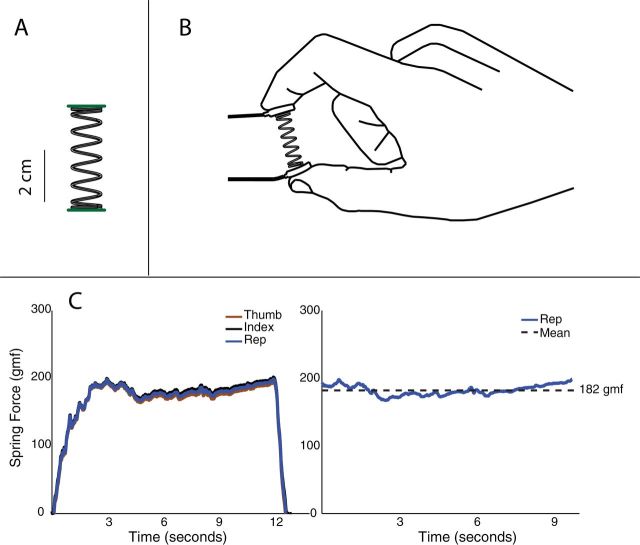
The instrumentation and experimental procedure for the SD test are described in detail in Dayanidhi et al. (13). Briefly, a helical compression spring (stiffness = 0.86 N/cm, length = 3.96cm, solid length = 0.6858cm, outer diameter = 0.9525cm, wire diameter = 0.0508cm, # 4268; Century Springs Corp., Los Angeles, CA) was used (Figure 1A). The spring was chosen to fit comfortably between the thumb and index finger and provide high resolution while requiring only very low forces (<3N; Figure 1B). Two miniature compression load cells (ELB4-10, Measurement Specialties, Hampton, VA) were mounted at the spring endcaps (Figure 1B). The data were sampled at 400 Hz using a custom written MATLAB program (Natick, MA) and a deadweight calibration procedure was used for conversion from voltage to force.
Figure 1.
Strength–dexterity test demonstrating the slender spring, the hardware for force data capture, and a representative trial. A) The spring had a stiffness of 0.86 N/cm, length of 3.96cm, whereas the maximum force required for compression of the spring was c. 3 N. The spring was chosen such that it would become unstable when compressed at low force levels. B) The instrumented spring with miniature load cells mounted to the end caps. C) An example time series of the force data from the thumb (brown) and index finger (black) and the average representative force (blue) along with the sustained compression plateau in gmf (right).
The goal of the task was to use the index finger and thumb of the dominant hand to maintain a sustained compression at their maximal level of instability. The slender spring for the task was such that increased compression would lead to an increase in instabilities (9,13). After a brief familiarization with the task, participants were asked to compress the spring as close as possible to the point beyond which the device will slip from their hand, that is, their maximal level of instability, and maintain that level of compression at a steady level for at least 3 seconds (Figure 1C). The participants were repeatedly encouraged to push their limits to ensure they were being tested to their true abilities. At least three trials with the compression maintained for at least 3 seconds at their maximal abilities were collected per participant.
In addition, pinch strength with the index finger and the thumb of the dominant hand using a tip-to-tip pinch was measured using a clinical pinch meter (B&L Engineering, Tustin, CA). To compare with the standardized tests of dexterity, we used one measure of “gross dexterity” (Box and Blocks test) (22) and one of “fine dexterity” (Nine Hole Peg test [9HP]) (4) in our middle-aged and older adults (n = 66). The former counts the number of blocks an individual can move across a partitioned box in 1 minute, whereas the latter measures the time it takes to pick up nine small, narrow pegs individually from a shallow well, fix them into holes, and then remove them one by one back into the well.
Data Reduction
As described in detail in Dayanidhi et al. (13), a custom MATLAB (Mathworks, Natick, MA) program was used to visually identify the sustained compression plateaus based on the force and force rate. The average of the thumb and index finger time series was used to create a representative compression force (Figure 1). The sustained compression plateau was defined as the period of at least 3 seconds for which the rate (first time derivative) of force was bounded within one standard deviation of the mean force rate for that same period. For each participant, as in Venkadesan et al. (9) and Dayanidhi et al. (13), the mean of the representative force during the sustained compression plateau (Figure 1C) was calculated for all attempts and three maximal values were averaged to create a metric for dexterous manipulation ability for the SD test, which we call Dynamic Manipulation Score, in units of gram force (gmf).
Lastly, we examined the data in the phase space (23) to characterize the dynamics and variability of how the participants controlled the instability during the sustained compression plateau (Phase portraits are an invaluable tool in studying dynamical systems. They consist of a plot of trajectories in the state space—in this case the fingertip forces vs their derivatives. This reveals dynamical characteristics such as whether an attractor, a repellor or limit cycle is present for the fingertip forces, and how strong are those tendencies.). As described previously (13), we plotted F versus F ˙ versus F ¨, where F is force, F ˙ is force rate (velocity) and F̈ is change in force rate (acceleration). As a measure of dispersion and strength of the control attractor produced by the neuromuscular system, that is, how strongly the system was able to maintain that current state, we calculated the sum of Euclidean distances of the trajectories in the phase space during the maximal sustained compression plateau normalized to the time of the plateau, that is, Euclidean distance/second. Greater dispersion could be considered an indication of a weaker attractor, that is, a weaker neuromuscular controller enforcing the constant sustained compression and consequently an inability to minimize variability in the current state.
Data Analysis
The independent variables were age distributions (18–34, 45–65, 66–89), whereas the dependent variables were the dynamic manipulation score, phase portrait dynamics, Nine Hole Peg test, Box and Blocks test, and the maximal pinch strength. To test the effect of age, we performed one-way ANOVAs with age distribution as the independent variable versus dynamic manipulation score and phase portrait dynamics. Linear regression models were created to evaluate shared variances between (i) age and dynamic manipulation score; (ii) pinch strength and dynamic manipulation score; (iii) dynamic manipulation score, Box and Blocks test, and Nine Hole Peg test; (iv) age and dynamic manipulation score, Box and Blocks test, Nine Hole Peg test, and pinch strength; and (v) phase portrait dynamics, Box and Blocks test, and Nine Hole Peg test. A stepwise regression model was used to evaluate percentage contribution to variance in age by dynamic manipulation score, Nine Hole Peg test, Box and Blocks test, and pinch strength.
Results
Change in Dexterous Manipulation Abilities With Age
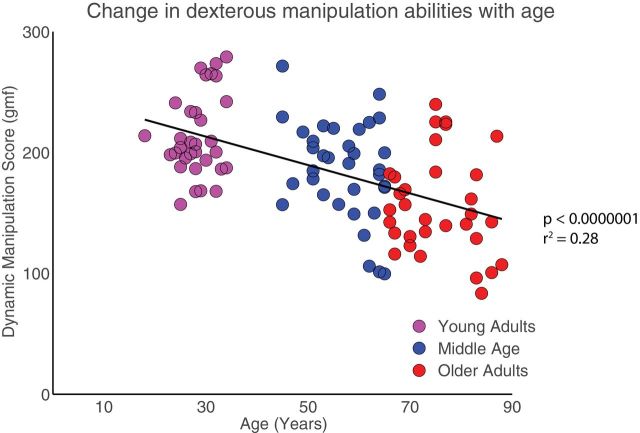
There was a negative association of dynamic manipulation score with age (p < .001, r 2 = .28, Figure 2). A one-way ANOVA revealed significant differences (p < .001, F 2,30 = 17.99) across the three age groups (18–34, 45–65, 66–89 years.). Scheffe’s post hoc test revealed significant pair wise differences among all three age groups. Dynamic manipulation score was reduced across the three age groups (mean ± SEM in gmf); 213.79 ± 6.8, 185.73 ± 6.6, and 156.15 ± 6.8, for the young, middle, and older ages, respectively.
Figure 2.
Differences in the dynamic manipulation score across the age groups. The 98 participants from 18–89 years show a decline across the age groups; young adults (magenta) middle-aged adults (blue) and older adults (red).
Relationship Between Dynamic Manipulation Score and Maximal Pinch Strength
We found no significant association (p = .07) between maximal pinch strength and dynamic manipulation score. Two participants detected to be outliers were not included for the analysis. Although including them does show a significant association (p < .05), the main result remains: only 7% of the variance (r 2 = .07) in dynamic manipulation can be explained by the variance in pinch strength.
Relationship Between Dynamic Manipulation Score and Standardized Tests of Dexterity
Similarly, no significant association was observed between the standardized tests (Box and Blocks, Nine Hole Peg) and the dynamic manipulation score (p = .21, Table 1). However, there was a correlation between the Box and Blocks and Nine Hole Peg tests (p < .001, r = .67) with a common variance of 46%. A multiple regression model of Box and Blocks, Nine Hole Peg, dynamic manipulation score, and pinch strength versus age was significant (p < .001, R 2 = .30), and a stepwise regression indicated that only the two metrics of finger dexterity (Nine Hole Peg and dynamic manipulation score) accounted for 28% of this variance with age. That is, measures of “fine manipulation” could explain most of the variance seen with age.
Table 1.
Correlation Matrix Between Age, Dynamic Manipulation Score, Nine Hole Peg Test and Box and Blocks Test
| Age | Dynamic Manipulation Score | Nine Hole Peg Test | Box and Blocks Test | |
|---|---|---|---|---|
| Age | 1.00 | −0.39* | 0.48* | −0.37* |
| Dynamic Manipulation Score | 1.00 | −0.23 | 0.15 | |
| Nine Hole Peg Test | 1.00 | −0.68* | ||
| Box and Blocks Test | 1.00 |
Notes: The positive values indicate an increase, whereas negative values indicate a decrease. The bottom half of the matrix is not shown for clarity. The first row shows that all the measures are significantly correlated with age, whereas our stepwise regression results indicate that the Nine Hole Peg Test and Dynamic Manipulation Score account for most of the variance in age (45–89 y).
*Significant correlation at p < .008, based on a Bonferroni correction.
Change in Dynamic Control of Instabilities With Age and Its Association With Standardized Tests of Dexterity
There were significant differences in the dispersion of the phase portraits, that is, Euclidean length, with age seen on a one-way ANOVA (p < .001, F 2,30 = 5.79). Scheffe’s post hoc test revealed the differences were only present between the younger (18–34, 23.93 ± 3.5) and middle (45–65, 34.88 ± 3.4) age group and between the younger and the older (66–89, 40.46 ± 3.5) age group (Supplementary Figure 1). The Euclidean length was correlated with the Nine Hole Peg test (p < .01, r = .32) but not with the Box and Blocks test.
Discussion
We find that individuals older than 65 were poorer in dexterous manipulation, compared with middle-aged and younger individuals, consistent with prior work on various aspects of hand function (6,15). However, our experimental paradigm now allows us to go beyond prior findings that focused on static force production and pick-and-place tasks because we detect poorer sensorimotor function for dynamic manipulation with the fingertips (8,9,12) even in those older than 45. Our novel application of the SD test in adult populations allows us to specifically detect poorer dynamical control of the fingertip force vectors of the thumb and index finger to stabilize a hand-held object. The clear increase in dispersion in our phase space analysis in both the middle-aged and older participants are indicative of deficits in neuromuscular control. Furthermore, our results suggest that these changes in neuromuscular control are functionally distinct from the loss of strength in healthy aging or the changes in upper extremity function detected by the Nine Hole Peg and Box and Blocks tests.
Our results extend in several ways the study by Cole (15) that showed individuals older than 73 had a compromised ability to direct static fingertip forces. Firstly, we show this decline is also true for the sensorimotor ability to dynamically regulate the magnitude and direction of fingertip force vectors during the manipulation of unstable objects. Secondly, we can in more detail point to changes in dexterous manipulation ability, specifically a reduction in dynamic manipulation score and increase in dispersion in the phase space that are seen even in middle age—which extends the work of Lindberg (17) and Marmon (24) into the dynamical domain. Thirdly, we also go beyond three limitations of the previous study by Cole (15) by (i) presenting a cross-sectional sample across the whole adult age range from 18 to 89 years; (ii) including a larger number of participants (n = 98 vs n = 20), and (iii) making the task a true dynamic manipulation task between the thumb and index finger, as opposed to a single digit–tracking task of producing static forces against a rigid surface.
SD Test Versus Standardized Tests of Dexterity
The standardized tests of dexterity used in this study (ie, Nine Hole Peg and Box and Blocks) do not share any variance in common with the dynamic manipulation score of the SD test. Our regression analyses show that most of the variance with age was explained just by the SD test and Nine Hole Peg test. Although the mean dispersion of the phase portraits did correlate with the Nine Hole Peg test, that shared variance was only 10%. This correlation might reflect reduced steadiness in the older participants (24). The similarity among these tests is only that the participants need to pick up an object, and our calculation of the dynamic manipulation score is not influenced by the ability to pick up and orient objects. Rather, it focuses on the ability to dynamically regulate fingertip force magnitude and direction to control instabilities once the object has been grasped. This is different from the static grasp of rigid objects in the standardized tests and is critical for manipulating the fragile, deformable, small, or compressible objects of daily life. During development, manipulation across a range of forces (0–80 N) have been shown to have some shared variance with the Box and Blocks test and to also have its own unique latent trait (10). As in the case of typically developing children (13), here we focus on manipulation with low-magnitude forces (0–3 N) common for many activities of daily living (25). Consequently, we conclude that the SD test captures some sensorimotor capabilities for dexterous manipulation that the standardized tests of dexterity do not. Moreover, it is arguable that those tests exaggerate the importance of time to complete the task as a surrogate for a number of factors including control of fingertip force direction, do not require a critical regulation of force, and have a number of confounds including sequence planning, appropriate spatial orientation, cognitive abilities, motivation, and the use of whole arm movements all of which might not be directly related to manipulation abilities. In contrast, the SD test infers the immediate appropriateness of dynamic regulation of force magnitude and direction at time scales directly relevant to the stability of the manipulation task.
Sensorimotor/Cortical Factors
The SD test has been shown to assess the integrative sensorimotor ability to produce dexterous manipulation involving tactile sensation, joint and muscle proprioception, and spinal cord and brain function (8,9,11,12). Therefore, much like we discuss in the case of development (13), there are a number of age-related changes in the sensorimotor system that can account for the observed decline. From early adulthood, there are some degenerative changes in brain volume, in cortical areas of the sensorimotor system (26), and in the diffuse cortical circuits associated with precision grasp behavior (11,12,27,28). In addition, slowing of central and peripheral (motor and sensory) conduction velocities have been observed in people older than 60 years of age (29), but its significance for dexterous manipulation is not known. Finally, even if changes in tactile sensibility and vision do play a role in the decline of manipulation (30)—with vision compensating to some extent for lack of tactile sensibility (9)—their impairments are unable to fully explain the declines in manipulation abilities (20). A limitation of this study is that we did not include data from tactile measurements nor did we systematically study the effect of vision—as we have done in young adults (9). Furthermore, our participants might have had underlying pathologies that were nonsymptomatic that might also influence our findings. Further study is needed to establish the relative contributions of age-related changes in central versus peripheral and sensory versus motor mechanisms to the clear qualitative change in neuromuscular control of fingertip force dynamics (Supplementary Figure 1).
Muscular and Motor Unit Factors
Tip-to-tip static pinch strength is relatively stable until around 60 years of age, followed by a gradual decline (31). But we detected poorer performance of the SD test starting in middle age. This reinforces the observation that the early change in dexterous manipulation capabilities we report are probably not related to static pinch strength. In addition, we have already observed poor correlation between pinch strength and dexterous manipulation score in prior studies in children (13) and adults (9).
Even fingertip forces of magnitudes approximately 2 N, such as that used for the SD test, can recruit c. 50% of the motor units of the first dorsal interosseous (32), perhaps to improve the resolution of control (33). Force steadiness in maintaining force even at low values has been observed to be worse in older adults (34), likely due to loss of alpha motor neurons and the subsequent reinnervation leading to increased number of muscle fibers per motor unit (1), poorer resolution of forces, and consequently increased signal-dependent noise. This partly contributes to changes in pick-and-place tests such as a Pegboard test (24). The reduction in force steadiness, by injecting noise into the already unstable task of compressing the spring, could potentially be a reason for decline in the dynamic manipulation score seen in the older people—and supports our findings of changes in the phase portrait during sustained compression (Supplementary Figure 1, Table 2).
Table 2.
Participant Descriptive Information and Summary of Results
| 18–34 | 45–65 | 66–89 | |
|---|---|---|---|
| Participants (n) | 32 | 34 | 32 |
| Mean age (y) | 28.38±3.69 | 57.06±6.58 | 75.6±7.15 |
| Sex distribution | 18 F, 14 M | 19 F, 15 M | 19 F, 13 M |
| Dynamic Manipulation score (mean ± SEM) | 213.79±6.8 | 185.73±6.6 | 156.15±6.8 |
| Phase Portrait Dynamics (mean ± SEM) | 23.93±3.5 | 34.88±3.4 | 40.46±3.5 |
Note: The number of participants, mean age, sex distribution, dynamic manipulation score, and phase portrait dynamics are shown for the three age groups: 18–34, 45–65, and 66–89.
Dynamical Analysis via Phase Portraits
The dynamical analysis during the sustained compression provides an indirect understanding of the stability and effectiveness of the neuromuscular controller. Previously, steadiness has been reported to be lower in older adults (but not in middle-aged adults) and to be associated with functional tasks (24). The SD test uses the inherent instability of the spring to challenge the brain–hand system to use fingertip forces to stabilize the system (9). Both middle-aged and older adults had a weaker attractor—evidenced by greater dispersion in the phase space (Supplementary Figure 1)—and consequently a less stable performance (ie, weaker corrective action and more likely to go unstable). In contrast, young adults were seen to have a stronger attractor, successful at maintaining a lower dispersion in the phase portrait (ie, stronger corrective action that reduced dispersion). Although the physiological basis and localization of these differences are not entirely known from the middle age onward, one can consider that the brain–hand system exhibits declining ability to enforce an attractor (or a change in control strategy) that has consequences to the maximal instability that can be sustained (35). Disambiguating the differential roles of muscle mechanics, spinal cord, and brain function for this dynamical ability requires careful studies combining, for example, electromyography (EMG), EMG-weighted average (36), Hoffmann’s reflex, and transcranial magnetic stimulation while participants control different levels of instability.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This work was funded in part by a grant from the National Institute on Disability and Rehabilitiation Research Grant (RERC 84-133E2008-8), National Science Foundation Grant (EFRI-COPN 0237258), and National Institutes of Health Grants (AR050520 and AR052345 to F.V.C.). However, those contents do not necessarily represent the policy of the Department of Education, and you should not assume endorsement by the Federal Government.
Conflict of Interest
F.J.V.-C. holds U.S. Patent No. 6,537,075 on some of the technology used but has no active or pending licensing agreements with any commercial entity.
Supplementary Material
Acknowledgments
We acknowledge the assistance of Kathleen Shanfield, Allison Chu, Juan Garibay, Wenhsin Hu, Emily Lawrence, Na-hyeon Ko, Isak Hägg, Novalie Lilja, and Phil Requejo with data collection and recruitment, and Nora Nelson and Jon Weisz with technical assistance. Sudarshan Dayanidhi is currently a postdoctoral fellow at the Muscle Physiology lab at University of California, San Diego.
References
- 1. Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–595 [DOI] [PubMed] [Google Scholar]
- 2. Close J, Ellis M, Hooper R, Glucksman E, Jackson S, Swift C. Prevention of falls in the elderly trial (PROFET): a randomised controlled trial. Lancet. 1999;353:93–97 [DOI] [PubMed] [Google Scholar]
- 3. Lange BS, Requejo P, Flynn SM, et al. The potential of virtual reality and gaming to assist successful aging with disability. Phys Med Rehabil Clin N Am. 2010;21:339–356 [DOI] [PubMed] [Google Scholar]
- 4. Oxford Grice K, Vogel KA, Le V, Mitchell A, Muniz S, Vollmer MA. Adult norms for a commercially available Nine Hole Peg test for finger dexterity. Am J Occup Ther. 2003;57:570–573 [DOI] [PubMed] [Google Scholar]
- 5. Desrosiers J, Hébert R, Bravo G, Rochette A. Age-related changes in upper extremity performance of elderly people: a longitudinal study. Exp Gerontol. 1999;34:393–405 [DOI] [PubMed] [Google Scholar]
- 6. Ranganathan VK, Siemionow V, Sahgal V, Yue GH. Effects of aging on hand function. J Am Geriatr Soc. 2001;49:1478–1484 [DOI] [PubMed] [Google Scholar]
- 7. Carmeli E, Patish H, Coleman R. The Aging Hand. J Gerontol A Biol Sci Med Sci. 2003;58:M146–M152 [DOI] [PubMed] [Google Scholar]
- 8. Valero-Cuevas FJ, Smaby N, Venkadesan M, Peterson M, Wright T. The strength-dexterity test as a measure of dynamic pinch performance. J Biomech. 2003;36:265–270 [DOI] [PubMed] [Google Scholar]
- 9. Venkadesan M, Guckenheimer J, Valero-Cuevas FJ. Manipulating the edge of instability. J Biomech. 2007;40:1653–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vollmer B, Holmström L, Forsman L, et al. Evidence of validity in a new method for measurement of dexterity in children and adolescents. Dev Med Child Neurol. 2010;52:948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holmström L, de Manzano O, Vollmer B, et al. Dissociation of brain areas associated with force production and stabilization during manipulation of unstable objects. Exp Brain Res. 2011;215:359–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mosier K, Lau C, Wang Y, Venkadesan M, Valero-Cuevas FJ. Controlling instabilities in manipulation requires specific cortical-striatal-cerebellar networks. J Neurophysiol. 2011;105:1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dayanidhi S, Hedberg A, Valero-Cuevas FJ, Forssberg H. Developmental improvements in dynamic control of fingertip forces last throughout childhood and into adolescence. J Neurophysiol. 2013;110:1583–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dayanidhi S, Kutch JJ, Valero-Cuevas FJ. Decrease in muscle contraction time complements neural maturation in the development of dynamic manipulation. J Neurosci. 2013;33:15050–15055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole KJ. Age-related directional bias of fingertip force. Exp Brain Res. 2006;175:285–291 [DOI] [PubMed] [Google Scholar]
- 16. Cole KJ, Rotella DL, Harper JG. Mechanisms for age-related changes of fingertip forces during precision gripping and lifting in adults. J Neurosci. 1999;19:3238–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindberg P, Ody C, Feydy A, Maier MA. Precision in isometric precision grip force is reduced in middle-aged adults. Exp Brain Res. 2009;193:213–224 [DOI] [PubMed] [Google Scholar]
- 18. Shinohara M, Latash ML, Zatsiorsky VM. Age effects on force produced by intrinsic and extrinsic hand muscles and finger interaction during MVC tasks. J Appl Physiol. 2003;95:1361–1369 [DOI] [PubMed] [Google Scholar]
- 19. Parikh PJ, Cole KJ. Handling objects in old age: forces and moments acting on the object. J Appl Physiol. 2012;112:1095–1104 [DOI] [PubMed] [Google Scholar]
- 20. Cole KJ, Rotella DL, Harper JG. Tactile impairments cannot explain the effect of age on a grasp and lift task. Exp Brain Res. 1998;121:263–269 [DOI] [PubMed] [Google Scholar]
- 21. Lowe BD. Precision grip force control of older and younger adults, revisited. J Occup Rehabil. 2001;11:267–279 [DOI] [PubMed] [Google Scholar]
- 22. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block test of manual dexterity. Am J Occup Ther. 1985;39:386–391 [DOI] [PubMed] [Google Scholar]
- 23. Hirsch MW, Smale S, Devaney RL. Differential Equations, Dynamical Systems and an Introduction to Chaos. 2nd ed. San Diego, CA: Academic Press/Elsevier; 2004 [Google Scholar]
- 24. Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43:560–567 [DOI] [PubMed] [Google Scholar]
- 25. Smaby N, Johanson ME, Baker B, Kenney DE, Murray WM, Hentz VR. Identification of key pinch forces required to complete functional tasks. J Rehabil Res Dev. 2004;41:215–224 [DOI] [PubMed] [Google Scholar]
- 26. Pieperhoff P, Hömke L, Schneider F, et al. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. J Neurosci. 2008;28:828–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623 [DOI] [PubMed] [Google Scholar]
- 28. Bonnard M, Galléa C, De Graaf JB, Pailhous J. Corticospinal control of the thumb-index grip depends on precision of force control: a transcranial magnetic stimulation and functional magnetic resonance imagery study in humans. Eur J Neurosci. 2007;25:872–880 [DOI] [PubMed] [Google Scholar]
- 29. Dorfman LJ, Bosley TM. Age-related changes in peripheral and central nerve conduction in man. Neurology. 1979;29:38–44 [DOI] [PubMed] [Google Scholar]
- 30. Johansson RS, Flanagan JR. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat Rev Neurosci. 2009;10:345–359 [DOI] [PubMed] [Google Scholar]
- 31. Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66:69–74 [PubMed] [Google Scholar]
- 32. Milner-Brown HS, Stein RB, Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973;228:285–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fuglevand AJ. Mechanical properties and neural control of human hand motor units. J Physiol. 2011;589(Pt 23):5595–5602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Galganski ME, Fuglevand AJ, Enoka RM. Reduced control of motor output in a human hand muscle of elderly subjects during submaximal contractions. J Neurophysiol. 1993;69:2108–2115 [DOI] [PubMed] [Google Scholar]
- 35. Newell KM, Liu YT, Mayer-Kress G. Time scales in motor learning and development. Psychol Rev. 2001;108:57–82 [DOI] [PubMed] [Google Scholar]
- 36. Kutch JJ, Kuo AD, Rymer WZ. Extraction of individual muscle mechanical action from endpoint force. J Neurophysiol. 2010;103:3535–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.