Abstract
We evaluated toxicity and outcomes for patients with peripheral lung tumors treated with stereotactic body radiation therapy (SBRT) in a dose-escalation and dose-convergence study. A total of 15 patients were enrolled. SBRT was performed with 60 Gy in 5 fractions (fr.) prescribed to the 60% isodose line of maximum dose, which was 100 Gy in 5 fr., covering the planning target volume (PTV) surface (60 Gy/5 fr. − (60%-isodose)) using dynamic conformal multiple arc therapy (DCMAT). The primary endpoint was radiation pneumonitis (RP) ≥ Grade 2 within 6 months. Toxicities were graded according to the Common Terminology Criteria for Adverse Events, version 4.0. Using dose–volumetric analysis, the trial regimen of 60 Gy/5 fr. − (60%-isodose) was compared with our institutional conventional regimen of 50 Gy/5 fr. − (80%-isodose). The enrolled consecutive patients had either a solitary peripheral tumor or two ipsilateral tumors. The median follow-up duration was 22.0 (12.0–27.0) months. After 6 months post-SBRT, the respective number of RP Grade 0, 1 and 2 cases was 5, 9 and 1. In the Grade 2 RP patient, the image showed an organizing pneumonia pattern at 6.0 months post-SBRT. No other toxicity was found. At last follow-up, there was no evidence of recurrence of the treated tumors. The target volumes of 60 Gy/ 5 fr. − (60%-isodose) were irradiated with a significantly higher dose than those of 50 Gy/5 fr. − (80%-isodose), while the former dosimetric parameters of normal lung were almost equivalent to the latter. SBRT with 60 Gy/5 fr. − (60%-isodose) using DCMAT allowed the delivery of very high and convergent doses to peripheral lung tumors with feasibility in the acute and subacute phases. Further follow-up is required to assess for late toxicity.
Keywords: stereotactic body ridiotherapy, lung cancer, dose-escalation study, prescription dose, Phase I study, homogeneity index
INTRODUCTION
Currently, stereotactic body radiotherapy (SBRT) is considered as a treatment option for patients with medically inoperable early-stage non-small-cell lung carcinoma (NSCLC) [1] as well as those with oligometastatic lung cancers [2, 3]. However, aspects of SBRT delivery (including the total dose, fraction size and number, and prescription) vary between studies.
Since 1998, we have used SBRT for peripheral lung tumors using dynamic conformal multiple arc therapy with a total dose of 50 Gy in 5 fr. prescribed to the 80% isodose line of the maximum dose covering the planning target volume (PTV) surface (50 Gy/5fr. − (80%-isodose)) [4] and reported good outcomes for patients with NSCLC [5]. However, there is room for refinement of the methods of SBRT delivery, especially for dose escalation. In fact, local control has been unsatisfactory in the subgroup of patients with NSCLC [6] and also in patients with pulmonary oligometastases from colorectal cancer [2]. In addition, statistically significant correlations between doses and local control rates or overall survival have been reported for NSCLC [7, 8]. Recently, we reassessed the optimal prescription isodose line fitting the PTV surface. As a result, we found that the 60% isodose plan leads to lower comparative dosimetric factors in normal lung tissue, with higher comparative mean PTV and internal target volume (ITV) doses achieved, along with good conformity index values. We concluded that the 60% isodose plan was the most efficient plan [9]. In addition, the practice guidelines of the American Association of Physicists in Medicine (AAPM) Task Group 101 have also accepted dose heterogeneity within the PTV in SBRT [10].
Based on this concept and the result of our optimal prescription isodose study, we consecutively initiated a dose-escalation and dose-convergence Phase I study using a total dose of 60 Gy in 5 fr. to assess toxicity and outcomes for patients with peripheral lung tumors. In this treatment method, the maximum dose in the PTV was 100 Gy in 5 fr., which is, as far as we are aware, the highest dose used to date in Phase I studies of SBRT; this dose has the potential to lead to higher local control.
METHODS
Patient eligibility
The treatment protocol and consent form for this Phase I study were approved by an institutional review board and by the ethics committee in our institution (2011-011). Eligible patients for this trial satisfied all of the following criteria: (i) patients had peripheral solitary or two ipsilateral pulmonary tumors which were diagnosed as non-small-cell lung cancer or metastatic lung tumors, clinically or pathologically; FDG-PET/CT was performed for staging within 90 days prior to treatment; (ii) there were no malignancies other than pulmonary lesions; (iii) treatment plans followed dose constraints of the organs at risk shown in (Table 1); (iv) there was no history of irradiation to the thoracic region or systemic chemotherapy; (v) the Eastern Cooperative Oncology Group Performance Status (ECOG-PS) was 0–2; the Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage was 0–2; (vi) there was no radiographical finding suspected as interstitial pneumonia or fibrosis on chest CT; no active infectious disease; no history of other cancer within 2 years; no systemic steroid therapy; no continuous or intermittent oxygen therapy; and no fever ≥38°C. All patients provided written informed consent.
Table 1.
Dose constraints of the organs at risk
| Organ at risk | Acceptable dose | Volume |
|---|---|---|
| Total lung | V20 ≦ 15% | |
| Spinal cord | 25 Gy/5 fractions | Max |
| Gastrointestinal tract | 25 Gy/5 fractions | ≦1 ml |
| Trachea, main bronchus | 50 Gy/5 fractions | ≦1 ml |
| Aorta | 60 Gy/5 fractions | ≦1 ml |
| Pulmonary artery | 50 Gy/5 fractions | ≦1 ml |
| Heart | 60 Gy/5 fractions | ≦10 ml |
| Brachial plexus | 50 Gy/5 fractions | ≦1 ml |
V20 = the ratio of lung volume irradiated ≧20 Gy to total lung.
Treatment
We have previously described our methods of SBRT delivery [4]. For the treatment-planning computed tomography (CT), long-scan-time CT was used in order to directly visualize the ITV after immobilizing the patient with a vacuum pillow and abdominal corset. The PTV was determined by adding a margin of 6–8 mm to the ITV. Multi-arc dynamic conformal radiation with eight arcs was planned by a radiation treatment-planning system (FOCUS XiO version 4.2.0–4.3.3, Computerized Medical Systems, St Louis, MO , USA) calculated with a multigrid superposition algorithm with heterogeneity correction. Just before each treatment, the isocenter was determined on in-room CT images under the same conditions as the treatment-planning CT. Then, the treatment was performed using X-rays from a 6-MV linear accelerator (Varian Medical Systems, Inc., Palo Alto, CA, USA). Dynamic conformal multiple arc irradiation was used for SBRT. In our previous analysis [9], the 60% isodose plan was considered as the best plan because normal lung tissue dose could be lowered while maintaining a high dose to the target. The mean normal lung dose of the 60% isodose plan was 23% lower than that of the 80% isodose plan. Therefore, the prescription was defined as the 60% isodose of the maximum doses and was planned to enclose the PTV by the 60% isodose line. Then, the dose covering 95% of the PTV was more than or equal to the prescribed dose. The prescribed dose was 60 Gy in 5 fr. over five consecutive days.
Follow-up and evaluation
All patients were monitored monthly on an outpatient basis with monthly interviews, chest radiographs and laboratory data including C-reactive protein (CRP) and lactate dehydrogenase (LDH). Chest imaging follow-up included high-resolution CT scans obtained at 1 and 3 months after SBRT and then at 3-month intervals, and chest X-rays were obtained at 2, 4 and 5 months after SBRT.
Tumor response was assessed by follow-up CT scans and according to the World Health Organization criteria during the time-course of follow-up [11]. Response was recorded as complete response if all measurable tumor disappeared, as near-complete response if the tumor almost disappeared with a residual scar, as partial if the product of the dimensions was reduced by >50%, and as progressive disease if the product increased by >20%.
Endpoints
The primary endpoint of the Phase I portion was to ascertain that the rate of radiation pneumonitis (RP) ≥ Grade 2 within 6 months after SBRT with this regimen was equivalent to that after SBRT with our conventional regimen (40–50 Gy/5 fr. − (80%-isodose)). In our previous study with our conventional regimen, we analyzed RP after SBRT. As a result, Grade ≥2 RP occurred in 28 (22%) of 133 patients [12]. We assumed that the RP rates were equivalent when the number of patients with RP ≥ Grade 2 was ≤5 (33%) of the eligible 15 patients.
Secondary endpoints were the rate of RP ≥ Grade 3, any other toxicities within 6 months after SBRT, and local progression-free survival rate. Toxicity was evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Dose-limiting toxicity was considered as an acute Grade ≥ 3 RP or other toxicities attributed to the therapy.
Dose–volume histogram analysis
We compared the dose–volume histograms (DVHs) of target volumes and the organs at risk between the trial regimen (60 Gy/5 fr. − (60%-isodose)) and the conventional regimen in our institution (50 Gy/5 fr. − (80%-isodose)). The DVH parameters were compared using the paired t-test. Analyses were carried out using IBM SPSS Statistics 20.0 (IBM, Inc., Armonk, NY, USA). Differences were regarded as statistically significant at P values < 0.05.
RESULTS
Patient characteristics
A total of 15 patients were enrolled between August 2011 and June 2012. Of these patients, 14 had a solitary pulmonary tumor (pathologically confirmed non-small-lung cancer (n = 7), clinically diagnosed lung cancer (n = 4), or a solitary pulmonary metastasis (n = 3)), and one had two ipsilateral metastases (n = 1). Patient and tumor characteristics are shown in Table 2. The median follow-up duration was 22.0 months (range, 12.0–27.0 months). The median age was 77 years (range, 57–88 years). The median tumor diameter was 2.1 cm (range, 0.9–2.9 cm).
Table 2.
Patient and tumor characteristics
| Median age | 77 (57–88) |
| Sex: male/female | 10/5 |
| Median follow up duration | 22.0 (12.0–27.0) |
| Operability: Yes/No | 1/14 |
| GOLD: normal/I/II | 10/1/4 |
| Smoking history: Yes/No | 12/3 |
| Pack-years | 24.0 (0–135) |
| Median maximum diameter of tumor (cm) | 2.1 (0.9–2.9) |
| ITV (ml) | 5.8 (1.0–12.4) |
| PTV (ml) | 28.9 (10.1–45.2) |
| SUVmax | 2.7 (0.8–7.1) |
| Disease | |
| Non-small-cell lung cancer | 7 |
| Clinically diagnosed lung cancer | 4 |
| Lung metastasis | |
| Solitary | 3 |
| Two | 1 |
GOLD = the Global Initiative for Chronic Obstructive Lung Disease, ITV = internal target volume, PTV = planning target volume, SUVmax = the maximum standard uptake value in 18F- fluorodeoxyglucose positron emission tomography.
Radiation pneumonitis and other toxicities
The number of patients with Grade 0, 1 and 2 RP was 5 (33%), 9 (60%) and 1 (7%), respectively. There was no Grade ≥ 3 RP. For all nine patients with Grade 1 RP, the opacity of the RP appeared 3.0–6.0 months after SBRT, corresponded to only the high-dose irradiated area, and shrank gradually. For the Grade 2 patient, the usual RP (Grade 1) in and near the PTV occurred 3.0 months after SBRT. Patchy consolidation in both lungs occurred 6.0 months after SBRT; however, the patient had no fever and no other systemic symptoms, respiratory symptoms or abnormal laboratory data, except for an elevated C-reactive protein level (4.8 (< 0.3: normal range)). Three days later, the consolidation got worse, and the C-reactive protein increased further (7.5). The patient was suspected of having lung injury of an organizing pneumonia pattern and was administered steroid therapy. Soon the opacity and C-reactive protein value improved. Steroids were tapered carefully with no exacerbation. From the time-course, we diagnosed her with organizing pneumonia, which may have been related to the SBRT. We graded this pneumonia as Grade 2 RP.
In the entire follow-up period, there were no other toxicities (nausea, fatigue, dermatitis, chest pain or rib fracture).
Response
Table 3 shows tumor response at the last follow-up. There was no progressive disease. Complete response, partial response, no change, and not evaluable were observed in three, seven, two and four patients, respectively. In the four patients regarded as not evaluable, the tumors were buried in consolidations of RP or fibrosis that had no change during follow-up.
Table 3.
Tumor response
| Response | Number | % |
|---|---|---|
| Complete response | 3 | 19 |
| Partial response | 7 | 44 |
| No change | 2 | 13 |
| Progressive disease | 0 | 0 |
| Not evaluable | 4 | 25 |
DVH analysis of target volume and organs at risk
Table 4 shows the results of the DVH analysis, which compared the trial regimen of 60 Gy/5 fr. − (60%-isodose) with the conventional regimen of 50 Gy/5 fr. − (80%-isodose) in our institution. Figure 1 shows a patient's CT image with the dose distribution of both the trial and conventional regimens. Figure 2 shows the same patient's DVH for the ITV, PTV, normal lung, and chest wall. Target volumes of the trial regimen were irradiated with significantly higher dose than those of the conventional regimen. In contrast, the normal lung ( = lung − ITV) dose of the trial regimen could be kept almost as low as the conventional regimen. The chest wall dose of the trial regimen was significantly higher.
Table 4.
DVH parameter comparison
| 60 Gy/5 fr. (60% isodose) | 50 Gy/5 fr. (80% isodose) | Δ (60–50) | P-value | ||
|---|---|---|---|---|---|
| ITV | (ml) | 5.7 (1.0–12.5) | |||
| Mean | (Gy) | 93.5 (89.8–94.6) | 60.3 (58.6–61.1) | 33.09 (30.1–34.7) | <0.01 |
| Minimum | (Gy) | 78.6 (73.2–88.1) | 55.44 (52.4–57.8) | 23.2 (19.5–30.2) | <0.01 |
| Maximum | (Gy) | 100 (100–100) | 62.5 (62.5–62.5) | 37.5 (37.5–37.5) | <0.01 |
| D95 | (Gy) | 85.4 (81.9–89.9) | 57.7 (56.00–59.6) | 27.5 (25.3–31.1) | <0.01 |
| PTV | (ml) | 29.0 (10.1–45.1) | |||
| Mean | (Gy) | 79.3 (74.5–82.1) | 56.4 (54.5–57.5) | 22.7 (20.0–25.8) | <0.01 |
| Minimum | (Gy) | 48.7 (39.2–58.1) | 45.6 (41.5–49.0) | 4.1 (−4.7–9.7) | <0.01 |
| Maximum | (Gy) | 100 (100–100) | 62.5 (62.5–62.5) | 37.5 (37.5–37.5) | <0.01 |
| D95 | (Gy) | 62.2 (60.0–65.8) | 50.9 (50.090–51.7) | 11.3 (8.4–15.0) | <0.01 |
| Total lung | (ml) | 3086 (2127–3997) | |||
| Lung—ITV | (ml) | 3080 (2121–3991) | |||
| Mean lung dose | (Gy) | 3.2 (1.5–6.3) | 3.0 (1.4–5.7) | 0.2 (−0.3–0.6) | <0.01 |
| V5 | (%) | 12.2 (4.4–26.6) | 12.7 (4.7–26. 7) | −0.1 (−2.2–1.2) | 0.65 |
| V10 | (%) | 8.0 (3.0–16.8) | 8.2 (2.7–17.0) | 0.3 (−1.2–3.1) | 0.23 |
| V15 | (%) | 5.8 (2.4–12.1) | 5.5 (2.2–12.1) | −0.1 (−0.8–0.9) | 0.66 |
| V20 | (%) | 4.3 (1.7–9.3) | 4.0 (1.5–8.7) | 0.1 (−0.6–0.8) | 0.26 |
| Chest wall | |||||
| V30 | (ml) | 29.1 (4.6–51.5) | 16.7 (4.00–42.7) | 6.4 (−0.7–19.5) | <0.01 |
| V40 | (ml) | 9.7 (0.7–23.1) | 5.2 (0.3–25.5) | 3.7 (−3.8–7.4) | <0.01 |
DVH = dose–volume histogram, fr. = fractions, Δ (60–50) = each gap value between regimens of 60 Gy/5 fr.(60% isodose) and 50 Gy/5 fr.(80% isodose), P-value = probability value, ITV = internal target volume, D95 = more than 95% of the planning target volume, PTV = planning target volume, Vn = normal lung volume receiving ≥ n Gy.
Fig. 1.
Axial and coronal plane computed tomography images with superimposed dose distribution curves of stereotactic body radiation therapy (SBRT). (A, B) Our institutional conventional regimen of 50 Gy in 5 fractions prescribed to the 80% isodose line of maximum dose covering the planning target volume (PTV) surface. The maximum dose in the PTV was 62.5 Gy in 5 fractions. (C, D) The trial regimen of 60 Gy in 5 fractions prescribed to the 60% isodose line of maximum dose covering the PTV surface. The maximum dose in PTV was 100 Gy in 5 fractions. Isodose lines from outer to inner represent 20 Gy, 40 Gy, 50 Gy, 60 Gy and 80 Gy of the maximal dose, respectively.
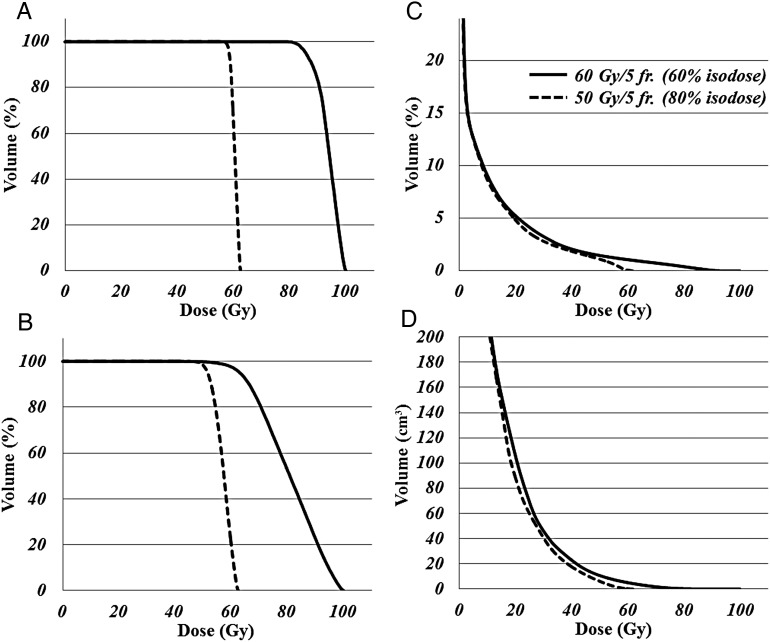
Fig. 2.
Dose–volume histograms for internal target volume (ITV) (A), planning target volume (PTV) (B), normal lung ( = lung minus internal target volume) (C), and chest wall (D), for the same patient as shown in Fig. 1. Mean PTV doses of the 60 Gy/5 fractions − (60% isodose) regimen and 50 Gy/5 fractions − (80% isodose) regimen were 93.7 Gy and 60.4 Gy, respectively. For the two regimens, the mean ITV was 80.5 Gy and 57.1 Gy, respectively, the volume irradiated > 20 Gy (V20) in the normal lung was 6.8% and 6.5%, respectively, and the V30 in the chest wall was 46.4 ml and 41.0 ml, respectively.
DISCUSSION
In this study we performed a Phase I study of dose-escalation and dose-convergence SBRT for peripheral lung tumors to demonstrate its feasibility in the acute phase following results demonstrating that the 60% isodose plan was the optimal plan [9]. Using this planning method, we tried to escalate the dose for the PTV while keeping the normal lung dose unchanged. This prescribed dose and dose-convergence was very high. The biological effective doses (BEDs) with α/β of 10 at the maximum dose point and the PTV periphery were 300 Gy10 and 132 Gy10, respectively. However, a Phase II study of SBRT with a similar BED for peripheral NSCLC revealed a high local control rate with limited toxicity [13]. The prescription dose was 66 Gy/3 fr. − (80% isodose); the BED10s at the maximum dose point and at the PTV periphery were 211 Gy10 and 309 Gy10, respectively. Based on our previous analysis of dose-convergence [9] and the previous results of dose-escalation, we performed a single arm trial of 60 Gy/5 fr. − (60%-isodose).
Radiation pneumonitis
Following radiotherapy (with or without chemotherapy) for locally advanced lung cancer, RP is the most critical toxicity and is dose–volume dependent [14, 15]. On the other hand, following SBRT for peripheral lung tumors, RP occurs less often. However, it is controversial whether RP is dose–volume dependent or not. Dose–volumetric factors have been reported to be significant predictors for RP of Grade ≥ 2 [16, 17]. In contrast, those factors have not been found to be a predictor for RP of Grade ≥ 3 [18]. In our previous study, the chest wall volume receiving 15 Gy (V15) was significantly higher in patients with Grade 2 RP than in patients with Grade 0–1 RP, while the V15 was almost equivalent in patients with Grade 1 RP and Grade 3 RP [12]. These results indicate that the mechanism or causes of low-grade RP might be different from those of high-grade RP.
In this study, there was only one Grade 2 RP and no other Grade ≥ 2 toxicities. Also, in a comparison of DVH parameters between the trial regimen of 60 Gy/5 fr. − (60%-isodose) with the conventional regimen of 50 Gy/5 fr. − (80%-isodose), there were no significant differences except for mean lung dose. The DVH comparison results supported the conclusion that the trial regimen did not increase the frequency of RP. In fact, there was only one case of Grade 2 atypical RP.
Organizing pneumonia
The only case of Grade 2 RP in this study represented lung injury of an organizing pneumonia pattern. There are some reports that organizing pneumonia after post-operative radiation for breast cancer occurrs in 1.8–2.3% of patients [19, 20]. However, there is only one report of organizing pneumonia after SBRT for lung tumors; the incidence was 5.2% [21]. We observed organizing pneumonia after SBRT in the current study for the first time among ∼700 previously treated cases. Therefore, we should follow up patients treated with this trial regimen carefully because this high-convergence dose might have an influence on the occurrence of organizing pneumonia.
Chest wall pain and rib fracture
During a follow-up after the treatment of peripheral lung tumors, chest wall pain and rib fracture were also critical toxicities, with the median interval to the onset of chest wall pain and/or rib fracture being 7–12 months [22–24]. They were also reported to be dose–volume dependent: Grade 3 chest wall toxicity was correlated with a chest wall volume receiving 30 Gy (V30) > 35 ml [22]; Grade 2 chest wall toxicity was correlated with V30 > 70 ml [23]; V40 was the most predictive of chest wall pain on multivariate analysis [24]. Additionally, skin dose should be considered in order to avoid acute skin toxicity [25]. In this analysis, there was a significant difference in V30 and V40 of the chest wall. Therefore, for tumors closely and broadly adjacent to the chest wall, we should look for the toxicities over a long-term follow-up and take dose reduction into consideration.
Phase I study of SBRT for lung tumors
There have been several dose-escalation Phase I studies of SBRT for lung tumors. In all such studies, SBRT was found to be safe and feasible [26–28], except in patients with prior thoracic radiation [26]. However, a subsequent Phase II study demonstrated that this regimen should not be used for patients with tumors near the central airways due to excessive toxicity [29].
The methods used in these dose-escalation studies varied considerably. Their fractionation and prescription dose ranged from 1–5 and 15–60 Gy, respectively. In addition, the methods of prescription also varied between studies: more than 95% of the PTV (D95) was covered within the median prescribed isodose line of 72% (range, 60–80%) [26]; D95 was covered by the 80% prescription isodose volume [27]; the median homogeneity index within the PTV was 1.16 (range, 1.02–1.36).
It is controversial whether the PTV should be irradiated homogeneously or inhomogeneously. Although SBRT is often administered while maintaining dose homogeneity within the PTV in Japan, dose heterogeneity within the PTV is prevailing in other countries [30] and is acceptable in SBRT according to the practice guidelines of the AAPM Task Group 101 [10]. By ignoring dose homogeneity within the PTV, tight conformity with steep and isotropic dose fall-off and high dose delivery to the target volume can be achieved in addition to a simultaneous reduction in the normal tissue dose [9]. In this current study, we respected the latter concept and revealed the feasibility of the dose-escalation and dose-convergence study.
Influence of dose escalation on local control and overall survival
In early stage NSCLC, recent data from both prospective and single institution clinical trials indicate that local control rates ≥ 88% can be achieved using SBRT [31]. In contrast, distant metastases constitute the predominant failure pattern following SBRT, a finding similar to that seen after surgery [31]. These outcomes suggest that higher dose may not necessarily lead to higher local control or overall survival. Furthermore, meta-analyses do not support the hypothesis of a positive dose–response relationship for tumor control and overall survival following SBRT for Stage I NSCLC [32, 33].
However, there are many other factors that might influence tumor control; these include methods of fixation and respiratory control, size of the PTV made from the gross tumor volume, homogeneity in the PTV, dose prescription point, treatment-planning strategy, and calculation algorithm. Those factors might have an additional influence on tumor control to that of the prescribed dose or BED, and the dose–response relationship might be biased by these factors. A large single-institution series suggests a positive dose–control relationship for SBRT [34]. Another single-institution series suggests that the local control rate can be improved by securing the minimum dose for the PTV [35].
There have been several reports of radio-resistant lung tumors with SBRT. The SUVmax on FDG-PET is a strong predictor of local recurrence for localized NSCLC after SBRT [6]. The local control rate of NSCLC for lower SUVmax ( <6.0) is significantly higher than for higher SUVmax (2-year; 93% and 42%, respectively). SBRT for T2 NSCLC compared with T1 lesions has a significantly lower local control rate (2-year; 70% and 90%, respectively) and trends toward a shorter survival duration (median; 16.7 months and 20 months, respectively) [36]. The local control in pulmonary oligometastases from colorectal cancer is significantly worse than that in oligometastases from other origins and primary lung cancers (2-year; 72% and 94%, respectively) [2]. For such radio-resistant lung tumors, a dose-escalation and dose-convergence regimen may contribute to high local control and consequently high overall survival.
Limitation
In this prospective trial, the occurrence of acute and subacute toxicity has been limited and manageable. Further follow-up from this trial and others will be required to assess for late toxicity, such as chest wall pain, rib fracture and symptomatic pulmonary fibrosis, and for survival characteristics. We are currently considering undertaking a Phase II study similar to this current study for peripheral lung tumors, and a challenging Phase I study for lung tumors near the central airways using the same convergent method but with a decreased dose.
CONCLUSION
SBRT with 60 Gy in 5 fr., prescribed to the 60% isodose line of the maximum dose covering the PTV surface using dynamic conformal multiple arc irradiation, has been shown to allow the delivery of very high and convergent doses to peripheral lung tumors. This treatment method is feasible in the acute and subacute phases. In addition, analysis of DVH parameters supports its feasibility. Further follow-up will be required to assess for late toxicity.
ACKNOWLEDGEMENTS
The content of this manuscript was presented at the 26th annual meeting of the Japanese Society for Therapeutic Radiology and Oncology. The Clinical Trial Registration number is UMIN000006172.
REFERENCES
- 1.Ettinger DS, Akerley W, Borghaei H, et al. NCCN Clinical Practice Guidelines in Oncology website. Non-small cell lung cancer, version 2.2013. National Comprehensive Cancer Network, 2013. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. (13 September 2013, date last accessed)
- 2.Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol. 2011;101:255–9. doi: 10.1016/j.radonc.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 3.Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72:398–403. doi: 10.1016/j.ijrobp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Takeda A, Kunieda E, Sanuki N, et al. Dose distribution analysis in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Int J Radiat Oncol Biol Phys. 2009;74:363–9. doi: 10.1016/j.ijrobp.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Takeda A, Sanuki N, Kunieda E, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys. 2009;73:442–8. doi: 10.1016/j.ijrobp.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 6.Takeda A, Yokosuka N, Ohashi T, et al. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT) Radiother Oncol. 2011;101:291–7. doi: 10.1016/j.radonc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Allibhai Z, Taremi M, Bezjak A, et al. The impact of tumor size on outcomes after stereotactic body radiation therapy for medically inoperable early-stage non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2013;87:1064–70. doi: 10.1016/j.ijrobp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 9.Oku Y, Takeda A, Kunieda E, et al. Analysis of suitable prescribed isodose line fitting to planning target volume in stereotactic body radiotherapy using dynamic conformal multiple arc therapy. Pract Radiat Oncol. 2011;2:46–53. doi: 10.1016/j.prro.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Takeda A, Ohashi T, Kunieda E, et al. Comparison of clinical, tumour-related and dosimetric factors in grade 0–1, grade 2 and grade 3 radiation pneumonitis after stereotactic body radiotherapy for lung tumours. Br J Radiol. 2012;85:636–42. doi: 10.1259/bjr/71635286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 14.Palma DA, Senan S, Tsujino K, et al. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–50. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo Y, Shibuya K, Nakamura M, et al. Dose–volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:e545–9. doi: 10.1016/j.ijrobp.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Barriger RB, Forquer JA, Brabham JG, et al. A dose–volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457–62. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 18.Fujino M, Shirato H, Onishi H, et al. Characteristics of patients who developed radiation pneumonitis requiring steroid therapy after stereotactic irradiation for lung tumors. Cancer J. 2006;12:41–6. doi: 10.1097/00130404-200601000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Ogo E, Komaki R, Fujimoto K, et al. A survey of radiation-induced bronchiolitis obliterans organizing pneumonia syndrome after breast-conserving therapy in Japan. Int J Radiat Oncol Biol Phys. 2008;71:123–31. doi: 10.1016/j.ijrobp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Katayama N, Sato S, Katsui K, et al. Analysis of factors associated with radiation-induced bronchiolitis obliterans organizing pneumonia syndrome after breast-conserving therapy. Int J Radiat Oncol Biol Phys. 2009;73:1049–54. doi: 10.1016/j.ijrobp.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 21.Murai T, Shibamoto Y, Nishiyama T, et al. Organizing pneumonia after stereotactic ablative radiotherapy of the lung. Radiat Oncol. 2012;7:123. doi: 10.1186/1748-717X-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlap NE, Cai J, Biedermann GB, et al. Chest wall volume receiving > 30 Gy predicts risk of severe pain and/or rib fracture after lung stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:796–801. doi: 10.1016/j.ijrobp.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 23.Mutter RW, Liu F, Abreu A, et al. Dose–volume parameters predict for the development of chest wall pain after stereotactic body radiation for lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:1783–90. doi: 10.1016/j.ijrobp.2011.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creach KM, El Naqa I, Bradley JD, et al. Dosimetric predictors of chest wall pain after lung stereotactic body radiotherapy. Radiother Oncol. 2012;104:23–7. doi: 10.1016/j.radonc.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe BS, Laser B, Kowalski AV, et al. Acute skin toxicity following stereotactic body radiation therapy for stage I non-small-cell lung cancer: who's at risk? Int J Radiat Oncol Biol Phys. 2008;72:1283–6. doi: 10.1016/j.ijrobp.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 26.Le QT, Loo BW, Ho A, et al. Results of a phase I dose-escalation study using single-fraction stereotactic radiotherapy for lung tumors. J Thorac Oncol. 2006;1:802–9. [PubMed] [Google Scholar]
- 27.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–55. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 28.Ernst-Stecken A, Lambrecht U, Mueller R, et al. Hypofractionated stereotactic radiotherapy for primary and secondary intrapulmonary tumors: first results of a phase I/II study. Strahlenther Onkol. 2006;182:696–702. doi: 10.1007/s00066-006-1577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–9. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 30.Nagata Y, Matsuo Y, Takayama K, et al. Current status of stereotactic body radiotherapy for lung cancer. Int J Clin Oncol. 2007;12:3–7. doi: 10.1007/s10147-006-0646-6. [DOI] [PubMed] [Google Scholar]
- 31.Senan S, Lagerwaard F. Stereotactic radiotherapy for stage I lung cancer: current results and new developments. Cancer Radiother. 2010;14:115–8. doi: 10.1016/j.canrad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Yang F, Li B, et al. Which is the optimal biologically effective dose of stereotactic body radiotherapy for Stage I non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol Biol Phys. 2011;81:e305–16. doi: 10.1016/j.ijrobp.2011.04.034. [DOI] [PubMed] [Google Scholar]
- 33.van Baardwijk A, Tome WA, van Elmpt W, et al. Is high-dose stereotactic body radiotherapy (SBRT) for stage I non-small cell lung cancer (NSCLC) overkill? A systematic review. Radiother Oncol. 2012;105:145–9. doi: 10.1016/j.radonc.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 34.McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2009;73:112–8. doi: 10.1016/j.ijrobp.2008.03.062. [DOI] [PubMed] [Google Scholar]
- 35.Shirata Y, Jingu K, Koto M, et al. Prognostic factors for local control of stage I non-small cell lung cancer in stereotactic radiotherapy: a retrospective analysis. Radiat Oncol. 2012;7:182. doi: 10.1186/1748-717X-7-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunlap NE, Larner JM, Read PW, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT) J Thorac Cardiovasc Surg. 2010;140:583–9. doi: 10.1016/j.jtcvs.2010.01.046. [DOI] [PubMed] [Google Scholar]