Abstract
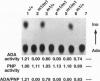
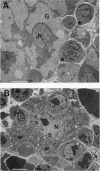
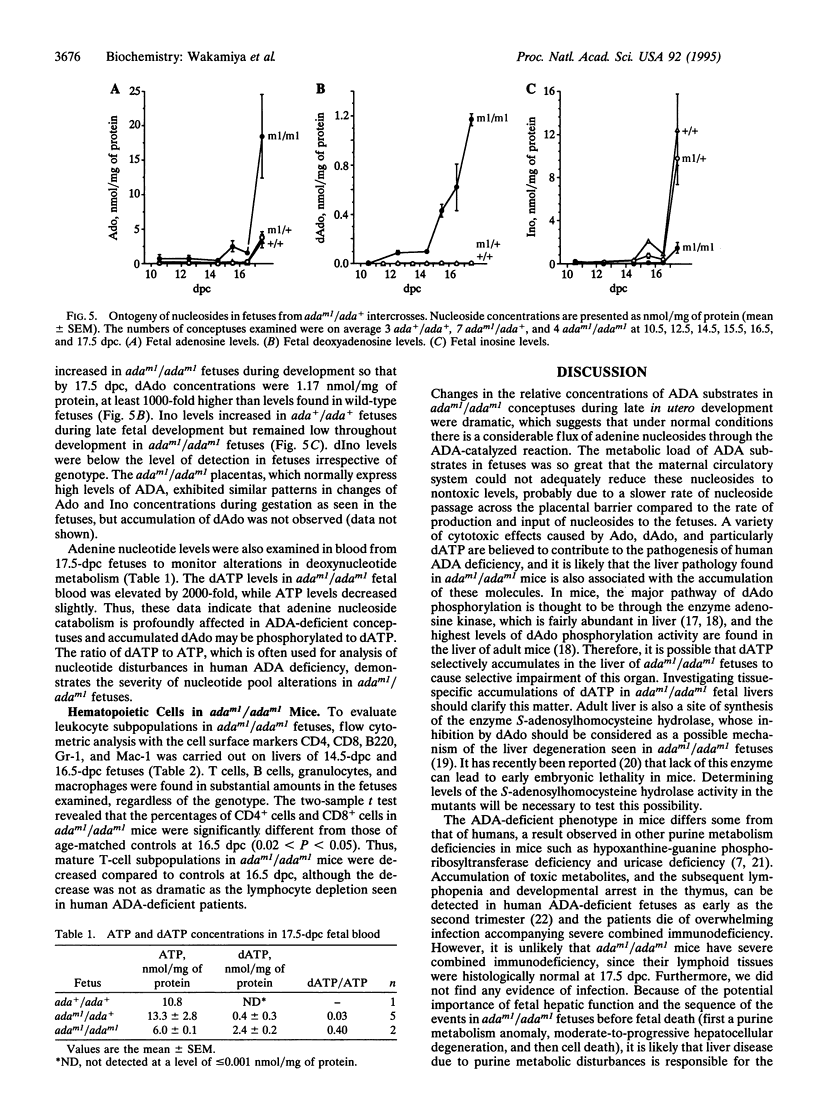
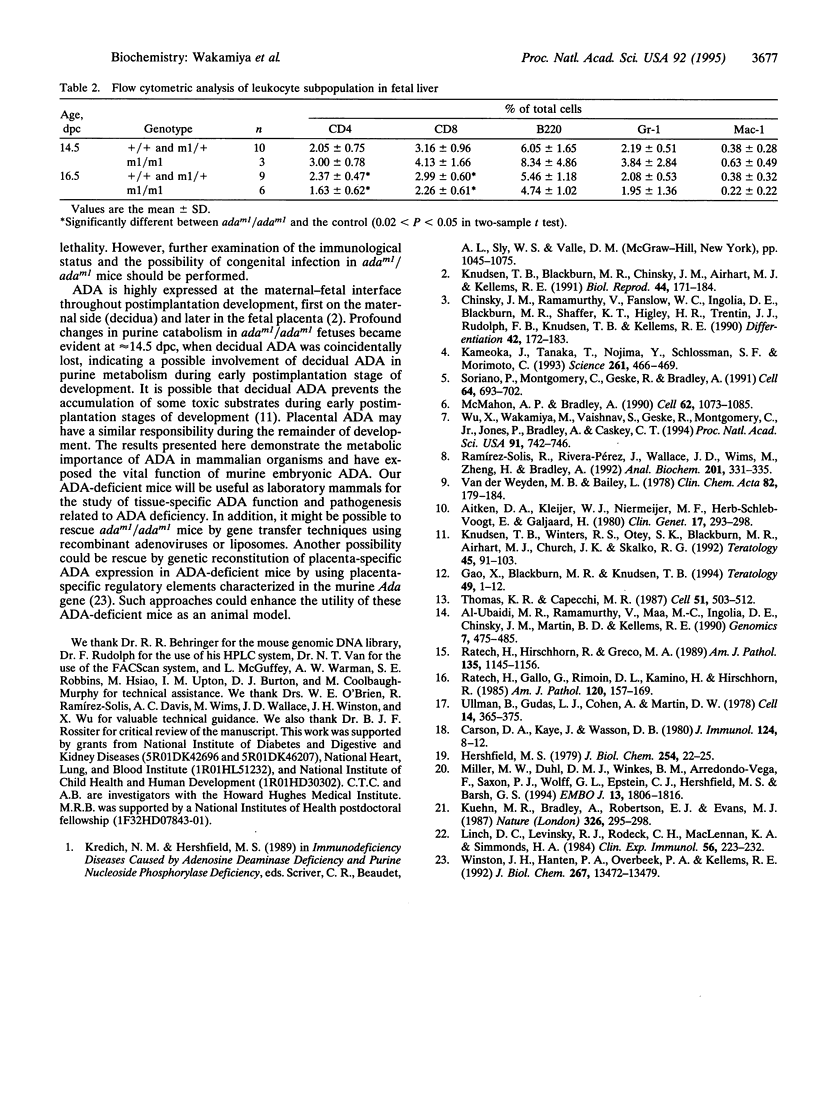
We have generated mice with a null mutation at the Ada locus, which encodes the purine catabolic enzyme adenosine deaminase (ADA, EC 3.5.4.4). ADA-deficient fetuses exhibited hepatocellular impairment and died perinatally. Their lymphoid tissues were not largely affected. Accumulation of ADA substrates was detectable in ADA-deficient conceptuses as early as 12.5 days postcoitum, dramatically increasing during late in utero development, and is the likely cause of liver damage and fetal death. The results presented here demonstrate that ADA is important for the homeostatic maintenance of purines in mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken D. A., Kleijer W. J., Niermeijer M. F., Herbschleb-Voogt E., Galjaard H. Prenatal detection of a probable heterozygote for ADA deficiency and severe combined immunodeficiency disease using a microradioassay. Clin Genet. 1980 Apr;17(4):293–298. doi: 10.1111/j.1399-0004.1980.tb00150.x. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Kaye J., Wasson D. B. Differences in deoxyadenosine metabolism in human and mouse lymphocytes. J Immunol. 1980 Jan;124(1):8–12. [PubMed] [Google Scholar]
- Chinsky J. M., Ramamurthy V., Fanslow W. C., Ingolia D. E., Blackburn M. R., Shaffer K. T., Higley H. R., Trentin J. J., Rudolph F. B., Knudsen T. B. Developmental expression of adenosine deaminase in the upper alimentary tract of mice. Differentiation. 1990 Feb;42(3):172–183. doi: 10.1111/j.1432-0436.1990.tb00759.x. [DOI] [PubMed] [Google Scholar]
- Gao X., Blackburn M. R., Knudsen T. B. Activation of apoptosis in early mouse embryos by 2'-deoxyadenosine exposure. Teratology. 1994 Jan;49(1):1–12. doi: 10.1002/tera.1420490103. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S. Apparent suicide inactivation of human lymphoblast S-adenosylhomocysteine hydrolase by 2'-deoxyadenosine and adenine arabinoside. A basis for direct toxic effects of analogs of adenosine. J Biol Chem. 1979 Jan 10;254(1):22–25. [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Knudsen T. B., Blackburn M. R., Chinsky J. M., Airhart M. J., Kellems R. E. Ontogeny of adenosine deaminase in the mouse decidua and placenta: immunolocalization and embryo transfer studies. Biol Reprod. 1991 Jan;44(1):171–184. doi: 10.1095/biolreprod44.1.171. [DOI] [PubMed] [Google Scholar]
- Knudsen T. B., Winters R. S., Otey S. K., Blackburn M. R., Airhart M. J., Church J. K., Skalko R. G. Effects of (R)-deoxycoformycin (pentostatin) on intrauterine nucleoside catabolism and embryo viability in the pregnant mouse. Teratology. 1992 Jan;45(1):91–103. doi: 10.1002/tera.1420450109. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- Linch D. C., Levinsky R. J., Rodeck C. H., Maclennan K. A., Simmonds H. A. Prenatal diagnosis of three cases of severe combined immunodeficiency: severe T cell deficiency during the first half of gestation in fetuses with adenosine deaminase deficiency. Clin Exp Immunol. 1984 May;56(2):223–232. [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Miller M. W., Duhl D. M., Winkes B. M., Arredondo-Vega F., Saxon P. J., Wolff G. L., Epstein C. J., Hershfield M. S., Barsh G. S. The mouse lethal nonagouti (a(x)) mutation deletes the S-adenosylhomocysteine hydrolase (Ahcy) gene. EMBO J. 1994 Apr 15;13(8):1806–1816. doi: 10.1002/j.1460-2075.1994.tb06449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Solis R., Rivera-Pérez J., Wallace J. D., Wims M., Zheng H., Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992 Mar;201(2):331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- Ratech H., Greco M. A., Gallo G., Rimoin D. L., Kamino H., Hirschhorn R. Pathologic findings in adenosine deaminase-deficient severe combined immunodeficiency. I. Kidney, adrenal, and chondro-osseous tissue alterations. Am J Pathol. 1985 Jul;120(1):157–169. [PMC free article] [PubMed] [Google Scholar]
- Ratech H., Hirschhorn R., Greco M. A. Pathologic findings in adenosine deaminase deficient-severe combined immunodeficiency. II. Thymus, spleen, lymph node, and gastrointestinal tract lymphoid tissue alterations. Am J Pathol. 1989 Dec;135(6):1145–1156. [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Ullman B., Gudas L. J., Cohen A., Martin D. W., Jr Deoxyadenosine metabolism and cytotoxicity in cultured mouse T lymphoma cells: a model for immunodeficiency disease. Cell. 1978 Jun;14(2):365–375. doi: 10.1016/0092-8674(78)90122-8. [DOI] [PubMed] [Google Scholar]
- Winston J. H., Hanten G. R., Overbeek P. A., Kellems R. E. 5' flanking sequences of the murine adenosine deaminase gene direct expression of a reporter gene to specific prenatal and postnatal tissues in transgenic mice. J Biol Chem. 1992 Jul 5;267(19):13472–13479. [PubMed] [Google Scholar]
- Wu X., Wakamiya M., Vaishnav S., Geske R., Montgomery C., Jr, Jones P., Bradley A., Caskey C. T. Hyperuricemia and urate nephropathy in urate oxidase-deficient mice. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):742–746. doi: 10.1073/pnas.91.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Ubaidi M. R., Ramamurthy V., Maa M. C., Ingolia D. E., Chinsky J. M., Martin B. D., Kellems R. E. Structural and functional analysis of the murine adenosine deaminase gene. Genomics. 1990 Aug;7(4):476–485. doi: 10.1016/0888-7543(90)90189-2. [DOI] [PubMed] [Google Scholar]
- van der Weyden M. B., Bailey L. A micromethod for determining adenosine deaminase and purine nucleoside phosphorylase activity in cells from human peripheral blood. Clin Chim Acta. 1978 Jan 2;82(1-2):179–184. doi: 10.1016/0009-8981(78)90041-4. [DOI] [PubMed] [Google Scholar]