Abstract
The steroidogenic acute regulatory (StAR) protein, a novel mitochondrial protein, is involved in the regulation of steroid hormone biosynthesis through its mediation of the intramitochondrial transport of the steroid substrate, cholesterol, to the cytochrome P450 cholesterol side chain cleavage (P450scc) enzyme. The expression of StAR protein is regulated by cAMP-dependent signaling in steroidogenic cells. During the course of our studies in mouse Leydig cells, we employ several methods for studying the regulation of StAR protein expression by human chorionic gonadotropin (hCG). A sensitive quantitative reverse transcription and polymerase chain reaction (RT-PCR) was utilized for determining StAR mRNA expression. Stimulation of mLTC-1 mouse Leydig tumor cells with hCG resulted in the coordinate regulation of StAR mRNA expression and progesterone accumulation in a time-response manner. The validity and accuracy of quantitative RT-PCR results in mLTC-1 cells were verified by a competitive PCR approach and were further confirmed in primary cultures of isolated mouse Leydig cells. Immunoblotting studies demonstrated an increase in the levels of the StAR protein in a concentration dependent manner following hCG stimulation in mLTC-1 cells. Northern hybridization analysis revealed three StAR transcripts, all of which were of sufficient size to encode functional StAR protein, and which were coordinately expressed in response to hCG. Collectively, the experimental approaches utilized in the present investigation allow for the demonstration and characterization of hCG mediated regulation of StAR mRNA and StAR protein expression in mouse Leydig cells.
Keywords: Leydig cells, Chorionic Gonadotropin, Methods
Introduction
The classical mechanism utilized in tropic hormone-responsive steroid synthesis occurs through an increase in intracellular cAMP, which, in turn, triggers a regulatory cascade resulting in the mobilization and delivery of cholesterol from the outer to the inner mitochondrial membrane (1-3). Indeed, the intramitochondrial transport of cholesterol is the rate-limiting and regulated step in steroidogenesis and is dependent on de novo protein synthesis, as inhibitors of protein synthesis have been shown to decrease the steroidogenic response (4-6). It has been found that the inhibitor-sensitive step is present in the mitochondria and that protein synthesis inhibitors have no effect on the activity of cytochrome P450 cholesterol side chain cleavage (P450scc) complexes or on cholesterol accumulation in the outer mitochondrial membranes (2, 7). Therefore, the acute production of steroids requires a trophic hormone induced, rapidly synthesized, and cycloheximide sensitive regulatory protein that is involved in the transfer of cholesterol from the outer to the inner mitochondrial membrane (2-3, 7-8). Among the candidate regulatory proteins proposed in this transfer, the steroidogenic acute regulatory (StAR) protein has essentially all the necessary characteristics for cholesterol delivery and the initiation of steroidogenesis (9-10). The purification, cloning, and expression of the novel mitochondrial protein, StAR, was carried out for the first time in MA-10 mouse Leydig tumor cells (11). Since then cDNA clones for StAR have been isolated from rat (12), human (13), ovine (14), bovine (15), porcine (16), equine (17), monkey (GenBank accession no. AY007224) and hamster (18), and a high degree of homology has been demonstrated among these species.
The endocrine regulation of Leydig cell function occurs predominantly through the action of luteinizing hormone (LH)/hCG, interacting with its cognate receptor and coupling to the adenylate cyclase signal transduction system. It has been demonstrated that LH, its “superagonist” hCG, and analogs of their second messenger, cAMP, (dibutyryl cAMP, 8-bromo-cAMP), coordinately increase StAR protein and StAR mRNA levels in a time frame that is consistent with increased steroid production in mouse Leydig cells (10, 19-21). Also, StAR expression has been predominantly associated with the steroid producing cells of the testis, adrenal, and ovary in the adult (20, 22-23). While cAMP mediated signaling is the principle mechanism regulating StAR expression, several other agents and/or signals appear to play critical roles in this regulatory process (24-25). It is noteworthy, however, that the inhibition of StAR protein expression at the transcriptional or translational level results in a dramatic inhibition of steroid biosynthesis (26-28). Furthermore, compelling evidence regarding the role of the StAR protein in the regulation of steroidogenesis has been further implicated by targeted disruption of the StAR gene and by the study of patients suffering from lipoid congenital adrenal hyperplasia, both of which result in severe impairment of adrenal and gonadal steroid synthesis due to mutations in the StAR gene (22-23, 29-30). Thus, StAR protein plays an indispensable role in steroid biosynthesis by controlling the rate-limiting step i.e. the delivery of cholesterol from the outer to the inner mitochondrial membrane in steroidogenic cells. The present studies utilized several methods in demonstrating the regulation of StAR protein expression by hCG, using the murine Leydig tumor cell line (mLTC-1) as an experimental model (31).
Materials and Methods
Cell culture and stimulation experiments
Mouse Leydig tumor cells (mLTC-1) were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in RPMI 1640 medium as described previously (31-32). Cells were cultured in growth medium at 37°C under a humidified atmosphere of 95% air and 5% CO2. During splitting, the viability of the cells was determined with 0.4% trypan blue (w/v) exclusion. Cells were sub-cultured at a density of 5 x 104 cells/well in 24-well plates or 1.5 x 105 cells/well in 6-well plates for different experiments and grown for 24 h prior to their use in experiments.
On the day preceding an experiment, cells were washed two times with 0.01 M phosphate-buffered saline (PBS) and replaced with serum-free RPMI medium. The additives, stimulators and/or inhibitors [hCG (CR-127, 14,900 IU/mg, NHPP, Bethesda, MD) at 0-500 ng/ml as indicated in specific experiments; actinomycin D and cycloheximide (Sigma-Aldrich, St. Louis, MO) at 10 μg/ml], were constituted fresh, diluted, and applied to cells in serum-free medium. Cells were processed for total RNA or mitochondrial protein isolation, and the accumulation of progesterone in the medium (in specific experiments) was determined by specific radioimmunoassay.
Isolation, preparation, and incubation of mouse Leydig cells
Testicular Leydig cells from adult mouse (C57BL/6 strain, 2-3 months of age) were isolated and cultured utilizing procedures described previously (33-36). Testes were de-capsulated, interstitial cells were isolated in Dulbecco’s Modified Eagle’s Medium (DMEM)/F12 (Gibco-BRL, Life Technologies™, Grand Island, NY) containing 25 mM Hepes, pH 7.4, 18 mM NaHCO3 and 0.2% BSA (w/v), and dispersed by collagenase [0.2% (w/v), 22 min, 34°C, 95% O2/5% CO2]. Cells were filtered through sterile nylon gauze (mesh 0.5-0.8 mm) and washed with DMEM/F12 medium to remove the collagenase. Following washing, cells were purified using continuous Percoll (Amersham Biosciences AB, Uppsala, Sweden) density gradient (range 1.01-1.126 kg/L) centrifugation. During centrifugation, cell types partitioned as a result of their individual buoyant densities, and the Leydig cells, which gathered at approximately 1.07 kg/L of Percoll, were collected, washed, and plated for further experiments (34-35). The purity of the Leydig cells was assessed histochemically by 3β-hydroxysteroid dehydrogenase staining and approximately 70-80% of the cells were found to be positive (33). These cells were sub-cultured in DMEM/F12 growth medium supplemented with 10000 U/L penicillin and 50 mg/L streptomycin, at a density of 5 x 105 cells/well in 6-well plates. Following 48 h of plating, these cells were used for experiments.
Extraction of RNA, and quantitative reverse transcription and polymerase chain reaction (RT-PCR) (Protocol I)
Total RNA was extracted from control and hCG stimulated cells using Trizol reagent (Invitrogen™ life technologies, Carlsbad, CA), as described previously (32, 37). The isolation and amplification of mLTC-1 cell StAR cDNA were carried out utilizing primers designed from the mouse StAR cDNA sequence (11). The sense primer, 5’-GACCTTGAAAGGCTCAGGAAGAAC-3’, and the antisense primer, 5’-TAGCTGAAGATGGACAGACTTGC-3’, spanned bases -51 to -27 and 931 to 908 respectively, relative to the first base of the translation initiation codon. The variation in RT-PCR efficiency was evaluated with the L19 ribosomal protein gene as an internal control, using the sense primer 5’-GAAATCGCCAATGCCAACTC-3’ (bases 154 to 173) and the antisense primer 5’-TCTTAGACCTGCGAGCCTCA-3’ (bases 559 to 540) (38).
RT and PCR of the target genes were run sequentially in the same assay tube as described previously (32, 35, 39). Briefly, total RNA (1-2 μg) from different experimental groups was reverse transcribed using avian myeloblastosis virus reverse transcriptase (AMV-RT; Promega Corporation, Madison, WI) and the antisense primers. StAR and L19 cDNAs generated were further amplified by PCR using the primer pairs listed above. The total reaction volume was 50 μl and contained 1 nM of each oligo primer, 200 μM of a deoxy-NTP mixture including [α32P]-CTP, 20 U RNasin, 5 U AMV-RT and 2.5-5 U Taq DNA polymerase in 1 x PCR buffer [20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 50% glycerol (v/v), 0.5% Nonidet-P40 (v/v) and 0.5% Tween-20 (v/v)]. The reaction was initiated at 50°C for 15 min (RT) followed by denaturation at 97°C for 5 min. Then PCR was performed using a variable number of cycles of amplification that were defined by denaturation at 96°C for 1.5 min, annealing at 55°C for 1.5 min and extension at 72°C for 3 min (PTC-100, Programmable™ Thermal Controller, MJ Research, Inc., Waltham, MA). The number of PCR cycles utilized was 19, which were optimized to be in the exponential phase of the PCR (21, 35). A final cycle of extension at 72°C for 16 min was also included. A 20-μl aliquot of each PCR product was analyzed by gel electrophoresis on a 1.2% agarose (w/v) gel. The molecular sizes of the amplified products (StAR and L19) were determined by comparison with the molecular weight markers (100 bp DNA ladder, Promega) run in parallel with RT-PCR products. The gels were then vacuum dried, exposed to X-ray films (Marsh Bio Products, Inc., Rochester, NY) at room temperature for 1 to 3 h, and the relative levels of the different signals were quantified using a computer-assisted image analysis system (Visage 2000, BioImage, Ann Arbor, MI). The integrated optical density (IOD) values for StAR in each band were normalized with the corresponding L19 expression, and the level of StAR mRNA in response to hCG was determined.
Quantitative competitive RT-PCR
This procedure was employed by generating a competitor of StAR from the mouse StAR cDNA (35). Briefly, full length StAR cDNA produced by RT-PCR was sub-cloned into the pGEM T-vector following the instructions of the manufacturer (Promega).
Analysis of restriction sites in the mouse StAR cDNA demonstrated the presence of two AvaI sites. Taking advantage of the two AvaI sites, the sub-cloned product was digested and separated in a 1.2% agarose (w/v) gel. Following electrophoresis, a 407-bp band consisting of the AvaI fragment from the full length StAR cDNA was excised from the gel. The remaining fragments (StAR cDNA sub-cloned into pGEM T-vector) were subjected to blunt-end ligation by the Klenow fragment of DNA polymerase I, and the strand was re-circularized by T4 DNA ligase (Promega). Thus, a StAR cDNA containing a 407-bp deletion was generated and inserted into the cloning site of the pGEM T-vector. The identity of all inserted fragments was tested by restriction endonuclease digestion and confirmed by sequencing. Transcription of the ApaI linearized template with the Sp6 RNA polymerase (Promega) generated a sense cRNA of approximately 570 bp in length, a fragment that was used as the StAR competitor. To perform competitive RT-PCR, decreasing concentrations of the StAR competitor (homologous cRNA, 1000-9.3 ng) were amplified together with a fixed amount of total RNA (1 μg from control and treated groups), using the same primer pairs utilized above. The molecular sizes of the RT-PCR products (StAR, StAR competitor and L19) were determined in 1.2% agarose (w/v) gels that were vacuum dried and following exposure to X-ray films, the bands were quantified as above (Visage 2000). The IOD values (StAR/StAR competitor) were normalized with the corresponding L19 expression, and the response of hCG on StAR mRNA expression was assessed.
Isolation of mitochondria and immunoblotting (Protocol II)
Isolation of mitochondria from mLTC-1 cells was performed by differential centrifugation (21, 37). Cells were washed with 0.01 mM PBS, scraped, and collected into a Tris-sucrose-EDTA buffer (10 mM Tris-HCl, pH 7.2, 250 mM sucrose, 0.1 mM EDTA) containing 1.0 mM phenyl methyl sulfonyl fluoride as a protease inhibitor. Cells were homogenized at 4°C (1200 rpm, 30 strokes) with a Potter-Elvehjem homogenizer. The homogenate was centrifuged at 600 x g for 25 min to remove broken cell debris and nuclei, and the resulting supernatant was further centrifuged at 9000 x g for 25 min. The pellet containing mitochondria was washed two times in the same buffer and recovered by centrifugation at 9000 x g for 10 min.
Mitochondrial protein (20 μg) was solubilized in sample buffer [25 mM Tris-Cl, pH 6.8, 1% sodium dodecyl sulphate (SDS, w/v), 5% β-mercaptoethanol (v/v), 10% glycerol (v/v) and 0.01% bromophenol blue (v/v)] and loaded onto a 12% SDS (w/v)-polyacrylamide gel electrophoresis (SDS-PAGE) (Mini Protean II System, Bio-Rad), as described by Laemmli (40), with minor modifications (21, 37). Electrophoresis was performed at 200 V for 1 h and the proteins were electrophoretically transferred onto Immuno-Blot™ PVDF Membrane (Bio-Rad, Hercules, CA). The membranes were incubated in a blocking buffer [Tris-buffered saline, TBS containing 0.2% Tween-20 (v/v) and 4% Carnation non-fat dry milk (w/v)] for 1 h at room temperature, and followed by incubation for an additional 1 h with anti-peptide antibodies (1:20000 dilution) generated in rabbits against an amino acid fragment (88-98) of the StAR protein (11). The membranes were washed three times (10 min each) in TBS buffer and incubated for 1 h at room temperature with horseradish peroxidase-labeled goat anti-rabbit IgG (1:20000) (Biosource International, Camarillo, CA). The membranes were washed again as above, and immunodetection of the StAR protein was performed with a Chemiluminescence Imaging Western Lightning Kit (PerkinElmer, Boston, MA). The membranes were exposed to X-ray films for 1-3 min (Marsh Bio Products), and the immuno-specific StAR band was quantified as above (Visage 2000).
Northern hybridization analysis (Protocol III)
Total RNA was isolated from different treatment groups using Trizol reagent (Invitrogen). Total RNA (15-20 μg) was resolved on 1.2% denaturing formaldehyde agarose (w/v) gels and transferred onto Hybond-N+ nylon membranes (Amersham Pharmacia Biotech, Piscataway, NJ) by employing the capillary transfer method. An antisense cRNA probe corresponding to bases -27 to 931, a NotI fragment of the StAR cDNA, was utilized in Northern analysis for detection of the StAR mRNA expression. This fragment was produced by in vitro transcription (Promega) with T7 RNA polymerase, dNTPs and [α32P]-UTP (specific activity, ~30 TBq/mmol; Amersham Pharmacia Biotech). The 32P-labeled probes were purified using Sephadex G-50 nick columns (Amersham Pharmacia Biotech). Pre-hybridization and hybridization were carried out under stringent conditions (21, 28). Pre-hybridization was carried out for 6 h at 65°C in a solution containing 50% formamide (v/v), 3 x SSC (150 mM NaCl, 50 mM sodium citrate, pH 7.0), 5 x Denhardt’s solution, 1% SDS (w/v), 0.1 mg/ml heat-denatured calf thymus DNA and 100 μg/ml yeast tRNA. Hybridization was performed at 66°C for 16 h in the same solution after addition of the 32P-labeled probe. In the case of the human glyceraldehyde-3-phosphate dehydrogenese (GAPDH) cDNA probe, pre-hybridization and hybridization were carried out at 42°C. The membranes were washed twice at room temperature for 20 min with 2 x SSC containing 0.1% SDS (w/v), followed by 2 h at 66°C with 0.1 x SSC and 0.1% SDS (w/v) until removal of the background counts. The membranes were then exposed to X-ray films (Marsh Bio Products) for 36-48 h at -80°C. To examine the variation in StAR mRNA expression, the membranes were subjected to re-hybridization with a GAPDH cDNA probe.
Statistical analysis
Statistical analysis was performed using one-way ANOVA followed by Fisher’s protected least significant differences test using the Statview Program (Abacus Concepts Inc., Berkeley, CA). Data represent the mean ± S.E., and P < 0.05 was considered to be statistically significant.
Results and Discussion
Assessment of hCG function in mouse Leydig cells, and the regulation of StAR expression
The steroidogenic acute regulatory (StAR) protein has been implicated in the regulation of steroid biosynthesis by virtue of its expression through cAMP dependent mechanisms in steroidogenic cells. Thus, StAR expression has been demonstrated to respond rapidly to various signals that regulate steroidogenesis in the adrenals and gonads (25, 41). LH/hCG plays a key role in regulating various Leydig cell functions including steroid biosynthesis through activation of multiple second messenger systems, as well as by intratesticular autocrine and paracrine impulses (42-44). In particular, the interaction of LH/hCG with its specific receptors on Leydig cell membranes is followed by a sequence of events that lead to intracellular modifications and the stimulation of steroidogenesis (45-46). Given the importance of the StAR protein in this process, the involvement of hCG in the regulation of StAR expression was investigated in mouse Leydig cells.
Treatment of mLTC-1 cells with hCG (50 ng/ml) resulted in a time dependent increase in the steady state levels of StAR mRNA and progesterone production (Fig. 1). hCG induced StAR mRNA expression was found to be significant (p < 0.05) at 1 h. The magnitude of the response reached a maximum of 6.8 ± 0.55 fold by 6 h, and thereafter StAR mRNA levels gradually declined. Although, the expression of StAR mRNA remained elevated over controls at all the time points studied. Progesterone accumulation in the media was measured at each time point, and was shown to parallel StAR mRNA levels (Fig. 1). The increase in progesterone in the media was significant within 1 h (p < 0.01), increased gradually to 37 ± 6.2 fold by 6 h, and then followed the pattern of StAR mRNA expression. In fact, following hCG stimulation, the time course of StAR mRNA expression and progesterone production demonstrate an intimate relationship between the two, an observation that is in agreement with previous findings (20, 47).
Fig. 1. Temporal response pattern of hCG stimulated StAR mRNA expression and progesterone production in mLTC-1 cells.
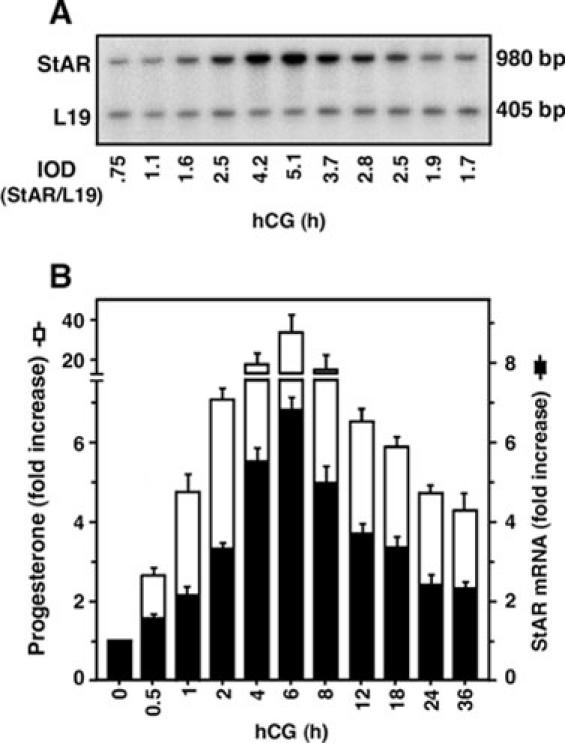
A representative autoradiogram illustrates StAR mRNA expression in response to hCG at different times (0-36 h), as determined by quantitative RT-PCR (panel A). Panel B illustrates the integrated optical density (IOD) of StAR mRNA expression at each time point after normalizing with the corresponding L19 bands (black bar). The production of progesterone in the media of the same samples was determined (white bar). Data represent the mean ± S.E. of three independent experiments.
In additional experiments, the accuracy and efficacy of the quantitative RT-PCR assay were verified by employing competitive PCR. To perform this, fixed amounts of total RNA (1 μg) obtained from control and hCG treated samples were allowed to compete with varying amounts of a StAR competitor (1000-9.3 ng). The results shown in Fig. 2 demonstrate that amplification of decreasing amounts of the competitor with fixed amounts of total RNA resulted in signals of decreasing intensity. In contrast, StAR mRNA expression decreased gradually as the concentrations of competitor increased, reflecting the specific nature of competitive PCR. The ratios of the intensities of StAR and StAR competitor in both cases were normalized with the corresponding L19 signals. The results of the competitive PCR clearly demonstrate that hCG induced a 6.7 fold increase in StAR mRNA expression when compared to control (horizontal line) (Fig. 2), an observation agreeing with and confirming the quantitative RT-PCR results (35).
Fig. 2. Schematic representation of the competitive RT-PCR to verify the quantitative accuracy of hCG induced StAR mRNA expression in mLTC-1 cells.
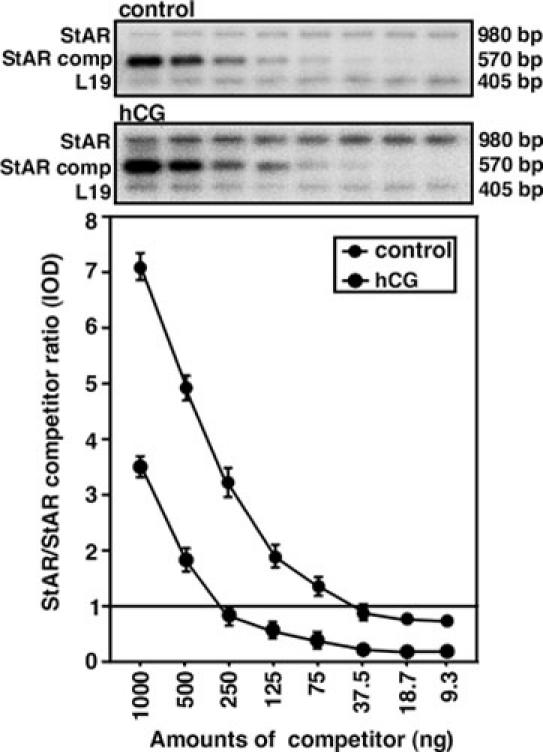
Decreasing amounts of StAR competitor (1000-9.3 ng) were incubated with a fixed amount of total RNA from control and hCG-treated samples, as described in Materials and Methods. The IOD ratio of StAR/StAR competitor from both control and hCG-stimulated groups was corrected for using the corresponding intensity of the L19 bands. The situation where the amplicon ratio is 1 (horizontal line) indicates hCG induced StAR mRNA expression when compared with two experimental groups. Data represent the mean ± S.E. of two independent experiments. StAR comp, StAR competitor.
Several lines of evidence demonstrate that StAR expression is regulated by cAMP-dependent mechanisms and that its expression is fundamentally associated with steroid producing cells (10, 20,22, 48). The present data are also consistent with previous studies that demonstrated stimulation of MA-10 cells with a cAMP analog coordinately increased StAR mRNA, StAR protein and steroid production (10, 20, 37). Also, in mouse Leydig cells, thyroid hormone has been reported to increase the levels of StAR protein and StAR mRNA, and their increased expression occurs in a time frame that parallels the acute production of steroids (28, 35, 49). It was also demonstrated that hCG and thyroid hormone (which mediate their actions through plasma membrane and nuclear receptor systems, respectively) have additive effects on StAR expression and steroidogenesis (35). Notably, a temporal relationship between the expression of the StAR gene and the capacity to produce steroid hormones has been demonstrated during development (20, 22-23). Never-theless, the mechanism of hormone action on StAR expression, in most cases, is due to a cAMP-mediated change in StAR transcription and/or StAR mRNA stability.
The functional relevance of hCG induced StAR mRNA expression in mLTC-1 cells was confirmed in primary cultures of isolated mouse Leydig cells. As determined by RT-PCR analysis (Fig. 3), stimulation of Leydig cells with hCG (50 ng/ml, 6 h) resulted in an approximate 2 fold increase in StAR mRNA expression when compared to controls. The accumulation of testosterone in the media was increased by 175% in response to hCG under a similar experimental paradigm (not illustrated), indicating the direct link between StAR expression and steroidogenesis. Taken together, these findings further strengthen the role of LH as a physiological regulator of various Leydig cell functions including steroidogenesis.
Fig. 3. StAR mRNA expression in response to hCG in isolated adult mouse Leydig cells.
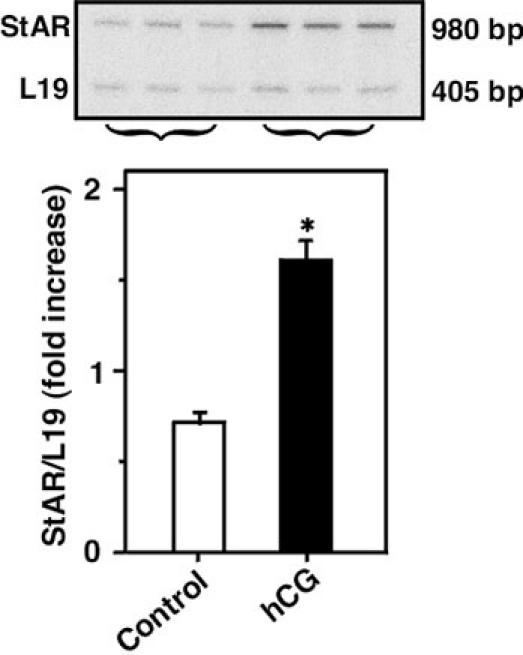
A representative autoradiogram shows RT-PCR analysis of StAR mRNA expression using 2 μg of total RNA from control and hCG (50 ng/ml, 6 h) treated groups (upper panel). IOD values (lower panel), *, p < 0.05, control vs. hCG.
Given the temporal pattern of hCG mediated StAR mRNA expression and steroid production, the pattern of the 30-kDa form of the StAR protein in response to hCG was investigated in mLTC-1 cells. Cells stimulated for 6 h with varying concentrations of hCG (0-500 ng/ml) demonstrated a dose-dependent increase in StAR protein levels (Fig. 4). The expression of StAR protein in response to hCG was detected (p < 0.05) at concentrations ≥ 5 ng/ml. The half-maximal stimulation occurred at 19 ng/ml, and the maximal increase at doses of ≥ 50 ng/ml (39). The involvement of hCG in StAR expression and steroidogenesis in Leydig cells has been demonstrated to be up-regulated by insulin-like growth factor–1 and also by prolactin (36, 50). In addition, extracellular Ca2+ has been shown to increase the stimulatory action of hCG on StAR expression and steroid production in mouse Leydig cells (21). Consistent with the current data, several studies have shown a striking correlation between the synthesis of steroids and the expression of StAR protein (10, 35, 51-52).
Fig. 4. Dose response pattern of hCG mediated StAR protein expression by immunoblotting.
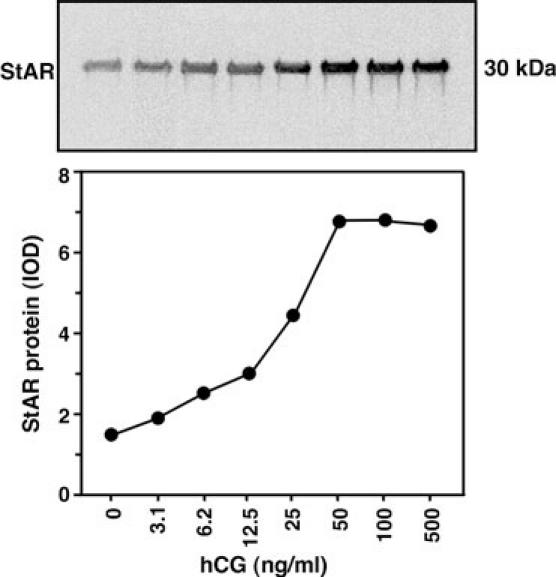
mLTC-1 cells were treated with varying doses of hCG (0-500 ng/ml) for 6 h, and a representative autoradiogram (and corresponding IOD values) illustrates the expression of the 30-kDa StAR protein. Data are representative of two independent experiments.
The roles of transcription and ongoing protein synthesis in hCG stimulated StAR expression were then evaluated. As illustrated in Fig. 5, mLTC-1 cells stimulated with hCG (50 ng/ml) for 6 h, or in the presence of actinomycin D (Act. D., inhibits RNA synthesis) and cycloheximide (Chx., inhibits protein synthesis), demonstrated an attenuation of StAR mRNA expression in response to hCG. A full length mouse StAR probe hybridized with three transcripts at 3.4, 2.7 and 1.6 kb, and all of them showed coordinated up-regulation by hCG. This observation is in agreement with previous findings that demonstrated the presence of three or four StAR transcripts of 3.4, 2.7, 1.6, and 1.4 kb in the mouse (20-21, 35, 53), and three of 7.4, 4.4, and 1.6 kb in the human (13). Act. D. and Chx. have been demonstrated to inhibit hCG and/or cAMP stimulated StAR expression and steroid production in MA-10 Leydig and human granulosa-lutein and thecal cells (26, 47-48). The present data also conform to the observation that inhibition of StAR expression at the level of transcription or translation results in a marked decrease in steroid synthesis (26-28, 48), and further demonstrates that StAR plays a crucial role in regulating steroid biosynthesis.
Fig. 5. StAR mRNA expression in mLTC-1 cells in response to hCG stimulation requires transcription and ongoing protein synthesis.
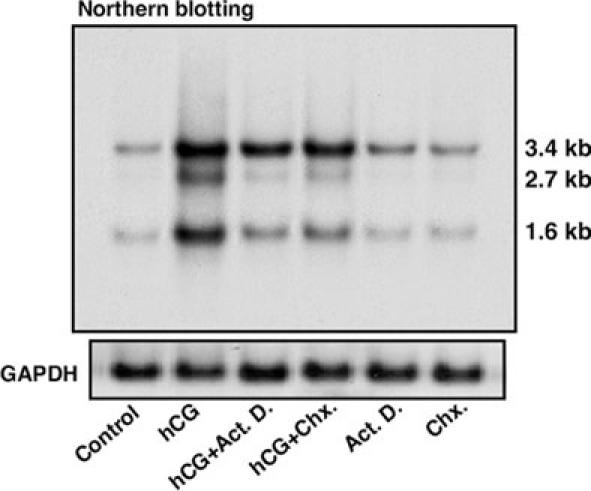
A representative autoradiogram of a Northern blot (upper panel) illustrates the apparent molecular sizes of the different StAR transcripts. The GAPDH mRNA expression demonstrates equal RNA loading (lower panel). Similar results were obtained from three independent experiments.
Trophic hormone or cAMP analog stimulation increases PKA activity, which then results in the induction of StAR protein expression. In mouse Leydig cells it was presently demonstrated that hCG was able to increase the expression of StAR mRNA, StAR protein and steroidogenesis in a time- and dose-responsive manner and did so utilizing a mechanism that required ongoing protein synthesis. While cAMP-mediated signaling regulates expression of the StAR gene, its promoter lacks a consensus cAMP response-element (CRE). The mechanism regulating StAR transcription in the absence of a canonical CRE has been demonstrated to be mediated by multiple DNA elements that are found to be located in a transcription factor-binding site-rich region in the StAR promoter (reviewed in Ref. 24). StAR expression has also been influenced by various compounds, including peptide and non-peptide hormones, growth factors, gonadotropin-releasing hormone, prostaglandins and steroids, acting through endocrine, autocrine and paracrine mechanisms (36, 43, 50, 54-56). It has also been demonstrated that phosphorylation of the StAR protein is critical in producing full cholesterol transferring activity (57-58). Moreover, several additional intracellular factors involved in various signaling pathways have also been demonstrated to be instrumental in regulating StAR expression, including calcium messenger systems, arachidonic acid metabolites, chloride ion channels, and mitogen activated protein kinase/extracellular signal-related kinases (21, 25, 59-64). Based on these many observations, it is highly likely that the combined action of multiple factors and signaling pathways are involved in controlling hCG mediated StAR expression and thus steroid biosynthesis in Leydig cells.
Acknowledgments
This investigation was supported in part by funds from NIH grant HD17481 and the Robert A. Welch Foundation to DMS, and the Sigrid Jusélius Foundation and Academy of Finland to ITH.
Appendix
Protocols
Protocol I: Quantitative RT-PCR
Isolate total RNA from mLTC-1 cells of different treatment groups with Trizol reagent (Invitrogen) or any commercially available product according to the manufacturer’s instructions.
At the end of extraction, resuspend total RNA in diethyl pyrocarbonate (DEPC)-treated water, and determine the purity of the RNA by scanning with a spectrophotometer at a wavelength of 220-320 nm. Dilute the total RNA in DEPC water (1-2 μg/5 μl) on ice and use it directly in the RT-PCR assay.
Resuspend the specific primer pairs (see Materials and Methods) in nuclease-free water for the isolation and amplification of StAR cDNA from mLTC-1 cells. Similarly, dilute the 2nd primer pairs (L19, as an internal control) to verify RT-PCR efficiency.
The total reaction volume should be 50 μl. Perform RT and PCR of the target genes sequentially in the same assay tube.
Prepare an incubation mixture which includes all the oligo primers (1 nM of each, 5 μl), 200 μM of a deoxy-NTP mixtures (GTP, TTP, ATP, and CTP) containing [α32P]-CTP (2 μl), 20 U RNasin (RNAse inhibitor. 0.5 μl), 5 U AMV-RT (0.5 μl) and 2.5-5 U Taq DNA polymerase (0.5-1.0 μl) in 1 x PCR buffer (see Materials and Methods). Adjust the total volume with nuclease-free water.
Add total RNA to the incubation mixture. Mix gently and add a drop or 25 μl of mineral oil (Sigma-Aldrich) to prevent evaporation during the RT-PCR. Mix again, centrifuge (800 rpm for 1 min at 4°C) and place the reaction tubes (0.5 ml thin-walled) in the thermal controller (MJ Research Inc.).
Run a program for RT and PCR at the same time using a programmable thermal controller (MJ Research Inc.). Start the reaction at 50°C for 15 min (RT) followed by denaturation at 97°C for 5 min. Following RT, run the PCR with a variable number of cycles that are defined by denaturation at 96°C for 1.5 min, annealing at 55°C for 1.5 min and extension at 72°C for 3 min.
Evaluate the varying numbers of PCR cycles (exponential, linear and plateau phases) for StAR expression and choose one that is in the exponential phase of the PCR. Under the present incubation conditions, 19 cycles were chosen for further analysis (31, 35). Utilize a final cycle of extension at 72°C for 16 min. Prepare an 1.2% agarose (w/v) gel following standard procedures to examine the PCR products by gel electrophoresis. Use 0.5 x TBE (0.05 M Tris-borate and 1.0 mM EDTA) as the running electrophoresis buffer.
Electrophorese an aliquot (20 μl) of each PCR product in the gel. The molecular sizes of the amplified products (approximately 980 bp for StAR and 405 bp for L19) were determined by comparison with the molecular weight markers (100 bp DNA ladder, Promega) run in parallel with the RT-PCR products.
Vacuum dry the gels and expose them to X-ray films (Marsh Bio Products) at room temperature for 1-3 h.
Quantify the relative levels of the different signals using an image analysis instrument (such as the Visage 2000), and normalize the expression of StAR mRNA in each band to the corresponding L19 expression.
Protocol II: Mitochondrial isolation, SDS-PAGE, and immunoblotting
Following treatment, wash mLTC-1 cells with 0.01 mM PBS. Collect cells for isolation of mitochondria by differential centrifugation in Tris-sucrose-EDTA buffer containing protease inhibitor (see Material and Methods).
Homogenize cells at 4°C (1200 rpm, 30 strokes) with a motor driven, glass-teflon homogenizer. Centrifuge the homogenate at 500 x g for 25 min to remove broken cell debris and nuclei. Centrifuge the resulting supernatant at 9000 x g for 25 min to pellet the mitochondria.
Wash the pellet two times by resuspension and centrifugation at 9000 x g for 10 min in Tris-sucrose-EDTA buffer. Resuspend the pellet, measure the protein content (65), and store at -80°C until use.
Cast a 12% SDS (w/v)-PAGE gel following a standard protocol according to the procedure of Laemmli (40).
Solubilize 20 μg of mitochondrial protein in sample buffer (see Materials and Methods) and load onto the 12% SDS (w/v)-PAGE (Mini Protean II System, Bio-Rad).
Perform electrophoresis at 200 V for 1 h.
Transfer the proteins electrophoretically onto Immuno-Blotä PVDF Membrane (Bio-Rad) at 100 V for 1 h.
Incubate the membranes in a blocking buffer [TBS with 0.2% Tween-20 (v/v) and 4% non-fat dry milk (w/v)] with slow shaking for about 1 h at room temperature or overnight at 4°C.
Incubate with anti-StAR peptide antibodies (1:20000) with slow shaking for 1 h.
Wash the membranes three times (10 min each) in TBS buffer and incubate for 1 h at room temperature with horseradish peroxidase-labeled goat anti-rabbit IgG (1:20000).
Wash the membranes again as described above. Detect the StAR protein using the Chemiluminescence Imaging Western Lightning Kit (PerkinElmer).
Expose the membranes for 1-3 min to X-ray films (Marsh Bio Products) and quantify the immuno-specific StAR band as described above.
Protocol III: Northern blotting
Isolate total RNA from mLTC-1 cells with Trizol reagent (Invitrogen) or any commercially available product according to the manufacturer’s instructions.
Prepare and cast a 1.2% denaturing formaldehyde agarose gel [agarose (1.2 gm, w/v), 10 x Mops (Sigma-Aldrich) buffer (10 ml), DEPC water (73 ml), formaldehyde (37%, v/v; 17 ml)] following standard protocol.
Incubate total RNA (15-20 μg) at 65°C for 5-8 min in a mixture containing de-ionized formamide (12.5 μl), 10 x Mops buffer (2.5 μl), formaldehyde (4 μl), ethidium-bromide (0.1 μl) and then chill on ice. Add 2.5 μl of 6 x loading dye (Promega).
Electrophorese the samples for 14-16 h in 1 x Mops buffer at 25 V.
Transfer the RNA onto a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech) using the capillary transfer method. Fix the RNA under UV light, dry the membranes and store at 4°C until use.
Prepare a cRNA probe, a NotI fragment of the mouse StAR cDNA, by in vitro transcription (see Materials and Methods). The reaction mixture should include 5 x labeling buffer (5 μl), DNA template (~100 ng), dNTPs (1 μl), nuclease-free BSA (1 μl), T7 RNA polymerase (1 μl), and [α32P]-UTP (2.5 μl). Adjust the total volume to 25 μl with nuclease-free water.
Incubate the mixture at 37°C for 1 h, add RQ1 RNAse-free DNAse (5 μl, Promega) and incubate again for 15 min. Purify the 32P-labeled probes using a Sephadex G-50 nick column by eluting with 0.5 M Tris-EDTA (pH 8.0) containing 20% SDS (w/v).
Pre-hybridize the membranes for 6 h at 65°C in a solution containing 50% formamide (v/v), 3 x SSC, 5 x Denhardt’s solution (see Molecular Cloning, Cold Spring Harbor Laboratory, 1989, Volume 2, pp 9.47-9.49) 1% SDS (w/v), 0.1 mg/ml heat-denatured calf thymus DNA and 100 μg/ml yeast tRNA, under stringent conditions.
Add 32P-labeled probe to the same solution and hybridize the membranes at 66°C for 16 h. Wash the membranes at room temperature for 20 min with 2 x SSC containing 0.1% SDS (w/v), followed by 2 h at 66°C with 0.1 x SSC and 0.1% SDS (w/v) until the background counts are removed.
Expose the membranes to X-ray film for 36-48 h at -80°C, and determine the level of StAR expression in the different transcripts.
To examine the variation in StAR mRNA expression, re-hybridize the same membranes with a cDNA probe for the human GAPDH. Use 42°C for both pre-hybridization and hybridization in the case of the GAPDH probe.
Assess GAPDH expression for verification of RNA loading.
References
- Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Privalle CT, Crivello JF, Jefcoate CR. Regulation of intramitochondrial cholesterol transfer to side-chain cleavage cytochrome P-450 in rat adrenal gland. Proc Natl Acad Sci USA. 1983;80:702–706. doi: 10.1073/pnas.80.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate CR, DiBartolomeis MJ, Williams CA, McNamara BC. ACTH regulation of cholesterol movement in isolated adrenal cells. J Steroid Biochem. 1987;27:721–729. doi: 10.1016/0022-4731(87)90142-7. [DOI] [PubMed] [Google Scholar]
- Ferguson JJ. Protein synthesis and adrenocorticotropin responsiveness. J Biol Chem. 1963;238:2754–2759. [PubMed] [Google Scholar]
- Garren LD, Ney RL, Davis WW. Studies on the role of protein synthesis in the regulation of corticosterone production by adrenocorticotropic hormone in vivo. Proc Natl Acad Sci USA. 1965;53:1443–1450. doi: 10.1073/pnas.53.6.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis WW, Garren LD. On the mechanism of action of adrenocorticotropic hormone. The inhibitory site of cycloheximide in the pathway of steroid biosynthesis. J Biol Chem. 1968;243:5153–5157. [PubMed] [Google Scholar]
- Simpson ER, McCarthy JL, Peterson JA. Evidence that the cycloheximide-sensitive site of adrenocorticotropic hormone action is in the mitochondrion. Changes in pregnenolone formation, cholesterol content, and the electron paramagnetic resonance spectra of cytochrome P-450. J Biol Chem. 1978;253:3135–3139. [PubMed] [Google Scholar]
- Stocco DM, Kilgore MW. Induction of mitochondrial proteins in MA-10 Leydig tumour cells with human choriogonadotropin. Biochem J. 1988;249:95–103. doi: 10.1042/bj2490095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WL. Mitochondrial specificity of the early steps in steroidogenesis. J Steroid Biochem Mol Biol. 1995;55:607–616. doi: 10.1016/0960-0760(95)00212-X. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Wells J, King SR, Stocco DM. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA-10 mouse Leydig tumor cells. Characterization of the steroidogenic acute regulatory protein (StAR). J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- Sandhoff TW, Hales DB, Hales KH, McLean MP. Transcriptional regulation of the rat steroidogenic acute regulatory protein gene by steroidogenic factor 1. Endocrinology. 1998;139:4820–4831. doi: 10.1210/endo.139.12.6345. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Holt JA, Driscoll D, Strauss JF III, Lin D, Miller WL, Patterson D, Clancy KP, Hart IM, Clark BJ, Stocco DM. Human steroidogenic acute regulatory protein: functional activity in COS-1 cells, tissue-specific expression, and mapping of the structural gene to 8p11.2 and a pseudogene to chromosome 13. Proc Natl Acad Sci USA. 1995;92:4778–4782. doi: 10.1073/pnas.92.11.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengel JL, Meberg BM, Turzillo AM, Nett TM, Niswender GD. Hormonal regulation of messenger ribonucleic acid encoding steroidogenic acute regulatory protein in ovine corpora lutea. Endocrinology. 1995;136:5423–5429. doi: 10.1210/endo.136.12.7588291. [DOI] [PubMed] [Google Scholar]
- Hartung S, Rust W, Balvers M, Ivell R. Molecular cloning and in vivo expression of the bovine steroidogenic acute regulatory protein. Biochem Biophys Res Commun. 1995;215:646–653. doi: 10.1006/bbrc.1995.2513. [DOI] [PubMed] [Google Scholar]
- Pilon N, Daneau I, Brisson C, Ethier JF, Lussier JG, Silversides DW. Porcine and bovine steroidogenic acute regulatory protein (StAR) gene expression during gestation. Endocrinology. 1997;138:1085–1091. doi: 10.1210/endo.138.3.5003. [DOI] [PubMed] [Google Scholar]
- Kerban A, Boerboom D, Sirois J. Human chorionic gonadotropin induces an inverse regulation of steroidogenic acute regulatory protein messenger ribonucleic acid in theca interna and granulosa cells of equine preovulatory follicles. Endocrinology. 1999;140:667–674. doi: 10.1210/endo.140.2.6499. [DOI] [PubMed] [Google Scholar]
- Fleury A, Ducharme L, LeHoux JG. In vivo effects of adrenocorticotrophin on the expression of the hamster steroidogenic acute regulatory protein. J Mol Endocrinol. 1998;21:131–139. doi: 10.1677/jme.0.0210131. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Sodeman TC. The 30-kDa mitochondrial proteins induced by hormone stimulation in MA- 10 mouse Leydig tumor cells are processed from larger precursors. J Biol Chem. 1991;266:19731–19738. [PubMed] [Google Scholar]
- Clark BJ, Soo SC, Caron KM, Ikeda Y, Parker KL, Stocco DM. Hormonal and developmental regulation of the steroidogenic acute regulatory protein. Mol Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- Manna PR, Pakarinen P, El-Hefnawy T, Huhtaniemi IT. Functional assessment of the calcium messenger system in cultured mouse Leydig tumor cells: regulation of human chorionic gonadotropin-induced expression of the steroidogenic acute regulatory protein. Endocrinology. 1999;140:1739–1751. doi: 10.1210/endo.140.4.6650. [DOI] [PubMed] [Google Scholar]
- Caron KM, Soo SC, Wetsel WC, Stocco DM, Clark BJ, Parker KL. Targeted disruption of the mouse gene encoding steroidogenic acute regulatory protein provides insights into congenital lipoid adrenal hyperplasia. Proc Natl Acad Sci USA. 1997;94:11540–11545. doi: 10.1073/pnas.94.21.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa T, Zhao L, Caron KM, Majdic G, Suzuki T, Shizawa S, Sasano H, Parker KL. Developmental roles of the steroidogenic acute regulatory protein (StAR) as revealed by StAR knockout mice. Mol Endocrinol. 2000;14:1462–1471. doi: 10.1210/mend.14.9.0515. [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, Handelsman DJ. Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology. 2004;145(4):1587–1593. doi: 10.1210/en.2003-1164. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Combs R, Hales KH, Hales DB, Stocco DM. Inhibition of transcription affects synthesis of steroidogenic acute regulatory protein and steroidogenesis in MA-10 mouse Leydig tumor cells. Endocrinology. 1997;138:4893–4901. doi: 10.1210/endo.138.11.5535. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Ranganathan V, Combs R. Post-translational regulation of steroidogenic acute regulatory protein by cAMP-dependent protein kinase A. Endocr Res. 2000;26:681–689. doi: 10.3109/07435800009048587. [DOI] [PubMed] [Google Scholar]
- Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology. 2001;142:319–331. doi: 10.1210/endo.142.1.7900. [DOI] [PubMed] [Google Scholar]
- Lin D, Sugawara T, Strauss JF III, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. Science. 1995;267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- Bose HS, Sugawara T, Strauss JF III, Miller WL. The pathophysiology and genetics of congenital lipoid adrenal hyperplasia. International Congenital Lipoid Adrenal Hyperplasia Consortium. N Engl J Med. 1996;335:1870–1878. doi: 10.1056/NEJM199612193352503. [DOI] [PubMed] [Google Scholar]
- Rebois RV. Establishment of gonadotropin-responsive murine leydig tumor cell line. J Cell Biol. 1982;94:70–76. doi: 10.1083/jcb.94.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manna PR, Huhtaniemi IT, Wang XJ, Eubank DW, Stocco DM. Mechanisms of epidermal growth factor signaling: regulation of steroid biosynthesis and the steroidogenic acute regulatory protein in mouse leydig tumor cells. Biol Reprod. 2002;67:1393–1404. doi: 10.1095/biolreprod.102.007179. [DOI] [PubMed] [Google Scholar]
- Mendelson C, Dufau M, Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'- monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975;250:8818–8823. [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Huhtaniemi I. Vasoactive intestinal peptide is an important endocrine regulatory factor of fetal rat testicular steroidogenesis. Endocrinology. 1998;139:1474–1480. doi: 10.1210/endo.139.4.5861. [DOI] [PubMed] [Google Scholar]
- Manna PR, Tena-Sempere M, Huhtaniemi IT. Molecular mechanisms of thyroid hormone-stimulated steroidogenesis in mouse Leydig tumor cells. Involvement of the steroidogenic acute regulatory (StAR) protein. J Biol Chem. 1999;274:5909–5918. doi: 10.1074/jbc.274.9.5909. [DOI] [PubMed] [Google Scholar]
- Manna PR, El-Hefnawy T, Kero J, Huhtaniemi IT. Biphasic action of prolactin in the regulation of murine Leydig tumor cell functions. Endocrinology. 2001;142:308–318. doi: 10.1210/endo.142.1.7899. [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Chan YL, Lin A, McNally J, Peleg D, Meyuhas O, Wood IG. The primary structure of rat ribosomal protein L19. A determination from the sequence of nucleotides in a cDNA and from the sequence of amino acids in the protein. J Biol Chem. 1987;262:1111–1115. [PubMed] [Google Scholar]
- Manna PR, Joshi L, Reinhold VN, Aubert ML, Suganuma N, Pettersson K, Huhtaniemi IT. Synthesis, purification and structural and functional characterization of recombinant form of a common genetic variant of human luteinizing hormone. Hum Mol Genet. 2002;11:301–315. doi: 10.1093/hmg/11.3.301. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Stocco D. Star protein and the regulation of steroid hormone biosynthesis. Annu Rev Physiol. 2001;63:193–213. doi: 10.1146/annurev.physiol.63.1.193. [DOI] [PubMed] [Google Scholar]
- Sullivan MH, Cooke BA. The role of Ca2+ in steroidogenesis in Leydig cells. Stimulation of intracellular free Ca2+ by lutropin (LH), luliberin (LHRH) agonist and cyclic AMP. Biochem J. 1986;236:45–51. doi: 10.1042/bj2360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JM. Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- Themmen APN, Huhtaniemi IT. Mutations of gonadotropins and gonadotropin receptors: elucidating the physiology and pathophysiology of pituitary-gonadal function. Endocr Rev. 2000;21:551–583. doi: 10.1210/edrv.21.5.0409. [DOI] [PubMed] [Google Scholar]
- Dufau ML. Endocrine regulation and communicating functions of the Leydig cell. Annu Rev Physiol. 1988;50:483–508. doi: 10.1146/annurev.ph.50.030188.002411. [DOI] [PubMed] [Google Scholar]
- Segaloff DL, Ascoli M. The lutropin/choriogonadotropin receptor ... 4 years later. Endocr Rev. 1993;14:324–347. doi: 10.1210/edrv-14-3-324. [DOI] [PubMed] [Google Scholar]
- Pescador N, Houde A, Stocco DM, Murphy BD. Follicle-stimulating hormone and intracellular second messengers regulate steroidogenic acute regulatory protein messenger ribonucleic acid in luteinized porcine granulosa cells. Biol Reprod. 1997;57:660–668. doi: 10.1095/biolreprod57.3.660. [DOI] [PubMed] [Google Scholar]
- Kiriakidou M, McAllister JM, Sugawara T, Strauss JF III. Expression of steroidogenic acute regulatory protein (StAR) in the human ovary. J Clin Endocrinol Metab. 1996;81:4122–4128. doi: 10.1210/jcem.81.11.8923870. [DOI] [PubMed] [Google Scholar]
- Manna PR, Roy P, Clark BJ, Stocco DM, Huhtaniemi IT. Interaction of thyroid hormone and steroidogenic acute regulatory (StAR) protein in the regulation of murine Leydig cell steroidogenesis. J Steroid Biochem Mol Biol. 2001;76:167–177. doi: 10.1016/S0960-0760(00)00156-4. [DOI] [PubMed] [Google Scholar]
- Lin T, Wang D, Hu J, Stocco DM. Upregulation of human chorionic gonadotrophin-induced steroidogenic acute regulatory protein by insulin-like growth factor-I in rat Leydig cells. Endocrine. 1998;8:73–78. doi: 10.1385/ENDO:8:1:73. [DOI] [PubMed] [Google Scholar]
- Krueger RJ, Orme-Johnson NR. Acute adreno-corticotropic hormone stimulation of adrenal corticosteroidogenesis. Discovery of a rapidly induced protein. J Biol Chem. 1983;258:10159–10167. [PubMed] [Google Scholar]
- Stocco DM, Chen W. Presence of identical mitochondrial proteins in unstimulated constitutive steroid-producing R2C rat Leydig tumor and stimulated nonconstitutive steroid-producing MA-10 mouse Leydig tumor cells. Endocrinology. 1991;128:1918–1926. doi: 10.1210/endo-128-4-1918. [DOI] [PubMed] [Google Scholar]
- Wang X, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275:20204–20209. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- Townson DH, Wang XJ, Keyes PL, Kostyo JL, Stocco DM. Expression of the steroidogenic acute regulatory protein in the corpus luteum of the rabbit: dependence upon the luteotropic hormone, estradiol-17 beta. Biol Reprod. 1996;55:868–874. doi: 10.1095/biolreprod55.4.868. [DOI] [PubMed] [Google Scholar]
- Irusta G, Parborell F, Peluffo M, Manna PR, Gonzalez-Calvar SI, Calandra R, Stocco DM, Tesone M. Steroidogenic Acute Regulatory Protein in Ovarian Follicles of Gonadotropin-Stimulated Rats Is Regulated by a Gonadotropin-Releasing Hormone. Agonist Biol Reprod. 2003;68:1577–1583. doi: 10.1095/biolreprod.102.009944. [DOI] [PubMed] [Google Scholar]
- Schwarzenbach H, Manna PR, Stocco DM, Chakrabarti G, Mukhopadhyay AK. Stimulatory Effect of Progesterone on the Expression of Steroidogenic Acute Regulatory Protein in MA-10 Leydig Cells. Biol Reprod. 2003;68:1054–1063. doi: 10.1095/biolreprod.102.009266. [DOI] [PubMed] [Google Scholar]
- Arakane F, King SR, Du Y, Kallen B, Walsh LP, Watari H, Stocco DM, Strauss JF III. Phosphorylation of steroidogenic acute regulatory protein (StAR) modulates its steroidogenic activity. J Biol Chem. 1997;272:32656–32662. doi: 10.1074/jbc.272.51.32656. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Ranganathan V, Combs R. Steroidogenic acute regulatory protein expression is dependent upon post-translational effects of cAMP-dependent protein kinase A. Mol Cell Endocrinol. 2001;173:183–192. doi: 10.1016/s0303-7207(00)00410-x. [DOI] [PubMed] [Google Scholar]
- Pezzi V, Clark BJ, Ando S, Stocco DM, Rainey WE. Role of calmodulin-dependent protein kinase II in the acute stimulation of aldosterone production. J Steroid Biochem Mol Biol. 1996;58:417–424. doi: 10.1016/0960-0760(96)00052-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Stocco DM. Cyclic AMP and arachidonic acid: a tale of two pathways. Mol Cell Endocrinol. 1999;158:7–12. doi: 10.1016/s0303-7207(99)00130-6. [DOI] [PubMed] [Google Scholar]
- Ramnath HI, Peterson S, Michael AE, Stocco DM, Cooke BA. Modulation of steroidogenesis by chloride ions in MA-10 mouse tumor Leydig cells: roles of calcium, protein synthesis, and the steroidogenic acute regulatory protein. Endocrinology. 1997;138:2308–2314. doi: 10.1210/endo.138.6.5162. [DOI] [PubMed] [Google Scholar]
- Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF III, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M003766200. [DOI] [PubMed] [Google Scholar]
- Gyles SL, Burns CJ, Whitehouse BJ, Sugden D, Marsh PJ, Persaud SJ, Jones PM. ERKs regulate cyclic AMP-induced steroid synthesis through transcription of the steroidogenic acute regulatory (StAR) gene. J Biol Chem. 2001;276:34888–34895. doi: 10.1074/jbc.M102063200. [DOI] [PubMed] [Google Scholar]
- Osman H, Murigande C, Nadakal A, Capponi AM. Repression of DAX-1 and induction of SF-1 expression. Two mechanisms contributing to the activation of aldosterone biosynthesis in adrenal glomerulosa cells. J Biol Chem. 2002;277:41259–41267. doi: 10.1074/jbc.M206595200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]


