Abstract
Herein we describe an episode of focal varicella-zoster virus (VZV) encephalitis in a healthy young man with neither rash nor radicular pain. The symptoms began with headaches and seizures, after which magnetic resonance imaging detected a single hyperintense lesion in the left temporal lobe. Because of the provisional diagnosis of a brain tumor, the lesion was excised and submitted for pathological examination. No tumor was found. But the tissue immunostained positively for VZV antigens, and wild-type VZV sequences were detected. In short, this case represents VZV reactivation, most likely in the trigeminal ganglion, in the absence of clinical herpes zoster.
Keywords: varicella-zoster virus, trigeminal ganglion, herpes zoster, latency, varicella vaccine, valacyclovir
In late adulthood, when immunity wanes, varicella-zoster virus (VZV) may reactivate from latency and cause the dermatomal exanthem known as herpes zoster (shingles) [1]. A less common form of VZV reactivation in the absence of a rash is known as zoster sine herpete [2]. In this report, we describe a most unusual case of VZV reactivation, which was first diagnosed after excision and examination of a suspected brain tumor within the left temporal lobe of an otherwise healthy 23-year-old male without a rash. Because the patient had recently received 2 varicella vaccinations, the question immediately was raised whether varicella vaccination had contributed to this serious VZV reactivation event.
CASE REPORT
The patient was a healthy 22-year-old man with a normal body mass index when he completed his college education in June 2012. Because of a requirement for new employment in a hospital, he was serotested and found to have a negative VZV antibody titer on 20 September 2012. As per regulation, he received his first varicella vaccination on 11 December 2012 and his second vaccination on 24 January 2013. He did not recall any side effects of the 2 varicella immunizations. Between 11 February 2013 and 15 February 2013, he developed intermittent headaches, mainly in the left temporal area, and took ibuprofen to reduce pain. On 16 February 2013, he had a headache earlier in the day and, later, a seizure that was witnessed by another person in the same room. He was taken to a local emergency department, where an initial assessment was negative.
On 19 February 2013, he was seen in follow-up by the neurology service at the Mayo Clinic; they found no remarkable findings but did order magnetic resonance imaging (MRI) and electroencephalography (EEG). The MRI demonstrated a small T2/FLAIR hyperintense and T1 mildly hypointense lesion (14 × 15 × 9 mm) involving the cortex and adjacent subcortical white matter centered at the left inferior temporal sulcus (Figure 1A). There was no associated restricted diffusion, hemorrhage, calcification, or contrast enhancement. A routine EEG recorded 2 subclinical left temporal lobe seizures; levetiracetam (500 mg twice daily) was prescribed to prevent further seizure activity. Differential considerations favored a neoplasm, such as a low-grade astrocytoma or oligodendroglioma.
Figure 1.
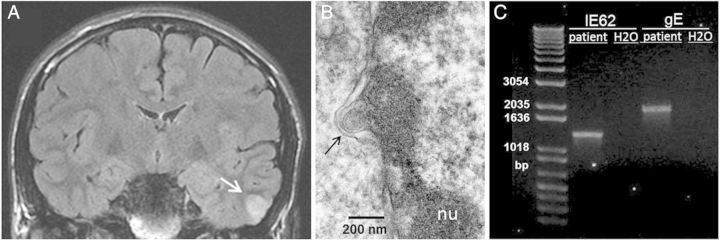
Studies of brain by magnetic resonance imaging (MRI), electron microscopy, and varicella-zoster virus (VZV) DNA amplification. A, Coronal MRI with lesion demarcated by an arrow. B, Electron micrograph showing single VZV capsid (black arrow) exiting the nucleus (nu) of an infected cell. The small ovoid structure adjacent to the capsid is a portion of the capsid that was displaced during sectioning. C, VZV DNA was extracted from the fresh frozen biopsy specimen from the patient's temporal lobe, and polymerase chain reaction amplifications of segments of VZV IE62 and VZV gE (ORF68) genes from the isolated DNA were performed. Amplifications from a water sample were negative.
On 12 March 2013, neurosurgeons performed an MRI-guided left temporal craniotomy and resected the lesion. He recovered quickly and was walking within 3 days of surgery; he had no further seizures. On 18 March 2013, the initial pathology report indicated an absence of tumor in the resected tissue. However, perivascular inflammation was present, involving many small leptomeningeal vessels. This finding suggested that a viral etiology could have caused the gliosis. On 21 March 2013, the patient developed recurrent headache that continued for another week. Because of the recent history of 2 varicella vaccinations, VZV immunostaining was performed on the brain tissue and was interpreted as positive on 22 March 2013. On 23 March 2013, the patient initiated valacyclovir treatment 1000 mg 3 times daily, even though he was greatly improved symptomatically and had already returned to work [3]. Subsequent hematologic screening studies on 25 March 2013 for a variety of autoimmune/rheumatology disorders were negative, but VZV serology was now positive. Serum immunoglobulin levels as well as absolute numbers of CD3+, CD4+, CD8+, CD19+, and CD16+CD56+ lymphocytes were within normal ranges.
Further examination of his childhood medical records disclosed that his older sister had varicella when the patient was 6 months old. The patient did not have an obvious varicella exanthem at that time. However, at 27 months of age, the patient developed a left-sided T6/T7 dermatomal rash. He was seen by his pediatrician and a dermatologist at the Mayo Clinic, both of whom documented in his medical record a clinical diagnosis of herpes zoster. The patient was not given any varicella vaccinations during his childhood. At a follow-up examination 1 year after the seizure episode, the patient was completely free of any central nervous system symptoms.
MATERIALS AND METHODS AND RESULTS
Neuropathology Studies
After neurosurgical excision, sections of the left temporal mass and cortex were fixed, processed and examined by hematoxylin and eosin and immunohistochemistry (IHC) with antibodies to Neu-N, GFAP, S-100, mutant IDH1 (IDH1 R132H), p53, Ki-67, and KP1. There was no evidence of tumor. The cortex showed severe gliosis and diffuse microglial activation without microglial nodule formation (Figure 2A and 2B). A chronic lymphocytic inflammatory infiltrate was present with a predominant perivascular distribution, involving cortex and small leptomeningeal vessels. The infiltrate was composed predominantly of CD3+ cells and only a few CD20+ cells. The findings were consistent with an inflammatory reactive process and raised the possibility of viral etiology, but no viral inclusions were observed. The edges of the excised brain tissue were free of inflammation.
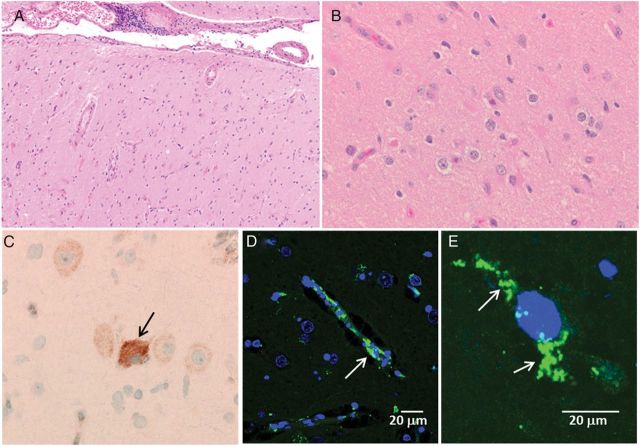
Figure 2.
Investigation of neuropathology in the temporal lobe by light and confocal microscopy. A, Low-power hematoxylin-eosin–stained image shows cortical gliosis and lymphocytes in leptomeninges. B, Higher-power hematoxylin-eosin–stained image again shows gliosis and some microglial activation but no viral inclusions. C, Varicella immunohistochemistry analysis with anti-varicella-zoster virus (VZV) monoclonal antibody (MAb) followed by horseradish peroxidase and 3,3′-diaminobenzidine immunostaining detected a positive neuron (brown color; arrow). D, Immunolabeling with characterized MAbs against VZV gE and IE62 antigens and fluoroprobe AlexaFluor 488 (Molecular Probes) illustrating a positive cell (green color; arrow). VZV-specific immunoreactivity was present mainly in the cytoplasm of the cells. Samples were viewed with Zeiss 710 confocal microscope at 400×. E, Another confocal microscopic image viewed under same conditions as for panel D, but at 1000×.
Confocal Microscopy
When the tissue was probed with a VZV antibody followed by IHC staining, there was an occasional positive cell (Figure 2C). To increase both sensitivity and resolution, another slide was incubated in a primary anti-VZV antibody mixture (mouse MAb 3B3 against a major VZV gE glycoprotein and mouse MAb 5C6 against a major VZV IE62 tegument protein); these 2 reagents were produced in the laboratory of C. Grose [4]. VZV antigens were easily identified in cells within the patient's brain tissue (Figure 2D and 2E). In the most inflamed areas, about 20% of the cells contained VZV gE/IE62 antigens. Since the patient is not blood group A, none of the immunostaining can be confounded by reaction of diagnostic antiviral antibody with this human antigen in brain cells [5]. A large panel of control immunostaining experiments is included in the Supplementary Materials.
Electron Microscopy
A separate small section of the patient's temporal lobe tissue biopsy specimen was fixed, dehydrated in ethanol, embedded in Epon, sectioned by ultramicrotomy, and viewed with a JEOL 1230 electron microscope, by previously described methods [6]. Although not plentiful, both capsids and enveloped viral particles characteristic of VZV in size and morphology were observed in the brain tissue (Figure 1B). These findings are remarkably similar to those published >40 years ago [7].
VZV DNA Sequencing
DNA was extracted from a section of the fresh frozen biopsy tissue specimen from the patient's temporal lobe, using the DNeasy spin column kit (Qiagen). The DNA was then subjected to real-time polymerase chain reaction (PCR), using Sybr Green and primers to VZV gE and IE62 and cellular GAPDH by previously described methods; control samples included DNA from VZV-infected cells (positive control) and DNA from a water sample (negative control) [8]. The PCR products were subjected to pyrosequencing at the University of Iowa DNA core facility (Figure 1C). Sequences from the 2 amplified VZV genes closely resembled those of clade 1 wild-type VZV strains, commonly found in North America (GenBank accession DQ479961); the sequences lacked any polymorphism found in VZV Oka vaccine virus (GenBank accession AB097932).
DISCUSSION
This case of VZV reactivation presenting as a single focal brain lesion suggestive on MRI as a low-grade brain tumor in a young adult is extraordinarily unusual [1, 9]. The simplest explanation is VZV reactivation without rash, most likely within a branch of the left trigeminal ganglion entering the temporal lobe along the carotid vasculature [10]. A possible alternative site is the olfactory lobe. This episode has some features of zoster sine herpete but lacks radicular pain that is traditionally associated with the word ‘zoster.’ Under this VZV reactivation scenario, the patient with likely varicella at age 6 months had clinical herpes zoster in the thoracic region at age 27 months followed by a second bout of VZV reactivation at age 23 years. The negative varicella serology at age 22 years can be attributed to the low sensitivity of the commercial enzyme-linked immunosorbent tests; for example, as many as one-fourth of people with low titers may have a false-negative result [11, 12].
Investigations over the past decade have established a link between clinically apparent VZV reactivation from the trigeminal ganglion and subsequent stroke in an elderly population [13]. But there are published case reports of likely VZV reactivation within a trigeminal ganglion with subsequent pathology other than stroke. For example, there is a case report of a 30-year-old man with AIDS and herpes zoster of the mandibular branch of the trigeminal ganglion who subsequently developed a demyelinating lesion in the ipsilateral caudal medulla [14]. VZV-positive antigens were detected at autopsy. Another report describes an immunocompetent 45-year-old woman with persistent pain in the maxillary distribution of the right trigeminal ganglion for 1 year; VZV DNA and VZV antigens were detected in a mass which was eventually removed from her affected trigeminal ganglion [2]. A third report describes a 39-year-old morbidly obese man with severe cardiomyopathy, who had 3 episodes of bilateral trigeminal ganglionitis with chronic pain but without rash before death [15]. Virologic studies at autopsy demonstrated VZV antigens and VZV DNA in both trigeminal ganglia, as well as in the meningeal artery. The fact that the VZV reactivation episode in our patient did not lead to further central nervous system pathology can be explained by 2 events: (1) as a young healthy adult, he developed a sufficiently rapid anti-VZV immune response, possibly primed by the 2 recent immunizations, which prevented further inflammation; and (2) the area of inflammation in the parenchyma of the temporal lobe was quickly removed by neurosurgical excision, before extension of infection occurred.
The final intriguing aspect of this unusual case was the proximity of the VZV reactivation in the temporal lobe with 2 varicella vaccinations during the preceding months. Since our virology sequencing data indicated that the VZV infection in the brain was caused by wild-type VZV, the most likely explanation was a coincidental association.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grant AI 89716 to C. G.)
Potential conflict of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hope-Simpson RE. The Nature of Herpes Zoster: a Long-Term Study and a New Hypothesis. Proc R Soc Med. 1965;58:9–20. [PMC free article] [PubMed] [Google Scholar]
- 2.Hevner R, Vilela M, Rostomily R, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–71. doi: 10.1016/s1474-4422(03)00506-4. [DOI] [PubMed] [Google Scholar]
- 3.Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(Suppl 1):S1–26. doi: 10.1086/510206. [DOI] [PubMed] [Google Scholar]
- 4.Jackson W, Yamada M, Moninger T, Grose C. Visualization and quantitation of abundant macroautophagy in virus-infected cells by confocal three-dimensional fluorescence imaging. J Virol Methods. 2013;193:244–50. doi: 10.1016/j.jviromet.2013.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerboni L, Sobel RA, Lai M, et al. Apparent expression of varicella-zoster virus proteins in latency resulting from reactivity of murine and rabbit antibodies with human blood group a determinants in sensory neurons. J Virol. 2012;86:578–83. doi: 10.1128/JVI.05950-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter JE, Henderson EP, Grose C. Enumeration of an Extremely High Particle to Pfu Ratio for Varicella Zoster Virus. J Virol. 2009;83:6917–21. doi: 10.1128/JVI.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esiri MM, Tomlinson AH. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- 8.Wagenaar TR, Chow VT, Buranathai C, Thawatsupha P, Grose C. The out of Africa model of varicella-zoster virus evolution: single nucleotide polymorphisms and private alleles distinguish Asian clades from European/North American clades. Vaccine. 2003;21:1072–81. doi: 10.1016/s0264-410x(02)00559-5. [DOI] [PubMed] [Google Scholar]
- 9.Gershon AA, Gershon MD. Pathogenesis and current approaches to control of varicella-zoster virus infections. Clin Microbiol Rev. 2013;26:728–43. doi: 10.1128/CMR.00052-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons T, Ruskell GL. Distribution and termination of trigeminal nerves to the cerebral arteries in monkeys. J Anat. 1988;159:57–71. [PMC free article] [PubMed] [Google Scholar]
- 11.Breuer J, Schmid DS, Gershon AA. Use and limitations of varicella-zoster virus-specific serological testing to evaluate breakthrough disease in vaccinees and to screen for susceptibility to varicella. J Infect Dis. 2008;197(Suppl 2):S147–51. doi: 10.1086/529448. [DOI] [PubMed] [Google Scholar]
- 12.Saiman L, LaRussa P, Steinberg SP, et al. Persistence of immunity to varicella-zoster virus after vaccination of healthcare workers. Infect Control Hosp Epidemiol. 2001;22:279–83. doi: 10.1086/501900. [DOI] [PubMed] [Google Scholar]
- 13.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8:731–40. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenblum MK. Bulbar encephalitis complicating trigeminal zoster in the acquired immune deficiency syndrome. Hum Pathol. 1989;20:292–5. doi: 10.1016/0046-8177(89)90140-8. [DOI] [PubMed] [Google Scholar]
- 15.Birlea M, Nagel MA, Khmeleva N, et al. Varicella-zoster virus trigeminal ganglioneuritis without rash. Neurology. 2014;82:90–2. doi: 10.1212/01.wnl.0000438228.48470.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.