Abstract
Background. It is not known if fluctuations in genital tract antiretroviral drug concentrations correlate with genital virus shedding in human immunodeficiency virus (HIV)–infected women on antiretroviral therapy (ART).
Methods. Among 20 HIV-infected women on ART (tenofovir [TFV], emtricitabine [FTC], and ritonavir-boosted atazanavir [ATV]) with suppressed plasma virus loads, blood and cervicovaginal samples collected twice weekly for 3 weeks were tested for antiretroviral concentrations, HIV-1 RNA, and proviral DNA.
Results. Cervicovaginal:plasma antiretroviral concentration ratios were highest for FTC (11.9, 95% confidence interval [CI], 8.66–16.3), then TFV (3.52, 95% CI, 2.27–5.48), and ATV (2.39, 95% CI, 1.69–3.38). Within- and between-person variations in plasma and genital antiretroviral concentrations were observed. Low amounts of genital HIV-1 RNA (<50 copies/mL) were detected in 45% of women at 16% of visits. Genital HIV-1 DNA was detected in 70% of women at 35% of visits. Genital virus detection was associated with higher concentrations of mucosal leukocytes but not with genital antiretroviral concentrations, menstrual cycle phase, bacterial vaginosis, genital bleeding, or plasma virus detection.
Conclusions. Standard doses of ART achieved higher genital than plasma concentrations across the menstrual cycle. Therapeutic ART suppresses genital virus shedding throughout the menstrual cycle, even in the presence of factors reported to increase virus shedding.
Keywords: female genital tract, HIV-1, pharmacology, viral shedding
Antiretroviral therapy (ART) decreases mother-to-child and sexual transmission and is associated with suppression of human immunodeficiency virus (HIV)–1 shedding in blood and genital secretions [1–3]. While genital and plasma HIV-1 shedding are strongly correlated [4, 5], some women on ART with undetectable plasma virus loads intermittently shed genital HIV-1 RNA [1, 6–10] and proviral DNA [11] at variable frequencies. Because evidence exists for compartmentalization of HIV-1 RNA production in the female genital tract [12, 13], the relationship between local drug concentrations and virus shedding is of particular interest. Despite reduced genital HIV-1 shedding with ART, the effect of genital compartmental antiretroviral concentrations and intermittent HIV-1 shedding is poorly understood, particularly in the presence of factors reported to increase genital HIV-1 shedding, such as bacterial vaginosis (BV), sexually transmitted infections [14–17], local inflammation [18, 19], and menstrual cycle phase [20–25].
Antiretroviral concentrations in female genital secretions are known to vary by drug [26, 27], and have potential impact on ART for prevention, preexposure prophylaxis, prevention of mother-to-child transmission, and prevention of local drug resistant virus. High degrees of variation in plasma antiretroviral concentrations over time have previously been reported [28] and may be altered in women [29], potentially due to physiologic changes over the menstrual cycle affecting properties such as protein binding and volume of distribution [30]. However, despite high between-person variability observed in genital aspirate concentrations [31], little is known about the magnitude and virologic consequences of such fluctuations in genital concentrations among women on long-term ART. As therapeutic ART is increasingly relied upon as a prevention tool, there is a need to understand if fluctuations in genital ART concentrations contribute to intermittent genital shedding. In order to better understand this relationship between genital concentration variability and HIV shedding, we measured genital antiretroviral concentrations and the frequency of genital HIV-1 RNA and DNA shedding throughout the menstrual cycle of women who received the same antiretroviral regimen and had undetectable plasma HIV-1 RNA levels.
METHODS
Study Population and Screening
HIV-1-infected women who reported regular menses (occurring within 22–35 day intervals) for 3 cycles with undetectable (<75 copies/mL) plasma HIV-1 RNA within 90 days, were receiving combination ART for ≥6 months and using standard doses of the same ART regimen (tenofovir disoproxil fumarate/emtricitabine and ritonavir-boosted atazanavir) for ≥30 days were recruited from the Grady Infectious Diseases Program (Atlanta, Georgia). Exclusion criteria were: <18 years old, pregnant, menopausal (absence of menses in 12 months), nonadherent (reported missed doses in last 3 days), BV (by Amsel's criteria), trichomonas infection (by wet mount examination), vaginal candidiasis (by potassium hydroxide staining of wet mount), genital ulcers, or purulent vaginal discharge. This protocol was approved by the Emory University and Centers for Disease Control and Prevention Institutional Review Boards and the Grady Research Oversight Committee. All participants provided informed consent.
At screening, baseline demographic, medical, sexual, and reproductive histories were collected. Blood was tested using a rapid plasma reagin test (Alere, Orlando, FL), Treponema pallidum particle agglutination assay (Seriodia TP·PA, Fujirebio, Inc, Tokyo, Japan), and herpes simplex virus type 2 antibody enzyme-linked immunoassay immunoglobin G (IgG) test (HerpeSelect 2, Focus Diagnostics, Cypress, CA), and clinician-collected vaginal swabs were tested for Neisseria gonorrhoeae and Chlamydia trachomatis nucleic acid (Gen-Probe APTIMA, San Diego, CA), and for BV and Candida infection by Gram stain.
Study Visits
Twenty eligible women were asked to return following the completion of their next menses for twice-a-week study visits for 3 weeks (6 visits total) during which paired blood and genital specimens were collected. Women were asked not to have sexual intercourse, douche, or use intravaginal products for ≥24 hours before visits and were scheduled for visits 24 hours after their preceding self-administered antiretroviral dose. Women who started menses before completing 6 visits were asked to return after the completion of menses for missed visits. Study visits were sequentially numbered based on the number of days from the start of the preceding menses. Women with symptoms of cervicovaginal infections during the study were tested and treated per standard of care in our clinic.
Blood was collected in 8 mL sodium citrate–containing CPT vacutainer tubes (BD, Franklin Lakes, NJ) and centrifuged into plasma and peripheral blood mononuclear cell (PBMC) fractions, which were stored at −80°C for HIV-1 RNA, DNA, reproductive hormone, and drug concentration analyses. During a speculum examination, cervicovaginal fluid was first collected for antiretroviral drug measurements using 3 TearFlo wicks (HUB Pharmaceuticals, Rancho Cucamonga, CA) applied to the ectocervix until saturated and stored at −80°C. Next, a cervicovaginal lavage (CVL) was done by directing 10 mL of phosphate-buffered saline toward the endocervix and vaginal walls. The CVL was allowed to pool in the posterior vaginal fornix before it was collected and tested for blood and leukocytes using Mutistix 8SG urinalysis strips (Siemens Healthcare, Los Angeles, CA) and for semen using the ABACard p30 antigen detection test (Abacus Diagnostics, West Hill, CA). BV was assessed using Nugent scoring of a CVL Gram stain. CVL was centrifuged into cell-free supernatant and cellular fractions and frozen at −80°C until HIV-1 RNA and DNA testing.
Laboratory Testing
Concentrations of tenofovir (TFV), emtricitabine (FTC), and atazanavir (ATV) were measured in the plasma and the TearFlo wicks using high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) using a Kinetex (2.1 × 100 mm) Reverse Phase C18 column (Sigma–Aldrich) as described by Kuklenyik et al [32] with appropriate modifications. Namely, 3 pooled TearFlo wicks were extracted with 70% acetonitrile and ATV concentrations were monitored using transitions m/z 705.3 to 168.2 and 335.3. For each specimen, the reported drug concentration was the average of 2 separate HPLC-MS/MS analyses. The dynamic ranges for both plasma and wicks for all 3 drugs were 10–2000 ng/mL, with 15% inter- and intraday coefficients of variation (CV). In our results, “genital concentrations” refer to cervicovaginal wick concentrations.
HIV-1 RNA detection in plasma and CVL cell-free supernatant was done using a combined methodology of E.Z.N.A. Viral RNA Kit (Omega Bio-Tek Inc., Norcross, GA) for nucleic acid extraction and the COBAS Amplicor HIV-1 Monitor version 1.5 (Roche Diagnostics, Indianapolis, IN) for amplification with a 50 copies/mL lower limit of quantification. HIV-1 RNA signals 2 standard deviations above background but below the limit of quantification were recorded as detectable but not quantifiable. HIV-1 DNA in PBMCs and CVL cells was detected using the qualitative Amplicor HIV-1 DNA Test, version 1.5 (Roche Diagnostics, Indianapolis, IN) per the manufacturer's protocol. Inconclusive specimens were treated as missing values in the analysis. Plasma estradiol and progesterone concentrations were measured using a radioimmunoassay (Siemens Healthcare) with lower limits of detection of 5 pg/mL and 0.1 ng/mL, respectively.
Statistical Analysis
To minimize variability due to time between measurement and dosing, only study visits where sampling occurred within 2 hours of the next scheduled dose (plasma trough concentration, C24h) were included in drug concentration analyses. A natural logarithm transformation was performed prior to analysis. Plasma and genital concentrations were estimated by menstrual cycle phase (follicular if the study visit occurred before the start of the progesterone rise) for each antiretroviral drug with repeated-measures analyses using mixed-effects linear models. For example, we used a means model using SAS Proc Mixed (v.9.3) to estimate the means and 95% confidence interval (CI) for genital concentrations by phase and compare the mean concentration differences (follicular minus luteal phase for the genital tract and separately for the plasma) using the model's paired t test; P value < .05 was considered statistically significant. The model provided estimates of the between- and within-subject variance (compound-symmetric variance-covariance form [33]).The within- and between-subject CVs were calculated as the square root of the respective variance component estimates. The estimated mean concentrations and 95% CIs were back transformed to the original scale and reported as geometric means and geometric mean ratios.
To explore the association between genital HIV-1 RNA shedding and concentrations, we compared the genital C24h estimated from the mixed-effects linear models by each visit's HIV-1 RNA shedding status: (1) among “shedders” (women with at least 1 episode of HIV-1 RNA detection), study visits where genital HIV-1 RNA was detected; (2) among “shedders,” study visits where genital HIV-1 RNA was not detected; and (3) study visits from “nonshedders” (women with no genital HIV-1 RNA detected). We similarly compared genital concentrations based on the visit's HIV-1 DNA shedding status using these groups.
Genital tract HIV-1 RNA and DNA shedding rates over 1 menstrual cycle were estimated using the generalized estimating equations (GEE) methodology to account for correlation between multiple measurements from the same participant [34]. Using GEE methods, we compared genital HIV-1 shedding rates and estimated the odds ratios for factors potentially associated with HIV-1 shedding with an exchangeable correlation binomial logit model using SAS Proc Genmod. Risk factors included: plasma HIV-1 RNA detection, genital tract leukocyte and blood counts, menstrual cycle phase (follicular phase for study visits occurring before the start of the progesterone rise, or if no progesterone rise was detected, for study visits occurring >14 days before menses), and the presence of BV (Nugent score 7–10 using CVL Gram stain).
RESULTS
Study Population
Participants had been diagnosed with HIV infection for a median of 9 years and had median nadir and current CD4 lymphocyte cell counts of 110 and 412 cells/mm3, respectively (Table 1). All women had received ART for prolonged periods (any ART, median 90 months; current ART, median 14 months). Seventeen (85%) women reported sexual activity in the past 6 months, predominantly with 1 HIV-negative partner. One (5%) woman was using hormonal contraception (depot medroxyprogesterone acetate). All women tested negative for gonorrhea and chlamydia at baseline, 2 (10%) tested positive for syphilis, 19 (95%) were herpes simplex virus type 2 IgG positive, 5 (25%) had asymptomatic Candida infection or BV noted on Gram stain, 5 (25%) reported dysplasia by their most recent Pap test, and 6 (30%) reported treatment for a vaginal infection within the preceding 30 days.
Table 1.
Baseline Demographic and Clinical Characteristics of Study Participants (N = 20)
| Characteristic | n (%) or median (range) |
|---|---|
| Age in years | 36 (26–48) |
| Race | |
| African American | 19 (95) |
| White | 1 (5) |
| HIV risk factor | |
| Heterosexual sex | 19 (95) |
| Unknown | 1 (5) |
| Years of HIV diagnosis | 9 (1–17) |
| Nadir CD4 cell count (cells/µL) | 110 (2–320) |
| Most recent CD4 cell count (cells/µL) | 412 (71–1189) |
| <200 | 2 (10) |
| 200–500 | 12 (60) |
| >500 | 6 (30) |
| ART history | |
| Months since first ART regimen | 90 (9–115) |
| Months on current ART regimen | 14 (3–41) |
| Sexually active in past 6 mo | 17 (85) |
| 1 sexual partner | 16 (94) |
| 2 sexual partners | 1 (6) |
| Partner HIV negative | 12 (71) |
| Current hormonal contraceptive use | 1 (5)a |
| Genital infections at screeningb | |
| Gonorrhea | 0 |
| Chlamydia | 0 |
| Syphilis | 2 (10) |
| HSV2 IgG positive | 19 (95) |
| Candida on Gram stain | 5 (25) |
| Bacterial vaginosis from vaginal Gram stain | 5 (25) |
| Dysplasia by most recent Pap smear | 5 (25) |
| Treatment of vaginal infection within 30 d | 6 (30) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; HSV2, herpes simplex virus type 2; IgG, immunoglobin G.
a Depot medroxyprogesterone acetate.
b Women with bacterial vaginosis by Amsel criteria, trichomonas or vaginal candidiasis by wet mount, abnormal vaginal discharge, or genital ulcers at screening visit were excluded.
Visit Characteristics and Menstrual Cycle Phase
Nineteen women completed all 6 study visits (Figure 1). Eight (6.7%) visits occurred outside the study window because the woman started menses before study completion. Two women were treated for symptomatic genital infections during the study period. CVL semen contamination was noted in 8 (6.7%) study visits. BV was determined by Nugent criteria during 60 of 108 (55.6%) study visits from 17 (85%) women with available CVL Gram stains. Median (interquartile range) CVL leukocyte and blood counts were 125 (15–700) cells/µL and 25 (10–100) cells/µL, respectively.
Figure 1.
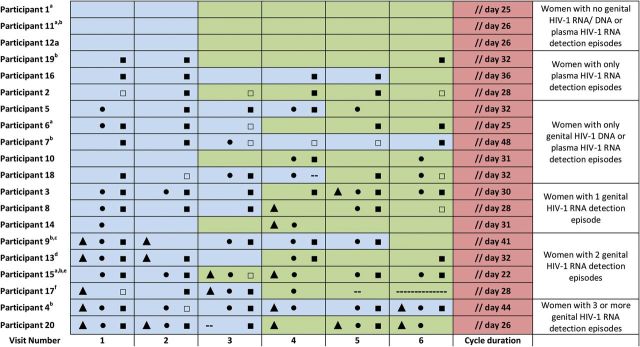
Timeline of study visits for 20 women over 1 menstrual cycle arranged from lowest to highest number of HIV-1 RNA detection episodes. Visits occurred twice weekly for 3 weeks following the week of menses and are numbered sequentially by order of time since preceding menses (visits 1–6). Cycle duration was defined as the duration from onset of menses until the onset of the next menses according to self-report. Follicular phase (blue) was determined as visits occurring between the end of menses and the start of the rise in serum progesterone. Luteal phase (green) was determined as any remaining days of the cycle until the onset of the next menses. Genital tract HIV-1 RNA detection (▴), genital HIV-1 DNA detection (•), plasma HIV-1 RNA detection <50 copies/mL / quantification ≥50 copies / mL) (▪)/□) are depicted for each study visit. Abbreviation: HIV, human immunodeficiency virus. a Menses began before study completion, so some visits occurred outside study window. b Did not have a rise in serum progesterone during the study period. Follicular phase was instead defined as visits occurring >14 days before the onset of the next menses. c Treated for symptomatic trichomonas infection on visit 5. d Treated for symptomatic vaginal yeast infection on visit 3. e Was receiving hormonal contraception (depot medroxyprogesterone acetate). f Completed only 5 study visits. -- indeterminate result.
The median menstrual cycle length was 30 (range, 21–47) days. Plasma progesterone concentrations increased during the study period as expected for an ovulatory cycle for 14 (70%) women at a median 22 (range, 8–27) days after the start of menses. Plasma estradiol concentrations were detectable during the study period for 18 (90%) women and peaked at a median 15 (range, 8–29) days after the start of menses.
Antiretroviral Drug Concentrations
Specimens were collected a median 24 (range, 11–53) hours before the previous self-reported antiretroviral dose; 96 (80.7%) visits from 19 (95%) women occurred within 22–26 hours of the previous dose and were included in drug concentration analyses. Plasma ATV concentration exceeded the recommended target trough concentration of 150 ng/mL [35] during 112 (94.1%) visits, suggesting adherence.
Genital concentrations exceeded plasma concentrations for all drugs studied (Table 2). The geometric mean ratio of genital to plasma C24h was 11.9 for FTC (95% CI, 8.66–16.3), 3.52 for TFV (95% CI, 2.27–5.48), and 2.39 for ATV (95% CI, 1.69–3.38). This pattern was relatively constant and was not significantly associated with menstrual cycle phase (Figure 2).
Table 2.
Genital and Plasma Antiretroviral Drug Concentrations (C24h)a and Within- and Between-person Variabilityb
| Antiretroviral drug |
|||
|---|---|---|---|
| FTC | TFV | ATV | |
| Geometric mean genital C24h, ng/mL (95% CI) | 903 (628–1299) | 244 (159–374) | 1440 (1020–2032) |
| Within-person CV, % | 66.1 | 81.9 | 82.4 |
| Between-person CV, % | 74.8 | 93.0 | 64.0 |
| Geometric mean plasma C24h, ng/mL (95% CI) | 76 (58–99) | 69 (54–88) | 601 (477–757) |
| Within-person CV, % | 47.4 | 41.2 | 70.7 |
| Between-person CV, % | 54.0 | 50.4 | 38.4 |
| Genital: plasma C24h geometric mean ratio (95% CI) | 11.9 (8.66–16.3) | 3.53 (2.27–5.48) | 2.39 (1.69–3.38) |
Abbeviations: ATV, atazanavir; C24h, concentration 24 hours after last dose; CI, confidence interval; CV, coefficient of variation; FTC, emtricitabine; TFV, tenofovir.
a 96 study visits from 19 subjects occurred within 2 hours of the next scheduled antiretroviral dose and were included in analysis.
b For natural log drug concentration data, the standard deviation is approximately equal to the CV in the original scale. Therefore, the within- and between-person CVs can be used as estimates of the within- and between-person standard deviations.
Figure 2.
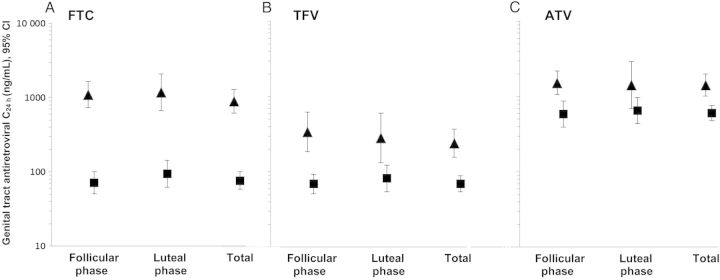
Mean genital (▴) and plasma (▪) and antiretroviral drug concentrations (C24h) by menstrual cycle phase (N = 63) and total (N = 96) for FTC (A), TFV (B), and ATV (C), N = 96. Abbreviations: ATV, atazanavir; CI, confidence interval; FTC, emtricitabine; TFV, tenofovir.
Variability in antiretroviral concentrations was expressed using within-person and between-person CVs. High CVs are indicative of a large amount of variability within- or between-persons. For all drugs, within- and between-person variability was higher for genital concentrations (exceeding 60%) than plasma concentrations (approximately 50% for all drugs except ATV). Between-person variability exceeded within-person variability for all drugs except ATV (Table 2).
HIV-1 RNA and DNA Detection
Over 1 menstrual cycle, HIV-1 RNA was detected in 69 (58.5%) plasma samples (95% CI, 45.1%–76.6%) from 16 (80%) women (Figure 1). Only 13 of these virus-positive plasma samples from 8 women had quantifiable virus loads (range 50–395 copies/mL). As expected, HIV-1 DNA was present in PBMCs for all patients at all visits.
Genital tract HIV-1 RNA was detected in 19 (16.1%) CVLs (95% CI, 8.8%–31.6%) from 9 (45%) women (all below the limit of quantification, <50 copies/mL); 7 (36.8%) occurred during visits without detectable plasma HIV-1 RNA and 10 (52.6%) occurred during visits with plasma HIV-1 RNA detectable below the limit of quantification (<50 copies/mL). Genital HIV-1 RNA was detected once in 3 women, twice in 4 women, and >twice in 2 women.
Genital HIV-1 DNA was detected in 42 (35.6%) CVLs (95% CI, 24.2%–52.9%) from 14 (70%) women. Genital HIV-1 DNA was detected at 14 (73.7%) study visits where genital HIV-1 RNA was detected. Genital HIV-1 DNA was detected once in 2 women, twice in 5 women, and >twice in 7 women.
Factors Associated With Genital HIV-1 RNA and DNA Detection
Concentration analyses included: (1) 18 visits where HIV-1 RNA shedding occurred among 10 “shedders”; (2) 26 visits where HIV-1 RNA shedding did not occur among 10 “shedders”; and (3) 51 visits among 10 “nonshedders” (Figure 3). Mean genital C24h did not significantly differ among these 3 types of study visits for any of the 3 drugs. Similarly, in analysis including (1) 34 visits where HIV-1 DNA shedding occurred among 13 “shedders”; (2) 31 visits where HIV-1 DNA shedding did not occur among 13 “shedders”; and (3) 31 visits among 6 “nonshedders,” mean genital C24h did not differ for any of the drugs. Among women with more than 1 genital HIV-1 RNA shedding episode, no pattern of plasma or genital drug concentration was observed in relationship to the shedding episode (Supplementary Figure 1).
Figure 3.
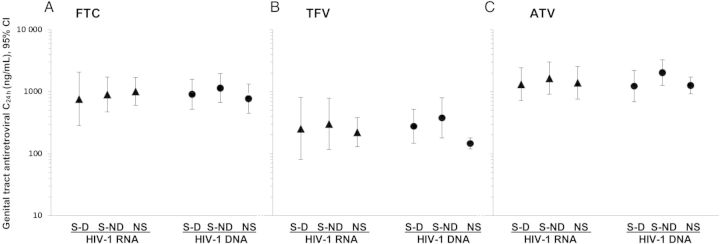
Mean genital antiretroviral drug concentration (C24h) and 95% CI by genital HIV-1 RNA (▴) and HIV-1 DNA (•) detection status at visit for FTC (A), TFV (B), and ATV (C), N = 96. Abbreviations: ATV, atazanavir; C24h, concentration 24 hours after last dose, CI, confidence interval; FTC, emtricitabine; HIV, human immunodeficiency virus; NS, visits from “nonshedders” (women without genital HIV-1 RNA/DNA detected during the study period); S-D, among “shedders” (women with at least 1 episode of genital HIV-1 RNA/DNA detection), visits with HIV-1 RNA/DNA detected; S-ND, among “shedders,” visits with HIV-1 RNA/DNA not detected; TFV, tenofovir.
Among women on ART, detection of ≥200 leukocytes/µL in the CVL was associated with detection of both genital HIV-1 RNA (rate ratio 2.38, 95% CI, 1.03–5.51) and DNA (rate ratio 2.41, 95% CI, 1.52–3.80, Supplementary Table 1). Genital HIV-1 RNA detection was associated with genital HIV-1 DNA detection (rate ratio 2.81, 95% CI, 1.39–5.64). A trend toward higher HIV-1 RNA and DNA detection rates was observed during visits with ≥200 blood cells/µL CVL and occurring during the follicular phase, but these were not statistically significant. Semen contamination of the CVL, BV, and plasma HIV-1 RNA were not associated with genital HIV-1 RNA or DNA detection (Supplementary Table 1).
DISCUSSION
Our study is the first to longitudinally assess the relationship between genital drug concentration and viral suppression in women on long-term ART using frequent sampling. We demonstrate that in a population of women on long-term ART with a commonly prescribed, first-line regimen, genital concentrations exceeded plasma concentrations for all active drugs in the regimen, and resulted in suppression of genital HIV-1 RNA shedding to very low or undetectable levels. Genital tract HIV-1 RNA shedding was uncommon, even in the presence of changes in endogenous reproductive hormone concentrations during the menstrual cycle, genital leukocytes, and asymptomatic BV, all of which have been reported to increase genital HIV-1 shedding risk [16, 36]. Given the direct relationship between cervicovaginal HIV-1 RNA levels and female-to-male sexual transmission [37], our findings lend support for ART as a tool for the prevention of sexual transmission of HIV, consistent with the findings of the clinical trial HPTN 052 [2].
With biweekly sampling over 1 menstrual cycle, our study is the first to report within- and between-person variability of antiretroviral concentrations in the female genital tract, demonstrating that genital concentrations are more variable than even plasma concentrations. Plasma virologic suppression despite high within-person plasma protease inhibitor concentration variability (median within-person CV 43.5%) has previously been described [28] and was observed in both the plasma and genital tract in our study. Possible contributors to high genital concentration variability include factors that affect plasma concentrations (ie, food effects, concomitant use of medications, medication timing, and genetic factors). However, additional factors may specifically contribute to mucosal variability, including douching, topical drug application, local drug interactions, local effects on drug transporters, and decreased precision of cervicovaginal sampling methods; these factors warrant further study as genital concentrations are increasingly relied upon to inform HIV prevention strategies.
Nonetheless, genital drug concentrations measured by cervicovaginal wick in our study remained high, were not affected by menstrual cycle phase, and were not associated with genital HIV-1 RNA or DNA detection. The high genital concentrations noted for TFV and FTC in our study are comparable to those reported previously [26, 27] and provide pharmacologic support for the successful clinical trials of TFV- and FTC-containing oral preexposure prophylaxis in heterosexual couples [38, 39]. However, we surprisingly found genital ATV concentrations that were higher (though within the measure of variability) than reported from studies with smaller sample sizes and measured from cervicovaginal aspirates [26, 27, 31]. Given the high within- and between-person variability in genital concentrations, concentration estimates could be impacted by sample size and/or frequency. Accumulation of ATV in the genital tract in women on long-term ART is plausible if differential protein binding or elimination characteristics in the blood versus genital tract are observed. In fact, a relationship between genital tract penetration and plasma protein binding has been previously noted for highly protein-bound drugs, including ATV [31]. High genital ATV concentrations in our study may have also been influenced by genital sampling method, use of an adherent population on long-term ART, and inclusion only of concentration measurements approximating C24 in the analysis, thus reducing variability due to dose timing. As genital concentrations are used to inform preexposure prophylaxis studies in women, our findings support future characterization of female genital concentrations with assessment across the dosing interval, repeated sampling per participant to account for variability, measurement of protein binding, and comparison of different collection methods.
The lack of association between genital virus detection and antiretroviral concentration supports the hypothesis that low-level genital tract virus is not due to incomplete antiviral efficacy of ART. One previous cross-sectional study measuring genital drug concentration and HIV-1 RNA suppression could not evaluate this question because only 1 study participant had detectable genital virus [27]. Another recent study found no relationship between the initial slopes of genital HIV-1 RNA and DNA decay and antiretroviral drug exposure in women initiating ART, suggesting that the maximal antiviral effect was attained in the genital tract with standard ART doses [11]. Further investigation is needed toward understanding the transmission potential of low-level cell-free or cell-associated virus shedding in the presence of antiretroviral drugs.
Consistent with earlier cross-sectional [1, 6, 7, 9, 10] and longitudinal [8, 11] studies, we detected low-level genital HIV-1 RNA and HIV-1 DNA at least once in 45% and 70% of women, respectively, mostly during visits with suppressed or near-suppressed plasma virus loads. We did not find an association between menstrual cycle phase and genital virus shedding among women on ART as has been noted in some studies of untreated viremic HIV-infected women [20–22], suggesting that any presumed endogenous hormonal effect could be blunted by ART. A major challenge in the assessment of menstrual cycle effects on biological outcomes in this population is misclassification of menstrual cycle phase by self-reported date, because women may have irregular or anovulatory cycles. We observed high variability in cycle length, poor correlation of phase defined by dates versus progesterone concentration, and 30% of women who did not exhibit the characteristic increase in plasma progesterone concentration; excluding women who did not have expected increases in plasma progesterone concentration from analysis did not alter our findings. However, our findings support the measurement of endogenous reproductive hormones in studies examining effects of the menstrual cycle in HIV-infected women.
Our study has some limitations. First, dilution of cervicovaginal fluid by CVL could underestimate genital tract HIV-1 RNA levels and may explain lower copy numbers than found in previous studies [8]. However, use of CVL enabled all cervicovaginal subcompartments (which may have differential genital tract shedding [8]) to be represented. Second, we did not use a quantitative HIV-1 DNA assay. Cervicovaginal HIV-1 DNA has previously been demonstrated only in low copy numbers (1 log10 copies/106 cells) in women on ART [11], thus quantitation was unlikely to alter our results. Third, except at the time of screening, we did not assess for cervicovaginal infections in asymptomatic women except BV. Only 2 women developed symptomatic vaginal infections during the study, and genital HIV-1 shedding was not affected in these women. Additionally, we recorded genital tract leukocytes at each visit, which is independently correlated with genital tract HIV-1 RNA levels in both the presence and absence of genital tract infections [18, 19]. Finally, we measured total extracellular trough drug concentrations, while free and/or intracellular drug exposure across the dosing interval may affect antiviral effect.
These limitations notwithstanding, our study demonstrated near complete suppression of genital HIV-1 RNA shedding through the menstrual cycle in the presence of highly variable genital concentrations. The strengths of our study include longitudinal assessment of drug concentrations and genital virus shedding using frequent sampling over 1 menstrual cycle, confirmation of cycle phase using hormone concentrations, uniformity of drug regimen (avoiding confounding by regimen potency or differential compartmental penetration), and measurement of cell-free and cell-associated virus in genital secretions (both of which may impact HIV-1 transmission [40]). Although the significance of low-level genital HIV-1 RNA and HIV-1 DNA remains unclear, our study provides evidence that high mucosal antiretroviral concentrations generally suppress local viral replication throughout the menstrual cycle in women on ART.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants, the Biomarkers Core Laboratory at the Yerkes National Primate Research Center (2P51RR000165-51) for estradiol and progesterone assays, the staff of the Center for AIDS Research at Emory University Clinical Research Core (P30AI050409), and Drs Kehmia Titanji, Wendy Armstrong, Angela Caliendo, and Jeffrey Lennox for their input.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or funding agencies.
Financial support. This work was supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2TR000455 and UL1TR000454).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Cu-Uvin S, Caliendo AM, Reinert SE, et al. HIV-1 in the female genital tract and the effect of antiretroviral therapy. AIDS. 1998;12:826–7. [PubMed] [Google Scholar]
- 2.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegfried NL, van der Merwe L, Brocklehurst P, et al. Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev. 2011;7:CD003510. doi: 10.1002/14651858.CD003510.pub2. DOI:003510.001002/14651858.CD14003510.pub14651853. [DOI] [PubMed] [Google Scholar]
- 4.Hart CE, Lennox JL, Pratt-Palmore M, et al. Correlation of human immunodeficiency virus type 1 RNA levels in blood and the female genital tract. J Infect Dis. 1999;179:871–82. doi: 10.1086/314656. [DOI] [PubMed] [Google Scholar]
- 5.Kovacs A, Wasserman SS, Burns D, et al. Determinants of HIV-1 shedding in the genital tract of women. Lancet. 2001;358:1593–601. doi: 10.1016/S0140-6736(01)06653-3. [DOI] [PubMed] [Google Scholar]
- 6.Coombs RW, Wright DJ, Reichelderfer PS, et al. Variation of human immunodeficiency virus type 1 viral RNA levels in the female genital tract: implications for applying measurements to individual women. J Infect Dis. 2001;184:1187–91. doi: 10.1086/323660. [DOI] [PubMed] [Google Scholar]
- 7.Cu-Uvin S, Caliendo AM, Reinert S, et al. Effect of highly active antiretroviral therapy on cervicovaginal HIV-1 RNA. AIDS. 2000;14:415–21. doi: 10.1097/00002030-200003100-00015. [DOI] [PubMed] [Google Scholar]
- 8.Cu-Uvin S, DeLong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24:2489–97. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 9.Neely MN, Benning L, Xu J, et al. Cervical shedding of HIV-1 RNA among women with low levels of viremia while receiving highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:38–42. doi: 10.1097/01.qai.0000248352.18007.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cu-Uvin S, Snyder B, Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir Immune Defic Syndr. 2006;42:584–7. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 11.Launay O, Tod M, Tschope I, et al. Residual HIV-1 RNA and HIV-1 DNA production in the genital tract reservoir of women treated with HAART: the prospective ANRS EP24 GYNODYN study. Antivir Ther. 2011;16:843–52. doi: 10.3851/IMP1856. [DOI] [PubMed] [Google Scholar]
- 12.Kelley CF, Sullivan ST, Lennox JL, et al. Lack of effect of compartmentalized drug resistance mutations on HIV-1 pol divergence in antiretroviral-experienced women. AIDS. 2010;24:1361–6. doi: 10.1097/QAD.0b013e3283394f3f. [DOI] [PubMed] [Google Scholar]
- 13.Kemal KS, Foley B, Burger H, et al. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycosylation. Proc Natl Acad Sci USA. 2003;100:12972–7. doi: 10.1073/pnas.2134064100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClelland RS, Wang CC, Overbaugh J, et al. Association between cervical shedding of herpes simplex virus and HIV-1. AIDS. 2002;16:2425–30. doi: 10.1097/00002030-200212060-00007. [DOI] [PubMed] [Google Scholar]
- 15.LeGoff J, Weiss HA, Gresenguet G, et al. Cervicovaginal HIV-1 and herpes simplex virus type 2 shedding during genital ulcer disease episodes. AIDS. 2007;21:1569–78. doi: 10.1097/QAD.0b013e32825a69bd. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LF, Lewis DA. The effect of genital tract infections on HIV-1 shedding in the genital tract: a systematic review and meta-analysis. Sex Transm Dis. 2008;35:946–59. doi: 10.1097/OLQ.0b013e3181812d15. [DOI] [PubMed] [Google Scholar]
- 17.Rotchford K, Strum AW, Wilkinson D. Effect of coinfection with STDs and of STD treatment on HIV shedding in genital-tract secretions: systematic review and data synthesis. Sex Transm Dis. 2000;27:243–8. doi: 10.1097/00007435-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Cummins JE, Christensen L, Lennox JL, et al. Mucosal innate immune factors in the female genital tract are associated with vaginal HIV-1 shedding independent of plasma viral load. AIDS Res Hum Retroviruses. 2006;22:788–95. doi: 10.1089/aid.2006.22.788. [DOI] [PubMed] [Google Scholar]
- 19.Anderson BL, Wang CC, Delong AK, et al. Genital tract leukocytes and shedding of genital HIV type 1 RNA. Clin Infect Dis. 2008;47:1216–21. doi: 10.1086/592303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benki S, Mostad SB, Richardson BA, et al. Cyclic shedding of HIV-1 RNA in cervical secretions during the menstrual cycle. J Infect Dis. 2004;189:2192–201. doi: 10.1086/421298. [DOI] [PubMed] [Google Scholar]
- 21.Money DM, Arikan YY, Remple V, et al. Genital tract and plasma human immunodeficiency virus viral load throughout the menstrual cycle in women who are infected with ovulatory human immunodeficiency virus. Am J Obstet Gynecol. 2003;188:122–8. doi: 10.1067/mob.2003.65. [DOI] [PubMed] [Google Scholar]
- 22.Reichelderfer PS, Coombs RW, Wright DJ, et al. Effect of menstrual cycle on HIV-1 levels in the peripheral blood and genital tract. AIDS. 2000;14:2101–7. doi: 10.1097/00002030-200009290-00005. [DOI] [PubMed] [Google Scholar]
- 23.Mostad SB, Jackson S, Overbaugh J, et al. Cervical and vaginal shedding of human immunodeficiency virus type 1-infected cells throughout the menstrual cycle. J Infect Dis. 1998;178:983–91. doi: 10.1086/515665. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva JM, Ellerbrock TV, Lennox JL, et al. The menstrual cycle does not affect human immunodeficiency virus type 1 levels in vaginal secretions. J Infect Dis. 2002;185:170–7. doi: 10.1086/338447. [DOI] [PubMed] [Google Scholar]
- 25.Goulston C, Stevens E, Gallo D, et al. Human immunodeficiency virus in plasma and genital secretions during the menstrual cycle. J Infect Dis. 1996;174:858–61. doi: 10.1093/infdis/174.4.858. [DOI] [PubMed] [Google Scholar]
- 26.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21:1899–907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwara A, Delong A, Rezk N, et al. Antiretroviral drug concentrations and HIV RNA in the genital tract of HIV-infected women receiving long-term highly active antiretroviral therapy. Clin Infect Dis. 2008;46:719–25. doi: 10.1086/527387. [DOI] [PubMed] [Google Scholar]
- 28.Nettles RE, Kieffer TL, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42:1189–96. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 29.Loutfy MR, Walmsley SL, Klein MB, et al. Factors affecting antiretroviral pharmacokinetics in HIV-infected women with virologic suppression on combination antiretroviral therapy: a cross-sectional study. BMC Infect Dis. 2013;13:256. doi: 10.1186/1471-2334-13-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kashuba AD, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998;34:203–18. doi: 10.2165/00003088-199834030-00003. [DOI] [PubMed] [Google Scholar]
- 31.Dumond JB, Nicol MR, Kendrick RN, et al. Pharmacokinetic modelling of efavirenz, atazanavir, lamivudine and tenofovir in the female genital tract of HIV-infected pre-menopausal women. Clin Pharmacokinet. 2012;51:809–22. doi: 10.1007/s40262-012-0012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuklenyik Z, Martin A, Pau CP, et al. Effect of mobile phase pH and organic content on LC-MS analysis of nucleoside and nucleotide HIV reverse transcriptase inhibitors. J Chromatogr Sci. 2009;47:365–72. doi: 10.1093/chromsci/47.5.365. [DOI] [PubMed] [Google Scholar]
- 33.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Oxford: Clarendon Press; 1994. p. 68. [Google Scholar]
- 34.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 35.Panel on Antiretroviral Guidelines for Adults and Adolescents. Department of Health and Human Services; Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed 20 November 2013. [Google Scholar]
- 36.Moreira C, Venkatesh KK, DeLong A, et al. Effect of treatment of asymptomatic bacterial vaginosis on HIV-1 shedding in the genital tract among women on antiretroviral therapy: a pilot study. Clin Infect Dis. 2009;49:991–2. doi: 10.1086/605540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001888. 77ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367:399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367:423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 40.Salle B, Brochard P, Bourry O, et al. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–44. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


