Abstract
Spontaneous bacterial peritonitis (SBP) is the most typical infection observed in cirrhosis patients. SBP is responsible for an in-hospital mortality rate of approximately 32%. Recently, pattern changes in the bacterial flora of cirrhosis patients have been observed, and an increase in the prevalence of infections caused by multi-resistant bacteria has been noted. The wide-scale use of quinolones in the prophylaxis of SBP has promoted flora modifications and resulted in the development of bacterial resistance. The efficacy of traditionally recommended therapy has been low in nosocomial infections (up to 40%), and multi-resistance has been observed in up to 22% of isolated germs in nosocomial SBP. For this reason, the use of a broad empirical spectrum antibiotic has been suggested in these situations. The distinction between community-acquired infectious episodes, healthcare-associated infections, or nosocomial infections, and the identification of risk factors for multi-resistant germs can aid in the decision-making process regarding the empirical choice of antibiotic therapy. Broad-spectrum antimicrobial agents, such as carbapenems with or without glycopeptides or piperacillin-tazobactam, should be considered for the initial treatment not only of nosocomial infections but also of healthcare-associated infections when the risk factors or severity signs for multi-resistant bacteria are apparent. The use of cephalosporins should be restricted to community-acquired infections.
Keywords: Spontaneous peritonitis, Multi-resistant bacteria, Cirrhosis, Infection, Ascites
Core tip: Spontaneous bacterial peritonitis (SBP) is the most typical infection observed in cirrhosis patients. The increasing trend of bacterial resistance development in cirrhotic patients with SBP has been associated with a low treatment efficacy of traditional therapy in nosocomial infections. The use of a broad empirical spectrum antibiotic has been suggested as an alternative. Cephalosporin use should be restricted to community-acquired infections, while changes are necessary with regard to empiric therapy recommendations. Broad-spectrum antimicrobial agents, such as carbapenems with or without glycopeptides or piperacillin-tazobactam, should be considered for the initial treatment of nosocomial infections.
INTRODUCTION
Chronic liver disease is one of the most common types of acquired immunodeficiency and bacterial infections are a common complication. In cirrhosis patients, specifically, bacterial infection represents the most frequent and worrisome complication. It is well established that 30% to 50% of cirrhotic patients either have bacterial infections when admitted to a hospital or acquire them during this period, and such infections are responsible for up to 25% of deaths in this patient population. In a recent systematic review of 178 studies including 11987 patients, an average mortality rate of 38% was observed. Using a meta-analysis approach, the study assessed mortality in relation to bacterial infection and found that infection increased the mortality risk approximately four-fold[1]. It is important to note that although the infection is often not the direct cause of death, it accelerates a negative outcome by promoting gastrointestinal bleeding, renal failure, and hepatic encephalopathy. When we assessed 541 consecutive hospital admissions (n = 426 patients), 25% of hepatic cirrhosis cases had infections, of which the most frequently observed were urinary tract infection, spontaneous bacterial peritonitis (SBP), respiratory infection, and skin infections. We observed an association between bacterial infections and the alcoholic etiology of liver disease, Child-Pugh class C, and the occurrence of upper gastrointestinal bleeding[2].
SBP is the most common form of infection seen in patients with cirrhosis[3-5]. Its incidence in hospitalized patients with chronic liver disease and ascites varies from 10%-30%. Approximately half of the cases already have SBP at the time of hospitalization, while the other half develop SBP during the hospitalization period[6]. The associated in-hospital mortality rate for SBP is approximately 32%. A high Child-Pugh score, low protein levels in the ascitic fluid, and the occurrence of upper gastrointestinal bleeding have been identified as risk factors for SBP development[3,4]. In our own hospital, 114 episodes of SBP from 1030 assessed hospitalizations of patients with cirrhosis and ascites were documented, corresponding to an incidence of 11.1% and an associated mortality of 21.9%. A majority of patients had community-acquired infections (61.4%), while the remaining patient population had hospital-acquired infections (37.7%)[7].
Until recently, guidelines from the International Ascites Club, the American Association for the Study of Liver Diseases, and the European Association for the Study of the Liver recommended treatment with third-generation cephalosporins for the majority of infections in patients with chronic liver disease due to their efficacy against the main etiologic agents (e.g., enterobacteria)[8-10]. Recently, however, studies have documented changes in the pattern of bacterial flora in cirrhosis patient infections, as well as an increase in infection prevalence attributed to multi-resistant bacteria[11-17]. Some of the findings highlighted an increase in the rate of infections caused by gram-positive cocci associated with invasive procedures during hospitalization[11]. Additionally, the widespread use of quinolones and other antibiotics in the prophylactic treatment of SBP in cirrhotic patients has also favored flora modifications and the development of bacterial resistance, directly affecting the current management of infections in this patient group[11,16].
SBP DIAGNOSIS
SBP is defined as an infection of the ascitic fluid in the absence of an intra-abdominal infection focus (Table 1)[8]. The primary symptoms for SBP are abdominal pain and fever[18]. Clinical identification, however, is often delayed as the infection presentation is generally atypical or with few symptoms. To achieve early diagnosis, diagnostic paracentesis should be performed on all cirrhotic patients with ascites at the time of hospital admission, regardless of the presence of symptoms, as well as in patients who display local or systemic signs of infection, hepatic encephalopathy, or sudden loss of renal function[6,8-10].
Table 1.
Characteristics of ascitic fluid in spontaneous bacterial peritonitis and variants
| Diagnosis/variants | Polymorphonuclear count | Culture |
| SBP culture positive | > 250 cells/mm3 | Positive |
| Neutrocytic ascites | > 250 cells/mm3 | Negative |
| Bacterascites | < 250 cells/mm3 | Positive |
SBP: Spontaneous bacterial peritonitis.
A diagnosis of SBP is made when the polymorphonuclear (PMN) count in the ascitic fluid is higher than 250 cells/mm3, with no evidence of an intra-abdominal infection source[10]. A PMN count higher than 250 cells/mm3 and a positive culture of ascitic fluid results in the diagnosis of culture-positive SBP. A PMN count higher than 250 cells/mm3 with a negative culture of ascitic fluid results in a diagnosis of culture-negative SBP or neutrocytic ascites. Because treatment cannot be delayed until the culture results are obtained, early empirical antibiotic therapy is initiated in patients with a PMN count > 250 cells/mm3 in the ascitic fluid, regardless of the clinical status[9].
Despite the use of sensitive methods for the culture and inoculation of ascitic fluid in bedside hemoculture bottles, approximately 60% of ascitic fluid samples with a PMN count > 250 cells/mm3 do not show evidence of bacterial growth[10,19]. This is likely due to the liberal use of prophylactic antibiotics and to the increasingly earlier diagnosis of SBP, as well as to the low bacteria population (1-2 bacteria/mL) in this infection[20]. Upon assessing different ascitic fluid culture techniques, our group confirmed the superiority of the bedside hemoculture bottle with material inoculation; the bacteriological exam tested positive in 63% of the cases[21].
Some patients can present with bacterascites (positive culture of ascitic fluid and a PMN count < 250 cells/mm3), which generally represents a transitory spontaneous colonization of the ascitic fluid[22]. Some studies have recommended antimicrobial treatment for patients with bacterascites, however this has not been our standard practice. We have found that the survival rate was similar between patients with bacterascites that were not administered antibiotic therapy and patients with sterile ascites, suggesting that the use of antibiotic therapy ab initio in such cases is not warranted[23]. We understand, however, that control paracentesis should be performed[10].
Secondary peritonitis is the main differential diagnosis for SBP, accounting for 5%-10% of the cases of peritonitis in patients with cirrhosis and ascites. It arises from perforation or inflammation of an intra-abdominal organ, and its mortality rate is significantly higher than that of SBP (66% vs 10%, respectively)[24]. A diagnosis of secondary peritonitis is likely when two out of three Runyon criteria are present: glucose < 50 mg/dL, proteins > 10 g/L, and lactate dehydrogenase > 225 mU/mL in the ascites fluid[9]. To differentiate between SBP and secondary peritonitis, measurements of carcinoembryonic antigen and alkaline phosphatase levels in ascitic fluid are useful. A previous study demonstrated that carcinoembryonic antigen levels > 5 ng/mL or alkaline phosphatase > 250 units/L were associated with a sensitivity of 92% and specificity of 88% in the differentiation between SBP and secondary peritonitis, while the presence of two out of three Runyon criteria was correlated with a sensitivity of 97% and a specificity of 56%. In suspected cases of secondary peritonitis, an early abdominal imaging exam should also be performed[25].
MICROBIOLOGICAL ASPECTS AND BACTERIAL RESISTANCE IN SBP
Aerobic gram-negative bacteria, such as Escherichia coli, are the most commonly found pathogens in SBP[10,26-29]. Importantly, approximately 30% of isolated gram-negative bacteria are resistant to quinolone[10]. Gram-positive bacteria have also been identified by studies as frequent germs in patients with cirrhosis and infections. Some studies also identified methicillin-resistant Staphylococcus aureus in up to 27% of SBP cases[22]. It is currently believed that this type of infection occurs because of the wide use of quinolones as prophylaxis for SBP, as well as the increasingly frequent use of invasive procedures for the treatment of cirrhosis complications[11].
A higher prevalence of extended spectrum beta-lactamase (ESBL)-producing bacteria has been described among nosocomial infections in cirrhotic patients. The production of ESBL is associated with the resistance to many antibiotics, including third-generation cephalosporins and monobactams. Frequently, ESBL-producing bacteria are carriers of genes that encode resistance to other antibiotics such as quinolones, tetracyclines, and antifolates, all of which are easily spread around hospitals[22].
The mortality rate among cirrhotic patients with infection by germs resistant to third-generation cephalosporins is greater than the rate among patients infected by pathogens that are sensitive to antimicrobials[4,13]. A study assessing the occurrence of SBP with hemoculture and/or positive culture of ascitic fluid found an average global resistance to third-generation cephalosporins of 21.5%, where 7.1% were found in community-acquired infections, 21.1% in healthcare-associated infections, and 40.9% in nosocomial infections[30]. Healthcare-associated infections were considered to be those diagnosed within the first 48 h after hospital admission in patients who had had contact with the health care system in the previous three months (e.g., emergency visits). The empirical treatment was considered adequate in 92.9% of community-acquired infections, 85.3% of healthcare-associated infections, and 69.7% of nosocomial infections. The mortality rate within 30 d was 28.2% in patients with community-acquired infections, 25.8% in healthcare-associated infections, and 54.2% in nosocomial infections. A Spanish study also showed that the development of infections by resistant bacteria was associated with an increase in the mortality rates[29]. Other studies have demonstrated that administration of the usual antibiotic therapy in cirrhotic patients with resistant bacterial infections was associated with an increase in septic shock and death rates[12,13,29,31]. This increase in bacterial resistance was not restricted to the European continent, as a study performed in the United States also showed a high rate of resistance (45%) to cephalosporins in patients with SBP[32]. Up to 22% of the germs isolated in nosocomial SBP are multi-resistant[27], defined as bacterial resistance to at least two of the three main classes of antimicrobials, including beta-lactams. Thus, it is important to revise the prevalence of the varied bacterial species in each institution, since the change of spectrum is very likely a global one, which greatly affects the therapy that should be administered.
SBP TREATMENT
A new diagnostic paracentesis is recommended 48 h after the beginning of treatment, in which at least a 25% reduction in the PMN count should be observed. If the PMN count is not reduced by 25%, the possibility of infection by bacteria resistant to the initial treatment or the occurrence of secondary bacterial peritonitis should be considered[10].
The use of third-generation cephalosporins has been implemented as the standard empirical antimicrobial treatment of SBP over the past several years, as they are well tolerated and active against enterobacteria and non-enterococcal streptococci[9,10]. When we assessed the efficacy of two antibiotic therapy regimens in cirrhotic patients treated for SBP, patients who received cefotaxime showed a lower hospital mortality rate when compared to patients who received other antibiotic regimens (28.1% vs 61.1%, respectively)[33]. Recent studies, however, have shown an increase in the prevalence of infections caused by multi-resistant bacteria, especially in nosocomial episodes, which has prompted a change in practice[12,34]. Thus, recommendations regarding SBP treatment should distinguish between episodes of nosocomial infections and those that are community-acquired[34,35]. Infections of nosocomial origin, patients with hospitalizations over the past three months, with long-term norfloxacin prophylaxis, a history of recent infection by multi-resistant bacteria and recent use of beta-lactams should be treated according to these new insights[16,29]. It is important to stress that healthcare-associated infections also present a higher risk for infection by multi-resistant bacteria, and active antibiotics against specific resistant bacteria should be used. Moreover, a new epidemiological classification has been suggested with regard to the places where infections are acquired: community-acquired infections, nosocomial, and healthcare-associated infections[12,30].
The efficacy of classically recommended therapy has been low in nosocomial infections (up to 40%). The complete resolution of infection by multi-resistant germs occurs less frequently than when other pathogens are involved (72% vs 90%, respectively). The hospital mortality rate associated with multi-resistant bacteria is twice as large as the mortality rate associated with other germs[12]. Similarly, mortality rates are higher in nosocomial infections when compared to community-acquired ones (58.7% vs 37.3%, respectively)[13].
In our experience, mortality rates between 1991 and 2000 were 18.6% for community-acquired infections and 27.9% for nosocomial infections among the 114 SBP episodes documented. Infections were controlled in 91.1% of the episodes and the mortality rate in this case was 16.7%. In cases where the infection was not controlled, however, the mortality rate was 80%[7]. Given the dramatic changes in the bacterial spectrum, however, the present numbers would likely be different. Thus, it is necessary to assess the prevalent microbiota in each hospital with regard to the use of empirical therapy.
Because there is evidence of increased mortality in the presence of multi-resistant germs and the smaller efficacy of standard therapies recommended up until now in hospital and healthcare-associated infections, some clinicians have suggested the empirical use of the widest spectrum antibiotics in these situations. Fernández et al[29] observed a failure rate of up to 60% in empirical therapy for nosocomial infections, and suggested that carbapenems and tigecycline should be used in the treatment of nosocomial SBP and spontaneous bacteremia. Pleguezuelo et al[16] suggested that a combination of penicillin and a beta-lactamase inhibitor, such as piperacillin-tazobactam, might represent an adequate alternative to the use of carbapenems in nosocomial infections. For these cases, third-generation cephalosporins might be inefficient in the treatment of a significant portion of infections in patients with cirrhosis. Currently, its use should be restricted to community-acquired infections, with changes being necessary with regard to recommendations for the empirical therapy of infections[12,34,36]. The current management of SBP with antibiotics is summarized in Figure 1.
Figure 1.
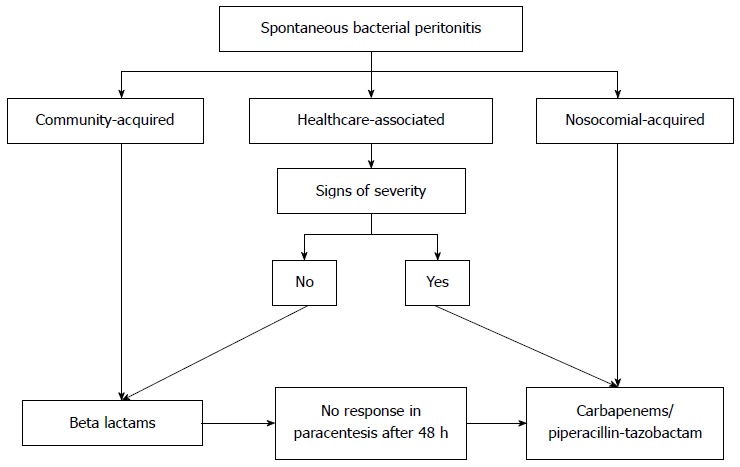
Use of antibiotics in spontaneous bacterial peritonitis.
It is worth noting that despite the proposals for new antibiotics to be instituted, the use of albumin should not be neglected. Renal failure is a frequent complication in patients with SBP and appears in approximately one-third of these patients. Renal failure is also considered a strong predictor of mortality during hospitalization[37]. When we assessed the occurrence of renal failure and its predictive factor in cirrhotic patients with SBP, we observed a permanent or progressive loss of renal function in 24% of the cases. Mortality in those with renal failure was 36.1%, and only 6.3% in patients with preserved renal function[38]. Intravenous albumin, given at a dose of 1.5 g/kg body weight on the first day and 1 g/kg of body weight on the third day of treatment, in addition to antibiotic therapy, reduced the incidence of renal failure from 33 to 10% and decreased the mortality rate from 29% to 10%[37]. The patients that benefited most from albumin treatment are those with creatinine levels > 1 mg/dL and total bilirubin > 4 mg/dL[39]. Thus, we believe that the administration of albumin in combination with antibiotic therapy is critical to the success of SBP treatment. It is important to note that albumin, in addition to boosting plasma volume, also has antioxidant, immunomodulatory, and detoxification properties, indicating that it should be seen as a drug and not a mere component of oncotic function[40]. A recent systematic review and meta-analysis evaluating albumin infusion recommended its use in all patients with SBP, whether or not they presented with higher risk factors[41].
It is important to highlight that the survival rate observed in patients with SBP is substantially smaller than in cirrhotic patients receiving a liver transplantation. Thus, liver transplantation must always be considered for those who survive an SBP episode. The association between the Model for End-Stage Liver Disease (MELD) score and the incidence of SBP must also be emphasized. A higher MELD score is associated with a higher SBP incidence (10% for each MELD point)[42,43]. The occurrence of SBP before liver transplantation does not influence patient survival, although it can put the patient at risk for major complications[44].
SBP PROPHYLAXIS
Patients who survive an episode of SBP have a high risk for recurrence (70% in the first year) and a reduced survival rate (30%-50% in the first year). Thus, cirrhotic patients who recovered from their first SBP episode are candidates for long-term prophylactic therapy with norfloxacin, and for liver transplantation assessment[6,8-10]. Prophylactic therapy with norfloxacin (400 mg/d) reduces the recurrence of SBP to approximately 20% in the first year, while the probability of SBP recurrence by a gram-negative microorganism is reduced from 60% to 3%[45,46].
Patients with cirrhosis and gastrointestinal bleeding have a higher frequency of bacterial infections, which favors a worse prognosis. The implementation of prophylactic antibiotics reduces the incidence of infections, re-bleeding, and mortality rates in this patient population[47-50]. In this context, norfloxacin (400 mg bid for seven days) has been used more commonly. A study demonstrated that endovenous ceftriaxone (1 g/d for seven days) was more effective than oral norfloxacin in the prevention of infections among patients with at least two of the following criteria: ascites, severe malnutrition, hepatic encephalopathy, or serum total bilirubin of > 3 mg/dL[51]. Thus, its use is recommended as a prophylactic therapy of choice for infections in patients with gastrointestinal bleeding and advanced cirrhosis[10]. It is worth noting that when comparing patients that received norfloxacin with those that received ceftriaxone, the former showed more frequent infections by gram-negative bacilli resistant to quinolones and by non-enterococcal streptococci[6]. This approach can be particularly useful in centers with high prevalence of germs resistant to quinolones.
Selective decontamination of the intestine with norfloxacin (400 mg/d orally) also seems to be efficient in the prevention of the first SBP episode in patients with low levels of proteins in the ascitic fluid[52]. In patients with ascitic fluid protein levels lower than 1.5 g/dL and advanced hepatic disease, the administration of prophylactic norfloxacin resulted in a reduction of the occurrence of SBP and hepatorenal syndrome during the first year, as well as an increase in survival rate over three months and over the first year[53]. However, the increasingly frequent number of bacterial infections caused by quinolone-resistant microorganisms and by gram-positive bacteria in cirrhotic patients should be kept in mind, as it may limit the efficacy of this approach[11].
More recently, studies have assessed the role of rifaximin in the prevention of SBP in cirrhotic patients because it is a non-absorbable antibiotic with a broad spectrum against gram-positive and gram-negative bacteria in the gastrointestinal tract, and has a low risk of bacterial resistance[54-56]. A recent study showed that patients who were treated long-term with rifaximin prophylactically had a smaller chance of developing SBP in comparison to those who did not receive it long-term (4.5% vs 46%, respectively)[55]. Moreover, no resistance to the drug was observed. Another study showed a 73% reduction in the risk for SBP in patients who used this antibiotic, and a survival rate free of liver transplantation that was greater than patients who did not use rifaximin (72% vs 57%, respectively)[56]. Thus, intestinal decontamination with rifaximin may be a promising alternative in SBP prevention, particularly if prophylactic use of quinolones constitutes a risk factor for infection by multi-resistant bacteria[29].
A brief commentary regarding prophylaxis without the use of antibiotics is also pertinent. A correlation between proton pump inhibitors and higher incidences of SBP has been suggested because the inhibitors favor enteric colonization, bacterial overgrowth and, ultimately, bacterial translocation among individuals with cirrhosis. Given that approximately 70% of patients with SBP use proton pump inhibitors without justification, it is clear that this medication should only be used in cirrhotic patients when it is indicated[57-59].
CONCLUSION
Taking into account the increasing evidence for multi-resistance among the bacteria that cause SBP and the lower efficacy of treatment with third-generation cephalosporins, new treatment recommendations have become necessary for the management of infections in cirrhotic patients.
The distinction between community-acquired infectious episodes, healthcare-associated or nosocomial infections, and the identification of risk factors for multi-resistant germs aid in the decision-making process for empirical antibiotic therapy choice. Broad-spectrum antimicrobial agents, such as carbapenems with or without glycopeptides or piperacillin-tazobactam, should be considered for the initial treatment, not only for nosocomial infections but also for healthcare-associated infections when signs of severity or risk factors for multi-resistant bacteria are present. It is crucial to identify the microbiological profile in each hospital to administer therapy early with the appropriate antibiotics, which will decrease the risk of bacterial mortality.
Footnotes
P- Reviewer: Avolio AW, Boin IF, Jin B, Lakatos PL S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246–1256, 1256.e1-5. doi: 10.1053/j.gastro.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 2.de Mattos AA, Coral GP, Menti E, Valiatti F, Kramer C. [Bacterial infection in cirrhotic patient] Arq Gastroenterol. 2003;40:11–15. doi: 10.1590/s0004-28032003000100003. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Tsao G. Bacterial infections in cirrhosis: treatment and prophylaxis. J Hepatol. 2005;42 Suppl:S85–S92. doi: 10.1016/j.jhep.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Heo J, Seo YS, Yim HJ, Hahn T, Park SH, Ahn SH, Park JY, Park JY, Kim MY, Park SK, et al. Clinical features and prognosis of spontaneous bacterial peritonitis in korean patients with liver cirrhosis: a multicenter retrospective study. Gut Liver. 2009;3:197–204. doi: 10.5009/gnl.2009.3.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinzello G, Simonetti RG, Craxì A, Di Piazza S, Spanò C, Pagliaro L. Spontaneous bacterial peritonitis: a prospective investigation in predominantly nonalcoholic cirrhotic patients. Hepatology. 1983;3:545–549. doi: 10.1002/hep.1840030411. [DOI] [PubMed] [Google Scholar]
- 6.Bonnel AR, Bunchorntavakul C, Reddy KR. Immune dysfunction and infections in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:727–738. doi: 10.1016/j.cgh.2011.02.031. [DOI] [PubMed] [Google Scholar]
- 7.Coral G, de Mattos AA, Damo DF, Viégas AC. [Prevalence and prognosis of spontaneous bacterial peritonitis. Experience in patients from a general hospital in Porto Alegre, RS, Brazil (1991-2000)] Arq Gastroenterol. 2002;39:158–162. doi: 10.1590/s0004-28032002000300005. [DOI] [PubMed] [Google Scholar]
- 8.Rimola A, García-Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, Inadomi JM. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142–153. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 9.Runyon BA. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 10.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Fernández J, Navasa M, Gómez J, Colmenero J, Vila J, Arroyo V, Rodés J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 12.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 13.Cheong HS, Kang CI, Lee JA, Moon SY, Joung MK, Chung DR, Koh KC, Lee NY, Song JH, Peck KR. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48:1230–1236. doi: 10.1086/597585. [DOI] [PubMed] [Google Scholar]
- 14.Park YH, Lee HC, Song HG, Jung S, Ryu SH, Shin JW, Chung YH, Lee YS, Suh DJ. Recent increase in antibiotic-resistant microorganisms in patients with spontaneous bacterial peritonitis adversely affects the clinical outcome in Korea. J Gastroenterol Hepatol. 2003;18:927–933. doi: 10.1046/j.1440-1746.2003.03086.x. [DOI] [PubMed] [Google Scholar]
- 15.Song KH, Jeon JH, Park WB, Park SW, Kim HB, Oh MD, Lee HS, Kim NJ, Choe KW. Clinical outcomes of spontaneous bacterial peritonitis due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella species: a retrospective matched case-control study. BMC Infect Dis. 2009;9:41. doi: 10.1186/1471-2334-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pleguezuelo M, Benitez JM, Jurado J, Montero JL, De la Mata M. Diagnosis and management of bacterial infections in decompensated cirrhosis. World J Hepatol. 2013;5:16–25. doi: 10.4254/wjh.v5.i1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida PR, Camargo NS, Arenz M, Tovo CV, Galperim B, Behar P. [Spontaneous bacterial peritonitis: impact of microbiological changes] Arq Gastroenterol. 2007;44:68–72. doi: 10.1590/s0004-28032007000100015. [DOI] [PubMed] [Google Scholar]
- 18.Chinnock B, Afarian H, Minnigan H, Butler J, Hendey GW. Physician clinical impression does not rule out spontaneous bacterial peritonitis in patients undergoing emergency department paracentesis. Ann Emerg Med. 2008;52:268–273. doi: 10.1016/j.annemergmed.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Soriano G, Esparcia O, Montemayor M, Guarner-Argente C, Pericas R, Torras X, Calvo N, Román E, Navarro F, Guarner C, et al. Bacterial DNA in the diagnosis of spontaneous bacterial peritonitis. Aliment Pharmacol Ther. 2011;33:275–284. doi: 10.1111/j.1365-2036.2010.04506.x. [DOI] [PubMed] [Google Scholar]
- 20.Runyon BA, Canawati HN, Akriviadis EA. Optimization of ascitic fluid culture technique. Gastroenterology. 1988;95:1351–1355. doi: 10.1016/0016-5085(88)90372-1. [DOI] [PubMed] [Google Scholar]
- 21.Mattos AA. Peritonite bacteriana espontânea: estudo comparativo de três técnicas no diagnóstico bacteriológico. Gastroenterologia e Endoscopia Digestiva. 1994;13:145–152. [Google Scholar]
- 22.Wiest R, Krag A, Gerbes A. Spontaneous bacterial peritonitis: recent guidelines and beyond. Gut. 2012;61:297–310. doi: 10.1136/gutjnl-2011-300779. [DOI] [PubMed] [Google Scholar]
- 23.Mattos AA, Buffé F, Mastalir ET, Silva DN. Sobrevida hospitalar dos pacientes cirróticos com bacterioascite. Gastroenterologia e Endoscopia Digestiva. 2000;19:117–120. [Google Scholar]
- 24.Soriano G, Castellote J, Alvarez C, Girbau A, Gordillo J, Baliellas C, Casas M, Pons C, Román EM, Maisterra S, et al. Secondary bacterial peritonitis in cirrhosis: a retrospective study of clinical and analytical characteristics, diagnosis and management. J Hepatol. 2010;52:39–44. doi: 10.1016/j.jhep.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Wiest R, Schoelmerich J. Secondary peritonitis in cirrhosis: “oil in fire”. J Hepatol. 2010;52:7–9. doi: 10.1016/j.jhep.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 26.Wu SS, Lin OS, Chen YY, Hwang KL, Soon MS, Keeffe EB. Ascitic fluid carcinoembryonic antigen and alkaline phosphatase levels for the differentiation of primary from secondary bacterial peritonitis with intestinal perforation. J Hepatol. 2001;34:215–221. doi: 10.1016/s0168-8278(00)00039-8. [DOI] [PubMed] [Google Scholar]
- 27.Ghassemi S, Garcia-Tsao G. Prevention and treatment of infections in patients with cirrhosis. Best Pract Res Clin Gastroenterol. 2007;21:77–93. doi: 10.1016/j.bpg.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Johnson DH, Cunha BA. Infections in cirrhosis. Infect Dis Clin North Am. 2001;15:363–371, vii. doi: 10.1016/s0891-5520(05)70150-1. [DOI] [PubMed] [Google Scholar]
- 29.Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, et al. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 30.Ariza X, Castellote J, Lora-Tamayo J, Girbau A, Salord S, Rota R, Ariza J, Xiol X. Risk factors for resistance to ceftriaxone and its impact on mortality in community, healthcare and nosocomial spontaneous bacterial peritonitis. J Hepatol. 2012;56:825–832. doi: 10.1016/j.jhep.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Acevedo J, Fernandez J, Castro M. Current efficacy of recommended empirical antibiotic therapy in patients with cirrhosis and bacterial infection. J Hepatol. 2009;50:S5. [Google Scholar]
- 32.Tandon P, Delisle A, Topal JE, Garcia-Tsao G. High prevalence of antibiotic-resistant bacterial infections among patients with cirrhosis at a US liver center. Clin Gastroenterol Hepatol. 2012;10:1291–1298. doi: 10.1016/j.cgh.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattos AA. Tratamento da peritonite bacteriana espontânea. Estudo retrospectivo. Gastroenterologia e Endoscopia Digestiva. 1996;15:201–206. [Google Scholar]
- 34.Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1–12. doi: 10.1016/S0168-8278(12)60002-6. [DOI] [PubMed] [Google Scholar]
- 35.Merli M, Lucidi C. Bacterial resistance in cirrhotic patients: an emerging reality. J Hepatol. 2012;56:756–757. doi: 10.1016/j.jhep.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Angeloni S, Leboffe C, Parente A, Venditti M, Giordano A, Merli M, Riggio O. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in the clinical practice. World J Gastroenterol. 2008;14:2757–2762. doi: 10.3748/wjg.14.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz-del-Arbol L, Castells L, Vargas V, Soriano G, Guevara M, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 38.Perdomo Coral G, Alves de Mattos A. Renal impairment after spontaneous bacterial peritonitis: incidence and prognosis. Can J Gastroenterol. 2003;17:187–190. doi: 10.1155/2003/370257. [DOI] [PubMed] [Google Scholar]
- 39.Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–1318. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Martinez R, Caraceni P, Bernardi M, Gines P, Arroyo V, Jalan R. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 41.Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11:123–130.e1. doi: 10.1016/j.cgh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 42.Obstein KL, Campbell MS, Reddy KR, Yang YX. Association between model for end-stage liver disease and spontaneous bacterial peritonitis. Am J Gastroenterol. 2007;102:2732–2736. doi: 10.1111/j.1572-0241.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 43.Musskopf MI, Fonseca FP, Gass J, de Mattos AZ, John JA, de Mello Brandão AB. Prognostic factors associated with in-hospital mortality in patients with spontaneous bacterial peritonitis. Ann Hepatol. 2012;11:915–920. [PubMed] [Google Scholar]
- 44.Mounzer R, Malik SM, Nasr J, Madani B, Devera ME, Ahmad J. Spontaneous bacterial peritonitis before liver transplantation does not affect patient survival. Clin Gastroenterol Hepatol. 2010;8:623–628.e1. doi: 10.1016/j.cgh.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 45.Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, Forné M, Miranda ML, Llach J, Salmerón JM. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: results of a double-blind, placebo-controlled trial. Hepatology. 1990;12:716–724. doi: 10.1002/hep.1840120416. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726–748. doi: 10.1053/gast.2001.22580. [DOI] [PubMed] [Google Scholar]
- 47.Bernard B, Grangé JD, Khac EN, Amiot X, Opolon P, Poynard T. Antibiotic prophylaxis for the prevention of bacterial infections in cirrhotic patients with gastrointestinal bleeding: a meta-analysis. Hepatology. 1999;29:1655–1661. doi: 10.1002/hep.510290608. [DOI] [PubMed] [Google Scholar]
- 48.Soares-Weiser K, Brezis M, Tur-Kaspa R, Leibovici L. Antibiotic prophylaxis for cirrhotic patients with gastrointestinal bleeding. Cochrane Database Syst Rev. 2002;(2):CD002907. doi: 10.1002/14651858.CD002907. [DOI] [PubMed] [Google Scholar]
- 49.Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY, Chang FY, Lee SD. Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology. 2004;39:746–753. doi: 10.1002/hep.20126. [DOI] [PubMed] [Google Scholar]
- 50.Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, Uribe M. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509–518. doi: 10.1111/j.1365-2036.2011.04746.x. [DOI] [PubMed] [Google Scholar]
- 51.Fernández J, Ruiz del Arbol L, Gómez C, Durandez R, Serradilla R, Guarner C, Planas R, Arroyo V, Navasa M. Norfloxacin vs ceftriaxone in the prophylaxis of infections in patients with advanced cirrhosis and hemorrhage. Gastroenterology. 2006;131:1049–1056; quiz 1285. doi: 10.1053/j.gastro.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 52.Novella M, Solà R, Soriano G, Andreu M, Gana J, Ortiz J, Coll S, Sàbat M, Vila MC, Guarner C, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology. 1997;25:532–536. doi: 10.1002/hep.510250306. [DOI] [PubMed] [Google Scholar]
- 53.Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818–824. doi: 10.1053/j.gastro.2007.06.065. [DOI] [PubMed] [Google Scholar]
- 54.Kalambokis GN, Mouzaki A, Rodi M, Tsianos EV. Rifaximin for the prevention of spontaneous bacterial peritonitis. World J Gastroenterol. 2012;18:1700–1702. doi: 10.3748/wjg.v18.i14.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlachogiannakos J, Viazis N, Vasianopoulou P, Vafiadis I, Karamanolis DG, Ladas SD. Long-term administration of rifaximin improves the prognosis of patients with decompensated alcoholic cirrhosis. J Gastroenterol Hepatol. 2013;28:450–455. doi: 10.1111/jgh.12070. [DOI] [PubMed] [Google Scholar]
- 56.Hanouneh MA, Hanouneh IA, Hashash JG, Law R, Esfeh JM, Lopez R, Hazratjee N, Smith T, Zein NN. The role of rifaximin in the primary prophylaxis of spontaneous bacterial peritonitis in patients with liver cirrhosis. J Clin Gastroenterol. 2012;46:709–715. doi: 10.1097/MCG.0b013e3182506dbb. [DOI] [PubMed] [Google Scholar]
- 57.Trikudanathan G, Israel J, Cappa J, O’Sullivan DM. Association between proton pump inhibitors and spontaneous bacterial peritonitis in cirrhotic patients - a systematic review and meta-analysis. Int J Clin Pract. 2011;65:674–678. doi: 10.1111/j.1742-1241.2011.02650.x. [DOI] [PubMed] [Google Scholar]
- 58.Deshpande A, Pasupuleti V, Thota P, Pant C, Mapara S, Hassan S, Rolston DD, Sferra TJ, Hernandez AV. Acid-suppressive therapy is associated with spontaneous bacterial peritonitis in cirrhotic patients: a meta-analysis. J Gastroenterol Hepatol. 2013;28:235–242. doi: 10.1111/jgh.12065. [DOI] [PubMed] [Google Scholar]
- 59.Goel GA, Deshpande A, Lopez R, Hall GS, van Duin D, Carey WD. Increased rate of spontaneous bacterial peritonitis among cirrhotic patients receiving pharmacologic acid suppression. Clin Gastroenterol Hepatol. 2012;10:422–427. doi: 10.1016/j.cgh.2011.11.019. [DOI] [PubMed] [Google Scholar]


