Abstract
Hepatitis B virus (HBV) continues to be a major cause of morbidity and mortality worldwide. It is estimated that about 350 million people throughout the world are chronically infected with HBV. Some of these people will develop hepatic cirrhosis with decompensation and/or hepatocellular carcinoma. For such patients, liver transplantation may be the only hope for cure or real improvement in quality and quantity of life. Formerly, due to rapidity of recurrence of HBV infection after liver transplantation, usually rapidly progressive, liver transplantation was considered to be contraindicated. This changed dramatically following the demonstration that hepatitis B immune globulin (HBIG), could prevent recurrent HBV infection. HBIG has been the standard of care for the past two decades or so. Recently, with the advent of highly active inhibitors of the ribose nucleic acid polymerase of HBV (entecavir, tenofovir), there has been growing evidence that HBIG needs to be given for shorter lengths of time; indeed, it may no longer be necessary at all. In this review, we describe genetic variants of HBV and past, present, and future prophylaxis of HBV infection during and after liver transplantation. We have reviewed the extant medical literature on the subject of infection with the HBV, placing particular emphasis upon the prevention and treatment of recurrent HBV during and after liver transplantation. For the review, we searched PubMed for all papers on the subject of “hepatitis B virus AND liver transplantation”. We describe some of the more clinically relevant and important genetic variations in the HBV. We also describe current practices at our medical centers, provide a summary and analysis of comparative costs for alternative strategies for prevention of recurrent HBV, and pose important still unanswered questions that are in need of answers during the next decade or two. We conclude that it is now rational and cost-effective to decrease and, perhaps, cease altogether, the routine use of HBIG during and following liver transplantation for HBV infection. Here we propose an individualized prophylaxis regimen, based on an integrated approach and risk-assessment.
Keywords: Cirrhosis, End-stage liver disease, Entecavir, Genetic variants, Hepatocellular carcinoma, Hepatitis B, Interferon alpha, Lamivudine, Liver transplantation, Tenofovir
Core tip: Hepatitis B viral (HBV) infection continues to be a major health problem world-wide. Recurrence of HBV following liver transplantation was a major problem in the 1980’s-1990’s, which led most insurers to refuse to cover costs of such transplants. This changed dramatically following the landmark demonstration that high-dose hepatitis B immune globulin (HBIG) could prevent recurrent infection. Recently, highly effective inhibitors of the HBV polymerase, with high barrier to resistance (entecavir, tenofovir) have become available, and they promise to decrease the need for HBIG and the costs and complexity of preventing recurrent HBV after liver transplantation.
INTRODUCTION
Infection with hepatitis B virus (HBV) continues to be a major cause of acute and chronic disease throughout the world, but most especially in East Asia, sub-Saharan Africa and Alaska. Recent estimates are that about 350 million people world-wide are chronically infected with HBV and that more than 1 million persons die each year due to advanced liver disease and/or hepatocellular carcinoma (HCC) caused by HBV infection. Other papers in this anniversary issue provide greater details regarding the epidemiology and prevalence of HBV infection, also reviewed in[1]. Fortunately, most adolescents or adults who contract acute HBV infection, chiefly from blood or blood products or from unprotected sex with infected persons, recover spontaneously from the infection. In contrast, neonates or young infants infected at or shortly after birth, usually due to vertical transmission from their mothers, do not mount immune responses to HBV or to infected hepatocytes. About 95% of these become immune-tolerant chronic carriers of the virus. Such children typically have no symptoms or signs of active hepatitis and are asymptomatic carriers of the virus.
Because of the high prevalence of chronic HBV infection and its proclivity for causing cirrhosis and HCC, it is not surprising that chronic hepatitis B (CHB) would be a leading indication for liver transplantation (LT), as this dramatic new, life-changing therapy was being introduced in the 1970’s and 1980’s throughout the world. However, due to the high frequency (above 90%) with which LT for CHB was followed by recurrent and rapidly progressive CHB with early graft failure, CHB was generally assessed as a contra-indication for LT, and, in the 1980’s and early 1990’s, United States Medicare and Medicaid and many private insurers refused to cover costs of LT for CHB.
This changed dramatically following the initial report of Samuel et al[2] from Europe, which established that hepatitis B immune globulin (HBIG), in sufficient doses and for long duration, could prevent recurrence of CHB in virtually all recipients of LT.
Thus, for the past 20 years, HBIG has been the cornerstone of prophylactic therapy. This is now beginning to change because of the advent of highly effective nucleoside and nucleotide inhibitors of the RNA polymerase (reverse transcriptase) of HBV called nucleos/tide analogues (NAs), especially tenofovir (TFV, Viread, Gilead) and entecavir (ETV, Baraclude, BMS).
In this paper, we present a review of the recent history of therapy of HBV during and after LT, a description of some of the major mutations of HBV that arise from the pressure of anti-viral therapy, and the promise of therapeutic regimens that, in the near future, will not include HBIG at all or that will involve HBIG for only a few months, rather than for indefinite durations. A number of unanswered questions remain and we suggest that carefully designed prospective randomized controlled trials should be carried out across the globe to answer these questions.
MOLECULAR VIROLOGY OF HBV AND ROLE OF SELECTED HBV MUTANTS IN POST-LT RECURRENCE
HBV is a member of the Hepadnaviridae family. Its partially double-stranded, circular desoxyribose nucleic acid (DNA) genome is contained in an icosahedron capsid, itself enveloped by a lipid bilayer decorated with three different surface proteins. Viral proteins that are clinically important include the following: envelope protein, hepatitis B surface antigen (HBsAg); a structural nucleocapsid core protein, hepatitis B core antigen (HBcAg); and a soluble nucleocapsid protein, hepatitis B e antigen (HBeAg) (Figure 1). Eight HBV genotypes have been recognized by a sequence divergence of more than 8% in the entire genome and designated by capital letters (A-H) in the order of discovery[3]. The predominant genotypes show geographical variations[3].
Figure 1.
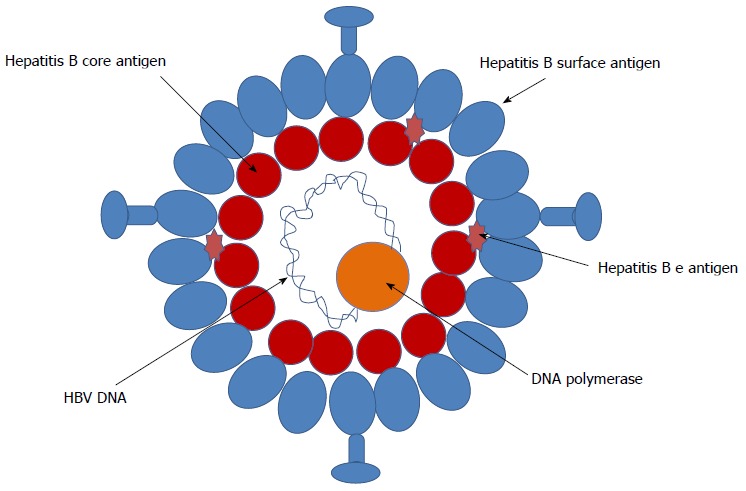
Schematic model of hepatitis B virus. HBV: Hepatitis B virus.
During the course of CHB, a proportion of infected persons spontaneously achieve significant reduction in virus replication with loss of HBeAg and seroconversion to its antibody, anti-HBe. Some of such HBeAg-negative subjects have persistent or intermittently high HBV replication associated with liver inflammation and ongoing fibrosis. This form of CHB, defined as HBeAg-negative CHB (e-negative CHB)[4], is mostly associated with mutations within the basal core promoter (BCP) and pre-core regions that result in reduction or prevention of HBeAg synthesis without affecting the replicative ability of the virus. The most well-known of these is a G to A mutation at nt 1896 in the pre-core region, which prevents HBeAg production[5]. Mutations within the BCP have also been reported in patients with e-negative CHB or fulminant hepatitis. The most commonly detected mutations within the BCP region are an A1762T transversion and a G1764A transition[6]. These BCP promoter double mutations, as well as pre-core mutation and HBV genotypes, are among the best known predictors of HCC risk. These promoter double mutations were also found to be an important predictor of post-LT clinical outcomes in patients with HBV-related HCC.
Recently, we identified another novel double promoter mutations known as T1764G1766 and suggested that this double mutant should be prevalent in genotype D of HBV isolates[7]. In this study performed on HBV-infected Iranians, we found that one third of subjects studied harbored HBV isolates containing these double mutations. We suggested that these mutations form a putative new binding site for the transcription factor hepatocyte nuclear factor 3 (HNF3)[7]. Furthermore, we showed that only patients harboring strains with A1757 had the T1764G1766 double mutations, and these mutations are likely to occur mainly in genotype D and possibly genotype E[7]. This mutational constraint depending on a single silent nucleotide polymorphism within the viral genome was among the first mutational constraints described in the HBV genome. These mutations were later found to be widespread in different regions of world where genotypes D, and E are more prevalent and are associated with increased risk of HCC[8-11]; the potential role of these mutations on post-LT HBV recurrence is yet to be explored.
There are only a few studies that have investigated the potential influences of precore or BCP mutants on outcome of liver transplantation. Angus et al[12] found that infection with precore mutant strains of HBV predisposes patients to severe recurrent disease and early graft loss following transplantation. In a recent follow-up study, performed on 78 consecutive patients who underwent LT because of HBV-related HCC, it was shown that BCP mutations independently predicted a shorter survival period free from HBV relapse[13]. Whole HBV genome sequencing of viral isolates from patients with recurrent HBV showed that infecting HBV strain(s) harbored T1753G/A1762T/G1764A triple mutations in the basal core promoter and the G1896A nonsense mutation in the pre-core region both pre- and post-transplant. After transplantation and therapy, several point mutations within the HBV genome emerged or became dominant. These mutations caused L426I/L526M/M550I triple mutations in the polymerase gene, and D144E mutation within the ‘‘a’’ determinant of HBsAg[14]. McMillan et al[15] showed that genotype D and accumulated mutations throughout the HBV precore/core gene, but not core promoter, were associated with severe recurrent disease post-transplantation. Mutations were found throughout the entire HBV core gene. However, at the amino acid level, clustering was observed in the B- and helper T-cell epitopes, as well as in nuclear localization signals.
Many related factors may be responsible for HBV recurrence, including recipient host factors (ethnic background, HLA type, pre-transplant HBV replication status, extra-hepatic foci of HBV), donor factors (compromised donor liver, HLA type and compatibilities, presence of donor HBV-specific lymphocyte), perioperative treatment (use of antiviral agents and immunosuppressants, drug resistance, viral mutations)[16]. Survival rates are known to be compromised after liver transplantation in patients who are HBeAg positive prior to liver transplant[17]. It is not surprising that viral replication is higher under drug-induced immunosuppression after liver transplantation. In particular, corticosteroids lead to increase replication of HBV and are thought to play a role in HBV recurrence after liver transplant. It has been shown that viral replication is stimulated by steroids that bind to the glucocorticoid responsive enhancer region of the HBV genome[18]. As a consequence it has been proposed that corticosteroids should be rapidly removed from immunosuppressive regimens to minimize the risk of HBV recurrence. Different HBV genotypes could potentially influence recurrence rates of HBV, but thus far differences in the recurrence rates among different HBV genotypes have not been observed[19,20].
Among numerous potential factors, HBV variants with antiviral drug-resistant mutations and/or HBIG-resistant mutations are main causes of HBV reinfections post-liver transplant[21]. While the use of NAs pre-transplant and combination of NAs and HBIG post-transplant have been shown to decrease the rate of HBV reinfection post-transplant to less than 10%, almost all cases of HBV reinfection are due to HBV variants with antiviral drug-resistant mutations and/or HBIG-resistant mutations[21]. Mutations associated with antiviral resistance may be classified as either primary (responsible for decreased susceptibility to the drug) or compensatory (responsible for restoring replication fitness of the mutant virus)[21].
Amino acid substitutions within the HBs antigen may produce conformational changes and influence the binding of neutralizing antibodies. Consequently, these HBV mutants have been shown to be able to escape from vaccine-induced antibody responses. The most frequent form of these substitutions is the sG145R mutation of HBsAg. The emergence of resistance to NAs is a major issue affecting long-term therapy with some of these agents. NAs directly inhibit the reverse transcriptase activity of the HBV RNA polymerase and may lead to emergence of HBV strains containing primary substitutions in the viral genome and associated resistance to NAs. Lamivudine (LAM) resistance occurs frequently and is observed in up to 80% of patients treated for 5 years[22]. These mutations lead to amino acid changes in the YMDD (Tyrosine-methionine-aspartic acid-aspartic acid, a four amino acid motif of HBV protein that is often mutated) motif of the viral reverse transcriptase (rt). Primary resistance mutations result in the replacement of the methionine by valine, leucine, or occasionally serine and are designated rtM204I/V/S[23]. Adefovir dipivoxil (ADV) treatment, which is an alternative therapy for LAM-resistant HBV, can also result in the selection of ADV-resistant variants like rtA181T/V or rtN236T. TFV is closely related to ADV. The primary mutations associated with ADV resistance can also decrease the efficacy of TFV[23].
A recent 53-mo follow-up study was done on 362 patients with CHB who underwent LT. None of the patients received HBIG. Half of the patients were on LAM, while 39% received ETV, and 12% were on combination therapy (LAM + ADV) at the time of transplant[24]. The virological relapse rates at 3 years were 17%, 0%, and 7% for LAM, ETV, and LAM + ADV. Forty-two patients had virological relapse, of which the majority had a HBV isolate harboring the YMDD mutation. These findings show the importance of using new agents with a high barrier to resistance for minimizing drug resistance[24].
Administration of HBIG that exerts an anti-HBs-mediated immune pressure on HBV is also associated with the emergence of immune escape HBV mutants. These HBsAg escape mutants harbor single or double point mutations that may significantly alter the immunological characteristics of HBsAg. Most escape mutations that influence HBsAg recognition by anti-HBs antibodies are located within the second ‘a’ determinant loop. Notably, HBsAg with an arginine replacement for glycine at amino acid 145 is considered the major immune escape mutant[25]. Schätzl et al[26] suggested that the high rate of reinfection in liver transplant recipients seems not to be associated with specific sequence variations in the major HBs gene, but shows a remarkable inter- and intra-individual variability. No correlation between heterogeneity in this gene and clinical outcome was present. In another study, Shen et al[27] found that mutations within the HBV DNA that encodes for HBV P and S proteins were factors affecting re-infection post-transplantation. In yet another study on 75 patients who received HBIG prophylaxis for more than 6 mo after liver transplantation from anti-HBc positive donors, it was shown that escape mutations from anti-HBs caused de novo activation of HBV under HBIG prophylaxis after liver transplantation[28].
Apart from molecular viral factors that were discussed above, the presence of HBV specific lymphocytes - in human liver grafts from HBV immune donors - plays a major role in HBV recurrence after liver transplantation. In a study by Luo et al[29], 48.6% of post-LT patients with chronic HBV infection showed a spontaneous anti-HBs production, which was significantly associated with a higher number of donor-derived T lymphocytes specific for HBsAg, and it was suggested that the presence of considerable numbers of donor-derived HB-specific immunocompetent cells in grafts may account for the adoptive transfer of HBV immunity through liver transplantation.
In summary, among numerous potential viral factors, HBV variants with antiviral drug-resistant mutations and HBIG-resistant mutations remain as major causes of HBV reinfections post-liver transplant while the role of other HBV mutants (e.g., BCP mutations or cytotoxic T lymphocyte escape mutations[30]) are yet to be adequately investigated.
MANAGEMENT OF HBV INFECTION IN THE PERI-TRANSPLANT PERIOD
Past
Major advances have been made in regards to the management of CHB pre- and post-liver transplantation. During the 1950’s, what is considered the early era of treating hepatitis B, corticosteroids were used for treatment. Their use was predicated on the knowledge even then that host immune responses (natural killer cells and cytotoxic T lymphocytes) were important in pathogenesis. In the 1960’s, landmark prospective controlled, randomized trials spearheaded by Dame Sheila Sherlock and her students[31,32], established that corticosteroids were of benefit for treatment of idiopathic autoimmune hepatitis. Such treatment was undoubtedly also given to many patients with infectious hepatitis, especially what then was called non-A, non-B hepatitis and which we now know was caused mainly by chronic hepatitis C. Such patients often showed improvements in the severity of hepatic inflammation, as measured biochemically and histologically, but at the expense of increased viral replication. Not surprisingly, the HBV infected patients treated with corticosteroids did not flare as well as those with autoimmune idiopathic hepatitis. A variety of other antiviral agents, (not specific for HBV) have been studied in HBV, including adenine arabinoside, acyclovir, zidovudine, foscarnet, ribavirin, and D-penicillamine. None of these agents proved to be useful or successful for HBV.
With development and growth of liver transplantation in the 1980’s-1990’s, chiefly fueled by improvement in surgical techniques and in immunosuppressant medications (cyclosporine, tacrolimus), it was expected that increasing numbers of patients with CHB, complicated by development of end-stage liver disease or hepatocellular carcinoma, would need to be considered for LT. It rapidly became clear that graft re-infection with HBV post LT was usual and led to rapidly progressive and fatal recurrent hepatitis B. These occurrences quickly fostered policies and practices that rendered active HBV infection a contraindication to LT in many centers[33]. Indeed, in the 1980’s through early 1990’s, United States Medicare and Medicaid, and many private insurers refused to cover LT for patients with active HBV infection.
Introduction of long-term intravenous (iv) high dose HBIG dramatically reduced the rate of post-transplant HBV recurrent infection and improved post-transplant survival[2]. This was a retrospective analysis from many European liver transplant centers. Because of these dramatic improvements in outcomes with HBIG use, transplant policies were again revised and it became acceptable to perform LT for HBV related liver disease[2,34]. HBV did recur as evidenced by the appearance of HBsAg escape mutants during long term prophylaxis with HBIG. Short term HBIG administration (< 6 mo) post-transplant dosing was also quite disappointing, with a very high HBV recurrence rate, similar to no immunoprophylaxis at all.
Interferon (IFN)-α was introduced in the late 1990’s as an antiviral agent for both HBV and HCV, but its use was quite limited, especially in those with decompensated liver disease[35]. Traditionally, it has had a limited role in the post-LT setting, most notably due to its numerous adverse effects/toxicities and the increased risk of graft rejection due to the immunostimulatory effects of IFN[36].
Lamivudine (LAM), an oral cytosine analogue, was introduced as the first nucleoside analogue for CHB. Compared to IFN, LAM was generally well tolerated and effective, making it a major advancement for the field of hepatology. LAM therapy improved liver function in many patients with CHB, making listing for LT in some cases no longer necessary. Initial reports of administration of LAM at 100 mg daily resulted in promising short term results[37], but subsequently virologic breakthroughs due to the emergence of resistant viral strains, as described above, occurred in 40%-50% of patients[38]. The emergence of HBV resistance to LAM is due chiefly to mutations within the YMDD motif of the HBV polymerase gene (see above). There are conflicting reports as to significance of these resistant mutants in the post-transplant setting.
In the post-transplant setting, LAM proved to be superior to HBIG at preventing recurrence of hepatitis B, because of its efficacy, ease of administration, and lower cost. Serum HBV-DNA levels usually became undetectable as assessed by hybridization assays after a mean of 12-25 mo of LAM treatment[39].
Adefovir dipivoxil (ADV) was approved for therapy of HBV infection several years after LAM. It is as active on wild-type virus as on LAM-resistant mutants, and therefore was approved as a first-line therapy, but also as a salvage therapy for patients with resistance to LAM[40,41]. Approximately 25% of patients had a decrease less than 2.2 log10 copies/mL after one year of ADV administration[42,43]. While there was initial enthusiasm following the introduction of ADV, this was tempered by its relatively weak potency. It has been shown to suppress levels of HBV more slowly than the NAs; LAM, telbivudine and the currently used TFV and ETV. Because of its relatively weaker potency, the recommendation was for use of ADV in the treatment of HBeAg-negative CHB infection. Patients who are HBeAg negative typically have lower rates of replication and levels of virus in serum, as compared to HBeAg-positive patients. Thus the potential for development of resistant mutants was diminished. Of note, ADV-resistant strains of HBV are usually susceptible to LAM, just as LAM-resistant strains are usually susceptible to ADV. A major concern with the long term use of ADV was the development of nephrotoxicity[44]. Patients with chronic liver disease are already at increased risk for developing renal insufficiency, as are patients who have undergone solid organ transplantations (heart, kidney, liver, lung) due to the use of nephrotoxic calcineurin inhibitors to prevent organ rejection. Thus, the manufacturer of ADV and the United States Food and Drug Administration issued a black box warning regarding risks of use of the drug in patients with advanced liver disease.
Present
Since the introduction of oral NAs as potent antiviral agents against HBV, a significant outcome benefit has been provided by using combination of HBIG plus NAs in post-LT patients, lowering HBV recurrence rates to 5%-10% and improving the 5-year graft survival to more than 80%. Using HBIG plus antivirals has been the standard of care for patients undergoing LT for acute fulminant or CHB disease over the past decade. The type of HBV prophylaxis regimens in post-LT patients has been evolving since ETV and TFV with more potent antiviral effect and higher resistant barrier became available.
Entecavir: 0.5 mg/d, an oral nucleoside analogue, was introduced in the mid-2000s as a potent anti-HBV agent. In comparison with LAM, ETV was associated with a higher rate of viral suppression (36% vs 67%) at 4 years of therapy in nucleoside-naïve patients with CHB and a very low rate of viral resistance (around 1%) up to 5 years of treatment[45,46]. ETV is not recommended in patients with LAM-resistance given that emergence of resistance to ETV can be as high as 50% in this group of patients[46]. ETV is both more potent than ADV with a higher rate of HBV DNA suppression resulting in an undetectable HBV DNA at 48 wk of treatment in 58% vs 19% patients on ETV and ADV, respectively[47]. Given the lack of nephrotoxicity, ETV is the preferred treatment option in both decompensated cirrhosis and after LT.
Tenofovir disoproxil fumarate: 300 mg/d, is a NA initially introduced for the treatment of human immunodeficiency virus (HIV) infection and subsequently approved for use in patients with CHB in 2008. It is available as tenofovir disoproxil fumarate (TFV) only or in combination with emtricitabine. The use of TFV in post-LT setting comes as a second choice following ETV due to the concern of nephrotoxicity associated with TFV. This is particularly important because of the considerable incidence of renal impairment in liver transplant patients due to calcineurin inhibitor use, peri-transplant acute kidney injury, as well as coexistence of diabetic and hypertensive nephropathy in these patients. The long term use of TFV in HIV patients has been associated with metabolic bone disease and osteomalacia[48]. This observation is relevant to post-LT patients who are already at higher risk of metabolic bone disease.
EFFECT OF HBIG PLUS LAM OR NEWER NAs COMBINED PROPHYLAXIS ON HBV RECURRENCE POST-LT
Despite dramatic reduction in post-LT HBV recurrence rate made by either HBIG or LAM monotherapy, due to emergence of drug resistance both options are clearly inferior to the combined prophylaxis. Combination of iv HBIG and LAM was first reported in 1998 to provide higher efficacy in HBV prophylaxis in a small group of post-LT patients[49]. Several reports subsequently showed that combination of HBIG and LAM brought the post-LT HBV recurrence rate to lower than 10%[50,51]. Later on, substitution of LAM with options that carried less risk of resistance including ADV or ADV plus LAM was studied in post-LT setting. A systematic review comparing patients who received combinations of HBIG and ADV with or without LAM with patients on HBIG and LAM, showed a lower rate of HBV recurrence post-LT in the group that received ADV (25% vs 6% respectively), although more patients in the HBIG + LAM group had high-risk factors for HBV recurrence, including detectable HBV DNA in serum at transplant[52]. Although, most centers have moved from using LAM or LAM plus ADV as the oral agents to the newer NAs, published outcome data still are limited.
A recent systematic review of data published in 2008-2012 by Cholongitas et al[53] compared patients who received combined prophylaxis regimens of HBIG [iv or intramuscular (im) with various durations and doses] plus older or newer NAs. Patients who received ADV or LAM + ADV were not included in the comparisons. All the patients who received ETV or TFV with or without another NA were included under the newer NAs group. This study concluded that patients on HBIG + LAM had a higher HBV recurrence rate (115/1889 or 6.1%) than patients who received HBIG plus newer NAs (3/303 or 1%). About 90% of all patients received indefinite HBIG.
Various strategies for routes and doses of HBIG administration exist. HBIG administration based on serum HBsAb titer with maintaining the titer above 50-100 IU/mL is less expensive than a fixed-dose HBIG protocol, however, it is time-consuming. A typical high dose iv HBIG protocol consists of a 10000 IU at anhepatic phase followed by daily (5000-10000 IU) for 1 wk, and then 5000 IU monthly for 6-12 mo and every 3 mo thereafter (Table 1). im HBIG has similar pharmacokinetics to iv HBIG and was shown to be more cost effective compared to high dose iv HBIG when it was combined with LAM[54]. Combination of LAM and low dose im HBIG (400-800 IU) daily for the first week post-LT and monthly thereafter was associated with 4% HBV recurrence at 5 year[50]. In another study using LAM plus im HBIG daily for 1 wk followed by weekly for 3 wk, then monthly thereafter, an 8% recurrence HBV was noted at 2 year in patients with low viremia (≤ 105 copies/mL) at transplant[55]. The combination of LAM plus low dose HBIG aiming to maintain HBsAb titer ≥ 100 IU/mL, revealed HBV recurrence of 8% at 5 year which was within the similar range of HBV recurrence rate in other fixed-dose HBIG + LAM combined protocols.
Table 1.
Three protocols for prevention of recurrent hepatitis B virus infection after liver transplantation
| HBIG-indefinite (HBIG continued for life) | HBIG 10000 IU iv during anhepatic phase; |
| then 5000 IU iv daily for 5 d; followed by 800 IU im monthly indefinitely | |
| Plus | |
| ETV or TFV indefinitely | |
| HBIG-light (HBIG for first 6 mo only) | HBIG 10000 IU iv during anhepatic phase; |
| then 5000 IU iv daily for 5 d; followed by 800 IU im monthly for 6 mo | |
| Plus | |
| ETV and/or TFV indefinitely | |
| HBIG-free (No HBIG) | ETV and/or TFV indefinitely |
ETV: Entecavir; HBIG: Hepatitis B immune globulin; im: Intramuscular; iv: Intravenous; TFV: Tenofovir. The appropriate annual costs of these are set out in Table 2.
EFFECT OF DISCONTINUATION OF HBIG FOLLOWING A COURSE OF COMBINED PROPHYLAXIS
Combination of HBIG and NAs is an effective regimen in preventing HBV recurrence, however, the cost and inconvenience of HBIG administration have led to evolution of HBIG protocols toward more limited durations. The most recent systematic review of four published studies on patients who remained on newer NAs after withdrawal of HBIG did not show statistically significant HBV recurrence rates in patients receiving newer NAs prophylaxis after withdrawal of HBIG compared to the group that remained on long term HBIG and LAM [3.9% (4/102) vs 6.1% (115/1889), respectively][53]. HBIG therapy was continued for a median 6 mo prior to withdrawal. In this study, the majority of patients (93/102) remained on TFV alone or TFV + emtricitabine/LAM after discontinuation of HBIG for a median follow up of 24 mo (range 11-31 mo). If HBV recurrence was defined based on a detectable HBV DNA instead of HBsAg positivity alone, only 0.9% (1/102) of patients in this group had HBV recurrence. One out of 10 patients who were on ETV alone during HBIG-free period had HBV recurrence. The study did not show statistically significant difference in HBV recurrence rate between those who remained on ETV or TFV monotherapy compared to those who received long term dual antiviral therapy [5.2% (1/19) vs 3.6% (3/83)]. Wesdorp et al[56] recently published the safety and efficacy of combination of NAs after withdrawal of HBIG in 16 patients who underwent LT for chronic HBV disease. All patients had undetectable HBV DNA at transplant and received HBIG for a minimum of 6 mo post-LT followed by TFV + emtricitabine dual therapy without HBIG for a mean duration of 2 years. No HBV recurrence was noted as defined by HBV DNA positivity in serum. Only 1 patient developed a positive HBsAg, however, with undetectable HBV DNA and no clinical evidence of hepatitis. The results of this study favor HBIG withdrawal and use of combined NA regimen, however, confidence in the conclusions is limited by the small size of study. Given the fact that nearly all patients (15/16) had a stable undetectable HBV DNA prior to cessation of HBIG-including regimen and start of the combination of TFV and emtricitabine, it is unclear that combination of NAs offers any benefit over NA monotherapy in patients with potentially a more favorable long term outcome. Teperman et al[57] studied the HBV recurrence rate in 40 patients who received combined dual NAs prophylaxis at a median follow up of 3.4 years post-LT. In this study, all patients received 24 wk of HBIG and subsequently were randomized to TFV plus emtricitabine combination with (18 patients) or without (19 patients) HBIG therapy. All the patients with post-LT recurrent HBV as well as those with likely higher risk for HBV recurrence such as those with history of HCC were excluded from the study. None of the patients in either arm of the study had HBV recurrence through 72 wk of follow up. Eighty-two percent of the patients had renal dysfunction (creatinine clearance less than 80 mL/min) at the pre-randomization stage, but serum creatinine remained stable throughout the study follow up.
In summary, small prospective and retrospective studies with rather short term follow up in those patients with lower risk for recurrence, reveal efficacy and safety of combination newer NAs regimen after a finite HBIG treatment. Limited data are available to compare the efficacy of NA combined therapy over monotherapy in such patients, therefore, larger and more definitive studies are necessary.
EFFECT OF HBIG-FREE PROPHYLAXIS WITH NEWER NAs ON HBV RECURRENCE POST-LT
Although studies have shown efficacy of HBIG withdrawal in post-LT setting, one of the key questions is whether HBIG can be omitted even during the early post-LT period. The results of a large cohort of 362 patients from Hong Kong showed a promising and effective outcome from an HBIG-free protocol using ETV alone with a 0% recurrence rate at 3 years post-transplant[24]. Extending results of their earlier study on 80 patients who underwent deceased or living donor liver transplant for chronic HBV disease followed for a median time of 26 mo post-LT on HBIG-free prophylaxis[24]. The study patients were heterogeneous with regards to the HBV recurrence risk as only 26% had an undetectable HBV DNA at transplant, 25% of patients had HCC, and 14/19 patients had LAM-resistant mutation. Interestingly, undetectable HBV DNA and HBsAg seroclearance at 2 years of follow up were achieved by ETV monotherapy in 98.8% and 91% of patients, respectively. In a series of 6 patients who had undetectable HBV DNA at transplant, a regimen of ETV monotherapy in addition to a single high dose of iv HBIG at anhepatic phase was shown to be effective[58]. Another smaller study also showed effective HBV prophylaxis via HBIG-free regimen using the combination of LAM plus ADV in patients with low risk of recurrence at transplant[59]. These studies suggest that HBIG-free regimens with newer NAs are effective in HBV prophylaxis post-LT and that monotherapy with NAs are not inferior to combined NAs regimens.
CONSIDERATION OF COSTS/COST EFFECTIVENESS/ALTERNATIVE STRATEGIES; ISSUES OF PUBLIC AND PRIVATE POLICY
The costs of HBV prophylaxis post-LT vary widely. Treatment has previously been estimated to be > $100000 in upper income countries for the first year post transplant and > $50000 for subsequent years[60-62]; however, these studies have cited older work by Lok in 2002 and Han in 2000 as a source for these estimates. Our estimates for the most expensive medication, HBIG, obtained through contemporary sources[63,64], indicate lower costs than noted in the literature (Table 2). Nonetheless, HBIG- containing regimens incur medication costs of upwards of $50000 in the first year in the United States. In low and middle income countries with far higher rates of HBV and lower access to treatment, higher costs may occur due to import fees and other charges. The high costs of HBIG present a barrier to HBV treatment, including LT, especially in low and middle income countries.
Table 2.
Approximate annual costs ($USD) for three post-transplant protocols for hepatitis B virus prophylaxis
| Costs for the first year | |||||||||
| HBIG-indefinite | HBIG 10K | 7051 | 5K IU | $17628 | 800 IU monthly | $6723 | ETV, TFV | $9900 | $41302 ($31059-$51545) |
| HBIG-light | HBIG 10K | 7051 | 5K IU | $17628 | 800 IU × 6 monthly | $3385 | ETV, TFV | $9900 | $37964 ($28549-$47379) |
| HBIG-free | None | None | None | None | None | None | ETV, TFV | $9900 | $9900 |
| Costs in the subsequent years | |||||||||
| HBIG-indefinite | None | None | None | None | 800 IU monthly | $6723 | ETV, TFV | $9900 | $16623 ($14097-$19149) |
| HBIG-light | None | None | None | None | None | None | ETV, TFV | $9900 | $9900 |
| HBIG-free | None | None | None | None | None | None | ETV, TFV | $9900 | $9900 |
ETV: Entecavir; HBIG: Hepatitis B immunoglobulin; iv: Intravenous; im: Intramuscular; 1K: 1000 units; 10K: 10000 units; TFV: Tenofovir; NAs: Nucleotide analogues. These costs will be approximately doubled if two NAs are administered.
In the United States, insurers may not cover the prohibitive costs of HBIG, all or in part, leading patients to have decreased access to treatment and increased risk of HBV recurrence. As already described, the three oral drugs that can potentially replace HBIG, i.e., NAs such as ETV, ADV, and TFV, offer another option for prophylaxis. Although those are more costly than LAM, each dramatically lowers the cost of care as compared to HBIG. For example, the estimated monthly price for NA in Canada was estimated to be between $600 and $900[65], or a mean yearly cost of $7200-$10800 per year.
Here we review the cost of three commonly used prophylaxis regimens in transplant centers in the United States (Table 1, Table 2): (1) HBIG-given indefinitely; and (2) HBIG-light, and c) HBIG-free (See the details of each protocol above). Using the cost estimates for United States in 2008 United States dollars made by Saab et al[60], Tanaka et al[65], and Di Paolo et al[66] augmented by published data from United States health facilities and the Centers for Disease Control, the cost for one unit HBIG iv is between $0.4 and $1.15 United States. In the same fashion, based on Tanaka et al[65], Tsai et al[67], Saab et al[60], and Ahn et al[68], the cost for one month of the ETV or TFV is between $600 and $1000. For the sake of simplicity, we disregarded the difference in cost of HBIG iv and that of HBIG im, assumed no cost difference between ETV and TFV prescriptions and used the mean cost estimates of each medication. Accordingly, the cost for the HBIG -indefinite protocol in the first year is approximately $41302 (range $31059 -$51545), for HBIG-light it is $37964 (range $28549-$47379) and for HBIG free-consisting of monotherapy, it is about $10000. In subsequent years, the total annual costs drop to about $17000 for HBIG -indefinite, and $10000 for HBIG-light and NA only treatment. We also estimated an interval for HBIG-free protocol to allow for the option of mono ($10000) or combination therapy (2 × $10000). From the perspective of cost it is evident that HBIG-free is associated with the lowest cost burden of the three options, however, the HBIG-light regimen also dramatically reduces the costs over the long term. Replacing HBIG, fully or partially, by any of the alternative protocols presented in this paper, would result in a drop of between 11%-75% in medication costs for patients in the first year after transplant[61,65,69]. The wide range of estimates is due to variable methods of cost calculation for HBIG available from the published sources. It’s important to note that we did not include any costs of administration of HBIG in our cost estimates. These costs can show marked variability, more so than the cost of the medications. In the United States, for example, the costs of drug infusion services are usually higher than those in most of the other high-income and middle-income countries where LT is performed.
It is evident from our approximate cost calculations that the dose and interval of HBIG administration are the most important determinants of cost projections, as also noted by Dan et al[61]. While the decision of the optimal protocol ideally is a clinical one, based on evidence of treatment efficacy, the high cost of HBIG affects the therapeutic decisions in particular in resource - poor settings. The route of drug administration (HBIG iv or HBIG im for example) can be a determinant of access to these medications as HBIG iv needs more services at higher costs compared to HBIG im administration. Also, small differences in the price of oral medications can significantly affect the long-term costs of care for the patients. We believe clinicians make implicit cost-effective decisions when the cost differences among available treatment options are large. This can be especially true in settings where third party payers refuse to cover certain expensive treatment strategies in the presence of less expensive options[70].
FUTURE: INDIVIDUALIZED PROPHYLACTIC THERAPY
The current state of HBV post-transplant prophylaxis is going through another phase of changes while transplant centers around the world are trying to either minimize the duration of HBIG therapy or take the HBIG-free approach (Table 3). In most centers, an HBV recurrence rate less than 5% at 3 years is expected post-LT in patients with CHB. A similar or better outcome is expected with any newer HBIG-light or HBIG-free protocols. We suggest a comprehensive approach, integrating risks related to patient, viral, and antiviral factors, cost and convenience of different protocols in the current era of post-LT HBV prophylaxis.
Table 3.
Proposed individualized protocols for prophylaxis of hepatitis B virus recurrence post- liver transplantation, based on risk of recurrence
| HBV recurrence risk | First 6 mo post-LT | Withdrawal of HBIG |
| High-risk: | ||
| Resistant mutations pre-LT | HBIG-Light | Combination of NAs |
| HCC at transplant | Plus | (ETV + TFV) |
| HDV/HBV co-infection | Monotherapy with NA (ETV or TFV) | |
| HIV/HBV co-infection | ||
| Non-adherence | ||
| Moderate-risk: | ||
| HbcAb + donors into HbsAb-recipients | HBIG-Light | Monotherapy with NA |
| Early post-LT renal dysfunction requiring | Plus | (ETV or TFV) |
| Indentation NA dose adjustment | Monotherapy with NA (ETV or TFV) | |
| Low risk: | ||
| Undetectable HBV DNA at transplant | HBIG-free | Monotherapy with NA |
| HbcAb + donors into HbsAb + recipients | Monotherapy with NA (ETV or TFV) | (ETV or TFV) |
| Unknown risk: | ||
| HBV naïve donor into HbcAb + recipient | None | None |
Ab: Antibody; ETV: Entecavir; HBIG: Hepatitis B immune globulin; HBc: Hepatitis B core; HBV: Hepatitis B virus; HCC: Hepatocellular carcinoma; HDV: Hepatitis D (delta agent); LT: Liver transplantation; NAs: Nucleotide analogues; TFV: Tenofovir; HIV: Human immunodeficiency virus.
Risk assessment
HBV replication status at transplant: Presence of a replicating virus pre-transplant is highly predictive of HBV recurrence. A serum HBV DNA ≥ 5 or 6 log10 copies/mL at transplant has been shown consistently to be associated with post-transplant HBV recurrence[24,50,71,72]. Growing data support the safety and efficacy of newer NA in patients with decompensated liver disease due to HBV, thus more patients are expected to benefit from achieving an undetectable HBV DNA in serum at transplant, which leads to a lower risk of HBV recurrence post-LT[73,74]. A randomized prospective study on 112 patients with decompensated HBV cirrhosis showed that the majority of patients who received TFV, emtricitabine/TFV, or ETV were able to achieve a low level of HBV DNA < 400 copies/mL (69 IU/mL) at week 48 (70%, 87%, and 72%, respectively)[74]. Fulminant hepatitis B on the other hand is associated with a lower risk of HBV recurrence[75].
LAM resistance: Presence of LAM-resistant HBV pre-transplant or emergence of drug resistance while on post-LT prophylaxis has been consistently predictive of HBV recurrence. Recognizing drug-resistance especially pre-transplant would determine the high-risk patients who potentially benefit from a conservative approach, including a combination of HBIG plus NAs. Combination of LAM plus either ADV or emtricitabine have shown efficacy with a low recurrence rate of HBV post-LT. Newer antiviral drugs used either alone or in combination are clearly superior to LAM and ADV in non-transplant patients, and as discussed earlier, single or combined regimens of newer NAs are also effective for HBV prophylaxis in post-LT setting when used with or without HBIG. No resistant HBV was detected at 3 year post-transplant in the study by Fung et al[24] in patients on ETV monotherapy. In this study, ETV monotherapy was superior to combination of LAM plus ADV with regards to recurrence rate.
Definition of HBV recurrence: Most studies have defined HBV recurrence as reappearance of HBsAg and/or HBV DNA post-transplant. It needs to be emphasized that, although reappearance of HBsAg has been considered the marker of viral recurrence, detection of HBV DNA is an important factor to determine post-transplant virologic relapse and prophylaxis failure as no definite hepatitis or graft dysfunction are always reported in the presence of HBsAg alone with an undetectable HBV DNA especially in patients on newer and potent antivirals.
Salvage therapy: With availability of newer drugs for salvage therapy as well as tools for testing resistance mutations to guide the treatment choice, the concern for HBV recurrence under HBIG-free or HBIG-light protocols due to emergence of drug-resistant HBV variants or inadequate viral suppression is much less, although not zero given case reports of graft failure subsequent to HBV recurrence despite initiation of salvage therapy[24,71]. Addition of TFV to LAM is preferred over ADV in cases with drug-resistant HBV recurrence. A combination of ETV plus TFV may represent a potential option for salvage therapy.
Association of HCC at transplant with HBV recurrence post-LT: Patients with HCC seem to have a higher risk for HBV recurrence than those patients without HCC. The reasons for this finding are currently unknown[76]. The study of Fung et al[24] showed a more than 7 fold higher risk of HBV recurrence in patients who had HCC at transplant. A recent retrospective study on 354 patients who underwent liver transplant for HBV and HCC found that patients who had HBV recurrence were 3.6 times more likely to develop HCC recurrence[77]. It is unclear if more aggressive HBV prophylaxis regimens have any benefit on long-term outcome of graft survival or HCC recurrence. In a rare case of recurrent extrahepatic HCC, persistent presence of HBsAg despite an aggressive HBV prophylaxis regimen led to further investigation for recurrent HCC and was used as a tumor marker[78].
Adverse profile of newer NAs with regards to post-transplant renal function: ETV is not associated with nephrotoxicity, hence, is the drug of choice of treatment in post-LT patients who are at risk of calcineurin inhibitor-induced renal dysfunction. ETV monotherapy in post-LT patients has been shown to be safe and without renal adverse effect[24,79]. According to the studies of long term use of antiviral drugs in HIV patients, TFV is known to cause nephrotoxicity due to tubular dysfunction[80]. In a majority of studies on post-LT patients, the combination of TFV and ETV/emtricitabine was tolerable and did not lead to discontinuation of treatment due to adverse renal effects[57,81]. In a study on 21 patients who remained on TFV plus emtricitabine for a median follow up of 31 mo, 3 patients developed reversible acute kidney injury of which 1 had possible TFV/emtricitabine-induced tubular necrosis on biopsy[82].
Hepatitis D or delta virus and HBV co-infection: The risk of HBV recurrence post-LT is lower in patients with HBV/hepatitis D or delta virus (HDV) co-infection rather than in those with HBV disease alone. These co-infected patients are at high risk (up to 80%) of HDV reinfection in their grafts; however, no clinical or histological hepatitis is seen in the absence of HBV recurrence and the 5-year graft survival is up to 88%[75,83]. The treatment options are limited if HBV recurs in these patients.
HBcAb-positive donors: Although the use of HBcAb positive grafts expands the donors available for LT, this can carry the risk of de novo hepatitis B in the graft. The prevalence of isolated positive HBcAb varies between 4.9%-25% among different populations in the United States[84]. The true risk of de novo hepatitis is unclear with likely highest rate in HBV naïve recipients (i.e., HBcAb negative, HbsAb negative), ranging from 33%-100% among those who do not receive antiviral prophylaxis[85]. A consensus about the optimal prophylaxis protocol to prevent de novo hepatitis B in recipients of HBcAb-positive grafts has not yet been emerged; however, the common current practice is to commence HBV prophylaxis in all recipients of HBcAb positive grafts. The data on the effectiveness of LAM monotherapy as the primary prophylactic regimen widely used to prevent de novo HBV is limited to small studies with short follow up. A recent retrospective study of 62 patients on LAM monotherapy for a median follow up of 5.3 years, of which 44% were HBV naïve, showed 8% rate of de novo HBV post-LT[85]. Another systematic review of 73 patients receiving LAM only and 110 patients receiving LAM + HBIG with a short follow up (median time of 25.4 mo and 31.1 mo, respectively) revealed that the rate of de novo HBV was 2.7% in LAM-only group vs 3.6% in LAM + HBIG group, regardless of the recipients HBV serology at transplant[81]. Given the known high risk of LAM-resistance, newer NAs are likely more effective than LAM monotherapy but have yet to be studied.
Patient compliance: HBIG-free prophylaxis may not be a suitable strategy in patients with poor compliance due to the risk of emergence of resistant or inadequate viral suppression.
In summary, pending results of well-designed, adequately powered prospective randomized controlled trials, we recommend an individualized approach to HBV prophylactic therapy utilizing risk-assessment profiles of patients (Table 3). In the current era most patients with HBV suppression pre-LT, are able to benefit from an HBIG-free regimen and only subgroups of high-risk category patients may need a combination of HBIG plus NA. Current data favor a finite period of regimens that include HBIG in patients with viremia at transplant. Most of the studies chose to withdraw HBIG after at least 6 mo post-LT in their protocols. The question that remains unanswered is whether HBIG can be discontinued at an earlier time point such as 3 mo post-LT when most transplant centers discontinue corticosteroid treatment as part of the early post-LT immunosuppression protocols. Patients with poor adherence to medications likely need to remain on either indefinite low dose HBIG or long term combined NA regimens. Patients with HDV or HIV co-infection have limited salvage therapy if they develop HBV recurrence, and thus may need more intensive prophylaxis. More comparative studies are needed to address whether patients with LAM-resistance should remain on monotherapy or combinations of newer NAs.
Unanswered questions/Need for more research
The excellent track record and now 20 years of clinical experience with HBIG during and after LT have rendered it the cornerstone of anti-viral therapy. In the United States and some other countries, survival for the first year after LT, both for the graft and for the patient, is of paramount importance to assure that centers performing LT will continue to be approved by Medicare, Medicaid, and private insurers. Unfortunately, this discourages the testing and adoption of new modes of therapy, such as the early use of TFV or ETV and early tapering of HBIG doses.
The government and private insurance authorities should revisit and liberalize their policies in this regard, to encourage the design and performance of adequately-powered multi-center trials of simpler and less expensive therapeutic regimens. We suggest that such trials should be sponsored by the insurers and by the pharmaceutical companies that might stand to gain if their drugs, rather than HBIG, were shown to be of equal or similar efficacy with appreciable savings in costs.
Although TFV and ETV currently are quite expensive, they are fairly simple small molecules, the prices of which will probably fall considerably when their current patents expire in a few years. Thus, in the future, it appears to us inevitable that we will markedly diminish our use of HBIG and increase our use of small molecule RNA polymerase inhibitors. With careful follow-up (perhaps, dependent more upon the behaviors of the patients being treated than the treating physicians), we believe that HBIG can safely have a much diminished role in prevention of recurrent HBV infection after LT.
ACKNOWLEDGMENTS
We thank Elizabeth Glaser of Brandeis University for her helpful comments on and editing of the section on costs of therapy. We thank Pamela Billings for assistance with typing the manuscript and aiding in its submission.
Footnotes
Supported by A grant (No. R15 HL 117199) and contract No. U01 DK 065201 from the United States National Institutes of Health (to Bonkovsky HL) and institutional funds from Carolinas HealthCare System (to Sendi H) and Beth Israel Deaconess Medical Center (to Ghaziani T)
P- Reviewer: Bayliss J, Chemin I, Wong GLH S- Editor: Nan J L- Editor: A E- Editor: Zhang DN
References
- 1.Chan HL, Wong VW. Boyer TD, Manns MP, Sanyal AJ, editors. Zakim Boyer’s Hepatology-A Textbook of Liver Disease. 6th ed. Phila, PA: Elsevier-Saunders; 2012. pp. 540–563. [Google Scholar]
- 2.Samuel D, Muller R, Alexander G, Fassati L, Ducot B, Benhamou JP, Bismuth H. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med. 1993;329:1842–1847. doi: 10.1056/NEJM199312163292503. [DOI] [PubMed] [Google Scholar]
- 3.Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289–309. doi: 10.1159/000080872. [DOI] [PubMed] [Google Scholar]
- 4.Hadziyannis SJ. Viral hepatitis: an overview. Br J Clin Pract. 1995;49:267–268. [PubMed] [Google Scholar]
- 5.Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, Thomas HC. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet. 1989;2:588–591. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Buckwold VE, Hon MW, Ou JH. Mechanism of suppression of hepatitis B virus precore RNA transcription by a frequent double mutation. J Virol. 1999;73:1239–1244. doi: 10.1128/jvi.73.2.1239-1244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sendi H, Mehrab-Mohseni M, Zali MR, Norder H, Magnius LO. T1764G1766 core promoter double mutants are restricted to Hepatitis B virus strains with an A1757 and are common in genotype D. J Gen Virol. 2005;86:2451–2458. doi: 10.1099/vir.0.81023-0. [DOI] [PubMed] [Google Scholar]
- 8.Asim M, Malik A, Sarma MP, Polipalli SK, Begum N, Ahmad I, Khan LA, Husain SA, Akhtar N, Husain S, et al. Hepatitis B virus BCP, Precore/core, X gene mutations/genotypes and the risk of hepatocellular carcinoma in India. J Med Virol. 2010;82:1115–1125. doi: 10.1002/jmv.21774. [DOI] [PubMed] [Google Scholar]
- 9.Elkady A, Tanaka Y, Kurbanov F, Oynsuren T, Mizokami M. Virological and clinical implication of core promoter C1752/V1753 and T1764/G1766 mutations in hepatitis B virus genotype D infection in Mongolia. J Gastroenterol Hepatol. 2008;23:474–481. doi: 10.1111/j.1440-1746.2008.05321.x. [DOI] [PubMed] [Google Scholar]
- 10.Khan A, Al Balwi MA, Tanaka Y, Hajeer A, Sanai FM, Al Abdulkarim I, Al Ayyar L, Badri M, Saudi D, Tamimi W, et al. Novel point mutations and mutational complexes in the enhancer II, core promoter and precore regions of hepatitis B virus genotype D1 associated with hepatocellular carcinoma in Saudi Arabia. Int J Cancer. 2013;133:2864–2871. doi: 10.1002/ijc.28307. [DOI] [PubMed] [Google Scholar]
- 11.Sunbul M, Sugiyama M, Kurbanov F, Leblebicioglu H, Khan A, Elkady A, Tanaka Y, Mizokami M. Specific mutations of basal core promoter are associated with chronic liver disease in hepatitis B virus subgenotype D1 prevalent in Turkey. Microbiol Immunol. 2013;57:122–129. doi: 10.1111/1348-0421.12011. [DOI] [PubMed] [Google Scholar]
- 12.Angus PW, Locarnini SA, McCaughan GW, Jones RM, McMillan JS, Bowden DS. Hepatitis B virus precore mutant infection is associated with severe recurrent disease after liver transplantation. Hepatology. 1995;21:14–18. [PubMed] [Google Scholar]
- 13.Wu TJ, Chan KM, Chou HS, Lee CF, Wu TH, Chen TC, Yeh CT, Lee WC. Liver transplantation in patients with hepatitis B virus-related hepatocellular carcinoma: the influence of viral characteristics on clinical outcome. Ann Surg Oncol. 2013;20:3582–3590. doi: 10.1245/s10434-013-3023-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Lee KH, Chang HY, Ahn SH, Tong S, Yoon YJ, Seong BL, Kim SI, Han KH. Evolution of hepatitis B virus sequence from a liver transplant recipient with rapid breakthrough despite hepatitis B immune globulin prophylaxis and lamivudine therapy. J Med Virol. 2003;71:367–375. doi: 10.1002/jmv.10503. [DOI] [PubMed] [Google Scholar]
- 15.McMillan JS, Bowden DS, Angus PW, McCaughan GW, Locarnini SA. Mutations in the hepatitis B virus precore/core gene and core promoter in patients with severe recurrent disease following liver transplantation. Hepatology. 1996;24:1371–1378. doi: 10.1002/hep.510240610. [DOI] [PubMed] [Google Scholar]
- 16.Wu LM, Xu X, Zheng SS. Hepatitis B virus reinfection after liver transplantation: related risk factors and perspective. Hepatobiliary Pancreat Dis Int. 2005;4:502–508. [PubMed] [Google Scholar]
- 17.Berenguer M, Wright TL. Treatment of recurrence of hepatitis B in transplant patients. J Hepatol. 2003;39 Suppl 1:S190–S193. doi: 10.1016/s0168-8278(03)00350-7. [DOI] [PubMed] [Google Scholar]
- 18.Riediger C, Berberat PO, Sauer P, Gotthardt D, Weiss KH, Mehrabi A, Merle U, Stremmel W, Encke J. Prophylaxis and treatment of recurrent viral hepatitis after liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii37–viii46. doi: 10.1093/ndt/gfm655. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ari Z, Ashur Y, Daudi N, Shmilovitz-Wiess H, Brown M, Sulkes J, Klein A, Mor E, Tur-Kaspa R, Shouval D. Genotype prevalence, viral load and outcome of hepatitis B virus precore mutant infection in stable patients and in patients after liver transplantation. Clin Transplant. 2004;18:415–422. doi: 10.1111/j.1399-0012.2004.00182.x. [DOI] [PubMed] [Google Scholar]
- 20.Girlanda R, Mohsen AH, Smith H, Sablon E, Yuen MF, O’Grady J, Muiesan P, Rela M, Heaton N, Norris S. Hepatitis B virus genotype A and D and clinical outcomes of liver transplantation for HBV-related disease. Liver Transpl. 2004;10:58–64. doi: 10.1002/lt.20004. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS. How to diagnose and treat hepatitis B virus antiviral drug resistance in the liver transplant setting. Liver Transpl. 2008;14 Suppl 2:S8–S14. doi: 10.1002/lt.21616. [DOI] [PubMed] [Google Scholar]
- 22.Lapiński TW, Pogorzelska J, Flisiak R. HBV mutations and their clinical significance. Adv Med Sci. 2012;57:18–22. doi: 10.2478/v10039-012-0006-x. [DOI] [PubMed] [Google Scholar]
- 23.Zoulim F, Locarnini S. Optimal management of chronic hepatitis B patients with treatment failure and antiviral drug resistance. Liver Int. 2013;33 Suppl 1:116–124. doi: 10.1111/liv.12069. [DOI] [PubMed] [Google Scholar]
- 24.Fung J, Chan SC, Cheung C, Yuen MF, Chok KS, Sharr W, Chan AC, Cheung TT, Seto WK, Fan ST, et al. Oral nucleoside/nucleotide analogs without hepatitis B immune globulin after liver transplantation for hepatitis B. Am J Gastroenterol. 2013;108:942–948. doi: 10.1038/ajg.2013.111. [DOI] [PubMed] [Google Scholar]
- 25.Cooreman MP, Leroux-Roels G, Paulij WP. Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen. J Biomed Sci. 2001;8:237–247. doi: 10.1007/BF02256597. [DOI] [PubMed] [Google Scholar]
- 26.Schätzl HM, Sieger E, Jilg W, Nitschko H, Zachoval R. Variability of the Hepatitis B Surface Protein in HBV-Infected Liver Transplant Recipients. J Biomed Sci. 1997;4:146–154. doi: 10.1007/BF02255643. [DOI] [PubMed] [Google Scholar]
- 27.Shen ZY, Zheng WP, Deng YL, Song HL. Variations in the S and P regions of the hepatitis B virus genome under immunosuppression in vitro and in vivo. Viral Immunol. 2012;25:368–378. doi: 10.1089/vim.2012.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda Y, Marusawa H, Egawa H, Okamoto S, Ogura Y, Oike F, Nishijima N, Takada Y, Uemoto S, Chiba T. De novo activation of HBV with escape mutations from hepatitis B surface antibody after living donor liver transplantation. Antivir Ther. 2011;16:479–487. doi: 10.3851/IMP1771. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Lo CM, Cheung CK, Lau GK, Fan ST, Wong J. Identification of hepatitis B virus-specific lymphocytes in human liver grafts from HBV-immune donors. Liver Transpl. 2007;13:71–79. doi: 10.1002/lt.20887. [DOI] [PubMed] [Google Scholar]
- 30.Sendi H, Mehrab-Mohseni M, Shahraz S, Norder H, Alavian SM, Noorinayer B, Zali MR, Pumpens P, Bonkovsky HL, Magnius LO. CTL escape mutations of core protein are more frequent in strains of HBeAg negative patients with low levels of HBV DNA. J Clin Virol. 2009;46:259–264. doi: 10.1016/j.jcv.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 32.Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnićk GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 33.Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000;32:1189–1195. doi: 10.1053/jhep.2000.19789. [DOI] [PubMed] [Google Scholar]
- 34.Webster G, Barnes E, Burroughs A, Dusheiko G. Organ transplantation and discrimination. Patients with hepatitis B should not be given low priority. BMJ. 2000;320:1600–1601. [PubMed] [Google Scholar]
- 35.Hoofnagle JH, Di Bisceglie AM, Waggoner JG, Park Y. Interferon alfa for patients with clinically apparent cirrhosis due to chronic hepatitis B. Gastroenterology. 1993;104:1116–1121. doi: 10.1016/0016-5085(93)90281-g. [DOI] [PubMed] [Google Scholar]
- 36.Wright HI, Gavaler JS, Van Theil DH. Preliminary experience with alpha-2b-interferon therapy of viral hepatitis in liver allograft recipients. Transplantation. 1992;53:121–124. doi: 10.1097/00007890-199201000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Grellier L, Mutimer D, Ahmed M, Brown D, Burroughs AK, Rolles K, McMaster P, Beranek P, Kennedy F, Kibbler H, et al. Lamivudine prophylaxis against reinfection in liver transplantation for hepatitis B cirrhosis. Lancet. 1996;348:1212–1215. doi: 10.1016/s0140-6736(96)04444-3. [DOI] [PubMed] [Google Scholar]
- 38.Fontana RJ, Keeffe EB, Han S, Wright T, Davis GL, Lilly L, Carey W, Fried M, Jensen D, Luketic V, et al. Prevention of recurrent hepatitis B infection following liver transplantation: experience in 112 North American patients. Hepatology. 1999;30:301a. [Google Scholar]
- 39.Papatheodoridis GV, Sevastianos V, Burroughs AK. Prevention of and treatment for hepatitis B virus infection after liver transplantation in the nucleoside analogues era. Am J Transplant. 2003;3:250–258. doi: 10.1034/j.1600-6143.2003.00063.x. [DOI] [PubMed] [Google Scholar]
- 40.Perrillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, Moorat A, Gardner S, Woessner M, Bourne E, et al. Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology. 2004;126:81–90. doi: 10.1053/j.gastro.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 41.Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df, et al. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91–101. doi: 10.1053/j.gastro.2003.10.051. [DOI] [PubMed] [Google Scholar]
- 42.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med. 2003;348:800–807. doi: 10.1056/NEJMoa021812. [DOI] [PubMed] [Google Scholar]
- 43.Marcellin P, Chang TT, Lim SG, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, et al. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N Engl J Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 44.Ha NB, Ha NB, Garcia RT, Trinh HN, Vu AA, Nguyen HA, Nguyen KK, Levitt BS, Nguyen MH. Renal dysfunction in chronic hepatitis B patients treated with adefovir dipivoxil. Hepatology. 2009;50:727–734. doi: 10.1002/hep.23044. [DOI] [PubMed] [Google Scholar]
- 45.Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, Lok AS, Han KH, Goodman Z, Zhu J, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354:1001–1010. doi: 10.1056/NEJMoa051285. [DOI] [PubMed] [Google Scholar]
- 46.Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503–1514. doi: 10.1002/hep.22841. [DOI] [PubMed] [Google Scholar]
- 47.Leung N, Peng CY, Hann HW, Sollano J, Lao-Tan J, Hsu CW, Lesmana L, Yuen MF, Jeffers L, Sherman M, et al. Early hepatitis B virus DNA reduction in hepatitis B e antigen-positive patients with chronic hepatitis B: A randomized international study of entecavir versus adefovir. Hepatology. 2009;49:72–79. doi: 10.1002/hep.22658. [DOI] [PubMed] [Google Scholar]
- 48.Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, Enejosa J. The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naïve HIV-1-infected patients. HIV Clin Trials. 2007;8:164–172. doi: 10.1310/hct0803-164. [DOI] [PubMed] [Google Scholar]
- 49.Markowitz JS, Martin P, Conrad AJ, Markmann JF, Seu P, Yersiz H, Goss JA, Schmidt P, Pakrasi A, Artinian L, et al. Prophylaxis against hepatitis B recurrence following liver transplantation using combination lamivudine and hepatitis B immune globulin. Hepatology. 1998;28:585–589. doi: 10.1002/hep.510280241. [DOI] [PubMed] [Google Scholar]
- 50.Gane EJ, Angus PW, Strasser S, Crawford DH, Ring J, Jeffrey GP, McCaughan GW. Lamivudine plus low-dose hepatitis B immunoglobulin to prevent recurrent hepatitis B following liver transplantation. Gastroenterology. 2007;132:931–937. doi: 10.1053/j.gastro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Loomba R, Rowley AK, Wesley R, Smith KG, Liang TJ, Pucino F, Csako G. Hepatitis B immunoglobulin and Lamivudine improve hepatitis B-related outcomes after liver transplantation: meta-analysis. Clin Gastroenterol Hepatol. 2008;6:696–700. doi: 10.1016/j.cgh.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cholongitas E, Goulis J, Akriviadis E, Papatheodoridis GV. Hepatitis B immunoglobulin and/or nucleos(t)ide analogues for prophylaxis against hepatitis b virus recurrence after liver transplantation: a systematic review. Liver Transpl. 2011;17:1176–1190. doi: 10.1002/lt.22354. [DOI] [PubMed] [Google Scholar]
- 53.Cholongitas E, Papatheodoridis GV. High genetic barrier nucleos(t)ide analogue(s) for prophylaxis from hepatitis B virus recurrence after liver transplantation: a systematic review. Am J Transplant. 2013;13:353–362. doi: 10.1111/j.1600-6143.2012.04315.x. [DOI] [PubMed] [Google Scholar]
- 54.Han SH, Martin P, Edelstein M, Hu R, Kunder G, Holt C, Saab S, Durazo F, Goldstein L, Farmer D, et al. Conversion from intravenous to intramuscular hepatitis B immune globulin in combination with lamivudine is safe and cost-effective in patients receiving long-term prophylaxis to prevent hepatitis B recurrence after liver transplantation. Liver Transpl. 2003;9:182–187. doi: 10.1053/jlts.2003.50002. [DOI] [PubMed] [Google Scholar]
- 55.Zheng S, Chen Y, Liang T, Lu A, Wang W, Shen Y, Zhang M. Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl. 2006;12:253–258. doi: 10.1002/lt.20701. [DOI] [PubMed] [Google Scholar]
- 56.Wesdorp DJ, Knoester M, Braat AE, Coenraad MJ, Vossen AC, Claas EC, van Hoek B. Nucleoside plus nucleotide analogs and cessation of hepatitis B immunoglobulin after liver transplantation in chronic hepatitis B is safe and effective. J Clin Virol. 2013;58:67–73. doi: 10.1016/j.jcv.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 57.Teperman LW, Poordad F, Bzowej N, Martin P, Pungpapong S, Schiano T, Flaherty J, Dinh P, Rossi S, Subramanian GM, et al. Randomized trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl. 2013;19:594–601. doi: 10.1002/lt.23628. [DOI] [PubMed] [Google Scholar]
- 58.Anders M, Orozco F, Goldaracena N, Quiñonez E, McCormack L, Mastai R. [Efficacy of the association of a single dose of hepatitis B hyperimmune gammaglobulin and entecavir in the prophylaxis of hepatitis B after liver transplantation: experience of a single center in Argentina] Acta Gastroenterol Latinoam. 2013;43:121–125. [PubMed] [Google Scholar]
- 59.Gane EJ, Patterson S, Strasser SI, McCaughan GW, Angus PW. Combination of lamivudine and adefovir without hepatitis B immune globulin is safe and effective prophylaxis against hepatitis B virus recurrence in hepatitis B surface antigen-positive liver transplant candidates. Liver Transpl. 2013;19:268–274. doi: 10.1002/lt.23600. [DOI] [PubMed] [Google Scholar]
- 60.Saab S, Ham MY, Stone MA, Holt C, Tong M. Decision analysis model for hepatitis B prophylaxis one year after liver transplantation. Liver Transpl. 2009;15:413–420. doi: 10.1002/lt.21712. [DOI] [PubMed] [Google Scholar]
- 61.Dan YY, Wai CT, Yeoh KG, Lim SG. Prophylactic strategies for hepatitis B patients undergoing liver transplant: a cost-effectiveness analysis. Liver Transpl. 2006;12:736–746. doi: 10.1002/lt.20685. [DOI] [PubMed] [Google Scholar]
- 62.Dan YY, Wai CT, Lee YM, Sutedja DS, Seet BL, Lim SG. Outcome of lamivudine-resistant hepatitis B virus is generally benign except in cirrhotics. World J Gastroenterol. 2005;11:4344–4350. doi: 10.3748/wjg.v11.i28.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.HBIG Product Information (U. S. Market), updated June. 2010. Available from: http://www.cdc.gov/hepatitis/PDFs/HBIG_Products&Distributors.pdf. [Google Scholar]
- 64.Sutter Health. Financial Matters: Liver Transplant Costs. Available from: http://www.cpmc.org/advanced/liver/patients/topics/finance.html.
- 65.Tanaka T, Benmousa A, Marquez M, Therapondos G, Renner EL, Lilly LB. The long-term efficacy of nucleos(t)ide analog plus a year of low-dose HBIG to prevent HBV recurrence post-liver transplantation. Clin Transplant. 2012;26:E561–E569. doi: 10.1111/ctr.12022. [DOI] [PubMed] [Google Scholar]
- 66.Di Paolo D, Tisone G, Piccolo P, Lenci I, Zazza S, Angelico M. Low-dose hepatitis B immunoglobulin given “on demand” in combination with lamivudine: a highly cost-effective approach to prevent recurrent hepatitis B virus infection in the long-term follow-up after liver transplantation. Transplantation. 2004;77:1203–1208. doi: 10.1097/01.tp.0000118904.63669.eb. [DOI] [PubMed] [Google Scholar]
- 67.Tsai N, Jeffers L, Cragin L, Sorensen S, Su W, Rosenblatt L, Tang H, Hebden T, Juday T. Cost-effectiveness of entecavir versus adefovir for the treatment of chronic hepatitis B in patients with decompensated cirrhosis from a third-party US payer perspective. Clinicoecon Outcomes Res. 2012;4:227–235. doi: 10.2147/CEOR.S31784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ahn JD, Cohen S. Oral antiviral therapy without long term maintenance hepatitis B immunoglobulin to prevent hepatitis B recurrence in liver transplant patients [abstract] J Hepatol. 2008;48 suppl 2:S73–S74. doi: 10.5812/kowsar.1735143X.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.PDR Staff. Red Book. 113th ed. Montvale, NJ: Thomson Healthcare; 2009. [Google Scholar]
- 70.Smith VE, Bruno CJ. The search for cost-effective treatment of chronic hepatitis B. J Manag Care Pharm. 2008;14:61–64. doi: 10.18553/jmcp.2008.14.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Degertekin B, Han SH, Keeffe EB, Schiff ER, Luketic VA, Brown RS, Emre S, Soldevila-Pico C, Reddy KR, Ishitani MB, et al. Impact of virologic breakthrough and HBIG regimen on hepatitis B recurrence after liver transplantation. Am J Transplant. 2010;10:1823–1833. doi: 10.1111/j.1600-6143.2010.03046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marzano A, Gaia S, Ghisetti V, Carenzi S, Premoli A, Debernardi-Venon W, Alessandria C, Franchello A, Salizzoni M, Rizzetto M. Viral load at the time of liver transplantation and risk of hepatitis B virus recurrence. Liver Transpl. 2005;11:402–409. doi: 10.1002/lt.20402. [DOI] [PubMed] [Google Scholar]
- 73.Lok AS, Trinh H, Carosi G, Akarca US, Gadano A, Habersetzer F, Sievert W, Wong D, Lovegren M, Cohen D, Llamoso C. Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology. 2012;143:619–628.e1. doi: 10.1053/j.gastro.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 74.Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, Horban A, Wang C, Kwan P, Buti M, Prieto M, Berg T, Kitrinos K, Peschell K, Mondou E, Frederick D, Rousseau F, Schiff ER. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62–72. doi: 10.1002/hep.23952. [DOI] [PubMed] [Google Scholar]
- 75.Samuel D, Feray C, Bismuth H. Hepatitis viruses and liver transplantation. J Gastroenterol Hepatol. 1997;12:S335–S341. doi: 10.1111/j.1440-1746.1997.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 76.Faria LC, Gigou M, Roque-Afonso AM, Sebagh M, Roche B, Fallot G, Ferrari TC, Guettier C, Dussaix E, Castaing D, et al. Hepatocellular carcinoma is associated with an increased risk of hepatitis B virus recurrence after liver transplantation. Gastroenterology. 2008;134:1890–1899; quiz 2155. doi: 10.1053/j.gastro.2008.02.064. [DOI] [PubMed] [Google Scholar]
- 77.Campsen J, Zimmerman M, Trotter J, Hong J, Freise C, Brown R, Cameron A, Ghobrial M, Kam I, Busuttil R, et al. Liver transplantation for hepatitis B liver disease and concomitant hepatocellular carcinoma in the United States With hepatitis B immunoglobulin and nucleoside/nucleotide analogues. Liver Transpl. 2013;19:1020–1029. doi: 10.1002/lt.23703. [DOI] [PubMed] [Google Scholar]
- 78.Hshieh TT, Sundaram V, Najarian RM, Hanto DW, Karp SJ, Curry MP. Hepatitis B surface antigen as a marker for recurrent, metastatic hepatocellular carcinoma after liver transplantation. Liver Transpl. 2012;18:995–998. doi: 10.1002/lt.23465. [DOI] [PubMed] [Google Scholar]
- 79.Perrillo R, Buti M, Durand F, Charlton M, Gadano A, Cantisani G, Loong CC, Brown K, Hu W, Lopez-Talavera JC, et al. Entecavir and hepatitis B immune globulin in patients undergoing liver transplantation for chronic hepatitis B. Liver Transpl. 2013;19:887–895. doi: 10.1002/lt.23690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaidan M, Lescure FX, Brochériou I, Dettwiler S, Guiard-Schmid JB, Pacanowski J, Rondeau E, Pialoux G, Girard PM, Ronco P, et al. Tubulointerstitial nephropathies in HIV-infected patients over the past 15 years: a clinico-pathological study. Clin J Am Soc Nephrol. 2013;8:930–938. doi: 10.2215/CJN.10051012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saab S, Desai S, Tsaoi D, Durazo F, Han S, McClune A, Holt C, Farmer D, Goldstein L, Busuttil RW. Posttransplantation hepatitis B prophylaxis with combination oral nucleoside and nucleotide analog therapy. Am J Transplant. 2011;11:511–517. doi: 10.1111/j.1600-6143.2010.03416.x. [DOI] [PubMed] [Google Scholar]
- 82.Stravitz RT, Shiffman ML, Kimmel M, Puri P, Luketic VA, Sterling RK, Sanyal AJ, Cotterell AH, Posner MP, Fisher RA. Substitution of tenofovir/emtricitabine for Hepatitis B immune globulin prevents recurrence of Hepatitis B after liver transplantation. Liver Int. 2012;32:1138–1145. doi: 10.1111/j.1478-3231.2012.02770.x. [DOI] [PubMed] [Google Scholar]
- 83.Samuel D, Zignego AL, Reynes M, Feray C, Arulnaden JL, David MF, Gigou M, Bismuth A, Mathieu D, Gentilini P. Long-term clinical and virological outcome after liver transplantation for cirrhosis caused by chronic delta hepatitis. Hepatology. 1995;21:333–339. [PubMed] [Google Scholar]
- 84.McQuillan GM, Coleman PJ, Kruszon-Moran D, Moyer LA, Lambert SB, Margolis HS. Prevalence of hepatitis B virus infection in the United States: the National Health and Nutrition Examination Surveys, 1976 through 1994. Am J Public Health. 1999;89:14–18. doi: 10.2105/ajph.89.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang MS, Olsen SK, Pichardo EM, Stiles JB, Rosenthal-Cogan L, Brubaker WD, Guarrera JV, Emond JC, Brown RS. Prevention of de novo hepatitis B in recipients of core antibody-positive livers with lamivudine and other nucleos(t)ides: a 12-year experience. Transplantation. 2013;95:960–965. doi: 10.1097/TP.0b013e3182845f97. [DOI] [PubMed] [Google Scholar]


