Abstract
Gastroesophageal reflux disease (GERD) is a condition that develops when the reflux of gastric contents into the esophagus leads to troublesome symptoms and/or complications. Heartburn is the cardinal symptom, often associated with regurgitation. In patients with endoscopy-negative heartburn refractory to proton pump inhibitor (PPI) therapy and when the diagnosis of GERD is in question, direct reflux testing by impedance-pH monitoring is warranted. Laparoscopic fundoplication is the standard surgical treatment for GERD. It is highly effective in curing GERD with a 80% success rate at 20-year follow-up. The Nissen fundoplication, consisting of a total (360°) wrap, is the most commonly performed antireflux operation. To reduce postoperative dysphagia and gas bloating, partial fundoplications are also used, including the posterior (Toupet) fundoplication, and the anterior (Dor) fundoplication. Currently, there is consensus to advise laparoscopic fundoplication in PPI-responsive GERD only for those patients who develop untoward side-effects or complications from PPI therapy. PPI resistance is the real challenge in GERD. There is consensus that carefully selected GERD patients refractory to PPI therapy are eligible for laparoscopic fundoplication, provided that objective evidence of reflux as the cause of ongoing symptoms has been obtained. For this purpose, impedance-pH monitoring is regarded as the diagnostic gold standard.
Keywords: Gastroesophageal reflux disease, Refractory gastroesophageal reflux disease, Laparoscopic fundoplication, Impedance-pH monitoring, Proton pump inhibitors
Core tip: The present review focuses on the definition and diagnosis of gastroesophageal reflux disease (GERD), the techniques and results of laparoscopic fundoplication, and the currently accepted indications for antireflux surgery. Proton pump inhibitor-refractory GERD is thoroughly considered, with a special regard to impedance-pH monitoring criteria useful for diagnosing this condition which currently represents the main indication for laparoscopic fundoplication.
DEFINITIONS
Gastroesophageal reflux disease (GERD) is currently defined as a condition that develops when the reflux of gastric contents into the esophagus leads to troublesome symptoms and/or complications[1-4]. GERD is the most common disease encountered by the gastroenterologist with a 20% prevalence in the adult population. It has been classified as the presence of reflux symptoms without erosions on endoscopic examination, i.e., nonerosive reflux disease (NERD) or reflux symptoms with mucosal breaks at endoscopic examination, i.e., erosive reflux disease (ERD)[3].
ERD is found in up to 20% of GERD patients and should be regarded as the most common complication of GERD rather than its principal manifestation[4]. Esophageal peptic ulcers and strictures have become quite uncommon in the proton pump inhibitor (PPI) era[4].
Barrett’s esophagus is a condition in which the stratified squamous esophageal epithelium is replaced by endoscopically detectable columnar metaplasia[5,6] : it occurs in 2% of the general adult population and represents the most dreaded complication of GERD because it predisposes to esophageal adenocarcinoma, the fastest growing cause of cancer mortality. There is still debate about the working definition of Barrett’s esophagus[5,6]. According to the American Gastroenterological Association, Barrett’s esophagus is a change in the distal esophageal epithelium of any length that can be recognized as columnar type mucosa at endoscopy and is confirmed to have intestinal metaplasia by biopsy of the tubular esophagus[5]. According to the British Society of Gastroenterology, only 1 cm or more of endoscopically visible columnar epithelium above the gastroesophageal junction dictates biopsy sampling, whereas the detection of intestinal metaplasia is not a prerequisite for the definition of Barrett’s esophagus but only for the necessity of endoscopic surveillance[6].
The typical reflux syndrome includes heartburn and regurgitation. Heartburn is the cardinal GERD symptom and is defined as a burning sensation in the retrosternal area, behind the breastbone. Regurgitation is defined as the perception of refluxed gastric content into the mouth or hypopharynx.
Atypical reflux syndromes include noncardiac chest pain (NCCP) and extraesophageal syndromes. NCCP consists of chest pain indistinguishable from ischemic cardiac pain in patients in whom cardiac disease has been carefully excluded: it can be caused by GERD, mainly when it is associated with heartburn, or by esophageal motility disorders. Extraesophageal syndromes include chronic cough, chronic asthma, chronic laryngitis, and dental erosions: a cause-and-effect relationship between extraesophageal syndromes and reflux can be shown in few patients only, more often in those also complaining of heartburn.
Associated syndromes include dyspepsia (epigastric pain/burn, post-prandial fullness, early satiety) and irritable bowel syndrome (abdominal pain or discomfort with disturbed bowel habit and/or bloating): no causal link with reflux has been shown but these syndromes can be found in up to 50% of patients with GERD.
DIAGNOSIS
ERD and Barrett’s esophagus are found at endoscopy in up to 30% of patients with the typical reflux syndrome. Accordingly, endoscopic assessment is recommended only in the presence of alarm symptoms (dysphagia, vomiting, anemia, involuntary weight loss) and the diagnosis of GERD is currently patient-centered, based on the presence of heartburn with/without regurgitation and on a positive response to a PPI therapeutic trial[1,3], given the extraordinary high efficacy of PPIs in providing rapid heartburn relief and mucosal healing in ERD. The current definition of NERD, i.e., reflux symptoms without erosions at endoscopic examination[3,4] does not completely fit with the definition of disease, however. A disease is a morbid entity characterized by at least two of three criteria: (1) recognized etiologic agent(s); (2) identifiable group of signs and symptoms; and (3) consistent anatomic alterations[4]. In patients with endoscopy-negative heartburn, i.e., without consistent anatomic alterations, to make a diagnosis of disease (NERD) we must rely upon the efficacy of PPIs in suppressing acid reflux-related symptoms, acid reflux being then identified as the etiologic agent of the typical reflux syndrome. However, between 10% and 30% of patients with heartburn remain symptomatic on standard and even high PPI dosages[7]. In these cases, direct reflux monitoring is warranted to distinguish patients with reflux-related PPI-unresponsive heartburn, i.e., refractory NERD from those with reflux-unrelated endoscopy-negative PPI-unresponsive heartburn, i.e., functional heartburn (FH)[8]. Direct reflux monitoring is also indicated in all other situations when the diagnosis of GERD is in question, i.e., NCCP and extraesophageal syndromes in patients not complaining of heartburn.
Traditional and wireless pH-monitoring have limited accuracy in studying reflux because they only detect reflux episodes with a pH < 4.0. Gastroesophageal refluxate contains a variety of noxious agents, including hydrochloric acid and pepsins. PPI therapy transforms the vast majority of acid refluxes into weakly acidic refluxes[9]. As the proteolytic activity of pepsins is essential for mucosal damage to occur and is maintained up to pH 6, weakly acidic refluxes can damage esophageal mucosa and have been implicated in the pathogenesis of ERD persisting despite PPI therapy[10].
Impedance-pH monitoring provides a complete assessment of reflux, allowing detection of acid, weakly acidic, and weakly alkaline refluxes, and should be regarded as the gold standard for distinguishing patients with reflux-related from those with reflux-unrelated syndromes[8,11]. In patients evaluated off PPI therapy, impedance-pH monitoring criteria afford a 12% diagnostic gain over pH-only criteria, allowing identification of NERD patients even when esophageal acid exposure is normal by means of a positive symptom-reflux association with nonacid refluxes[12]. However, off-PPI impedance-pH criteria to distinguish NERD from FH have not yet been validated by outcome studies in the clinical setting of PPI refractoriness. By adding quantitative analysis of impedance-pH parameters to symptom-reflux association, a subdivision of PPI-refractory patients into refractory NERD and FH can be obtained by on-PPI impedance-pH monitoring[13] which is substantiated by pathophysiological findings peculiar to GERD[14] as well as by outcome data[15-18]. In this respect, a recently developed new quantitative parameter, namely the post-reflux swallow-induced peristaltic wave index, allows a clear-cut separation of NERD from FH in patients evaluated off- as well as on-PPI therapy[19]. By adding quantitative analysis of on-PPI impedance-pH parameters to symptom-reflux association, NERD was diagnosed in two thirds of endoscopy-negative PPI-refractory patients as opposed to less than half of cases using symptom-reflux association only[13]. Therefore, on-PPI impedance-pH monitoring represents a cost-effective diagnostic strategy in patients with PPI-unresponsive typical reflux syndrome, validated by outcome studies[15-18]. On the other hand, given the low probability that NCCP and respiratory symptoms are due to reflux in the absence of heartburn, patients with atypical symptoms only should be assessed with impedance-pH monitoring after PPI withdrawal[3,11].
MEDICAL MANAGEMENT
PPIs represent the mainstay of medical treatment in GERD[1,3]. They provide the most rapid symptomatic relief and heal esophagitis in the highest percentage of patients[1,3], transforming the vast majority of acid refluxes into weakly acidic refluxes[9]. However, typical GERD symptoms recur within 1 year in more than 90% of patients after PPI-withdrawal, in many of them within few days. Moreover, up to 30% of patients with heartburn[7] and even more patients with regurgitation[20] fail to respond, either partially or completely, to PPI therapy at standard and even high dosages. Laparoscopic fundoplication is the only treatment modality currently recommended for overcoming PPI failures[1-3].
SURGICAL MANAGEMENT
Fundoplication is the standard surgical treatment for GERD. The question of laparoscopic vs open surgery is no longer relevant. Randomized studies and meta-analyses have shown that laparoscopic fundoplication should be preferred over the open alternative: efficacy is comparable but mortality is lower (0.04% vs 0.2%) and cosmesis is undoubtedly better[21].
Many studies have shown that laparoscopic fundoplication is highly effective in curing PPI-responsive GERD, long-term postoperative assessment consisting of symptom evaluation[21-26]: persistent relief of heartburn and regurgitation has been reported in 90% and 80% of patients at 10-year[22-24] and 20-year follow-up[21,25,26], respectively, with less than one half of those few patients with recurrent heartburn having evidence of abnormal reflux[22].
The Nissen fundoplication, consisting of a total (360°) wrap, is the most commonly performed antireflux operation. Dysphagia and gas bloating are the primary causes of dissatisfaction despite general reflux alleviation[27]. Aiming to reduce postoperative dysphagia and gas bloating, a variety of procedures in which the fundus is only partially wrapped have been proposed, including the Toupet fundoplication, consisting of a posterior (270°) wrap, and the Dor fundoplication, consisting of an anterior (180°) wrap. Recent studies suggest that anterior fundoplication is as effective as total fundoplication in terms of reflux and heartburn/regurgitation control with less dysphagia and gas bloat[28-30]. Likewise, at 6-12 mo after intervention, similar efficacy on heartburn/regurgitation and reflux parameters have been reported when Toupet and Nissen fundoplications have been compared but with less dysphagia and gas bloat with the former (3% and 23%) than with the latter (7% and 36%)[31-33]: however, it should be noted that such differences are minor and that with both the techniques the fundoplication was performed by fixing the wrap to the anterior wall of the esophagus. The Nissen procedure can also be carried out without anchoring the fundoplication: no wrap slipping, negligible gas bloating, and cumulative incidence of postoperative dysphagia quite similar to that of the Toupet procedure, in conjunction with normal reflux parameters and sustained symptom remission in the vast majority of cases have been reported[15-18]. To prevent postoperative dysphagia, other key technical issues must be considered including division of the short gastric vessels whenever deemed necessary in order to adequately mobilize the esophagus and to make the fundoplication tension-free, and insertion of a 52-Fr bougie into the esophagus during construction of the wrap[2,15-18]. Finally, the reported differences in postoperative side-effects favoring the partial wrap disappear after 2 decades of follow-up[25].
WHO IS SUITABLE FOR LAPAROSCOPIC FUNDOPLICATION?
Controversies in indications for laparoscopic fundoplication are reflected by most recent guidelines (Table 1).
Table 1.
Indications for laparoscopic fundoplication
| AGA | Patients with esophagitis who are intolerant of PPI therapy |
| Patients with symptoms of the esophageal GERD syndrome poorly controlled by PPI therapy, especially in the setting of persistent troublesome regurgitation | |
| Carefully selected patients with extraesophageal GERD syndromes in whom a reflux causality has been established to the greatest degree possible | |
| SAGES | Patients who have failed medical management (inadequate symptom control, severe regurgitation not controlled with acid suppression, or medication side-effects) |
| Patients who opt for surgery despite successful medical management | |
| Patients who have complications of GERD (e.g., Barrett’s esophagus, peptic stricture) | |
| Patients who have extra-esophageal manifestations (asthma, hoarseness, cough, chest pain, aspiration) | |
| ACG | Surgical therapy is a treatment option for long-term therapy in GERD patients |
| Surgical therapy is generally not recommended in patients who do not respond to PPI therapy | |
| Refractory patients with objective evidence of ongoing reflux as the cause of symptoms should be considered for additional antireflux therapies, which may include surgery | |
| Surgery should generally not be performed to treat extraesophageal symptoms of GERD in patients who do not respond to acid suppression with a PPI |
PPI: Proton pump inhibitor; GERD: Gastroesophageal reflux disease; AGA: American Gastroenterological Association; SAGES: Society of Gastrointestinal and Endoscopic Surgeons; ACG: American College of Gastroenterology.
PPI-responsive typical GERD
According to the American Gastroenterological Association, GERD patients who are well maintained on medical therapy have nothing to gain from laparoscopic fundoplication and should be advised against surgery[1]. On the other hand, guidelines from the American College of Gastroenterology state that surgical therapy is a treatment option for long-term therapy in GERD patients[3]. A recent multicenter trial showed that most PPI-responsive GERD patients achieve and remain in heartburn remission at 5 years with laparoscopic fundoplication or esomeprazole in a dose-escalating manner when required, with a higher prevalence of dysphagia and gas-bloating and a lower prevalence of regurgitation in the surgically-treated patients[34]. In advising laparoscopic fundoplication to PPI-responsive GERD patients, side effects and complications of laparoscopic fundoplication[35] and the excellent safety profile of PPIs[36] should be taken into account. Fundic gland polyps are a frequent complication of prolonged PPI therapy but are considered potentially harmful only when larger than 1 cm[37]. Therefore, in PPI-responsive patients the current consensus is to advise antireflux surgery only for those few patients who develop untoward side-effects or complications from PPI therapy (Table 2).
Table 2.
Side-effects and complications of proton pump inhibitors
| Headache (< 2%) |
| Diarrhea (< 2%) |
| Malabsorption of magnesium, calcium, vitamin B12, iron (doubtful) |
| Increased risk of Clostridium difficile colitis in antibiotic users (doubtful) |
| Increased risk of pneumonia (doubtful) |
| Acute interstitial nephritis (extremely rare) |
| Drug-drug interactions (doubtful) |
| Accelerated progression of Helicobacter pylori gastritis (doubtful) |
| Formation of gastric fundic gland polyps (potentially harmful when > 1 cm) |
As far as endoscopic treatment modalities are concerned, the usage of current endoscopic therapies cannot be recommended as an alternative to medical or traditional surgical therapy[3]. A laparoscopically implanted sphincter augmentation device has recently been proposed[38] but more data on long-term efficacy, safety, and costs are required before widespread usage can be recommended[3].
Before surgical intervention impedance-pH monitoring is warranted, always preceded by esophageal manometry to rule out severe esophageal motility disorders[1-3]. Impedance-pH monitoring should be performed during PPI withdrawal and only after symptoms have recurred in order to assess symptom-reflux association (Figure 1) and identify patients with esophageal hypersensitivity (positive symptom-reflux association with normal reflux parameters) because they can benefit from laparoscopic fundoplication[39]. In patients with negative impedance-pH results FH is the most likely diagnosis, PPIs resumption should be postponed, and only watchful follow-up is warranted.
Figure 1.
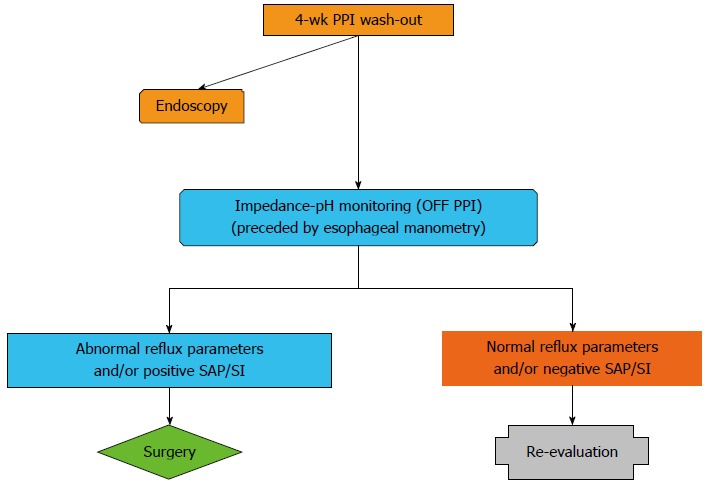
Diagnostic algorithm for proton pump inhibitor responsive patients with typical reflux symptoms. Endoscopy should be performed in uninvestigated patients and in patients with previous detection of reflux esophagitis or Barrett’s esophagus. Impedance-pH monitoring should be performed during proton pump inhibitor (PPI) withdrawal after symptoms have recurred. SAP: Symptom association probability; SI: Symptom index.
PPI-refractory typical GERD
Currently, PPI resistance is the real challenge in GERD[40]. It has been claimed that up to 30% of patients with heartburn fail to respond symptomatically, either partially or completely, to a standard dose of PPI, and high dosages may be effective in less than one half of them[7]. Furthermore, regurgitation persists in many patients despite PPI therapy[20], often awakening patients at night. Management of PPI-refractory GERD patients is a challenging task. Baclofen could be helpful as add-on therapy with PPIs, but its use is limited by poor tolerability[7] and it is not approved for GERD management. Transoral incisionless fundoplication proved significantly less effective than laparoscopic fundoplication in inducing symptom remission and improving reflux parameters in refractory GERD[16]. Currently, patients with typical GERD unresponsive to PPIs are considered eligible for laparoscopic fundoplication[1-3,40]. However, outcome data in this particular clinical setting are scanty and controlled trials are lacking as effective alternative treatment modalities to surgery are not available. Nevertheless, the results of well-designed observational studies do not systematically overestimate the magnitude of the effects of treatment as compared with those in randomized, controlled trials[41,42].
In order to assess the efficacy of laparoscopic fundoplication in PPI-refractory GERD, postoperative PPI usage is a poorly reliable parameter: PPIs are widely used for dyspeptic symptoms too, and post-surgical usage is associated with objective evidence of reflux recurrence in only a minority of cases[22]. Heartburn and regurgitation are the typical reflux symptoms but they have a number of non-reflux related causes[4] There is a general lack of studies validating reflux questionnaires measuring heartburn and regurgitation in response to surgical treatment[43]. As impedance-pH monitoring allows detection of all types of reflux, both acid and nonacid, off and on PPI therapy, it represents the ideal test to evaluate PPI-refractory patients in the pre- and post-operative setting[11]. In a recent study, normal reflux parameters and sustained symptom remission at 3-year follow-up, i.e., GERD cure, was achieved with laparoscopic fundoplication in 90% of patients with PPI-refractory GERD as diagnosed by on-PPI impedance-pH monitoring[18]. Interestingly, this study shows that weakly acidic refluxes are the main determinants of PPI refractoriness: preoperatively, positive symptom/reflux indexes and abnormal reflux parameters were mainly associated with weakly acidic refluxes; postoperatively, persistent remission of heartburn/regurgitation was associated with total/subtotal abolition of weakly acidic refluxes[18]. Furthermore, at postoperative off-PPI assessment the percentage of esophageal acid exposure time decreased significantly, despite restored gastric acidity, a result showing that laparoscopic fundoplication acts not only by reducing the number of transient lower esophageal sphincter relaxations accompanied by reflux, but also by reducing the volume of the postprandial acid pocket at the gastroesophageal junction[18]. According to this study, refractory GERD as diagnosed by on-PPI impedance-pH monitoring can be cured by laparoscopic fundoplication.
On-PPI impedance-pH monitoring is warranted in all PPI-refractory patients before laparoscopic fundoplication (Figure 2) in order to establish a cause-and-effect relationship between reflux and heartburn/regurgitation persisting despite PPI therapy; indeed, no reflux pattern can be demonstrated associated with PPI failure at off-PPI testing[44]. Impedance-pH monitoring should always be preceded by esophageal manometry to rule out severe esophageal motility disorders. Surgery is indicated in patients with abnormal impedance-pH parameters and/or positive symptom-reflux associations whereas in those with negative results FH is the most likely diagnosis, and only re-evaluation after PPI withdrawal is warranted.
Figure 2.
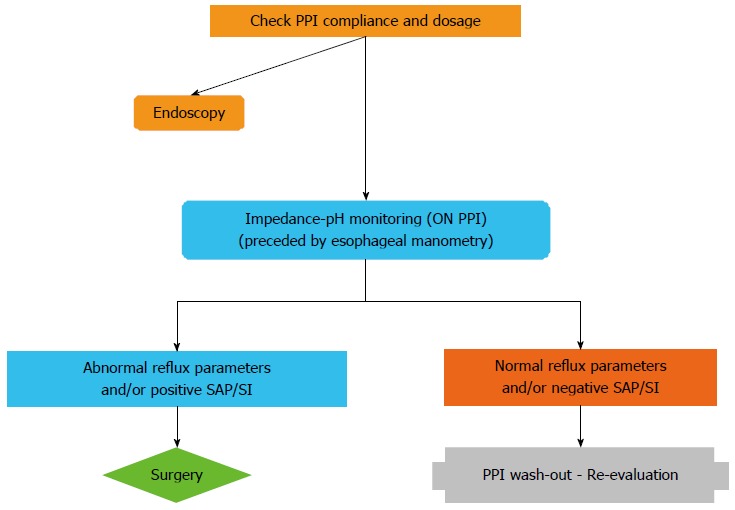
Diagnostic algorithm for proton pump inhibitor refractory patients with typical reflux symptoms. Endoscopy should be performed in uninvestigated patients and in patients with previous detection of reflux esophagitis or Barrett’s esophagus. Impedance-pH monitoring should be performed during ongoing proton pump inhibitor (PPI) treatment. SAP: Symptom association probability; SI: Symptom index.
Atypical GERD
NCCP in patients with endoscopic or pH-monitoring evidence of GERD tends to improve, but not resolve, with PPI therapy, whereas GERD-negative patients have little or no response[45].
PPIs for treatment of extraesophageal syndromes in patients without heartburn are considered ineffective[1] and a PPI trial is recommended to treat extraesophageal symptoms only in patients who also have typical symptoms[3]. Only small observational studies suggest some benefit of antireflux surgery for highly selected patients with cough and asthma[1].
The main problem in the clinical setting of extraesophageal GERD is that currently available diagnostic tools to establish GERD as the cause of atypical symptoms have serious limitations[3]. Ambulatory reflux monitoring can confirm the presence of GERD but it is difficult to establish a causal link between reflux and atypical symptoms by means of symptom-reflux association.
Given these limitations, there is lack of consensus for performing laparoscopic fundoplication in patients with NCCP or extraesophageal syndromes in the absence of typical GERD symptoms[1-3].
Barrett’s esophagus
Recently, uncontrolled reflux exposure as expressed by a high number of weakly acidic refluxes at impedance-pH monitoring during high-dosage PPI therapy has been implicated in the pathogenesis of persistent esophageal intestinal metaplasia in patients with dysplastic Barrett’s esophagus undergoing radiofrequency ablation[46]. In a small case-control series, patients with Barrett’s esophagus who had laparoscopic fundoplication in conjunction with endoluminal radiofrequency ablation were more likely to achieve durable ablation of intestinal metaplasia in comparison with patients who were treated with PPI therapy[47]. These data are inconclusive, however, and the current goal of laparoscopic fundoplication for Barrett’s esophagus remains relief of symptoms in patients unresponsive to PPI therapy[5,6].
CONCLUSION
Laparoscopic fundoplication is highly effective in curing GERD. However, considering its side effects laparoscopic fundoplication should be advised only for those few PPI-responsive GERD patients who develop side-effects or complications from PPI therapy. Currently, the main indication for laparoscopic fundoplication is represented by PPI-refractory GERD, provided that objective evidence of reflux as the cause of ongoing symptoms has been obtained by impedance-pH monitoring.
Footnotes
P- Reviewer: Antoniou SA, Niu CY, Rosemurgy AS S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Kahrilas PJ, Shaheen NJ, Vaezi MF. American Gastroenterological Association Institute technical review on the management of gastroesophageal reflux disease. Gastroenterology. 2008;135:1392–1413, 1413.e1-5. doi: 10.1053/j.gastro.2008.08.044. [DOI] [PubMed] [Google Scholar]
- 2.Stefanidis D, Hope WW, Kohn GP, Reardon PR, Richardson WS, Fanelli RD. Guidelines for surgical treatment of gastroesophageal reflux disease. Surg Endosc. 2010;24:2647–2669. doi: 10.1007/s00464-010-1267-8. [DOI] [PubMed] [Google Scholar]
- 3.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328; quiz 329. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 4.Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920; quiz 1943. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Spechler SJ, Sharma P, Souza RF, Inadomi JM, Shaheen NJ. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–e52; quiz e13. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S, et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7–42. doi: 10.1136/gutjnl-2013-305372. [DOI] [PubMed] [Google Scholar]
- 7.Sifrim D, Zerbib F. Diagnosis and management of patients with reflux symptoms refractory to proton pump inhibitors. Gut. 2012;61:1340–1354. doi: 10.1136/gutjnl-2011-301897. [DOI] [PubMed] [Google Scholar]
- 8.Savarino E, Zentilin P, Savarino V. NERD: an umbrella term including heterogeneous subpopulations. Nat Rev Gastroenterol Hepatol. 2013;10:371–380. doi: 10.1038/nrgastro.2013.50. [DOI] [PubMed] [Google Scholar]
- 9.Frazzoni M, Savarino E, Manno M, Melotti G, Mirante VG, Mussetto A, Bertani H, Manta R, Conigliaro R. Reflux patterns in patients with short-segment Barrett’s oesophagus: a study using impedance-pH monitoring off and on proton pump inhibitor therapy. Aliment Pharmacol Ther. 2009;30:508–515. doi: 10.1111/j.1365-2036.2009.04063.x. [DOI] [PubMed] [Google Scholar]
- 10.Frazzoni M, Conigliaro R, Melotti G. Weakly acidic refluxes have a major role in the pathogenesis of proton pump inhibitor-resistant reflux oesophagitis. Aliment Pharmacol Ther. 2011;33:601–606. doi: 10.1111/j.1365-2036.2010.04550.x. [DOI] [PubMed] [Google Scholar]
- 11.Pandolfino JE, Vela MF. Esophageal-reflux monitoring. Gastrointest Endosc. 2009;69:917–930, 930.e1. doi: 10.1016/j.gie.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 12.Savarino E, Zentilin P, Tutuian R, Pohl D, Casa DD, Frazzoni M, Cestari R, Savarino V. The role of nonacid reflux in NERD: lessons learned from impedance-pH monitoring in 150 patients off therapy. Am J Gastroenterol. 2008;103:2685–2693. doi: 10.1111/j.1572-0241.2008.02119.x. [DOI] [PubMed] [Google Scholar]
- 13.Frazzoni M, Conigliaro R, Mirante VG, Melotti G. The added value of quantitative analysis of on-therapy impedance-pH parameters in distinguishing refractory non-erosive reflux disease from functional heartburn. Neurogastroenterol Motil. 2012;24:141–146, e87. doi: 10.1111/j.1365-2982.2011.01800.x. [DOI] [PubMed] [Google Scholar]
- 14.Frazzoni M, De Micheli E, Zentilin P, Savarino V. Pathophysiological characteristics of patients with non-erosive reflux disease differ from those of patients with functional heartburn. Aliment Pharmacol Ther. 2004;20:81–88. doi: 10.1111/j.1365-2036.2004.01998.x. [DOI] [PubMed] [Google Scholar]
- 15.Frazzoni M, Conigliaro R, Melotti G. Reflux parameters as modified by laparoscopic fundoplication in 40 patients with heartburn/regurgitation persisting despite PPI therapy: a study using impedance-pH monitoring. Dig Dis Sci. 2011;56:1099–1106. doi: 10.1007/s10620-010-1381-4. [DOI] [PubMed] [Google Scholar]
- 16.Frazzoni M, Conigliaro R, Manta R, Melotti G. Reflux parameters as modified by EsophyX or laparoscopic fundoplication in refractory GERD. Aliment Pharmacol Ther. 2011;34:67–75. doi: 10.1111/j.1365-2036.2011.04677.x. [DOI] [PubMed] [Google Scholar]
- 17.Frazzoni M, Conigliaro R, Colli G, Melotti G. Conventional versus robot-assisted laparoscopic Nissen fundoplication: a comparison of postoperative acid reflux parameters. Surg Endosc. 2012;26:1675–1681. doi: 10.1007/s00464-011-2091-5. [DOI] [PubMed] [Google Scholar]
- 18.Frazzoni M, Piccoli M, Conigliaro R, Manta R, Frazzoni L, Melotti G. Refractory gastroesophageal reflux disease as diagnosed by impedance-pH monitoring can be cured by laparoscopic fundoplication. Surg Endosc. 2013;27:2940–2946. doi: 10.1007/s00464-013-2861-3. [DOI] [PubMed] [Google Scholar]
- 19.Frazzoni M, Manta R, Mirante VG, Conigliaro R, Frazzoni L, Melotti G. Esophageal chemical clearance is impaired in gastro-esophageal reflux disease--a 24-h impedance-pH monitoring assessment. Neurogastroenterol Motil. 2013;25:399–406, e295. doi: 10.1111/nmo.12080. [DOI] [PubMed] [Google Scholar]
- 20.Kahrilas PJ, Howden CW, Hughes N. Response of regurgitation to proton pump inhibitor therapy in clinical trials of gastroesophageal reflux disease. Am J Gastroenterol. 2011;106:1419–1425; quiz 1426. doi: 10.1038/ajg.2011.146. [DOI] [PubMed] [Google Scholar]
- 21.Dallemagne B, Perretta S. Twenty years of laparoscopic fundoplication for GERD. World J Surg. 2011;35:1428–1435. doi: 10.1007/s00268-011-1050-6. [DOI] [PubMed] [Google Scholar]
- 22.Broeders JA, Rijnhart-de Jong HG, Draaisma WA, Bredenoord AJ, Smout AJ, Gooszen HG. Ten-year outcome of laparoscopic and conventional nissen fundoplication: randomized clinical trial. Ann Surg. 2009;250:698–706. doi: 10.1097/SLA.0b013e3181bcdaa7. [DOI] [PubMed] [Google Scholar]
- 23.Ross SB, Gal S, Teta AF, Luberice K, Rosemurgy AS. Late results after laparoscopic fundoplication denote durable symptomatic relief of gastroesophageal reflux disease. Am J Surg. 2013;206:47–51. doi: 10.1016/j.amjsurg.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Dallemagne B, Weerts J, Markiewicz S, Dewandre JM, Wahlen C, Monami B, Jehaes C. Clinical results of laparoscopic fundoplication at ten years after surgery. Surg Endosc. 2006;20:159–165. doi: 10.1007/s00464-005-0174-x. [DOI] [PubMed] [Google Scholar]
- 25.Mardani J, Lundell L, Engström C. Total or posterior partial fundoplication in the treatment of GERD: results of a randomized trial after 2 decades of follow-up. Ann Surg. 2011;253:875–878. doi: 10.1097/SLA.0b013e3182171c48. [DOI] [PubMed] [Google Scholar]
- 26.Engström C, Cai W, Irvine T, Devitt PG, Thompson SK, Game PA, Bessell JR, Jamieson GG, Watson DI. Twenty years of experience with laparoscopic antireflux surgery. Br J Surg. 2012;99:1415–1421. doi: 10.1002/bjs.8870. [DOI] [PubMed] [Google Scholar]
- 27.Humphries LA, Hernandez JM, Clark W, Luberice K, Ross SB, Rosemurgy AS. Causes of dissatisfaction after laparoscopic fundoplication: the impact of new symptoms, recurrent symptoms, and the patient experience. Surg Endosc. 2013;27:1537–1545. doi: 10.1007/s00464-012-2611-y. [DOI] [PubMed] [Google Scholar]
- 28.Cao Z, Cai W, Qin M, Zhao H, Yue P, Li Y. Randomized clinical trial of laparoscopic anterior 180° partial versus 360° Nissen fundoplication: 5-year results. Dis Esophagus. 2012;25:114–120. doi: 10.1111/j.1442-2050.2011.01235.x. [DOI] [PubMed] [Google Scholar]
- 29.Broeders JA, Broeders EA, Watson DI, Devitt PG, Holloway RH, Jamieson GG. Objective outcomes 14 years after laparoscopic anterior 180-degree partial versus nissen fundoplication: results from a randomized trial. Ann Surg. 2013;258:233–239. doi: 10.1097/SLA.0b013e318278960e. [DOI] [PubMed] [Google Scholar]
- 30.Broeders JA, Roks DJ, Ahmed Ali U, Watson DI, Baigrie RJ, Cao Z, Hartmann J, Maddern GJ. Laparoscopic anterior 180-degree versus nissen fundoplication for gastroesophageal reflux disease: systematic review and meta-analysis of randomized clinical trials. Ann Surg. 2013;257:850–859. doi: 10.1097/SLA.0b013e31828604dd. [DOI] [PubMed] [Google Scholar]
- 31.Broeders JA, Bredenoord AJ, Hazebroek EJ, Broeders IA, Gooszen HG, Smout AJ. Reflux and belching after 270 degree versus 360 degree laparoscopic posterior fundoplication. Ann Surg. 2012;255:59–65. doi: 10.1097/SLA.0b013e31823899f8. [DOI] [PubMed] [Google Scholar]
- 32.Koch OO, Kaindlstorfer A, Antoniou SA, Luketina RR, Emmanuel K, Pointner R. Comparison of results from a randomized trial 1 year after laparoscopic Nissen and Toupet fundoplications. Surg Endosc. 2013;27:2383–2390. doi: 10.1007/s00464-013-2803-0. [DOI] [PubMed] [Google Scholar]
- 33.Broeders JA, Mauritz FA, Ahmed Ali U, Draaisma WA, Ruurda JP, Gooszen HG, Smout AJ, Broeders IA, Hazebroek EJ. Systematic review and meta-analysis of laparoscopic Nissen (posterior total) versus Toupet (posterior partial) fundoplication for gastro-oesophageal reflux disease. Br J Surg. 2010;97:1318–1330. doi: 10.1002/bjs.7174. [DOI] [PubMed] [Google Scholar]
- 34.Galmiche JP, Hatlebakk J, Attwood S, Ell C, Fiocca R, Eklund S, Långström G, Lind T, Lundell L. Laparoscopic antireflux surgery vs esomeprazole treatment for chronic GERD: the LOTUS randomized clinical trial. JAMA. 2011;305:1969–1977. doi: 10.1001/jama.2011.626. [DOI] [PubMed] [Google Scholar]
- 35.Richter JE. Gastroesophageal reflux disease treatment: side effects and complications of fundoplication. Clin Gastroenterol Hepatol. 2013;11:465–471; quiz e39. doi: 10.1016/j.cgh.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DA, Oldfield EC. Reported side effects and complications of long-term proton pump inhibitor use: dissecting the evidence. Clin Gastroenterol Hepatol. 2013;11:458–464; quiz e37-e38. doi: 10.1016/j.cgh.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 37.Shaib YH, Rugge M, Graham DY, Genta RM. Management of gastric polyps: an endoscopy-based approach. Clin Gastroenterol Hepatol. 2013;11:1374–1384. doi: 10.1016/j.cgh.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ganz RA, Peters JH, Horgan S, Bemelman WA, Dunst CM, Edmundowicz SA, Lipham JC, Luketich JD, Melvin WS, Oelschlager BK, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med. 2013;368:719–727. doi: 10.1056/NEJMoa1205544. [DOI] [PubMed] [Google Scholar]
- 39.Broeders JA, Draaisma WA, Bredenoord AJ, de Vries DR, Rijnhart-de Jong HG, Smout AJ, Gooszen HG. Oesophageal acid hypersensitivity is not a contraindication to Nissen fundoplication. Br J Surg. 2009;96:1023–1030. doi: 10.1002/bjs.6684. [DOI] [PubMed] [Google Scholar]
- 40.Cicala M, Emerenziani S, Guarino MP, Ribolsi M. Proton pump inhibitor resistance, the real challenge in gastro-esophageal reflux disease. World J Gastroenterol. 2013;19:6529–6535. doi: 10.3748/wjg.v19.i39.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benson K, Hartz AJ. A comparison of observational studies and randomized, controlled trials. N Engl J Med. 2000;342:1878–1886. doi: 10.1056/NEJM200006223422506. [DOI] [PubMed] [Google Scholar]
- 42.Concato J, Shah N, Horwitz RI. Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med. 2000;342:1887–1892. doi: 10.1056/NEJM200006223422507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bytzer P. Assessment of reflux symptom severity: methodological options and their attributes. Gut. 2004;53 Suppl 4:iv28–iv34. doi: 10.1136/gut.2003.034298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zerbib F, Belhocine K, Simon M, Capdepont M, Mion F, Bruley des Varannes S, Galmiche JP. Clinical, but not oesophageal pH-impedance, profiles predict response to proton pump inhibitors in gastro-oesophageal reflux disease. Gut. 2012;61:501–506. doi: 10.1136/gutjnl-2011-300798. [DOI] [PubMed] [Google Scholar]
- 45.Kahrilas PJ, Hughes N, Howden CW. Response of unexplained chest pain to proton pump inhibitor treatment in patients with and without objective evidence of gastro-oesophageal reflux disease. Gut. 2011;60:1473–1478. doi: 10.1136/gut.2011.241307. [DOI] [PubMed] [Google Scholar]
- 46.Krishnan K, Pandolfino JE, Kahrilas PJ, Keefer L, Boris L, Komanduri S. Increased risk for persistent intestinal metaplasia in patients with Barrett’s esophagus and uncontrolled reflux exposure before radiofrequency ablation. Gastroenterology. 2012;143:576–581. doi: 10.1053/j.gastro.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Connell K, Velanovich V. Effects of Nissen fundoplication on endoscopic endoluminal radiofrequency ablation of Barrett’s esophagus. Surg Endosc. 2011;25:830–834. doi: 10.1007/s00464-010-1270-0. [DOI] [PubMed] [Google Scholar]


