Abstract
Medical therapy for type 2 diabetes mellitus is ineffective in the long term due to the progressive nature of the disease, which requires increasing medication doses and polypharmacy. Conversely, bariatric surgery has emerged as a cost-effective strategy for obese diabetic individuals; it has low complication rates and results in durable weight loss, glycemic control and improvements in the quality of life, obesity-related co-morbidity and overall survival. The finding that glucose homeostasis can be achieved with a weight loss-independent mechanism immediately after bariatric surgery, especially gastric bypass, has led to the paradigm of metabolic surgery. However, the primary focus of metabolic surgery is the alteration of the physio-anatomy of the gastrointestinal tract to achieve glycemic control, metabolic control and cardio-metabolic risk reduction. To date, metabolic surgery is still not well defined, as it is used most frequently for less obese patients with poorly controlled diabetes. The mechanism of glycemic control is still incompletely understood. Published research findings on metabolic surgery are promising, but many aspects still need to be defined. This paper examines the proposed mechanism of diabetes remission, the efficacy of different types of metabolic procedures, the durability of glucose control, and the risks and complications associated with this procedure. We propose a tailored approach for the selection of the ideal metabolic procedure for different groups of patients, considering the indications and prognostic factors for diabetes remission.
Keywords: Metabolic surgery, Gastrointestinal surgery, Type-2 diabetes mellitus, Glucolipotoxicity, Efficacy, Prognostic factor
Core tip: The success of bariatric surgery in obese diabetic individuals [body mass index, body mass index (BMI) > 35 kg/m2] has led to the paradigm of metabolic surgery for the treatment of type 2 diabetes mellitus, including patients with a BMI < 35 kg/m2. The mechanisms of metabolic gastrointestinal surgery are thought to depend on the dramatic entero-hormonal changes after physio-anatomical re-arrangement of the gastrointestinal tract. However, data have shown that weight loss is still the cornerstone of diabetes remission. This review will discuss the current recommendations regarding the use of gastrointestinal surgery for glycemic and metabolic control in diabetic individuals with a BMI < 35 kg/m2.
INTRODUCTION
Amidst the current worldwide epidemic of type 2 diabetes mellitus (T2DM), the global diabetes health burden is projected to reach 522 million in 2030[1], with much of this increase occurring in developing countries. Asia’s population is predicted to become a hotspot of the “metabolically obese” phenotype (normal body weight with increased abdominal adiposity) that is characterized by a relatively young age of onset with a low body mass index (BMI)[2]. In China specifically, the estimated prevalence of diabetes in 2010 was 11.6%, and that of pre-diabetes was 50.1%, accounting for up to 113.9 million Chinese adults with diabetes and 493.4 million with pre-diabetes[3]. Furthermore, given the increasing prevalence of obesity worldwide, the epidemic of diabetes has become an even larger economic and social public health challenge. The free fatty acids derived from visceral adipose tissue impair insulin sensitivity and β-cell function (lipotoxicity), leading to metabolic syndrome (MetS) and T2DM.
Long-term glycemic control of diabetes can halt the progression of the disease, prevent complications and reduce cardiovascular risk[4]. There are still, however, major unmet needs that are not addressed by intensified multifactorial interventions due to the need for continual monitoring and increasing doses over time with disease progression[5]. Most diabetic patients are unable to achieve adequate glucose control with medical therapy in the long term due to time-consuming medical interventions[6,7]. In the United States, only 50% of diabetic patients are able to achieve glycated haemoglobin (HbA1c) < 7% with medical therapy as recommended by the American Diabetic Association[7].
In contrast, bariatric surgery has been shown to induce the remission of diabetes or to reduce the need for medications with durable long-term results in morbidly obese patients[8,9], thereby providing a potentially cost-effective approach to treating T2DM. Considering weight loss-independent mechanisms for diabetes improvement, metabolic gastrointestinal surgery is now being performed for mildly obese or even overweight patients (BMI < 35 kg/m2), with a focus on diabetes rather than obesity. The aims of metabolic surgery are glycemic control, metabolic control and cardio-metabolic risk reduction.
Therefore, this review will examine the current use of gastrointestinal surgery for the metabolic control of T2DM. Data on patients with a BMI < 35 kg/m2 will be discussed in depth to reflect the current research focus.
LITERATURE SEARCH
An extensive literature search of English-language journals was performed using the following databases from 1987 to October 2013: MEDLINE/Pubmed, Embase and the Cochrane database.
The MeSH headings used included the following: “metabolic surgery”, “type 2 diabetes mellitus”, “gastrointestinal surgery”, “non-obese”, “BMI < 35 kg/m2”, “ obesity”, “diabetes remission”, “HbA1c”, “glucolipotoxicity” and “prognostic factor”. The terms were searched with combination of “OR” and “AND”. All abstracts identified were read to ensure that they met the inclusion criteria of any form of metabolic surgery for T2DM. Additional articles found in the references of the selected articles were reviewed to determine if they met the inclusion criteria. Critically, articles were reviewed for data on changes in BMI, HbA1c, type of surgery, reported remission and complications.
TYPES OF METABOLIC SURGERY
Bariatric surgery denotes any surgical procedure that is aimed at the reduction of excess weight. The conventional bariatric operations are divided as follows: restrictive type [laparoscopic adjustable gastric banding (LAGB) or laparoscopic sleeve gastrectomy (LSG)], malabsorptive type [bilio-pancreatic division with duodenal switch (BPD/DS)] and combination type [roux-en-Y gastric bypass (RYGB) or mini gastric bypass]. Interestingly, both bypass and restrictive procedures were reported to cause diabetes remission, but through different mechanisms, depending on the degree of the physio-anatomical alteration of the gastro-intestinal tract[9]. Essentially, the rapid induction of a negative energy balance after surgery, limited foregut bypass, rapid nutrient delivery to the hindgut and decreased adipocyte mass may all play important roles in durable weight loss, decreased satiety, improved insulin resistance and resolution of the pro-inflammatory state associated with obesity.
ROLE OF OBESITY IN METS AND T2DM
Obesity, particularly central obesity, is closely associated with insulin resistance and T2DM[10]. Long-standing obesity will eventually lead to glucose intolerance due to stress failure of the beta cells in predisposed individuals[11]. Biologically, as adipose tissue is an active endocrine and immune tissue, the pathogenic nature of adipose tissue depends on how the positive calorie balance results in “sick fat’ in genetically and environmentally susceptible individuals rather than on the total amount of fat mass[12,13]. This adipose dysfunction will result in pathogenic adipocyte deposits with lipotoxicity, resulting in cardiovascular disease and mortality[14] (Figure 1). Visceral fat, the most metabolically active adipose tissue deposit, releases various pathogenic mediators into the portal system, leading to hepatic-mediated metabolic disease (dyslipidemia and T2DM)[14]. High levels of circulating free fatty acids are also “lipotoxic” to various organs, especially the pancreas and liver, leading to metabolic disease. Therefore, effective weight loss after bariatric surgery that reduces metabolically active “sick fat” will improve metabolic disease and lead to the resolution of T2DM[15].
Figure 1.
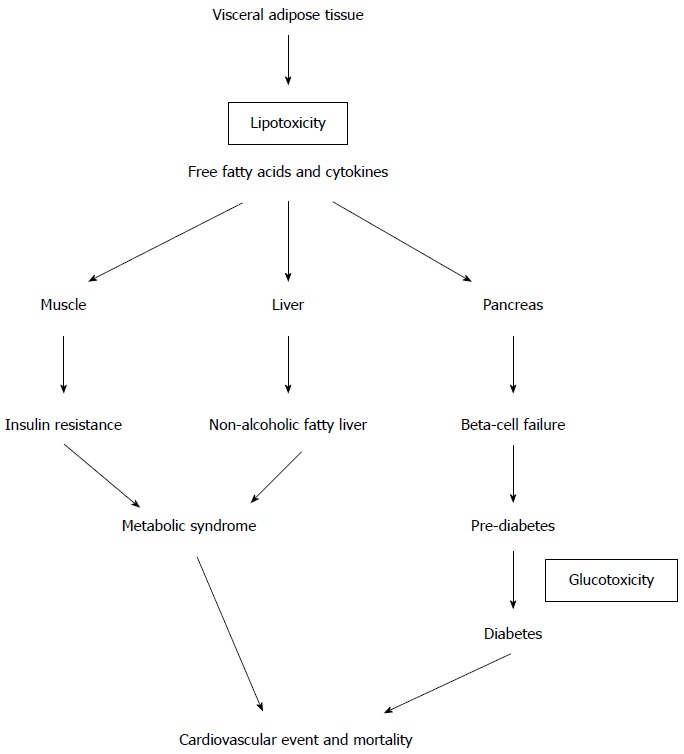
How lipotoxicity causes type 2 diabetes mellitus and metabolic syndrome.
MECHANISM OF GLYCEMIC CONTROL AFTER GASTROINTESTINAL SURGERY
Although various types of bariatric procedures have been used to treat T2DM, the physiology of remission is still incompletely understood[16]. Diabetes remission results from improvements in both insulin resistance and β-cell dysfunction, but the degree of their improvement also depends on the type of surgery performed. To elucidate the molecular mechanism of diabetes control, it is critical to understand which part of the typical anatomical rearrangement of gastrointestinal surgery is essential for the glycemic control of T2DM.
The restrictive procedure (LAGB) is thought to achieve glycemic control purely through weight loss without an entero-hormonal effect. Therefore, the remission of diabetes is slow and occurs in parallel with gradual weight loss. In contrast, the most investigated metabolic procedure is RYGB, which exhibits significant hormonal changes after surgery; the glycemic control is acute and immediate via an anti-diabetic weight-independent mechanism, even without significant weight loss after surgery. Moreover, the magnitude of metabolic control is much greater than the expected degree of weight loss, which might be a clue that the altered hormonal milieu of gut-hormone release explains the metabolic disease control[8]. Generally, immediately after RYGB, acute calorie restriction, usually 200-300 kcal/d, leads to improved glucose tolerance due to improved hepatic insulin sensitivity and reduced hepatic glycogen stores[17]. Later, the incretin effect due to hormonal changes is responsible for further improvements in insulin secretion and sensitivity[18]. For those patients with obesity and lipotoxicity, significant subsequent weight loss will account for improved pancreatic function and decreased insulin resistance due to resolution of the pro-inflammation state[13]. Based on this observation, several plausible mechanisms are possibly responsible for the early and long-term glycemic normalization after bariatric surgery. The most prominent glycemic effects are the decrease in insulin resistance and the increase in early phase insulin release[18,19]. Nevertheless, the interaction of multiple organ-related pathways involving the brain, gut, liver, pancreas, muscle, adipose tissues and other undiscovered tissues may result in dramatic resolution of the diabetic state. The main suggested hypotheses of the possible related mechanisms are discussed below.
Calorie restriction and weight loss
Calorie restriction and subsequent weight loss can have potent effects on insulin sensitivity due to either surgical or medical interventions. Studies have shown that the effect of 48 h of acute calorie restriction (approximately 1100 kcal/d) could result in improved hepatic insulin sensitivity with reduced hepatic gluconeogenesis; however, there is no effect on muscle insulin sensitivity unless greater weight loss is achieved in obese subjects[20]. Similarly, the static measure of the HOMA-IR score improves within days after bariatric surgery when weight loss is minimal[21], but greater weight loss (> 20%) after RYGB or LAGB is required to improve skeletal muscle insulin sensitivity[22-24]. RYGB and BPD produced a significant decrease in insulin resistance at 2 wk after surgery[21]. However, the effect of BPD on muscle sensitivity is unique and possibly related to massive lipid malabsorption, as this surgery results in a rapid improvement in muscle insulin sensitivity despite minimal weight loss (< 10%)[25,26] and minimal improvement thereafter despite greater weight loss[27,28]. Interestingly, one study has shown that despite similar weight losses of 30% after RYGB and LAGB, the RYGB cohort had higher rates of diabetes remission (72% vs 17%) at 2 years[29]. This result clearly suggests that in addition to weight loss, there is a weight-independent anti-diabetes mechanism in diabetes remission after RYGB.
Foregut effect
The “foregut hypothesis” proposes that the exclusion of the duodenum and proximal jejunum from the transit of nutrients may prevent the secretion of a putative signal that promotes insulin resistance and T2DM, suggesting that a yet unidentified inhibitory product from the proximal bowel causes metabolic changes (anti-incretin). This hypothesis was based on an animal study by Rubino et al[30], which supported the foregut hypothesis as a dominant mechanism in improving glucose homeostasis after RYGB. In their study, duodenal-jejunal bypass (DJB) (gastrojejunostomy + duodenal exclusion as in RYGB) greatly improved diabetes in Goto-Kakizaki rats, an animal model of non-obese T2DM. Conversely, a simple gastrojejunostomy without excluding nutrient flow through the proximal intestine did not improve diabetes. Interestingly, when the authors reestablished the normal gastroduodenal route but preserved the gastrojejunostomy, the glycemic abnormality returned in the DJB-treated rats. Thus, the authors proposed that excluding the proximal intestine from nutrient flow would improve glucose tolerance and suggested that a putative signal originating in the foregut might be involved in the pathophysiology of T2DM. Similarly, strong support for the foregut hypothesis came from the use of an endoluminal sleeve to endoscopically achieve DJB, which produced significant weight loss, resolution of T2DM and improvement in the cardiovascular risk factor profile[31]; these findings might open new avenues for diabetes research. Interestingly, Zhang et al[32] investigated the role of Billoth-II gastrojejunostomy, with exclusion of the proximal gut (almost similar to RYGB), in glucose metabolism and gut hormone modulations in non-obese Chinese gastric cancer patients. They found that blood glucose tolerance was improved in the non-obese T2DM patients but worsened in the non-diabetic patients. All patients had comparable weight loss. These results suggested that the role of the proximal gut in the pathophysiology of T2DM and the glycemic effect is weight independent. Clinically, there is strong evidence from randomized trials by Lee et al[33] and Schauer et al[34] showing that gastric bypass is superior to sleeve gastrectomy without duodenal exclusion for diabetes remission in patients with poorly controlled diabetes.
Hindgut effect
The “hindgut hypothesis” proposes that diabetes control results from the expedited delivery of nutrients to the distal bowel, thus producing a physiologic signal that improves glucose homeostasis. The potential mediators of this effect are glucagon-like peptide-1 (GLP-1), GIP (incretin effect) and peptide YY (non-incretin). The rapid delivery of nutrients has been demonstrated to stimulate the “L” cells in the distal intestine to secrete incretin, thus enhancing insulin secretion and insulin sensitivity[35]. GLP-1, which is suppressed in T2DM, is an insulinotropic hormone secreted from the gut in response to luminal glucose[36]. It acts mainly as an incretin, promoting post-prandial insulin release and improving pancreatic β-cell function[37]. Furthermore, GLP-1 exerts proliferative and anti-apoptotic effects on pancreatic β-cells[38]. Interesting results have been recently reported with the use of GLP-1 agonists, now widely used in the treatment of T2DM[39]. A novel procedure with ileal interposition has been used as an “ileal brake” to induce the early release of GLP-1 with favorable results[40]. In contrast, peptide YY is an anorexic hormone co-secreted with GLP-1 from intestinal L cells in response to nutrients. It acts to decrease food intake due to faster satiation and may reduce insulin resistance. Most studies have shown the elevation of peptide YY together with GLP-1 in response to nutrients after RYGB, which is not observed after LAGB.
Ghrelin effect
Ghrelin is an orexigenic gut hormone and has stimulatory effects on growth hormone release. It is mainly secreted from the gastric fundus and displays an ultradian rhythm with an increase before meals and a decrease after meals. However, its levels are reduced in obese individuals compared with normal weight individuals. When this gastric segment is disconnected and isolated from food contact, fatigue in ghrelin hormone production is hypothesized to develop, which stops the ghrelin effects. Geloneze et al[41] demonstrated that ghrelin levels decrease dramatically in patients who have undergone RYGB. Ghrelin has also been shown to have diabetogenic effects[42] because ghrelin administration in humans suppresses insulin secretion, even in the setting of ghrelin-induced hyperglycemia[43]. However, published studies on ghrelin levels after gastric bypass are inconsistent and have shown that the levels increase, decrease or do not change after surgery[44]. These inconsistent findings might be due to variations in the surgical resection/isolation of the gastric fundus or to differences in the time of measurement of the ghrelin levels due to the physiological fluctuations of hormone levels. However, ghrelin is undoubtedly decreased after sleeve gastrectomy and, over the long term, may play an important role in the sustainable effect of weight loss after sleeve gastrectomy[45].
Role of bile acid
Bile acids are a key stimulus for the farnesoid X receptor in the liver[46,47], affecting hepatic metabolism and G-protein-coupled bile acid-activated receptors (TGR5) of the enteroendocrine L-cells[48,49] and promoting the release of incretin. Therefore, bile acids play an important role in glucose homoeostasis. Post-operative increases in circulating bile acids have been suggested to contribute to the metabolic benefits of bariatric surgery; however, their mechanisms remain undefined. Recent human clinical trials with the bile acid sequestrant colesevelam have shown its effectiveness in improving glycemic control in patients with T2DM[50]. The re-route of nutrients due to altered physio-anatomy after gastric bypass may also affect the enterohepatic recirculation of bile acids and contribute to improved glycemic control[51]. Therefore, manipulation of bile acid homeostasis might be an attractive approach for T2DM.
Role of microbiota
The microbiota of the intestine can affect the control of energy metabolism by increasing the energy harvested from the diet; thus, changes in the gut microbiota might contribute to the epidemics of obesity and T2DM in human evolution[52]. However, the pathophysiology remains poorly understood. Probiotics, which include lactic acid bacteria, have been investigated extensively for their beneficial role in the alteration of gut microbiota by affecting the inflammatory state of the human body. Their role in T2DM was recently explored in an animal model. Naito et al[53] reported that Lactobacillus casei in diet-induced obese mice has the potential to prevent obesity-associated metabolic abnormalities by improving insulin resistance. The weight-gain suppressive effect of Lactobacillus in high calorie-fed mice has also been reported; thus, it may be an alternative method for treating obesity[54]. However, the translation of these beneficial animal study results into humans has still not been achieved.
EFFICACY OF GLYCEMIC CONTROL IN T2DM FOR BMI > 35 KG/M2 VS BMI < 35 KG/M2
Multiple studies have demonstrated impressive improvements in T2DM after bariatric surgery in morbidly obese patients. In a meta-analysis of bariatric surgery involving 3188 patients with T2DM, Buchwald et al[9] reported an overall 78% rate of complete diabetes remission that lasted at least 2 years. The weight loss and diabetes resolution were greatest for patients undergoing BPD/DS, followed by RYGB, and least for gastric restrictive procedures, such as vertical gastric banding and LAGB[9], with remission rates of 95%, 80%, 79% and 56%, respectively. The procedures producing greater excess weight loss led to higher remission rates. Moreover, in the morbidly obese population, there are compelling data on long term cardiovascular risk reduction and mortality based on the longitudinal prospective Swedish Obese Subjects study[55,56]. The majority of patients underwent a gastric restrictive procedure rather than a gastric bypass procedure in this study.
Nevertheless, there is level 1 evidence showing the efficacy of bariatric surgery over medical therapy in T2DM, based on 4 randomized clinical trials (RCT) that directly compared bariatric surgery with a non-surgical intervention for the treatment of T2DM (Table 1). However, none of these trials exclusively enrolled patients with diabetes with a BMI < 35 kg/m2.
Table 1.
Randomized controlled trials that directly compared the effectiveness of bariatric procedures with medical management for the treatment of type 2 diabetes
| Trial | Dixon et al[57] | Schauer et al[34] | Mingrone et al[105] | Ikramuddin et al[58] |
| Intervention arms | LAGB; standard medical management | RYGB; LSG; medical management | RYGB; BPD; medical management | RYGB with medical management; medical management |
| Follow-up duration (yr) | 2 | 1 | 2 | 1 |
| Baseline characteristics | ||||
| n | 60 | 150 | 60 | 120 |
| Mean BMI (kg/m2) | 37 (30-40) | 36 (27-43) | 45 (35-60) | 34 (30-40) |
| Duration of T2DM (yr) | < 2 | > 8 | 6 | 9 |
| Mean HbA1c | 7.8% | 9.2% | 8.7% | 9.6% |
| Primary end-point | FBG < 126 mg/dL with HbA1c < 6.2% without pharmacotherapy | HbA1c < 6% with or without pharmacotherapy | FBG < 100 mg/dL with HbA1c < 6.5% without pharmacotherapy | CV risk reduction with HbA1c < 7% with LDL cholesterol < 100 mg/dL and systolic BP < 130 mmHg |
| End results | ||||
| Diabetes remission | LAGB 73%; medical arm 13% | RYGB 42%; LSG 37%; medical arm 12% | BPD 95%; RYGB 75%; medical arm 0% | RYGB 75%; medical arm 32% |
| Mean HbA1c | LAGB 6.0%; medical arm 7.2% | RYGB 6.4%; LSG 6.6%; medical arm 7.5% | BPD 4.9%; RYGB 6.3%; medical arm 7.7% | RYGB 6.3%; medical arm 7.8% |
| Mean BMI (kg/m2) | LAGB 29.5; medical arm 36.6 | RYGB 26.8; LSG 27.2; medical arm 34.4 | BPD 29.1; RYGB 29.3; medical arm 43.1 | RYGB 25.8 medical arm 31.6 |
| Mean excess weight loss | LAGB 62%; medical arm 4.3% | RYGB 88%; LSG 81%; medical arm 13% | BPD 69%; RYGB 68%; medical arm 9% | 1RYGB 26.1 medical arm 7.9% |
| Major surgical complications | 2 revision surgeries (6.7%) due to pouch enlargement; 1 required band removal due to persistent regurgitation | 4 re-operations (4.0%) due to intra-abdominal hematoma, vomiting, cholecystectomy, and feeding access after gastric leak | 2 re-operations (5.0%) due to incisional hernia and bowel obstruction. More nutritional risk in BPD than in RYGB | 2 re-operations (3.3%) due to anastomotic leak. Surgical group had 50% more serious events with greater nutritional deficiency than medical arm |
| Mortality | Nil | Nil | Nil | Nil |
| Comment | This trial involved patients with early (< 2 yr) diabetes of mild severity (HbA1c < 7.8%), but only 22% of patients had BMI 30-35. This trial supported early surgical intervention for early obese T2DM | This trial involved patients with more advanced diabetes (> 8 years and HbA1c 9.2%), with 34% of patients having BMI < 35. This trial supported surgical intervention in more severe T2DM | This trial involved severely obese diabetic patients and showed the greater effectiveness of bariatric surgery over medical therapy in poorly controlled T2DM | This trial showed the potential benefits and risks of adding RYGB to the best medical therapy in achieving therapeutic goals for diabetes (49% vs 19%). This trial had a higher proportion of BMI 30-35 (59%) |
Mean weight loss. T2DM: Type 2 diabetes mellitus; BMI: Body mass index; LAGB: Laparoscopic adjusted gastric banding; RYGB: Roux-en-Y gastric bypass; LSG: Laparoscopic sleeve gastrectomy; BPD: Bilio-pancreatic diversion; FBG: Fasting blood glucose; HbA1c: Glycosylated hemoglobin; LDL: Low density lipoprotein; CV: Cardiovascular.
The results of a study by Dixon et al[57] strongly supported early surgical intervention in early obese diabetes. Interestingly, in their study subgroup with a BMI of 30-35 kg/m2 (22% of the total cohort), there was evidence that glycemic control and weight loss were as efficacious as those in the higher BMI group. However, the Schauer et al[34] study demonstrated that bariatric surgery was even effective in more advanced T2DM patients. The most recent multicenter RCT by Ikramuddin et al[58] showed that there was a benefit of adding RYGB to optimal medical therapy instead of using medical therapy alone in achieving diabetes therapeutic goals (49% vs 19%) for cardiovascular risk reduction. This study included a higher proportion of the cohort with a BMI of 30-35 (59%), suggesting that metabolic surgery might play a very important role in cardio-metabolic risk reduction in patients with a BMI < 35 kg/m2 and T2DM. All these trials included insufficient numbers of patients, used different diagnostic criteria for diabetes remission and had only 1-2 years of follow-up; therefore, cardiovascular risk reduction and the durability of glycemic control still remain to be defined using longer follow-up data in the future.
Currently, emerging data have shown good outcomes after metabolic surgery aiming to treat T2DM in less obese diabetics with a BMI < 35 kg/m2[18,59-62]. The extraordinary glucose, lipid and blood pressure control reported in the published series[63] also suggest good long-term effects on the cardiovascular risk profile and mortality.
Lee et al[61] reported a prospective multicenter study of metabolic surgery involving 200 Asian patients with T2DM and a BMI < 35 kg/m2, with an end point of T2DM remission, which was defined by a fasting plasma glucose < 110 mg/dL and HbA1C < 6.0%. In that study, 172 patients underwent gastric bypass, 24 underwent sleeve gastrectomy, and 4 underwent adjustable banding. At 1 year, the mean BMI decreased from 28.5 to 23.4 kg/m2, and HbA1C decreased to 6.3%. T2DM remission was achieved in 72.4% of the patients. Diabetes remission was significantly higher in patients with a duration of diabetes < 5 years and a BMI > 30 kg/m2 who underwent gastric bypass surgery.
The longest prospective study on patients with T2DM and a BMI < 35 kg/m2 and who underwent metabolic surgery was published by Cohen et al[64] In that study, 66 patients with a BMI of 30-35 kg/m2, a disease duration of 12.5 ± 7.4 years and a baseline HbA1c 9.7 ± 1.5% underwent RYGB. At 6 years, with 100% follow-up, durable diabetic remission (defined as HbA1c < 6.5% without medication) was achieved in 66% of patients, with glycemic improvement in 11% of patients and HbA1c reduced to 5.9%. There was no mortality, major morbidity or excessive weight loss. The improvement of hypertension, dyslipidemia and glycemia translated to a 50%-84% predicted 10-year cardiocerebral risk reduction.
Regarding the efficacy of metabolic surgery, two randomized clinical trials directly compared different bariatric procedures in a diabetic population. Lee et al[33] randomized 60 diabetic patients with a mean BMI of 30.3 to undergo either gastric bypass or sleeve gastrectomy. At 1 year, the gastric bypass group had a higher rate of diabetes remission, with HbA1c < 6.5% (93% vs 47%), a significantly higher reduction in weight (BMI 22.8 kg/m2 vs 24.4 kg/m2), HbA1c change (4.2% vs 3.0%) and fasting glucose level change (99.3 mg/dL vs 140.1 mg/dL). Similarly, Schauer et al[34] published a randomized trial with 3 different arms of gastric bypass, sleeve gastrectomy and medical therapy for diabetic patients; patients in the gastric bypass group lost more weight than those in the sleeve gastrectomy group (-29.4 kg vs -25.1 kg) at 1 year. Although there was no difference in glucose levels, 30% of patients in the sleeve gastrectomy group still needed medication to achieve the target level of HbA1c < 6.0%.
Although the best evidence is based on 1-2 years of short-term results from available randomized trials[33,34,57,58], metabolic surgery for the treatment of T2DM in patients with a BMI of 30-35 kg/m2 is promising. These data also indicate that gastric bypass is an effective metabolic surgery because patients who underwent gastric bypass had more weight loss and higher diabetes remission rates than individuals who underwent restrictive-type procedures. Post-operative weight loss[65,66] and a shorter duration of diabetes[61] are still powerful predictors of early remission after metabolic surgery. Therefore, surgical results are not independent of initial BMI or weight loss. Caution is still required when performing metabolic surgery for low BMI patients.
INDICATIONS FOR METABOLIC SURGERY
Current guidelines[67,68] strongly recommend that conventional bariatric surgery be considered for the treatment of T2DM in surgical candidates with poorly controlled diabetes and a BMI > 35 kg/m2. However, according to the international diabetes federation (IDF) position statement from March 2011, surgery should be considered as an alternative treatment option in patients with a BMI between 30 and 35 when the diabetes cannot be adequately controlled with an optimal medical regimen, especially in the presence of other major cardiovascular disease risk factors. In Asians, the BMI cut-off point is 2.5 points lower compared with Western populations to reflect the higher obesity risk profile in Asian populations[69]. Historically, that report was the first to include bariatric surgery in a diabetes treatment algorithm. For individual diabetic patients with a BMI < 30 kg/m2, some data have shown encouraging results[18,33,59,61,62,66,70-73]. As shown by our study, good outcomes could be achieved, especially for patients with some degree of weight loss, good β-cell function and central obesity[61,66].
However, with metabolic surgery, the BMI may not be considered an appropriate selection criterion, as it does not reflect the metabolic profile of at-risk patients. The BMI was originally derived from the Caucasian population and may not be applicable to other populations, especially Asian populations[74]. Moreover, BMI does not necessarily reflect the fat composition and distribution in the body, which affect metabolic risk[74]. Studies have shown that insulin resistance is secondary to an increase in hepatic triglyceride content and visceral adiposity[75,76], of which BMI is not a good indicator. T2DM individuals have been shown to have altered adipose tissue distribution with more visceral adipose tissue and intermuscular adipose tissue than subcutaneous tissue[76]. In other words, BMI is a poor indicator of metabolically significant obesity[74]. Therefore, BMI should not be used as the sole criterion for metabolic surgery[77]. Suitable criteria that reflect the metabolic profile of patients are needed for the appropriate selection of patients for metabolic surgery.
TAILORED APPROACH FOR THE SELECTION OF THE APPROPRIATE METABOLIC SURGERY
The selection of patients for metabolic surgery should be matched to an ideal risk-benefit profile to obtain the optimal outcomes of metabolic control. Therefore, we propose a tailored approach for the selection of metabolic surgery for poorly controlled T2DM.
LAGB is ideal for patients with early, newly diagnosed T2DM (< 2 years) and a BMI > 30 kg/m2, as the mechanisms of glycemic control are calorie restriction and effective weight loss. The RCT by Dixon et al[57] has proven its role in early T2DM. LAGB results in more limited weight reduction with higher probability of repeated interventions due to band erosion and slippage but carries a minimal risk of long-term complications such as malnutrition and internal hernias.
LSG is suitable for individuals with a BMI > 27 kg/m2 but with a duration of DM < 5 years and good C-peptide levels indicative of good pancreatic β-cell function[73]. The metabolic effect is related to greater improvement in insulin resistance secondary to calorie restriction and weight loss rather than an increase in insulin secretion. Although the metabolic effect due to the hindgut effect with the elevation of GLP-1 and peptide YY has been reported, the long-term glycemic amelioration effect is still unknown. C-peptide > 3 ng/mL is the most important predictor of successful treatment[73,78]. This procedure has the risk of acute complications such as leakage, bleeding and stricture due to very long stapler lines but carries minimal long-term nutritional risks.
RYGB, the most investigated metabolic procedure, is ideal for individuals with poorly controlled DM with a BMI > 27 kg/m2. Studies have confirmed the superiority of gastric bypass over restrictive procedures due to the bypass of the hormonally active foregut and early nutrient stimulation of the hindgut[33,34,79]. This procedure should be reserved for those with more advanced and long-standing T2DM. The surgical risks are acute complications such as anastomotic leakage and bleeding, and the long-term risks are malnutrition and internal hernias. For those subjects with a high operative risk, mini-gastric bypass is likely the best alternative due to the shorter surgical time and only one anastomosis.
BPD, which is a purely malabsorptive procedure with superior glycemic control[9] compared with the above metabolic procedures, may not be appropriate in lower-BMI individuals due to the high complication rates and long-term malnutrition risk. Instead, the novel procedure of duodenal-jejunal bypass with sleeve gastrectomy (DJB-SG)[80,81] should be considered, although the long-term results have not yet been reported (refer to new perspectives).
PATIENT SELECTION
The long-term risk of T2DM is highly associated with elevated HbA1c levels, not a high BMI[82]. Therefore, considering the potential benefits of metabolic surgery and the risk of surgery in the BMI < 35 kg/m2 population, the selection of the ideal candidate should be based on the presence of MetS with poorly controlled T2DM. In other words, the selection criteria should be specific for diabetes and MetS.
The diabetes-specific criteria should include HbA1c, diabetes duration and C-peptide levels. Reducing HbA1c below 7% has been shown to reduce microvascular complications, and a reduction soon after the diagnosis of diabetes is associated with a long-term reduction in macrovascular disease[68]. Therefore, the cut-off point of HbA1c is 7%, which indicates that poorly controlled glycemia is an appropriate selection criterion for metabolic surgery. Moreover, performing surgery on individuals with a short duration of diabetes, especially < 5 years, and a C-peptide > 2.9 ng/dL will produce better long-term outcomes due to better preserved pancreatic β-cell function[61,66,83].
For metabolic-specific criteria, waist circumference (WC) is most likely a pragmatic clinical consideration. Central obesity is associated with the increasing prevalence of MetS with insulin resistance, which is driven by the strong relationship between WC and increasing adiposity. If diabetes is not already present, MetS is a strong predictor of T2DM, which is five times more likely to occur in individuals with this syndrome[84]. Therefore, WC is a surrogate of metabolic disease with insulin resistance. WC as defined by the IDF, which is ethnically specific[85], should be part of the clinical criteria for the selection of patients for metabolic surgery.
Given the operative risk of cardiovascular complications in this higher-risk group compared with the conventional bariatric population, metabolic surgery should be avoided in patients with any recent major cardiovascular event.
COMPLICATIONS
MetS is a constellation of syndromes, including T2DM, hypertension, hyperlipidemia and central obesity, that lead to increased cardiovascular disease. Although bariatric surgery is relatively safe, with a complication risk of 0.1%-0.3%, which is considered low even in the era of laparoscopic surgery and is similar to the complication risk of laparoscopic cholecystectomy[86], the presence of MetS might be a risk factor for adverse events in bariatric surgery. In a study based on the Bariatric Outcomes Longitudinal Database, the national database for the American Society for Metabolic and Bariatric Surgery’s Bariatric Surgery Center of Excellence Program, MetS patients had a higher incidence of early adverse outcomes compared with non-MetS patients[87]. Patients with MetS tend to be older males with more visceral fat (central obesity), a higher incidence of obstructive sleep apnea and an American Society of Anesthesiologists category of Class III or greater that may lead to surgical difficulty with high peri-operative risk. The MetS patients had significantly higher incidences of serious complications (2.4% vs 1.0%) and mortality 90 d after bariatric surgery (0.3% vs 0.1%) compared with non-MetS patients. This finding is expected because individuals with MetS who already have a heightened pro-inflammatory state will not able to defend themselves against complications due to limited organ reserve. This risk is especially true for poor-health T2DM patients with impaired organ function.
Generally, the mortality and morbidity rates are low, most likely due to the standardization of surgical techniques and peri-operative care; however, post-operative complications can be serious and must be addressed promptly by surgeons who are familiar with these problems. For overweight patients with a BMI > 25 kg/m2, there is no risk of excessive weight loss that will move them into the normal weight category[16]. Given the risk of hypoglycemia immediately after metabolic surgery due to acute calorie restriction in diabetic patients, close monitoring of glucose levels with the appropriate withdrawal of anti-diabetes medications is essential post-operatively.
Metabolic surgery is a life-changing operation with associated risks and complications, especially long-term nutritional deficiencies. The surgical risk is not negligible, as shown in Table 1. Surgical intervention was associated with 50% more serious events with greater nutritional deficiency than medical therapy[58]. Therefore, patients should be counselled regarding life-style modifications, especially the restrictions related to eating after surgery and the commitment to life-long follow-up to prevent potential complications. For lower BMI individuals, the risk of each metabolic procedure needs to be considered in light of the individual risk-benefit ratio with respect to potential reductions in diabetes-associated mortality and morbidity and improvements in the quality of life.
DURABILITY OF THE GLYCEMIC EFFECT
The durability and degree of glycemic control in different surgical procedures remain uncertain. Bypass procedures have been reported to have a higher degree of glycemic control and resolution of associated comorbidities[9]. RYGB is more effective than LSG with respect to glycemic control[33]. A small-scale prospective study in mildly obese patients (class 1 obesity) with long term follow-up showed that RYGB achieved 88% diabetic remission over 6 years, with glycemic improvement in 11% of patients[64]. However, there is a substantial rate of relapse within 5 years after the initial diabetes remission[88,89], which might suggest that metabolic surgery slows but does not prevent the progressive loss of the insulin secretory capacity of the pancreas. In relapsed patients, it is unclear how a period of good control after metabolic surgery will affect the long-term morbidity and mortality rates. Although the large ACCORD[90], ADVANCE[91] and UKPDS 33[92] trials have suggested that tight glucose reduction has a microvascular benefit but not a macrovascular benefit, the legacy effect, as shown by the UKPDS study, is that the benefit of a brief period of intensive glycemic control in patients with T2DM might extend into subsequent years, with a reduced risk of macrovascular disease and mortality[93]. Clearly, longer term follow-ups in larger series are needed to clarify this important issue.
PROGNOSTIC FACTORS
Currently, there is no consensus regarding how to choose appropriate T2DM patients for metabolic surgery because various surgeries have different remission rates with different mechanisms[94]. Offering surgery only to those patients who are young and in the early stages of diabetes with better preserved pancreatic β-cell mass may result in better outcomes. In addition, the variation of a patient’s metabolic profile in poorly controlled diabetes might affect the reported remission rate in various published metabolic surgery results. Therefore, the ability to predict diabetes remission after metabolic surgery has great implications for choosing the type of surgical procedure that is best suited for particular patients and for evaluating the risk-benefit profile and the likelihood of success after surgery so that optimal metabolic surgery outcomes can be achieved. Perhaps, more importantly, we could avoid the unnecessary risk of surgery without a clear clinical benefit in patients who are not expected to respond well to metabolic surgery.
Generally, the possible predictors of diabetes remission that have been studied may produce better outcomes after metabolic surgery and might be related to younger age, better pancreatic β-cell function (C-peptide level), lower disease severity (duration of diabetes, intensity of medication and insulin dosage), lower obesity (BMI), the amount of weight loss, the high sensitivity C-reactive protein and insulin resistance[18,61,65,78,83,95-97].
Dixon et al[66] studied the factors that influence glycemic control following gastric bypass surgery in patients with T2DM and a BMI < 30 kg/m2. A baseline BMI of < 27 kg/m2 and C-peptide of < 2.0 ng/mL were associated with a poor glycemic response. A higher baseline BMI, shorter disease duration (< 7 years) and higher fasting C-peptide (> 2.4 ng/mL) with post-surgery weight loss (> 16%) were associated with excellent glycemic control. DiGiorgi et al[88] reported that those patients who required insulin or oral medication before RYGB were more likely to experience improvement rather than resolution. The patients with recurrence or worsening associated with a lower preoperative BMI regained a greater percentage of lost weight, had a greater weight loss failure rate and had greater postoperative glucose levels. However, all these factors might be inter-related, and it is difficult to interpret them without a clinically meaningful grading system to categorize and help select the most appropriate patients for metabolic surgery.
In Asia, Lee et al[98] has advocated a simple but validated multidimensional grading system for metabolic surgery (diabetic surgery score) to stratify T2DM patients with regard to their metabolic status and to predict the outcome of metabolic surgery (Table 2). Patients with a higher diabetic surgery score have a higher T2DM remission rate (from 33% for score 0 to 100% for score 10); a 1-point increase in the score translates to an absolute 6.7% increase in the success rate. However, in patients with low scores (1-3), low remission rates (0%-33%) are expected. Therefore, this score might be used as a tool to achieve realistic expectations of the surgical outcome and might be used in future research to stratify the metabolic profile of patients according to the probability of diabetes remission instead of according to BMI alone. However, this grading system was derived from an Asian population, which is prone to diabetes at relatively younger ages with lower BMIs; therefore, the system may not be applicable to Western populations.
Table 2.
Diabetes surgery score system
| Variable | Score | |||
| 0 | 1 | 2 | 3 | |
| Age (yr) | ≥ 40 | < 40 | ||
| BMI (kg/m2) | < 30 | 30-39 | 40-49 | ≥ 50 |
| C-peptide (ng/mL) | < 2 | 2-3.9 | 4-6 | > 6 |
| Duration of T2DM (yr) | > 10 | 5-10 | 2-4.9 | < 2 |
Total possible score ranges from 0 to 10. BMI: Body mass index; T2DM: Type 2 diabetes mellitus (data adapted from Lee et al[98]).
NEW PERSPECTIVES
Several investigations of metabolic procedures were introduced in recent years. As newer metabolic procedures, the efficacy, durability and safety of these procedures have yet to be established.
Duodenal-jejunal bypass
The duodenal-jejunal bypass stomach-sparing operation was introduced as a procedure that could induce the remission of diabetes without weight loss in lean diabetic patients. The operation is based on the work of Rubino and colleagues in Goto-Kakizaki genetically diabetic lean rats[30]. Early human trials are encouraging[70,99]. Moreover, a novel procedure, DJB-SG, designed as a variant of the mini-duodenal switch by a combination of duodenal-jejunal bypass and sleeve gastrectomy, has shown good outcomes in the treatment of T2DM[80,81].
The EndoBarrier is a fluoropolymer sleeve that is reversibly fixed to the duodenal bulb and extends 80 cm into the small bowel, usually terminating in the proximal jejunum[31]. This endoscopically inserted device facilitates weight loss through malabsorption by preventing the mixture of pancreatic juice and chyme. It also results in improved glycemic control by activating the hormonal triggers based on the foregut bypass theory. To date, the favorable outcomes have been based on short-term studies alone[31]. The EndoBarrier has been reported to be implanted for 52 wk in patients with T2DM, with statistically significant reductions in fasting blood glucose, fasting glucose and HbA1c by the end of the 52 wk in 16 out of 22 patients[100]. Currently, its role in the pre-operative control of glycemia and weight loss, compared with a very low calorie diet, in morbidly obese patients still needs to be defined. Although it is not a permanent procedure, it is non-invasive and could be used to explore, at the molecular level, the role of duodenal-jejunal dysfunction in the pathogenesis of T2DM.
Ileal interposition
Ileal interposition with the aim of early exposure of the hindgut to nutrients in order to increase the secretion of GLP-1, is still in the early stages of human trials[40,101]. It was first described by DePaula as an operation that is specifically performed for the treatment of T2DM in non-morbidly obese patients[102]. It functions as a neuroendocrine brake to increase the early phase of insulin secretion after meals, which is characteristically absent in T2DM[103].
Metabolic surgery as a new clinical pathway
As most metabolic surgery is performed as part of a conventional bariatric program, Rubino et al[104] suggested that the separation of the clinical pathway of metabolic surgery from conventional bariatric surgery might have far-reaching clinical implications in terms of patient expectations, specific outcome measures, the involvement of appropriate multidisciplinary teams and the level of care. Moreover, the demographics of the referred patients were different from those of the conventional bariatric program, as they were much older males with a higher prevalence of severe diabetes, MetS and cardiovascular disease[105]. These demographics might place these patients at higher risk compared with conventional bariatric surgery patients, which would necessitate more intense and detailed peri-operative care. Moreover, with the name change to diabetic/metabolic surgery, public perceptions may change because obesity is currently perceived as a life-style problem that requires behavioral therapy, but MetS and diabetes are perceived as chronic and deadly diseases that need to be treated effectively. This change in perception might encourage patients to seek surgery and might improve access to gastrointestinal metabolic surgery. Finally, an integrated and multidisciplinary approach to metabolic surgery, especially one that involves endocrinologists and surgeons, is necessary for addressing the issues and challenges in this evolving field.
CONCLUSION
Metabolic surgery is the most effective therapy for glycemic control in poorly controlled T2DM. Further prospective longer-term studies are needed, especially in mildly obese T2DM patients (BMI < 35 kg/m2). For successful outcomes after surgery, the selection of patients with higher BMIs and better β-cell function will result in higher remission rates. We proposed a staging system to elucidate the metabolic profile of patients that might help more effectively select patients for surgery and result in better outcomes. Further research is required to establish new guidelines and indications for metabolic surgery in less obese or lean patients.
In conclusion, T2DM can be considered a gastrointestinal disease that can be surgically treated. Interventional diabetology may be a new modality for diabetes care in the future. Novel surgical procedures may help elucidate the underlying pathophysiology and mechanism of T2DM, thus producing a better future for individuals with this deadly disease.
Footnotes
P- Reviewer: Gómez-Sáez J, Maleki AR, Romeo C, Sumi S S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 4.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 5.U.K. prospective diabetes study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. U.K. Prospective Diabetes Study Group. Diabetes. 1995;44:1249–1258. [PubMed] [Google Scholar]
- 6.Liebl A, Mata M, Eschwège E. Evaluation of risk factors for development of complications in Type II diabetes in Europe. Diabetologia. 2002;45:S23–S28. doi: 10.1007/s00125-002-0863-0. [DOI] [PubMed] [Google Scholar]
- 7.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care. 2006;29:531–537. doi: 10.2337/diacare.29.03.06.dc05-1254. [DOI] [PubMed] [Google Scholar]
- 8.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 10.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 11.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bays HE, González-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther. 2008;6:343–368. doi: 10.1586/14779072.6.3.343. [DOI] [PubMed] [Google Scholar]
- 13.Bays HE, Laferrère B, Dixon J, Aronne L, González-Campoy JM, Apovian C, Wolfe BM. Adiposopathy and bariatric surgery: is ‘sick fat’ a surgical disease? Int J Clin Pract. 2009;63:1285–1300. doi: 10.1111/j.1742-1241.2009.02151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 15.Pontiroli AE, Frigè F, Paganelli M, Folli F. In morbid obesity, metabolic abnormalities and adhesion molecules correlate with visceral fat, not with subcutaneous fat: effect of weight loss through surgery. Obes Surg. 2009;19:745–750. doi: 10.1007/s11695-008-9626-4. [DOI] [PubMed] [Google Scholar]
- 16.Rubino F, Schauer PR, Kaplan LM, Cummings DE. Metabolic surgery to treat type 2 diabetes: clinical outcomes and mechanisms of action. Annu Rev Med. 2010;61:393–411. doi: 10.1146/annurev.med.051308.105148. [DOI] [PubMed] [Google Scholar]
- 17.Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE, Marks-Shulman PA, Abumrad NN. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee WJ, Chong K, Chen CY, Chen SC, Lee YC, Ser KH, Chuang LM. Diabetes remission and insulin secretion after gastric bypass in patients with body mass index & lt; 35 kg/m2. Obes Surg. 2011;21:889–895. doi: 10.1007/s11695-011-0401-6. [DOI] [PubMed] [Google Scholar]
- 19.Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- 20.Kirk E, Reeds DN, Finck BN, Mayurranjan SM, Patterson BW, Klein S. Dietary fat and carbohydrates differentially alter insulin sensitivity during caloric restriction. Gastroenterology. 2009;136:1552–1560. doi: 10.1053/j.gastro.2009.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao RS, Yanagisawa R, Kini S. Insulin resistance and bariatric surgery. Obes Rev. 2012;13:316–328. doi: 10.1111/j.1467-789X.2011.00955.x. [DOI] [PubMed] [Google Scholar]
- 22.Campos GM, Rabl C, Peeva S, Ciovica R, Rao M, Schwarz JM, Havel P, Schambelan M, Mulligan K. Improvement in peripheral glucose uptake after gastric bypass surgery is observed only after substantial weight loss has occurred and correlates with the magnitude of weight lost. J Gastrointest Surg. 2010;14:15–23. doi: 10.1007/s11605-009-1060-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muscelli E, Mingrone G, Camastra S, Manco M, Pereira JA, Pareja JC, Ferrannini E. Differential effect of weight loss on insulin resistance in surgically treated obese patients. Am J Med. 2005;118:51–57. doi: 10.1016/j.amjmed.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Camastra S, Gastaldelli A, Mari A, Bonuccelli S, Scartabelli G, Frascerra S, Baldi S, Nannipieri M, Rebelos E, Anselmino M, et al. Early and longer term effects of gastric bypass surgery on tissue-specific insulin sensitivity and beta cell function in morbidly obese patients with and without type 2 diabetes. Diabetologia. 2011;54:2093–2102. doi: 10.1007/s00125-011-2193-6. [DOI] [PubMed] [Google Scholar]
- 25.Mari A, Manco M, Guidone C, Nanni G, Castagneto M, Mingrone G, Ferrannini E. Restoration of normal glucose tolerance in severely obese patients after bilio-pancreatic diversion: role of insulin sensitivity and beta cell function. Diabetologia. 2006;49:2136–2143. doi: 10.1007/s00125-006-0337-x. [DOI] [PubMed] [Google Scholar]
- 26.Ferrannini E, Mingrone G. Impact of different bariatric surgical procedures on insulin action and beta-cell function in type 2 diabetes. Diabetes Care. 2009;32:514–520. doi: 10.2337/dc08-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guidone C, Manco M, Valera-Mora E, Iaconelli A, Gniuli D, Mari A, Nanni G, Castagneto M, Calvani M, Mingrone G. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- 28.Rosa G, Mingrone G, Manco M, Euthine V, Gniuli D, Calvani R, Calvani M, Favuzzi AM, Castagneto M, Vidal H. Molecular mechanisms of diabetes reversibility after bariatric surgery. Int J Obes (Lond) 2007;31:1429–1436. doi: 10.1038/sj.ijo.0803630. [DOI] [PubMed] [Google Scholar]
- 29.Pournaras DJ, Osborne A, Hawkins SC, Vincent RP, Mahon D, Ewings P, Ghatei MA, Bloom SR, Welbourn R, le Roux CW. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- 30.Rubino F, Forgione A, Cummings DE, Vix M, Gnuli D, Mingrone G, Castagneto M, Marescaux J. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel SR, Hakim D, Mason J, Hakim N. The duodenal-jejunal bypass sleeve (EndoBarrier Gastrointestinal Liner) for weight loss and treatment of type 2 diabetes. Surg Obes Relat Dis. 2013;9:482–484. doi: 10.1016/j.soard.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XJ, Xiao Z, Yu HL, Zhang XX, Cheng Z, Tian HM. Short-term glucose metabolism and gut hormone modulations after Billroth II gastrojejunostomy in nonobese gastric cancer patients with type 2 diabetes mellitus, impaired glucose tolerance and normal glucose tolerance. Arch Med Res. 2013;44:437–443. doi: 10.1016/j.arcmed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143–148. doi: 10.1001/archsurg.2010.326. [DOI] [PubMed] [Google Scholar]
- 34.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. doi: 10.1056/NEJMoa1200225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, Karkavitsas N. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258:976–982. doi: 10.1097/SLA.0b013e3182774522. [DOI] [PubMed] [Google Scholar]
- 36.Orskov C, Wettergren A, Holst JJ. Secretion of the incretin hormones glucagon-like peptide-1 and gastric inhibitory polypeptide correlates with insulin secretion in normal man throughout the day. Scand J Gastroenterol. 1996;31:665–670. doi: 10.3109/00365529609009147. [DOI] [PubMed] [Google Scholar]
- 37.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- 38.Drucker DJ. Glucagon-like peptide-1 and the islet beta-cell: augmentation of cell proliferation and inhibition of apoptosis. Endocrinology. 2003;144:5145–5148. doi: 10.1210/en.2003-1147. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz S, DeFronzo RA. Is incretin-based therapy ready for the care of hospitalized patients with type 2 diabetes?: The time has come for GLP-1 receptor agonists! Diabetes Care. 2013;36:2107–2111. doi: 10.2337/dc12-2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinoco A, El-Kadre L, Aquiar L, Tinoco R, Savassi-Rocha P. Short-term and mid-term control of type 2 diabetes mellitus by laparoscopic sleeve gastrectomy with ileal interposition. World J Surg. 2011;35:2238–2244. doi: 10.1007/s00268-011-1188-2. [DOI] [PubMed] [Google Scholar]
- 41.Geloneze B, Tambascia MA, Pilla VF, Geloneze SR, Repetto EM, Pareja JC. Ghrelin: a gut-brain hormone: effect of gastric bypass surgery. Obes Surg. 2003;13:17–22. doi: 10.1381/096089203321136539. [DOI] [PubMed] [Google Scholar]
- 42.Cummings DE, Shannon MH. Roles for ghrelin in the regulation of appetite and body weight. Arch Surg. 2003;138:389–396. doi: 10.1001/archsurg.138.4.389. [DOI] [PubMed] [Google Scholar]
- 43.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 44.Tymitz K, Engel A, McDonough S, Hendy MP, Kerlakian G. Changes in ghrelin levels following bariatric surgery: review of the literature. Obes Surg. 2011;21:125–130. doi: 10.1007/s11695-010-0311-z. [DOI] [PubMed] [Google Scholar]
- 45.Patrikakos P, Toutouzas KG, Gazouli M, Perrea D, Menenakos E, Papadopoulos S, Zografos G. Long-term plasma ghrelin and leptin modulation after sleeve gastrectomy in Wistar rats in comparison with gastric tissue ghrelin expression. Obes Surg. 2011;21:1432–1437. doi: 10.1007/s11695-011-0426-x. [DOI] [PubMed] [Google Scholar]
- 46.Neuschwander-Tetri BA. Farnesoid x receptor agonists: what they are and how they might be used in treating liver disease. Curr Gastroenterol Rep. 2012;14:55–62. doi: 10.1007/s11894-011-0232-6. [DOI] [PubMed] [Google Scholar]
- 47.Teodoro JS, Rolo AP, Palmeira CM. Hepatic FXR: key regulator of whole-body energy metabolism. Trends Endocrinol Metab. 2011;22:458–466. doi: 10.1016/j.tem.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Stepanov V, Stankov K, Mikov M. The bile acid membrane receptor TGR5: a novel pharmacological target in metabolic, inflammatory and neoplastic disorders. J Recept Signal Transduct Res. 2013;33:213–223. doi: 10.3109/10799893.2013.802805. [DOI] [PubMed] [Google Scholar]
- 49.Parker HE, Wallis K, le Roux CW, Wong KY, Reimann F, Gribble FM. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br J Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi CP, Loke SC. Colesevelam for Type 2 diabetes mellitus: an abridged Cochrane review. Diabet Med. 2014;31:2–14. doi: 10.1111/dme.12295. [DOI] [PubMed] [Google Scholar]
- 51.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kootte RS, Vrieze A, Holleman F, Dallinga-Thie GM, Zoetendal EG, de Vos WM, Groen AK, Hoekstra JB, Stroes ES, Nieuwdorp M. The therapeutic potential of manipulating gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:112–120. doi: 10.1111/j.1463-1326.2011.01483.x. [DOI] [PubMed] [Google Scholar]
- 53.Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa F. Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-induced obesity mice. J Appl Microbiol. 2011;110:650–657. doi: 10.1111/j.1365-2672.2010.04922.x. [DOI] [PubMed] [Google Scholar]
- 54.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–714. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 55.Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, Wedel H, Ahlin S, Anveden Å, Bengtsson C, Bergmark G, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 56.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 57.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 58.Ikramuddin S, Korner J, Lee WJ, Connett JE, Inabnet WB, Billington CJ, Thomas AJ, Leslie DB, Chong K, Jeffery RW, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. doi: 10.1001/jama.2013.5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shah SS, Todkar JS, Shah PS, Cummings DE. Diabetes remission and reduced cardiovascular risk after gastric bypass in Asian Indians with body mass index & lt; 35 kg/m(2) Surg Obes Relat Dis. 2010;6:332–338. doi: 10.1016/j.soard.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang CK, Shabbir A, Lo CH, Tai CM, Chen YS, Houng JY. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25-35. Obes Surg. 2011;21:1344–1349. doi: 10.1007/s11695-011-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16:45–51; discussion 51-52. doi: 10.1007/s11605-011-1740-2. [DOI] [PubMed] [Google Scholar]
- 62.Scopinaro N, Adami GF, Papadia FS, Camerini G, Carlini F, Fried M, Briatore L, D’Alessandro G, Andraghetti G, Cordera R. Effects of biliopanceratic diversion on type 2 diabetes in patients with BMI 25 to 35. Ann Surg. 2011;253:699–703. doi: 10.1097/SLA.0b013e318203ae44. [DOI] [PubMed] [Google Scholar]
- 63.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, Li Z, Shekelle PG. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309:2250–2261. doi: 10.1001/jama.2013.4851. [DOI] [PubMed] [Google Scholar]
- 64.Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care. 2012;35:1420–1428. doi: 10.2337/dc11-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kadera BE, Lum K, Grant J, Pryor AD, Portenier DD, DeMaria EJ. Remission of type 2 diabetes after Roux-en-Y gastric bypass is associated with greater weight loss. Surg Obes Relat Dis. 2009;5:305–309. doi: 10.1016/j.soard.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Dixon JB, Hur KY, Lee WJ, Kim MJ, Chong K, Chen SC, Straznicky NE, Zimmet P. Gastric bypass in Type 2 diabetes with BMI & lt; 30: weight and weight loss have a major influence on outcomes. Diabet Med. 2013;30:e127–e134. doi: 10.1111/dme.12107. [DOI] [PubMed] [Google Scholar]
- 67.Rubino F, Kaplan LM, Schauer PR, Cummings DE. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 68.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- 70.Cohen R, Caravatto PP, Correa JL, Noujaim P, Petry TZ, Salles JE, Schiavon CA. Glycemic control after stomach-sparing duodenal-jejunal bypass surgery in diabetic patients with low body mass index. Surg Obes Relat Dis. 2012;8:375–380. doi: 10.1016/j.soard.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 71.Geloneze B, Geloneze SR, Chaim E, Hirsch FF, Felici AC, Lambert G, Tambascia MA, Pareja JC. Metabolic surgery for non-obese type 2 diabetes: incretins, adipocytokines, and insulin secretion/resistance changes in a 1-year interventional clinical controlled study. Ann Surg. 2012;256:72–78. doi: 10.1097/SLA.0b013e3182592c62. [DOI] [PubMed] [Google Scholar]
- 72.Lee HC, Kim MK, Kwon HS, Kim E, Song KH. Early changes in incretin secretion after laparoscopic duodenal-jejunal bypass surgery in type 2 diabetic patients. Obes Surg. 2010;20:1530–1535. doi: 10.1007/s11695-010-0248-2. [DOI] [PubMed] [Google Scholar]
- 73.Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664–669. doi: 10.1016/j.surg.2009.10.059. [DOI] [PubMed] [Google Scholar]
- 74.Pories WJ, Dohm LG, Mansfield CJ. Beyond the BMI: the search for better guidelines for bariatric surgery. Obesity (Silver Spring) 2010;18:865–871. doi: 10.1038/oby.2010.8. [DOI] [PubMed] [Google Scholar]
- 75.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009;106:15430–15435. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, Pi-Sunyer FX, Heshka S. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89:807–814. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sjöholm K, Anveden A, Peltonen M, Jacobson P, Romeo S, Svensson PA, Sjöström L, Carlsson LM. Evaluation of current eligibility criteria for bariatric surgery: diabetes prevention and risk factor changes in the Swedish obese subjects (SOS) study. Diabetes Care. 2013;36:1335–1340. doi: 10.2337/dc12-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee WJ, Chong K, Ser KH, Chen JC, Lee YC, Chen SC, Su YH, Tsai MH. C-peptide predicts the remission of type 2 diabetes after bariatric surgery. Obes Surg. 2012;22:293–298. doi: 10.1007/s11695-011-0565-0. [DOI] [PubMed] [Google Scholar]
- 79.Thaler JP, Cummings DE. Minireview: Hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–2525. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 80.Kasama K, Tagaya N, Kanehira E, Oshiro T, Seki Y, Kinouchi M, Umezawa A, Negishi Y, Kurokawa Y. Laparoscopic sleeve gastrectomy with duodenojejunal bypass: technique and preliminary results. Obes Surg. 2009;19:1341–1345. doi: 10.1007/s11695-009-9873-z. [DOI] [PubMed] [Google Scholar]
- 81.Lee WJ, Lee KT, Kasama K, Seiki Y, Ser KH, Chun SC, Chen JC, Lee YC. Laparoscopic single-anastomosis duodenal-jejunal bypass with sleeve gastrectomy (SADJB-SG): short-term result and comparison with gastric bypass. Obes Surg. 2014;24:109–113. doi: 10.1007/s11695-013-1067-z. [DOI] [PubMed] [Google Scholar]
- 82.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 83.Dixon JB, Chuang LM, Chong K, Chen SC, Lambert GW, Straznicky NE, Lambert EA, Lee WJ. Predicting the glycemic response to gastric bypass surgery in patients with type 2 diabetes. Diabetes Care. 2013;36:20–26. doi: 10.2337/dc12-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stern MP, Williams K, González-Villalpando C, Hunt KJ, Haffner SM. Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care. 2004;27:2676–2681. doi: 10.2337/diacare.27.11.2676. [DOI] [PubMed] [Google Scholar]
- 85.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 86.Buchwald H, Estok R, Fahrbach K, Banel D, Sledge I. Trends in mortality in bariatric surgery: a systematic review and meta-analysis. Surgery. 2007;142:621–632; discussion 632-635. doi: 10.1016/j.surg.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 87.Inabnet WB, Winegar DA, Sherif B, Sarr MG. Early outcomes of bariatric surgery in patients with metabolic syndrome: an analysis of the bariatric outcomes longitudinal database. J Am Coll Surg. 2012;214:550–556; discussion 556-557. doi: 10.1016/j.jamcollsurg.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 88.DiGiorgi M, Rosen DJ, Choi JJ, Milone L, Schrope B, Olivero-Rivera L, Restuccia N, Yuen S, Fisk M, Inabnet WB, et al. Re-emergence of diabetes after gastric bypass in patients with mid- to long-term follow-up. Surg Obes Relat Dis. 2010;6:249–253. doi: 10.1016/j.soard.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 89.Chikunguwo SM, Wolfe LG, Dodson P, Meador JG, Baugh N, Clore JN, Kellum JM, Maher JW. Analysis of factors associated with durable remission of diabetes after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2010;6:254–259. doi: 10.1016/j.soard.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 90.Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 92.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 93.Chalmers J, Cooper ME. UKPDS and the legacy effect. N Engl J Med. 2008;359:1618–1620. doi: 10.1056/NEJMe0807625. [DOI] [PubMed] [Google Scholar]
- 94.Pournaras DJ, Aasheim ET, Søvik TT, Andrews R, Mahon D, Welbourn R, Olbers T, le Roux CW. Effect of the definition of type II diabetes remission in the evaluation of bariatric surgery for metabolic disorders. Br J Surg. 2012;99:100–103. doi: 10.1002/bjs.7704. [DOI] [PubMed] [Google Scholar]
- 95.Schauer PR, Burguera B, Ikramuddin S, Cottam D, Gourash W, Hamad G, Eid GM, Mattar S, Ramanathan R, Barinas-Mitchel E, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484; discussion 84-85. doi: 10.1097/01.sla.0000089851.41115.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall TC, Pellen MG, Sedman PC, Jain PK. Preoperative factors predicting remission of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery for obesity. Obes Surg. 2010;20:1245–1250. doi: 10.1007/s11695-010-0198-8. [DOI] [PubMed] [Google Scholar]
- 97.Chen SB, Lee YC, Ser KH, Chen JC, Chen SC, Hsieh HF, Lee WJ. Serum C-reactive protein and white blood cell count in morbidly obese surgical patients. Obes Surg. 2009;19:461–466. doi: 10.1007/s11695-008-9619-3. [DOI] [PubMed] [Google Scholar]
- 98.Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC, Lee YC, Ser KH. Predicting success of metabolic surgery: age, body mass index, C-peptide, and duration score. Surg Obes Relat Dis. 2013;9:379–384. doi: 10.1016/j.soard.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 99.Geloneze B, Geloneze SR, Fiori C, Stabe C, Tambascia MA, Chaim EA, Astiarraga BD, Pareja JC. Surgery for nonobese type 2 diabetic patients: an interventional study with duodenal-jejunal exclusion. Obes Surg. 2009;19:1077–1083. doi: 10.1007/s11695-009-9844-4. [DOI] [PubMed] [Google Scholar]
- 100.de Moura EG, Martins BC, Lopes GS, Orso IR, de Oliveira SL, Galvão Neto MP, Santo MA, Sakai P, Ramos AC, Garrido Júnior AB, et al. Metabolic improvements in obese type 2 diabetes subjects implanted for 1 year with an endoscopically deployed duodenal-jejunal bypass liner. Diabetes Technol Ther. 2012;14:183–189. doi: 10.1089/dia.2011.0152. [DOI] [PubMed] [Google Scholar]
- 101.DePaula AL, Stival AR, DePaula CC, Halpern A, Vencio S. Surgical treatment of type 2 diabetes in patients with BMI below 35: mid-term outcomes of the laparoscopic ileal interposition associated with a sleeve gastrectomy in 202 consecutive cases. J Gastrointest Surg. 2012;16:967–976. doi: 10.1007/s11605-011-1807-0. [DOI] [PubMed] [Google Scholar]
- 102.DePaula AL, Macedo AL, Rassi N, Machado CA, Schraibman V, Silva LQ, Halpern A. Laparoscopic treatment of type 2 diabetes mellitus for patients with a body mass index less than 35. Surg Endosc. 2008;22:706–716. doi: 10.1007/s00464-007-9472-9. [DOI] [PubMed] [Google Scholar]
- 103.Kahn SE. Beta cell failure: causes and consequences. Int J Clin Pract Suppl. 2001;(123):13–18. [PubMed] [Google Scholar]
- 104.Rubino F, Shukla A, Pomp A, Moreira M, Ahn SM, Dakin G. Bariatric, metabolic, and diabetes surgery: what’s in a name? Ann Surg. 2014;259:117–122. doi: 10.1097/SLA.0b013e3182759656. [DOI] [PubMed] [Google Scholar]
- 105.Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]


