Abstract
Laparoscopic rectal surgery continues to be a challenging operation associated to a steep learning curve. Robotic surgical systems have dramatically changed minimally invasive surgery. Three-dimensional, magnified and stable view, articulated instruments, and reduction of physiologic tremors leading to superior dexterity and ergonomics. Therefore, robotic platforms could potentially address limitations of laparoscopic rectal surgery. It was aimed at reviewing current literature on short-term clinical and oncological (pathological) outcomes after robotic rectal cancer surgery in comparison with laparoscopic surgery. A systematic review was performed for the period 2002 to 2014. A total of 1776 patients with rectal cancer underwent minimally invasive robotic treatment in 32 studies. After robotic and laparoscopic approach to oncologic rectal surgery, respectively, mean operating time varied from 192-385 min, and from 158-297 min; mean estimated blood loss was between 33 and 283 mL, and between 127 and 300 mL; mean length of stay varied from 4-10 d; and from 6-15 d. Conversion after robotic rectal surgery varied from 0% to 9.4%, and from 0 to 22% after laparoscopy. There was no difference between robotic (0%-41.3%) and laparoscopic (5.5%-29.3%) surgery regarding morbidity and anastomotic complications (respectively, 0%-13.5%, and 0%-11.1%). Regarding immediate oncologic outcomes, respectively among robotic and laparoscopic cases, positive circumferential margins varied from 0% to 7.5%, and from 0% to 8.8%; the mean number of retrieved lymph nodes was between 10 and 20, and between 11 and 21; and the mean distal resection margin was from 0.8 to 4.7 cm, and from 1.9 to 4.5 cm. Robotic rectal cancer surgery is being undertaken by experienced surgeons. However, the quality of the assembled evidence does not support definite conclusions about most studies variables. Robotic rectal cancer surgery is associated to increased costs and operating time. It also seems to be associated to reduced conversion rates. Other short-term outcomes are comparable to conventional laparoscopy techniques, if not better. Ultimately, pathological data evaluation suggests that oncologic safety may be preserved after robotic total mesorectal excision. However, further studies are required to evaluate oncologic safety and functional results.
Keywords: Surgical procedures, Minimally invasive, Rectal neoplasms, Robotics, Colorectal surgery
Core tip: Laparoscopic oncologic rectal surgery remains a challenging procedure. Robotic systems aim at overcoming the limits of conventional laparoscopic techniques. The evidence on robotic and robotic-assisted rectal cancer surgery is rapidly increasing. Currently, published studies have demonstrated exciting evidence regarding similar or improved short-term outcomes after robotic rectal surgery when compared to laparoscopic conventional techniques. Moreover, robotic surgery seems to be oncologic safe. Further studies are required to evaluate the long-term oncologic and functional results of robotic over laparoscopic surgery for rectal cancer treatment.
INTRODUCTION
Laparoscopic colorectal surgery was first described in 1991[1]. In the past two decades, it has progressively expanded. Since it was proven to be as safe and effective as open surgery[2], it was recognized as a reliable alternative to conventional surgery. It has become the standard of care for benign and malignant colonic diseases mainly due to the fact that laparoscopic colectomy is consistently associated to early postoperative outcomes, such as less postoperative pain, reduced postoperative morbidity, shorter length of stay, and earlier return to normal activities[3,4]. Moreover, it was also demonstrated that laparoscopic colectomy has oncological results comparable to open surgery[5-8].
However, the adoption of laparoscopic colectomy remains disappointing in most countries for several reasons, but mainly because it represents a challenging procedure associated to a steep learning curve[9-11]. Moreover, although laparoscopic access has been widely accepted for colonic surgery, there are several limitations associated to the laparoscopic approach to colorectal diseases. The fulcrum effect results in reduced motion range, especially inside the pelvis. A poorly trained camera operator may lead to an unstable bidimensional view leading to a reduction in the dissection accuracy required to properly approach Waldeyer’s and Dennonvillier’s fascias.
Total mesorectal excision (TME) has long been established as the standard surgical technique[12], laparoscopic TME remains a technically demanding procedure. The reported high conversion rates and involvement of circumferential resection margins[13] are thought to reflect the high level of difficulty associated to laparoscopic TME.
The Da Vinci robotic surgical system (Intuitive Surgical Inc., Sunnyvale, California, United States) has dramatically changed minimally invasive surgery. A robotic-assisted approach could potentially overcome some of the limitations of conventional laparoscopic rectal surgery. Robotic system enables the surgeon to control a three-dimensional, high-definition, 10-fold magnification vision steady camera. It provides wristed motion for endoscopic instruments (7 o of freedom, 180o articulation, and 540o rotation). Motion scaling results in reduced physiological tremors, superior dexterity, and far greater ergonomic comfort[14]. Therefore, robotic systems are particularly designed for operations conducted within a small anatomical field in which high precision is demanded, such as cardiac surgery, prostate surgery and rectal surgery. Although robotic-assisted operations have been utilized for years in other surgical specialties, it was not until 2002 that Weber et al[15] reported the first two cases of robotic-assisted colectomies.
Case series, comparative, and multicenter studies have demonstrated that robotic rectal surgery is feasible, effective and safe for minimally invasive TME. However, evidence of its clinical superiority regarding short-term outcomes over conventional rectal surgery conducted by expert surgeons is still lacking. Moreover, long-term oncological safety remains to be demonstrated. Ultimately, robotic surgery is expensive, which results in a major impediment to greater spread of its use. Therefore, currently, there are two multicenter randomized controlled trials comparing robotic versus laparoscopic surgery for rectal cancer: the ROLARR and the ACOSOG-Z6051. However, at this moment, both trials are recruiting. Although the abovementioned limitations potentially associated to robotic rectal surgery, two recent systematic reviews followed by meta-analysis have concluded that robotic-assisted surgery decreases conversion rate when compared to a conventional laparoscopic approach for rectal cancer surgery and is also associated to reduced blood loss[16,17].
In the present study, it was aimed at reviewing the rapidly expanding current available literature on short-term clinical and immediate oncological (pathological) outcomes after robotic rectal cancer surgery in comparison with standard laparoscopic rectal surgery or conventional resections, and to provide a perspective on the use of robotics for the curative surgical treatment of rectal cancer.
LITERATURE REVIEW
A systematic review of the electronic literature examining robotics for rectal cancer surgery was performed. Two reviewers (Araujo SEA and Seid VE) conducted a search of electronic databases (PubMed, Google Scholar and Embase) for the period 2002 to 2014. The search strategy included the terms “robot”, “robotic”, “Da Vinci”, “rectum”, “rectal surgery”, “proctectomy”, “anterior resection”, and “abdominoperineal excision”. No other search restrictions were applied. Then, an additional manual search was conducted in the reference list of all relevant selected publications to prevent article loss by the search strategy. The last search was performed on March 2014.
Case series, comparative studies, and randomized controlled trials were all selected. The definition of oncologic rectal surgery included: anterior resection, low anterior resection, TME, coloanal anastomosis, intersphincteric, and abdominoperineal resections. The exclusion criteria were: review articles or letters, studies on robotic surgery for colon cancer or benign colonic disease, animal experiments, case reports, studies using only robotic camera holders (AESOP 3000; Computer Motion, Santa Barbara, California, United States) or the not commercially available Zeus Surgical System (Computer Motion, Santa Barbara, California, United States), studies with inappropriate data or not written in English language.
The following parameters were extracted to a specific protocol: name of first author, year of publication, country, study design, surgical technique, number of patients, neoadjuvant treatment, type of TME, operating time, estimated blood loss, conversion rate, overall morbidity rate, anastomotic complications, positivity of circumferential resection margins (CRMs), extent of distal resection margins (DRMs), and mean number of lymph nodes harvested.
QUALITY OF THE ASSEMBLED EVIDENCE
Published data on robotic oncologic rectal surgery comprise case series, nonrandomized retrospective and prospective comparative studies, and one randomized trial[18]. In these cases, bias associated to the evidence come from the unknown criteria used to recruit patients, and also from the quality of data collection. Although it is highly expected that experienced colorectal surgeons undertook all procedures, the expertise of the minimally invasive surgical team with laparoscopy and robotic approaches is seldom reported on the evaluated studies.
PATIENTS AND OPERATIONS
The electronic search followed by manual review identified 68 abstracts (Figure 1). After excluding 21 duplicates, 49 papers were reviewed. Seventeen articles were excluded. Eleven papers were on robotic surgery for colon cancer, and six papers comprised patients operated on for benign colonic diseases. Thirty-two studies[18-49] were suitable for inclusion in the systematic review. Seventeen studies[19-21,24,28-40] represented case series, 14[22,23,25-27,41-49] were comparative studies, and there was only one randomized controlled trial[18] (Table 1).
Figure 1.
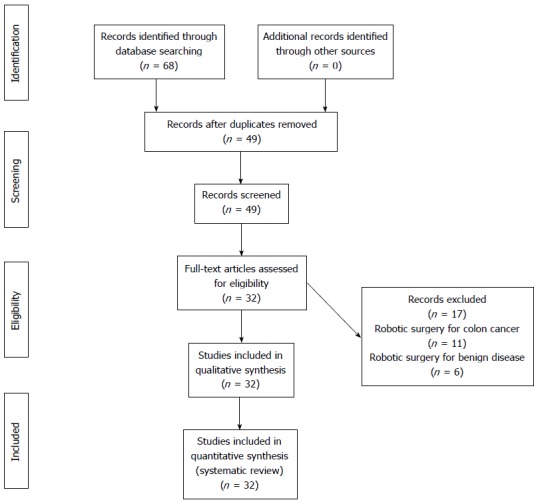
Preferred reporting items for systematic reviews and meta-analyses diagram.
Table 1.
Use of robotics in rectal cancer surgery (case series, comparative, and randomized studies)
| Ref. | Year | Country | Study design | Surgical technique | Number of patients | Neoadjuvant treatment |
TME operation |
||
| Anterior resection | Intersphincteric resection | Abdomino- perineal resection | |||||||
| Hellan et al[28] | 2007 | United States | Case series | Hybrid | 39 | 33 | 22 | 11 | 6 |
| Choi et al[29] | 2009 | Korea | Case series | Hybrid | 13 | NA | 13 | ||
| Choi et al[19] | 2009 | Korea | Case series | Totally robotic | 50 | NA | 40 | 8 | 2 |
| Ng et al[30] | 2009 | Singapore | Case series | Hybrid | 8 | NA | 8 | ||
| deSouza et al[31] | 2010 | United States | Case series | Hybrid | 44 | 31 | 30 | 6 | 8 |
| Pigazzi et al[32] | 2010 | United States, Italy, Korea | Case series | Hybrid | 143 | 93 | 80 | 32 | 31 |
| Baek et al[33] | 2010 | United States | Case series | Hybrid | 64 | 55 | 34 | 18 | 12 |
| Zimmern et al[34] | 2010 | United States | Case series | Hybrid | 58 | 23 | 47 | 11 | |
| Kang et al[20] | 2011 | Korea | Case series | Totally robotic | 269 | 72 | 265 | 4 | |
| Hybrid | 120 | 118 | 2 | ||||||
| Koh et al[35] | 2011 | Singapore | Case series | Hybrid | 19 | 2 | 18 | 1 | |
| Leong et al[21] | 2011 | Korea | Case series | Totally robotic | 29 | 11 | 29 | ||
| Marecik et al[36] | 2011 | United States | Case series | Hybrid | 5 | 4 | 5 | ||
| Alimoglu et al[24] | 2012 | Turkey | Case series | Totally robotic | 7 | 4 | 7 | ||
| Kang et al[37] | 2012 | United States | Case series | Hybrid | 6 | 5 | 6 | ||
| Karahasanoglu et al[38] | 2012 | Turkey | Case series | Hybrid | 30 | 7 | 27 | 3 | |
| Park et al[39] | 2012 | United States | Case series | Hybrid | 30 | 20 | 5 | 19 | 6 |
| Du et al[40] | 2013 | China | Case series | Hybrid | 22 | NA | 22 | ||
| Pigazzi et al[41] | 2006 | United States | Comparative | Hybrid | 6 | 2 | 6 | ||
| Baik et al[18] | 2008 | Korea | Randomized | Hybrid | 18 | 0 | 18 | ||
| Patriti et al[42] | 2009 | Italy | Comparative | Hybrid | 29 | 7 | 29 | ||
| Baik et al[43] | 2009 | Korea | Comparative | Hybrid | 56 | 5 | 56 | ||
| Bianchi et al[22] | 2010 | Italy | Comparative | Totally robotic | 25 | 13 | 18 | 7 | |
| Popescu et al[44] | 2010 | Romania | Comparative | Hybrid | 38 | NA | 30 | 0 | 8 |
| Kim et al[23] | 2010 | Korea | Comparative | Totally robotic | 100 | 14 | 100 | ||
| Park et al[45] | 2010 | Korea | Comparative | Hybrid | 41 | 14 | 29 | 12 | |
| Park et al[46] | 2011 | Korea | Comparative | Hybrid | 52 | 12 | 52 | ||
| Baek et al[47] | 2011 | United States | Comparative | Hybrid | 41 | 33 | 33 | 2 | 6 |
| Kwak et al[48] | 2011 | Korea | Comparative | Hybrid | 59 | 8 | 54 | 5 | |
| Kim et al[25] | 2012 | Korea | Comparative | Totally robotic | 100 | 34 | 55 | 45 | |
| Park et al[49] | 2013 | Korea | Comparative | Hybrid | 40 | 32 | 40 | ||
| Kang et al[26] | 2013 | Korea | Comparative | Totally robotic | 165 | 39 | 165 | ||
| D’Annibale et al[27] | 2013 | Italy | Comparative | Totally robotic | 50 | 34 | 50 | ||
TME: Total mesorectal excision; NA: Not available.
Regarding the distribution of papers according to the publication year (Figure 1), the number of publications on minimally invasive robot-assisted rectal surgery is increasing. In 2006 and 2007, the systematic review returned only one paper per year. Some authors rather not consider these experiences for evaluation, since they represent initial case series. In the years 2010, 2011, 2012, and 2013 it was observed, respectively, eight, seven, five, and three published papers. Although global numbers seem to be diminishing, an increase in the publication of studies comparing robotic-assisted and conventional laparoscopic approaches for rectal surgery may be observed, starting in 2010.
A total of 1776 patients with rectal cancer underwent minimally invasive robotic or robotic-assisted treatment in 32 studies. Of these, 956 patients were operated on a case series design study. Only 795 patients of eight studies[19-27] were operated on using a totally robotic approach. The mean number of patients operated on with robotic assistance for publication was 55.5 (5-379). Only 125 (7%) patients undergoing robotic TME underwent sphincter ablation operations. Among the 1651 patients who underwent sphincter preserving-operations, 227 (13.7%) were submitted to an intersphincteric dissection prior to coloanal anastomosis.
OPERATING TIME
For the total of 1776 patients with rectal cancer who underwent robotic or robotic-assisted surgical treatment in 32 studies, the mean operating time ranged from 192 to 385 min (Table 2). For the 887 patients operated on using a laparoscopic approach in the selected comparative studies, the mean operating time ranged from 158 to 297 min. In the two studies[25,26] using a cohort of patients undergoing TME through an open approach, the results of this particular group of patients were not considered in the present review.
Table 2.
Use of robotics in rectal cancer surgery - clinical outcomes
| Ref. | Type of procedure | Number of patients | Mean operating time (min) | Estimated blood loss (mL) | Length of stay (d) | Conversion rate (%) | Overall complication rate | Anastomotic complications |
| Hellan et al[28] | RTME | 39 | 285 (180-540) | 200 (25-6000) | 4 | 2.6 | 12.8% | 12.1% |
| Choi et al[29] | RTME | 13 | 260 (210-390) | NA | 7 | 0 | 23% | 7.7% |
| Choi et al[19] | RTME | 50 | 304 (190-405) | NA | 9.2 (5-24) | 0 | 18% | 8.3% |
| Ng et al[30] | RTME | 8 | 192 (145-2500 | NA | 5 (4-30) | 0 | 0% | 0% |
| deSouza et al[31] | RTME | 44 | 347 (155-510) | 250 (50-1000) | 5 (3-36) | 4.5 | 4.5% | 4.5% |
| Pigazzi et al[32] | RTME | 143 | 297 (90-660) | 283 (0-6000) | 8.3 (2-33) | 4.9 | 41.3% | 10.5% |
| Baek et al[33] | RTME | 64 | 270 (150-540) | 200 (20-6000) | 5 (2-33) | 9.4 | 35.9% | 7.7% |
| Zimmern et al[34] | RTME | 58 | 350 | 250 | 6 | 1.7 | 24.1% | 3.4% |
| Kang et al[20] | RTME | 389 | 305.4 ± 111.5/ 339.3 ± 127.41 | NA | 8.7 ± 3.0/ 17.6 ± 13.31 | 0/11 | 19% | 7% |
| Koh et al[35] | RTME | 19 | 316 (232-444) | NA | 6.4 (3-21) | 0 | 14.3% | 0% |
| Leong et al[21] | RTME | 29 | 325 (235-435) | 50 (50-1000) | 9 (5-15) | 0 | 10.3% | 31% |
| Marecik et al[36] | RTME | 5 | 343 (270-442) | 230 (100-400) | 5.8 (5-7) | 0 | 20% | 0%2 |
| Alimoglu et al[24] | RTME | 7 | NA | NA | 8.1 (5-10) | 0 | 28.6% | 0%2 |
| Kang et al[37] | RTME | 6 | 335 (267-452) | 250 (150-400) | 5 (4-7) | 0 | 50% | 0%2 |
| Karahasanoglu et al[38] | RTME | 22 | 270 (175-480) | 50 (20-100) | 4 (4-20) | 0 | 13.3% | 3.3% |
| RPME | 8 | |||||||
| Park et al[39] | RTME | 30 | 369 (306-410) | 100 (75-200) | 4 (3-6) | 0 | 36.6% | 4.2% |
| Du et al[40] | RTME | 22 | 220 (152-286) | 33 (10-70) | 7.8 (7-13) | 0 | 4.5% | 0% |
| Pigazzi et al[41] | RTME | 6 | 264 (192-318) | 104 | 4.5 (3-11) | 0 | 16.6% | 0% |
| LTME | 6 | 258 (198-312) | 150 | 3.6 (3-6) | 0 | 16.6% | 0% | |
| Baik et al[18] | RTME | 18 | 203 (149-315) | NA | 7 (5-10)4 | 0 | 22.2% | 0% |
| LTME | 18 | 196 (114-297) | NA | 9 (6-12)4 | 11.1 | 5.5% | 0% | |
| Patriti et al[42] | RTME | 29 | 202 ± 12 | 137 ± 156 | 11.9 (6-29) | 04 | 30.6% | 6.8% |
| LTME | 37 | 208 ± 7 | 127 ± 169 | 9.6 (5-37) | 18.94 | 18.9% | 2.7% | |
| Baik et al[43] | RTME | 56 | 178 (120-315) | NA | 5 (5-10)4 | 04 | 5.4%4 | 1.7% |
| LTME | 57 | 179 (100-360) | NA | 6 (4-16)4 | 10.54 | 19.3%4 | 7% | |
| Bianchi et al[22] | RTME | 25 | 240 (170-420) | NA | 6.5 (4-15) | 0 | 16% | 4% |
| LTME | 25 | 237 (170-545) | NA | 6 (4-20) | 4 | 24% | 8% | |
| Popescu et al[44] | RTME | 38 | 208 (180-300)4 | 1004 | 8.1 ± 4.5 | 5.2 | 15.7% | 5.2% |
| LTME | 84 | 182 (140-220)4 | 1504 | 8.4 ± 3.5 | 10.5 | 15.3% | 7.1% | |
| Kim et al[23] | RTME | 100 | 385.3 ± 102.64 | NA | 11.7 ± 6.7 | 2 | 20% | 8.2% |
| LTME | 100 | 297.3 ± 83.74 | NA | 14.4 ± 10 | 3 | 27% | 11.1% | |
| Park et al[45] | RTME | 41 | 231.9 ± 61.44 | NA | 9.9 | 0 | 19.3% | 9.7% |
| LTME | 82 | 168.6 ± 49.34 | NA | 9.4 | 0 | 29.3% | 7.3% | |
| Park et al[46] | RTME | 52 | 233 ± 52.4 | NA | 10 ± 19.2 | 0 | 10% | 9.6% |
| LTME | 123 | 158 ± 49.2 | NA | 15 ± 12.2 | 0 | 15% | 5.6% | |
| Baek et al[47] | RTME | 41 | 296 (150-520) | 200 (20-2000) | 6.5 (2-33) | 7.3 | 22% | 8.6% |
| LTME | 41 | 315 (174-584) | 300 (17-1000) | 6.6 (3-20) | 22 | 26.8% | 2.9% | |
| Kwak et al[48] | RTME | 59 | 270 (241-325)4 | NA | NA | 0 | 32.2% | 13.5% |
| LTME | 59 | 228 (177-254)4 | NA | NA | 3.4 | 27.1% | 10.1% | |
| Kim et al[25] | RTME | 100 | 188 ± 454 | NA | 7.1 ± 2.1 | 0 | 26% | 2% |
| OTME | 100 | 103 ± 234 | NA | 6.9 ± 1.5 | 03 | 27% | 4% | |
| Park et al[49] | RTME | 40 | 235.5 ± 57.54 | 45.7 ± 40 | 10.6 ± 4.2 | 0 | 15% | 7.5% |
| LTME | 40 | 185.4 ± 72.84 | 59.2 ± 35.8 | 11.3 ± 3.6 | 0 | 12.5% | 5% | |
| Kang et al[26] | RTME | 165 | 309.7 ± 115.24 | 133 ± 192.34 | 10.8 ± 5.54 | 0.6 | 20.6% | 7.3% |
| LTME | 165 | 277.8 ± 81.94 | 140.1 ± 216.44 | 13.5 ± 9.24 | 1.8 | 27.9% | 10.8% | |
| OTME | 165 | 252.6 ± 88.14 | 275.4 ± 368.84 | 16 ± 8.64 | 03 | 24.8% | 3.4% | |
| D’Annibale et al[27] | RTME | 50 | 270 (240-315) | NA | 8 (7-11) | 0 | 10% | 10% |
| LTME | 50 | 280 (240-350) | NA | 10 (8-14) | 12 | 22% | 14% |
Non-complicated/complicated cases;
Abdominoperineal resections only;
Conventional (open) approach;
The original publication as statistically significant (P < 0.05). RTME: Robotic total mesorectal excision; LTME: Laparoscopic total mesorectal excision; RPME: Robotic partial mesorectal excision; OTME: Open total mesorectal excision; NA: Not available.
Although the overall results of operating time in the study of Patriti et al[42] have not demonstrated significant differences after robotic and conventional laparoscopy, operative times after robotic surgery with TME with and without sphincter preservation were significantly higher. In the reports of Popescu et al[44], Kim et al[23], Park et al[45], Kwak et al[48], Kim et al[25], Park et al[49], and Kang et al[26], operating times were significantly longer after operations with robotic assistance.
ESTIMATED BLOOD LOSS
The estimated blood loss values were not available in 14 publications. For the remaining 17 studies in this systematic review, mean estimated blood loss after oncologic robotic rectal surgery varied from 33 to 283 mL. For the comparative studies where data after laparoscopic operations have also been available, mean estimated blood loss was between 127 and 300 mL (Table 2). Only in the reports of Popescu et al[44] (100 mL vs 150 mL) and Kang et al[26] (133 mL vs 140 mL), the estimated blood loss was significantly reduced after robotic rectal surgery when compared to the laparoscopic approach.
LENGTH OF STAY
The mean length of stay was not available for only one comparative study[48]. Among rectal cancer patients undergoing robotic surgery, the length of stay for 29 studies ranged from 4 to 10 d (Table 2). For the comparative studies, mean length of hospital stay after laparoscopic rectal surgery ranged from 6 to 15 d (Table 2). In three studies[18,23,46], one of them a small randomized trial[18], which enrolled a total of 84 patients, the mean length of stay was significantly reduced after robotic surgery when compared to laparoscopic access.
CONVERSION
Conversion rates were available for all 32 studies included in this systematic review. Among 1776 patients with rectal cancer undergoing robotic or robotic-assisted surgical treatment in this systematic review, the mean conversion rate varied between 0% and 9.4%. For the laparoscopic cases, mean conversion to open surgery was between 0% and 22% (Table 2).
In three comparative studies[27,42,43] included in this review, robotic surgery for rectal cancer was significantly associated to a lower conversion rate. This three studies included 279 patients. In the publication of Patriti et al [42], the majority of patients in the robotic group had previous abdominal surgery and low rectal cancer requiring a TME. In addition, more patients in the robotic group had undergone neoadjuvant chemoradiation when compared to the laparoscopic group. In the second study where conversion was reduced after a robotic approach[43], conversion was null in the robotic group. In the laparoscopic group, the rate was 10.5% (6 cases). The reasons for conversion in the laparoscopic group were hemorrhage from the lateral pelvic wall, failure to progress due to a severe narrow pelvis, and rectal perforation.
POSTOPERATIVE COMPLICATIONS (OVERALL)
For the 32 studies included in this systematic review, the overall morbidity after robotic or robotic-assisted rectal cancer surgery ranged from 0% to 41.3% (Table 2). The overall postoperative complication rate after laparoscopic treatment of rectal cancer on 14 comparative studies and 1 randomized controlled trial varied between 5.5% and 29.3% (Table 2).
In only one comparative study[43], where 56 and 57 patients underwent a robotic and laparoscopic low anterior resection respectively, postoperative morbidity was significantly reduced after robotic surgery (5.4% vs 19.3%).
ANASTOMOTIC COMPLICATIONS
Among the 32 studies included in this review, the occurrence of complications of colorectal or coloanal anastomosis was not available on three publications dealing exclusively with sphincter-ablative rectal operations[24,36,37] (Table 2).
After robotic or robotic-assisted sphincter preserving rectal surgery, the mean occurrence of anastomotic complications varied between 0% and 13.5%. After laparoscopic sphincter-preserving surgery, it varied between 0% and 11.1% (Table 2). In one recent study[26], matched robotic, laparoscopic and open rectal cancer cases were compared comprising a total of 674 recruited patients. In this study, robotic surgery was significantly associated to less anastomotic complications (7.3%) when compared to the laparoscopic (10.8%) approach.
IMMEDIATE ONCOLOGICAL OUTCOMES
CRM positivity results after TME or tumor-specific mesorectal excision was available for all studies in this review with the exception of one case series[34], two comparative studies[41,44], and one randomized trial[18]. The mean frequency of CRM positivity after robotic rectal cancer surgery for 1656 operated on rectal cancer patients in the present review was between 0% and 7.5%. After laparoscopic rectal surgery, for 879 patients, it ranged from 0% to 8.8% (Table 3). Only in two recent comparative studies[26,27], the involvement of CRM showed a significant decrease after robotic rectal surgery when compared to the laparoscopic access. In the study of Kang et al[26], 495 patients submitted to robotic, laparoscopic, or open rectal surgery were retrospectively selected and matched according to clinical characteristics. No significant differences in baseline characteristics were observed among the matched cohort with 165 pairs of patients. CRM involvement was observed in 4.2%, 6.7%, and 10.3% of patients undergoing robotic, laparoscopic, or open access TME, respectively. In the experience of D’Annibale et al[27], 50 patients underwent robotic TME and were retrospectively compared to 50 patients undergoing laparoscopic TME. In spite of CRM involvement could have depended on pathology site and extension, the authors stated that robot-assisted surgery allowed them to achieve a complete and oncologic adequate resection of the specimen due to articulation of the instruments and the 3-D magnified vision.
Table 3.
Use of robotics in rectal cancer surgery - immediate oncological outcomes
| Ref. | Type of procedure | Number of patients | Positivity of circumferential resection margin (%) | Distal resection margins, cm | Mean/median number of lymph nodes harvested |
| Hellan et al[28] | RTME | 39 | 0 | 2.6 (0.4-7.5) | 13 (7-28) |
| Choi et al[29] | RTME | 13 | 0 | 4.7 ± 1.8 | 24.6 ± 16.7 |
| Choi et al[19] | RTME | 50 | 2 | 1.9 ± 1.1 | 20.6 ± 10 |
| Ng et al[30] | RTME | 8 | 0 | > 2 | 15 (2-26) |
| deSouza et al[31] | RTME | 44 | 0 | NA | 14 (5-45) |
| Pigazzi et al[32] | RTME | 143 | 0.7 | 2.9 ± 1.8 | 14.1 (1-39) |
| Baek et al[33] | RTME | 64 | 0 | 3.4 (0.2-10) | 14.5 (3-28) |
| Zimmern et al[34] | RTME | 58 | NA | NA | 11.8-15.3 |
| Kang et al[20] | RTME | 389 | 3.6 | NA | 15.7 ± 10 |
| Koh et al[35] | RTME | 19 | 5.3 | 0.8-1 | 17.8 ± 7.1 |
| Leong et al[21] | RTME | 29 | 6.8 | 0.8 (0-4) | 16 (1-44) |
| Marecik et al[36] | RTME | 5 | 0 | -1 | 12.4 |
| Alimoglu et al[24] | RTME | 7 | 0 | -1 | 16 (14-21) |
| Kang et al[37] | RTME | 6 | 0 | -1 | 20 (7-58) |
| Karahasanoglu et al[38] | RTME | 30 | 0 | 4 (2-8) | 15 (3-38) |
| Park et al[39] | RTME | 30 | 0 | NA | 20 (14-25) |
| Du et al[40] | RTME | 22 | 0 | 2.6 (1-5.5) | 14.3 (8-27) |
| Pigazzi et al[41] | RTME | 6 | NA | 3.8 (1.8-9) | 14 (9-28) |
| LTME | 6 | NA | 3.5 (2.2-5) | 17 (9-39) | |
| Baik et al[18] | RTME | 18 | NA | 4.0 ± 1.1 | 18 (6-49) |
| LTME | 18 | NA | 3.7 ± 1.1 | 22 (9-42) | |
| Patriti et al[42] | RTME | 29 | 0 | 2.1 ± 0.9 | 10.3 ± 4 |
| LTME | 37 | 0 | 4.5 ± 7.2 | 11.2 ± 5 | |
| Baik et al[43] | RTME | 56 | 7.2 | 4 (1-7) | 17.5 (4-43) |
| LTME | 57 | 8.8 | 3 (1-9) | 17 (4-53) | |
| Bianchi et al[22] | RTME | 25 | 0 | 2 (1.5-4.5) | 18 (7-34) |
| LTME | 25 | 4 | 2 (1.8-3.5) | 17 (8-37) | |
| Popescu et al[44] | RTME | 38 | NA | Negative | 11.7 ± 3.8 |
| LTME | 84 | NA | Negative | 11.1 ± 3.2 | |
| Kim et al[23] | RTME | 100 | 3 | 2.7 ± 1.9 | 14.7 ± 9.7 |
| LTME | 100 | 2 | 2.6 ± 1.8 | 16.6 ± 9.1 | |
| Park et al[45] | RTME | 41 | 1.2 | 2.1 ± 1.4 | 17.3 ± 7.7 |
| LTME | 82 | 7.3 | 2.3 ± 1.5 | 14.2 ± 8.9 | |
| Park et al[46] | RTME | 52 | 1.9 | 2.8 ± 1.9 | 19.4 ± 10.2 |
| LTME | 123 | 2.4 | 3.2 ± 2.1 | 15.9 ± 10.1 | |
| Baek et al[47] | RTME | 41 | 4.9 | 3.6 (0.4-10) | 13.1 (3-33) |
| LTME | 41 | 2.4 | 3.8 (0.4-11) | 16.2 (3-33) | |
| Kwak [48] | RTME | 59 | 1.7 | 2.2 (1.5-3) | 20 (12-27) |
| LTME | 59 | 0 | 2 (1.2-3.5) | 21 (14-28) | |
| Kim et al[25] | RTME | 100 | 1 | 2.7 ± 1.72 | 20 ± 6.9 |
| OTME | 100 | 1 | 1.9 ± 1.32 | 19.6 ± 8.5 | |
| Park et al[49] | RTME | 40 | 7.5 | 1.4 ± 0.9 | 12.9 ± 7.5 |
| LTME | 40 | 5 | 1.3 ± 0.9 | 13.3 ± 8.6 | |
| Kang et al[26] | RTME | 165 | 4.22 | 1.9 ± 1.4 | 15 ± 9.4 |
| LTME | 165 | 6.72 | 2 ± 1.4 | 15.6 ± 9.1 | |
| OTME | 165 | 10.32 | 2.2 ± 1.7 | 17.4 ± 10.9 | |
| D’Annibale et al[27] | RTME | 50 | 02 | 3 (2-7) | 16.5 (11-44) |
| LTME | 50 | 122 | 3 (1-6) | 13.8 (4-29) |
Abdominoperineal resections only;
The original publication as statistically significant (P < 0.05). RTME: Robotic total mesorectal excision; LTME: Laparoscopic total mesorectal excision; RPME: Robotic partial mesorectal excision; OTME: Open total mesorectal excision; NA: Not available.
In the present review, data regarding the extent of lymphadenectomy associated to robotic rectal surgery was available for all 32 selected studies (1776 rectal cancer patients). The mean number of retrieved lymph nodes after robotic rectal surgery was between 10.3 and 20. After laparoscopic surgery, the mean number of retrieved lymph nodes among 987 operated on patients was between 11.1 and 21). In all comparative, and in the randomized study, there was no difference in the extent of lymphadenectomy due to a robotic or laparoscopic approach. In the study of D’Annibale et al[27], the mean number of lymph nodes retrieved after robotic TME was 16.5 (range 11-44), and was 13.8 (4-29) after laparoscopic TME. In this paper, there was no difference in the extent of the lymphadenectomy (P = 0.053) (Table 2). However, in the discussion session, the authors stated that there was no difference in the extent of lymphadenectomy among the groups (P = 0.073).
Regarding the extent of DRM, among the 17 comparative studies, the information was not available for four studies[20,31,34,39] due to not declared reasons, for three studies comprising only patients submitted to robotic abdominoperineal excisions[24,36,37] and for one comparative study[44]. For 1199 patients undergoing robotic rectal cancer, surgery, the mean value of DRM varied between 0.8 and 4.7 cm. For 903 patients undergoing laparoscopic sphincter-preserving rectal cancer surgery, the mean value for DRM ranged from 1.9 to 4.5 cm. In all selected comparative studies, there was no difference for DRM value between robotic and laparoscopic cases. However, in one study[25] comparing 100 matched robotic to open cases, mean DRM was longer (2.7 vs 1.9; P = 0.001) after robotic approach.
DISCUSSION
It was demonstrated that laparoscopic colorectal surgery is as safe and effective as open surgery regarding early postoperative outcomes. These data are at this time mature and reflect a modest but significant benefit on short-term outcomes when compared to conventional colectomies[50]. Long-term oncological results also demonstrate that laparoscopic surgery is entirely equivalent to laparotomy regarding oncologic safety[6].
Similarly, laparoscopic rectal resection is fully feasible[51]. However, laparoscopic TME remains a technically demanding procedure associated with a steep learning curve, high conversion and positive CRM rates[11,13]. The 16% rate of CRM involvement reported in the MRC CLASICC trial has been advocated as an indirect evidence of increased technical difficulty associated to laparoscopic TME[13]. Particular features of laparoscopic TME include the limited dexterity of non-articulating instruments attached to a fulcrum effect especially in the narrow male pelvis or when dealing with bulky tumors, and loss of three-dimensional view.
Robotic surgery for the management of rectal cancer remains a highly controversial issue. The current robotic surgical platform provides a stable, three-dimensional, high-definition, 10-fold magnification vision, intuitive articulated instrument manipulation, tremor elimination, superior dexterity, and high precision of the movement of the robotic arms. Therefore, robotic surgical systems may be particularly suited for deep and precise pelvic surgery, obviating some of the limitations of conventional laparoscopic surgery as required during TME operations. The first robotic colectomy was reported in 2002[15]. Since then, the number of publications on robotic colorectal surgery has markedly increased[52]. In 2004, D’Annibale et al[53] reported on 52 cases of robotic-assisted colon and rectal surgery. In this report, 10 cases of robotic-assisted anterior resection were included. The first paper on robotic TME included 6 cases of rectal cancer and was published in 2006[41].
The high costs associated with the currently available robotic platform have limited the implementation of this technology. Therefore, before robotic surgery can be accepted as the preferred approach for rectal cancer surgery, it should be confirmed that the technology provides superior short-term outcomes and equal or improved oncologic and functional results in comparison with other approaches. Due to the lack of evidence from controlled randomized trials, a systematic review of the currently available literature might help to critically assess such an argument. The interest on the matter is elevated and the first TME operation was reported in 2004[53]. Nevertheless, since then there is only one very small randomized controlled trial[18] comparing robotic to conventional laparoscopic rectal surgery, published in 2008. On the other hand, we could find at least two non-systematic reviews on robotic surgery for colorectal diseases[54,55], and one systematic review on robotic surgery for rectal cancer[56]. The available published data on robotic colon and rectal surgery comprise case reports, case series, and non-randomized retrospective and prospective comparative studies. All of these studies are, by their very nature, subject to significant bias derived from patient selection, and quality of reported data extraction. However, this concept did not prevent the publication of meta-analyses on robotic colorectal surgery[52], and on robotic rectal surgery[16,17,57]. Because of the lack of available evidence from ongoing prospective randomized trials (ROLLAR[58] and ACOSOG-Z6051[59]), systematic reviews as conducted in the present paper represent the most organized way to evaluate current evidence on robotics for rectal malignancy.
Before the present study design was accomplished, the findings of two recent meta-analyses[16,17] on robotic surgery for rectal cancer demonstrated non-similar results but the same limitations. Both meta-analyses included seven studies. In both, four studies[22,42,43,48] were present. Yang et al[17] found that robotic proctectomy is associated with increased operative time, less estimated blood loss, lower conversion rate, and higher hospital costs. Memon et al[16] were able to confirm only the findings of lower conversion associated with robotic surgery when compared to conventional laparoscopy. Meta-analyses of robotics for rectal surgery have several limitations. Non-randomized, retrospective or prospective, not case-matched or matched studies are biased. Moreover, the number of studies in the analysis is small, precluding subgroup analysis. In the present study, we calculated the mean number of robotic cancer cases being 55.5 (range, 5-379). Ultimately, uncontrolled variables included in biased studies lead to under- or over-estimation of risk effects[17].
In the present systematic review, it could be observed that robotic TME is associated with a prolonged operating time when compared to laparoscopic TME[23,26,44,45,48,49]. The extended time taken to dock the robot may be an important issue on the longer operating time. In the present review, it was observed that 795 patients of 8 studies[19-27] were operated on using a totally robotic approach. In the hybrid technique, the abdominal part of minimally invasive TME (inferior mesenteric vein and artery division, splenic flexure and left colon mobilization) is accomplished using conventional laparoscopic techniques. And the robot is used for the pelvic TME part of the operation. Most surgeons use the hybrid technique to avoid robot repositioning/re-docking. One time docking could also be done for a fully robotic technique by changing some trocars or arms. In the fully robotic technique reported by several authors[19,60,61], the robotic approach is used for the abdominal and pelvic parts of the operation. To the present moment, no study has compared a hybrid to a fully robotic technique.
There is no current significant evidence that a robotic approach to rectal cancer may reduce estimated blood loss. Only in the small report by Popescu et al[44] and Kang et al[26], intraoperative blood loss was significantly reduced after robotic rectal surgery. Popescu et al[44] have reported on only 38 robotic rectal cancer cases. Moreover, in the study of Kang et al[26], the difference between mean estimated blood loss after robotic (133 mL) and laparoscopic (140 mL) cases was very small. Ultimately, it must be remembered that estimating blood loss in a retrospective design may be imprecise.
Regarding hospital stay, in only three studies[18,23,46] involving 239 patients operated with robotic assistance, a shorter hospital stay could be observed. In all other selected comparative studies there was no significant difference. Moreover, Yang et al[17] and Memon et al[16] independently looked at the same data using meta-analytical techniques and could detect no difference. Variability between comparative retrospective studies may include differences in discharge criteria. Certainly, a shorter duration of hospitalization produced by robotic access to the rectum when compared to laparoscopy remains to be demonstrated.
Robotic surgery may overcome limitations derived from conventional laparoscopic TME. High-definition three-dimensional view and superior dexterity due to motion scale and articulated instruments may contribute to superior visualization and a more precise pelvic dissection. In the present systematic review, conversion to laparotomy after robotic rectal surgery (0%-9.4%) seems to be reduced when compared to laparoscopy (0%-22%). This result was directly observed in three[7,42,43] comparative studies included in this review and may be due to the ability to perform fine dissection in a narrow surgical field. On the other hand, it is important to highlight that none of the studies were randomized neither blinded. Therefore, these results must be interpreted with caution since selection bias may have inadvertently favored robotic cases, since robotics was the technology under evaluation.
There is no current evidence regarding a role for robotic surgery in reducing postoperative or anastomotic complications in all comparative studies selected in this review. In only one small study[43] comprising 56 and 57 patients undergoing a low anterior robotic and laparoscopic rectal resection, respectively, postoperative complications were higher in the laparoscopic group. The authors believe that the higher incidence of anastomotic complications observed in the laparoscopic group may be due to a difficulty in the perpendicular rectal transection leading to an increased number of stapler firings which was reported to be significantly related to anastomotic leakage. However, the number of stapler firings were not accrued in the study[62].
Although most specialists performing minimally invasive TME are highly experienced laparoscopic surgeons, performing precise dissection in the pelvis is especially difficult. On this matter, robotic surgery may provide some advantages. The oncologic outcomes after surgery for rectal cancer rely on the quality of mesorectal excision. Regarding CRM involvement in the present review, available data on robotic cases from case series and comparative studies indicate a (+)CRM from 0% to 7.5%; and from 0% to 8.8% after laparoscopic cases from comparative studies. However, there was no difference regarding (+)CRM rates for 10 comparative studies[22,23,25,42,43,45-49] included in this review. Three meta-analyses support these findings[16,17,23]. Only for the two most recent comparative studies[26,27], (+)CRM was more frequent after a laparoscopic approach.
Regarding the extent of dissected lymph nodes among TME specimens in this systematic review, the results were available for all 32 included studies. There was no significant difference between a robotic and laparoscopic approach in the total number of lymph nodes extracted. Despite the numerous variables determining the number of lymph nodes in the surgical specimen after TME, it is likely that the oncological efficacy and safety of robotic surgical treatment of rectal cancer may be improved after more experienced surgeons foster robotic technique.
Robotics is a minimally invasive technology with advantages over conventional laparoscopic surgery. It has been reported to enhance a surgeon’s ability to perform difficult cases such as a low rectal anastomosis in an obese patient. The approach proposed by Prasad et al[63] represents a clear example of robotic technology enhanced capabilities. In this approach, after completion of robotic TME, the distal rectum is divided, the specimen is passed through the low rectal (anal) stump, and, with the aid of the robotic movements, a purse-string suture is placed on the distal rectal stump, allowing a single-stapled transanal anastomosis to be performed. However, one of the unique drawbacks of robotic surgery is the loss of tactile sensation (haptic feedback). In the initial experience, it may lead to organ injury and perforation. Only through carefully observing robotic instrumentations effect on the tissue, the surgeon can offset the lack of tactile sensation. External robotic arms collisions remain another important issue in robotic surgery. Patient positioning, port placement, and arms adjustment are crucial for robotic surgery.
Undoubtedly, the most important obstacle to widespread implementation of robotic technology includes the high start-up and maintenance costs (US$1-2.5 million per robot, and approximately US$160000 annually for upkeep)[55]. Baek et al[64] reported that robotic surgery is more expensive than laparoscopic surgery for rectal cancer. In their study, total hospital charges were approximately 1.5 times higher in the robotic group compared with the laparoscopic group. Given the high costs involved in surgical treatment using robotics, it is imperative that the cost-effectiveness of robotic rectal cancer surgery be determined based on oncological outcomes and functional results of forthcoming studies.
When evaluating the learning curve of a new technology for oncologic rectal cancer surgery, long-term oncological and functional outcomes (voiding and sexual function) must be addressed. To date, no prospective studies have evaluated long-term functional outcomes of robotic rectal cancer surgery. Kim et al[65] have demonstrated that urinary function was recovered over 6 mo after laparoscopic TME compared to over three months after robotic TME. In the same study, patients operated on using a robotic approach also exhibited a shorter recovery time for erectile function (6 mo vs 12 mo) when compared to laparoscopic TME. Luca et al[66] have assigned that a better preservation of voiding and sexual functions derive from superior movement of articulated robotic instruments, and from a more precise dissection in the narrow pelvis, with accurate identification of the anatomical planes and smaller neural components.
CONCLUSION
In conclusion, it was observed that more frequently, very experienced minimally invasive surgeons are performing robotic rectal cancer surgery. However, the quality of the assembled evidence does not support strong conclusions about most of the parameters of interest. Robotic rectal cancer surgery is still associated to increased cost and operating time. In the setting of the selected patients that characterizes this review, robotic rectal cancer surgery is associated to reduced conversion rates when compared to conventional laparoscopic techniques. Other short-term outcomes are comparable to conventional laparoscopic techniques, if not better. Furthermore, pathological data evaluation suggests that oncologic safety may be preserved after robotic TME. However, further studies are required to evaluate oncologic safety and functional results associated to robotic rectal cancer surgery.
Footnotes
P- Reviewer: Pescatori M, Sgourakis G S- Editor: Ding Y L- Editor: A E- Editor: Wang CH
References
- 1.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 2.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 3.Gaertner WB, Kwaan MR, Madoff RD, Willis D, Belzer GE, Rothenberger DA, Melton GB. The evolving role of laparoscopy in colonic diverticular disease: a systematic review. World J Surg. 2013;37:629–638. doi: 10.1007/s00268-012-1872-x. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui MR, Sajid MS, Khatri K, Cheek E, Baig MK. Elective open versus laparoscopic sigmoid colectomy for diverticular disease: a meta-analysis with the Sigma trial. World J Surg. 2010;34:2883–2901. doi: 10.1007/s00268-010-0762-3. [DOI] [PubMed] [Google Scholar]
- 5.Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, Yang X, Gao P, Zhang K, Yuan Y, et al. Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22:e39–e43. doi: 10.1016/j.suronc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Kuhry E, Schwenk W, Gaupset R, Romild U, Bonjer J. Long-term outcome of laparoscopic surgery for colorectal cancer: a cochrane systematic review of randomised controlled trials. Cancer Treat Rev. 2008;34:498–504. doi: 10.1016/j.ctrv.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;(2):CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKay GD, Morgan MJ, Wong SK, Gatenby AH, Fulham SB, Ahmed KW, Toh JW, Hanna M, Hitos K. Improved short-term outcomes of laparoscopic versus open resection for colon and rectal cancer in an area health service: a multicenter study. Dis Colon Rectum. 2012;55:42–50. doi: 10.1097/DCR.0b013e318239341f. [DOI] [PubMed] [Google Scholar]
- 9.Tekkis PP, Senagore AJ, Delaney CP, Fazio VW. Evaluation of the learning curve in laparoscopic colorectal surgery: comparison of right-sided and left-sided resections. Ann Surg. 2005;242:83–91. doi: 10.1097/01.sla.0000167857.14690.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichenbach DJ, Tackett AD, Harris J, Camacho D, Graviss EA, Dewan B, Vavra A, Stiles A, Fisher WE, Brunicardi FC, et al. Laparoscopic colon resection early in the learning curve: what is the appropriate setting? Ann Surg. 2006;243:730–735; discussion 735-737. doi: 10.1097/01.sla.0000220039.26524.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cecconello I, Araujo SE, Seid VE, Nahas SC. Laparoscopic total mesorectal excision: early and late results. Asian J Endosc Surg. 2011;4:99–106. doi: 10.1111/j.1758-5910.2011.00090.x. [DOI] [PubMed] [Google Scholar]
- 12.Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 13.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 14.Lanfranco AR, Castellanos AE, Desai JP, Meyers WC. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber PA, Merola S, Wasielewski A, Ballantyne GH. Telerobotic-assisted laparoscopic right and sigmoid colectomies for benign disease. Dis Colon Rectum. 2002;45:1689–1694; discussion 1695-1696. doi: 10.1007/s10350-004-7261-2. [DOI] [PubMed] [Google Scholar]
- 16.Memon S, Heriot AG, Murphy DG, Bressel M, Lynch AC. Robotic versus laparoscopic proctectomy for rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19:2095–2101. doi: 10.1245/s10434-012-2270-1. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Ma Y. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19:3727–3736. doi: 10.1245/s10434-012-2429-9. [DOI] [PubMed] [Google Scholar]
- 18.Baik SH, Ko YT, Kang CM, Lee WJ, Kim NK, Sohn SK, Chi HS, Cho CH. Robotic tumor-specific mesorectal excision of rectal cancer: short-term outcome of a pilot randomized trial. Surg Endosc. 2008;22:1601–1608. doi: 10.1007/s00464-008-9752-z. [DOI] [PubMed] [Google Scholar]
- 19.Choi DJ, Kim SH, Lee PJ, Kim J, Woo SU. Single-stage totally robotic dissection for rectal cancer surgery: technique and short-term outcome in 50 consecutive patients. Dis Colon Rectum. 2009;52:1824–1830. doi: 10.1007/DCR.0b013e3181b13536. [DOI] [PubMed] [Google Scholar]
- 20.Kang J, Min BS, Park YA, Hur H, Baik SH, Kim NK, Sohn SK, Lee KY. Risk factor analysis of postoperative complications after robotic rectal cancer surgery. World J Surg. 2011;35:2555–2562. doi: 10.1007/s00268-011-1270-9. [DOI] [PubMed] [Google Scholar]
- 21.Leong QM, Son DN, Cho JS, Baek SJ, Kwak JM, Amar AH, Kim SH. Robot-assisted intersphincteric resection for low rectal cancer: technique and short-term outcome for 29 consecutive patients. Surg Endosc. 2011;25:2987–2992. doi: 10.1007/s00464-011-1657-6. [DOI] [PubMed] [Google Scholar]
- 22.Bianchi PP, Ceriani C, Locatelli A, Spinoglio G, Zampino MG, Sonzogni A, Crosta C, Andreoni B. Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc. 2010;24:2888–2894. doi: 10.1007/s00464-010-1134-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim NK, Kang J. Optimal Total Mesorectal Excision for Rectal Cancer: the Role of Robotic Surgery from an Expert’s View. J Korean Soc Coloproctol. 2010;26:377–387. doi: 10.3393/jksc.2010.26.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alimoglu O, Atak I, Kilic A, Caliskan M. Robot-assisted laparoscopic abdominoperineal resection for low rectal cancer. Int J Med Robot. 2012;8:371–374. doi: 10.1002/rcs.1432. [DOI] [PubMed] [Google Scholar]
- 25.Kim JC, Yang SS, Jang TY, Kwak JY, Yun MJ, Lim SB. Open versus robot-assisted sphincter-saving operations in rectal cancer patients: techniques and comparison of outcomes between groups of 100 matched patients. Int J Med Robot. 2012;8:468–475. doi: 10.1002/rcs.1452. [DOI] [PubMed] [Google Scholar]
- 26.Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257:95–101. doi: 10.1097/SLA.0b013e3182686bbd. [DOI] [PubMed] [Google Scholar]
- 27.D’Annibale A, Pernazza G, Monsellato I, Pende V, Lucandri G, Mazzocchi P, Alfano G. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–1895. doi: 10.1007/s00464-012-2731-4. [DOI] [PubMed] [Google Scholar]
- 28.Hellan M, Anderson C, Ellenhorn JD, Paz B, Pigazzi A. Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol. 2007;14:3168–3173. doi: 10.1245/s10434-007-9544-z. [DOI] [PubMed] [Google Scholar]
- 29.Choi GS, Park IJ, Kang BM, Lim KH, Jun SH. A novel approach of robotic-assisted anterior resection with transanal or transvaginal retrieval of the specimen for colorectal cancer. Surg Endosc. 2009;23:2831–2835. doi: 10.1007/s00464-009-0484-5. [DOI] [PubMed] [Google Scholar]
- 30.Ng KH, Lim YK, Ho KS, Ooi BS, Eu KW. Robotic-assisted surgery for low rectal dissection: from better views to better outcome. Singapore Med J. 2009;50:763–767. [PubMed] [Google Scholar]
- 31.deSouza AL, Prasad LM, Marecik SJ, Blumetti J, Park JJ, Zimmern A, Abcarian H. Total mesorectal excision for rectal cancer: the potential advantage of robotic assistance. Dis Colon Rectum. 2010;53:1611–1617. doi: 10.1007/DCR.0b013e3181f22f1f. [DOI] [PubMed] [Google Scholar]
- 32.Pigazzi A, Luca F, Patriti A, Valvo M, Ceccarelli G, Casciola L, Biffi R, Garcia-Aguilar J, Baek JH. Multicentric study on robotic tumor-specific mesorectal excision for the treatment of rectal cancer. Ann Surg Oncol. 2010;17:1614–1620. doi: 10.1245/s10434-010-0909-3. [DOI] [PubMed] [Google Scholar]
- 33.Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251:882–886. doi: 10.1097/SLA.0b013e3181c79114. [DOI] [PubMed] [Google Scholar]
- 34.Zimmern A, Prasad L, Desouza A, Marecik S, Park J, Abcarian H. Robotic colon and rectal surgery: a series of 131 cases. World J Surg. 2010;34:1954–1958. doi: 10.1007/s00268-010-0591-4. [DOI] [PubMed] [Google Scholar]
- 35.Koh DC, Tsang CB, Kim SH. A new application of the four-arm standard da Vinci® surgical system: totally robotic-assisted left-sided colon or rectal resection. Surg Endosc. 2011;25:1945–1952. doi: 10.1007/s00464-010-1492-1. [DOI] [PubMed] [Google Scholar]
- 36.Marecik SJ, Zawadzki M, Desouza AL, Park JJ, Abcarian H, Prasad LM. Robotic cylindrical abdominoperineal resection with transabdominal levator transection. Dis Colon Rectum. 2011;54:1320–1325. doi: 10.1097/DCR.0b013e31822720a2. [DOI] [PubMed] [Google Scholar]
- 37.Kang CY, Carmichael JC, Friesen J, Stamos MJ, Mills S, Pigazzi A. Robotic-assisted extralevator abdominoperineal resection in the lithotomy position: technique and early outcomes. Am Surg. 2012;78:1033–1037. [PubMed] [Google Scholar]
- 38.Karahasanoglu T, Hamzaoglu I, Baca B, Aytac E, Erguner I, Uras C. Robotic surgery for rectal cancer: initial experience from 30 consecutive patients. J Gastrointest Surg. 2012;16:401–407. doi: 10.1007/s11605-011-1737-x. [DOI] [PubMed] [Google Scholar]
- 39.Park IJ, You YN, Schlette E, Nguyen S, Skibber JM, Rodriguez-Bigas MA, Chang GJ. Reverse-hybrid robotic mesorectal excision for rectal cancer. Dis Colon Rectum. 2012;55:228–233. doi: 10.1097/DCR.0b013e31823c0bd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Du XH, Shen D, Li R, Li SY, Ning N, Zhao YS, Zou ZY, Liu N. Robotic anterior resection of rectal cancer: technique and early outcome. Chin Med J (Engl) 2013;126:51–54. [PubMed] [Google Scholar]
- 41.Pigazzi A, Ellenhorn JD, Ballantyne GH, Paz IB. Robotic-assisted laparoscopic low anterior resection with total mesorectal excision for rectal cancer. Surg Endosc. 2006;20:1521–1525. doi: 10.1007/s00464-005-0855-5. [DOI] [PubMed] [Google Scholar]
- 42.Patriti A, Ceccarelli G, Bartoli A, Spaziani A, Biancafarina A, Casciola L. Short- and medium-term outcome of robot-assisted and traditional laparoscopic rectal resection. JSLS. 2009;13:176–183. [PMC free article] [PubMed] [Google Scholar]
- 43.Baik SH, Kwon HY, Kim JS, Hur H, Sohn SK, Cho CH, Kim H. Robotic versus laparoscopic low anterior resection of rectal cancer: short-term outcome of a prospective comparative study. Ann Surg Oncol. 2009;16:1480–1487. doi: 10.1245/s10434-009-0435-3. [DOI] [PubMed] [Google Scholar]
- 44.Popescu I, Vasilescu C, Tomulescu V, Vasile S, Sgarbura O. The minimally invasive approach, laparoscopic and robotic, in rectal resection for cancer. A single center experience. Acta Chir Iugosl. 2010;57:29–35. doi: 10.2298/aci1003029p. [DOI] [PubMed] [Google Scholar]
- 45.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010;17:3195–3202. doi: 10.1245/s10434-010-1162-5. [DOI] [PubMed] [Google Scholar]
- 46.Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240–248. doi: 10.1007/s00464-010-1166-z. [DOI] [PubMed] [Google Scholar]
- 47.Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc. 2011;25:521–525. doi: 10.1007/s00464-010-1204-x. [DOI] [PubMed] [Google Scholar]
- 48.Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum. 2011;54:151–156. doi: 10.1007/DCR.0b013e3181fec4fd. [DOI] [PubMed] [Google Scholar]
- 49.Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48–55. doi: 10.1007/s00464-012-2405-2. [DOI] [PubMed] [Google Scholar]
- 50.Abraham NS, Byrne CM, Young JM, Solomon MJ. Meta-analysis of non-randomized comparative studies of the short-term outcomes of laparoscopic resection for colorectal cancer. ANZ J Surg. 2007;77:508–516. doi: 10.1111/j.1445-2197.2007.04141.x. [DOI] [PubMed] [Google Scholar]
- 51.Aziz O, Constantinides V, Tekkis PP, Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW, Heriot AG. Laparoscopic versus open surgery for rectal cancer: a meta-analysis. Ann Surg Oncol. 2006;13:413–424. doi: 10.1245/ASO.2006.05.045. [DOI] [PubMed] [Google Scholar]
- 52.Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. doi: 10.1007/s11605-014-2469-5. [DOI] [PubMed] [Google Scholar]
- 53.D’Annibale A, Morpurgo E, Fiscon V, Trevisan P, Sovernigo G, Orsini C, Guidolin D. Robotic and laparoscopic surgery for treatment of colorectal diseases. Dis Colon Rectum. 2004;47:2162–2168. doi: 10.1007/s10350-004-0711-z. [DOI] [PubMed] [Google Scholar]
- 54.Pucci MJ, Beekley AC. Use of robotics in colon and rectal surgery. Clin Colon Rectal Surg. 2013;26:39–46. doi: 10.1055/s-0033-1333660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirnezami AH, Mirnezami R, Venkatasubramaniam AK, Chandrakumaran K, Cecil TD, Moran BJ. Robotic colorectal surgery: hype or new hope? A systematic review of robotics in colorectal surgery. Colorectal Dis. 2010;12:1084–1093. doi: 10.1111/j.1463-1318.2009.01999.x. [DOI] [PubMed] [Google Scholar]
- 56.Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56:253–262. doi: 10.1097/DCR.0b013e3182694595. [DOI] [PubMed] [Google Scholar]
- 57.Trastulli S, Farinella E, Cirocchi R, Cavaliere D, Avenia N, Sciannameo F, Gullà N, Noya G, Boselli C. Robotic resection compared with laparoscopic rectal resection for cancer: systematic review and meta-analysis of short-term outcome. Colorectal Dis. 2012;14:e134–e156. doi: 10.1111/j.1463-1318.2011.02907.x. [DOI] [PubMed] [Google Scholar]
- 58.Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H, et al. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27:233–241. doi: 10.1007/s00384-011-1313-6. [DOI] [PubMed] [Google Scholar]
- 59.Nandakumar G, Fleshman JW. Laparoscopy for rectal cancer. Surg Oncol Clin N Am. 2010;19:793–802. doi: 10.1016/j.soc.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Hellan M, Stein H, Pigazzi A. Totally robotic low anterior resection with total mesorectal excision and splenic flexure mobilization. Surg Endosc. 2009;23:447–451. doi: 10.1007/s00464-008-0193-5. [DOI] [PubMed] [Google Scholar]
- 61.Park YA, Kim JM, Kim SA, Min BS, Kim NK, Sohn SK, Lee KY. Totally robotic surgery for rectal cancer: from splenic flexure to pelvic floor in one setup. Surg Endosc. 2010;24:715–720. doi: 10.1007/s00464-009-0656-3. [DOI] [PubMed] [Google Scholar]
- 62.Kim JS, Cho SY, Min BS, Kim NK. Risk factors for anastomotic leakage after laparoscopic intracorporeal colorectal anastomosis with a double stapling technique. J Am Coll Surg. 2009;209:694–701. doi: 10.1016/j.jamcollsurg.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 63.Prasad LM, deSouza AL, Marecik SJ, Park JJ, Abcarian H. Robotic pursestring technique in low anterior resection. Dis Colon Rectum. 2010;53:230–234. doi: 10.1007/DCR.0b013e3181bc9db0. [DOI] [PubMed] [Google Scholar]
- 64.Baek SJ, Kim SH, Cho JS, Shin JW, Kim J. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg. 2012;36:2722–2729. doi: 10.1007/s00268-012-1728-4. [DOI] [PubMed] [Google Scholar]
- 65.Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485–2493. doi: 10.1245/s10434-012-2262-1. [DOI] [PubMed] [Google Scholar]
- 66.Luca F, Valvo M, Ghezzi TL, Zuccaro M, Cenciarelli S, Trovato C, Sonzogni A, Biffi R. Impact of robotic surgery on sexual and urinary functions after fully robotic nerve-sparing total mesorectal excision for rectal cancer. Ann Surg. 2013;257:672–678. doi: 10.1097/SLA.0b013e318269d03b. [DOI] [PubMed] [Google Scholar]


