Abstract
Bloating is one of the most common and bothersome symptoms complained by a large proportion of patients. This symptom has been described with various definitions, such as sensation of a distended abdomen or an abdominal tension or even excessive gas in the abdomen, although bloating should probably be defined as the feeling (e.g. a subjective sensation) of increased pressure within the abdomen. It is usually associated with functional gastrointestinal disorders, like irritable bowel syndrome, but when bloating is not part of another functional bowel or gastrointestinal disorder it is included as an independent entity in Rome III criteria named functional bloating. In terms of diagnosis, major difficulties are due to the lack of measurable parameters to assess and grade this symptom. In addition, it is still unclear to what extent the individual patient complaint of subjective bloating correlates with the objective evidence of abdominal distension. In fact, despite its clinical, social and economic relevance, bloating lacks a clear pathophysiology explanation, and an effective management endorsement, turning this common symptom into a true challenge for both patients and clinicians. Different theories on bloating etiology call into questions an increased luminal contents (gas, stools, liquid or fat) and/or an impaired abdominal empting and/or an altered intra-abdominal volume displacement (abdomino-phrenic theory) and/or an increased perception of intestinal stimuli with a subsequent use of empirical treatments (diet modifications, antibiotics and/or probiotics, prokinetic drugs, antispasmodics, gas reducing agents and tricyclic antidepressants). In this review, our aim was to review the latest knowledge on bloating physiopathology and therapeutic options trying to shed lights on those processes where a clinician could intervene to modify disease course.
Keywords: Bloating, Functional gastro-intestinal disorders, Irritable bowel syndrome, Constipation, Diarrhea
Core tip: Bloating is one of the most common and bothersome symptoms complained by a large proportion of patients, mostly with functional gastrointestinal disorders, but when bloating is not part of another functional bowel or gastrointestinal disorder it is included as an independent entity in Rome III criteria named functional bloating. Despite its clinical, social and economic relevance, bloating lacks a clear pathophysiology explanation and an effective management endorsement. Moreover, it is unclear to what extent subjective complaint of bloating correlates with the objective evidence of abdominal distension. The aim of this paper is to review the latest knowledge on bloating and to explore future research directions to improve its management.
BACKGROUND
There is no consensus concerning the definition of bloating which is a term very ambiguous both in English and in other languages. Patients refer bloating when they feel a full and tight abdomen, or a sensation of a swollen/distended abdomen, or an abdominal pressure or wall tension, or even the sensation of excessive gas in the abdomen. Furthermore, other complains such as burping and belching which reflect the expulsion of excess gas from the stomach, nausea, crampy, gurgling, or rumbling stomach; or needing to go to the bathroom are misleading with bloating from patient’s perspective. The definition of bloating should probably be regarded as meaning that there is a feeling (subjective sensation) of increased pressure within the abdomen, whereas abdominal distension should be reserved to an actual (objective) increase in diameter of the abdominal area. While often considered closely related, bloating is not always accompanied by visible distention[1,2]. However, irrespective of whether bloating is or is not accompanied by distension, patients usually refer to it as bloating and currently even clinicians tend to use the term bloating to cover both features.
Bloating is also reported in organic diseases such as enteropathogenic infections, malabsorptive conditions, acute or subacute bowel ischemia, and even neoplastic diseases. Bloating has been shown to be even useful for the early diagnosis of ovarian cancer and falls into a specific ovarian cancer symptoms index to its earlier detection, while it remains an unspecific signal for gastro-intestinal tumors[1-4].
Bloating is one of the most common and bothersome complaints in a large proportion of patients with various functional gastro-intestinal disorders (FGIDs), namely: functional dyspepsia (FD)[5,6], irritable bowel syndrome (IBS)[5-7], and functional constipation[5,6,8]. Up to 80% of patients with constipation complain of bloating and abdominal distension[9,10]. However, bloating in the absence of visible distension is frequently reported by patients with diarrhea[11]. Bloating is one of the principal symptoms of postprandial distress syndrome, one of the two subgroups of FD, where it is often located in the upper abdomen and precipitated by meals[6,12].
When bloating is not part of another functional bowel or gastroduodenal disorder, functional bloating is also included as an independent entity in Rome III criteria[13]. In details, its diagnostic criteria include recurrent feeling of bloating or visible distension at least 3 d a month in the last 3 mo with symptom onset at least 6 mo prior to diagnosis[13]. The name has been changed from functional abdominal bloating in Rome I and II criteria to functional bloating in Rome III criteria[13].
Epidemiologically, one in six to one in five healthy individual reported bloating in population based studies both in western[14,15] and east countries[16]. This prevalence dramatically increases, being reported by up to 96% in IBS patients[7]. Also, the majority of those patients (60%) rate bloating as their most bothersome symptom, scoring worse than abdominal pain[17].
Finally, there is some evidence documenting the association between bloating and quality of life[18]. However, only abdominal pain and diarrhea, but not bloating and other IBS related symptoms, were independently associated with reduced quality of life[19,20].
Typically, the symptom is variably reported over the months and fluctuates in intensity over daytime, particularly in the postprandial period, being worse at the end of the day and improving at night-time[11,21]. Changes in abdominal girth are greater in patients with IBS, and these patients are more likely to be symptomatic[22]. The severity of bloating may, therefore, vary from very mild to severe and uncomfortable[15]. In addition, most of the patients report typical symptom exacerbation after eating, no matter the size of the meal assumed[22,23]. High-fiber food and supplements, fatty meals and dairy products are frequently reported as offending[24,25]. Bloating symptoms are described more frequently by women[11,26,27] with a female to male ratio of 2:1[26,27], but this may be due to divergent health-seeking behaviors[28].
Despite its clinical, social and economic relevance[29], the pathophysiology of bloating and abdominal distension is complicated and incompletely understood, and no treatment is reported as universally effective. However, with the increasing knowledge of these disorders due to more objective ways of assessing it in combination with the availability of several new therapies over the past decade[30], we aim to review the latest knowledge on patophysiological mechanisms of bloating, and to explore present and future research directions aimed on improving its management.
We reviewed the literature of mechanisms and treatment interventions for abdominal bloating based on a PubMed search on the following terms; “abdominal bloating,” “intestinal gas and IBS,” “distension and IBS” and “FGID.”
PATHOPHYSIOLOGY
Bloating, like many other abdominal symptoms, is probably a heterogeneous condition produced by a combination of pathophysiological mechanisms that differ among individuals.
Different theories on bloating/abdominal distension etiology have been postulated (Figure 1), incriminating time after time as (1) increased luminal contents (gas, stools, liquid or fat); (2) impaired abdominal empting (e.g., defective propulsion, obstructed evacuation); (3) altered intra-abdominal volume displacement (abdomino-phrenic theory); and/or (4) increased perception of intestinal stimuli (sensory dysfunction or psychological factors).
Figure 1.
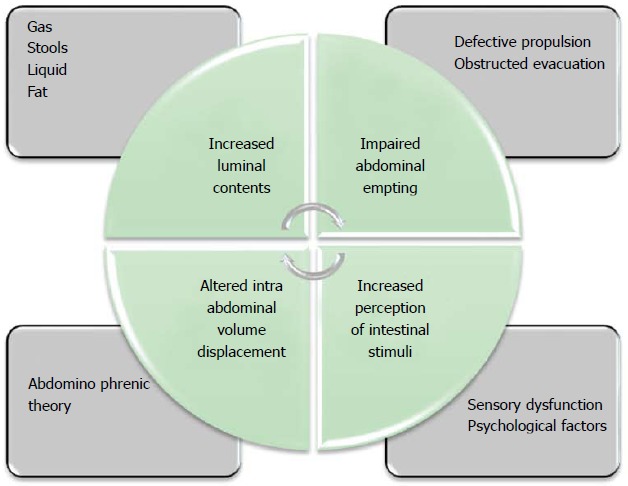
Possible underlining mechanism behind bloating.
Increased luminal contents
Gas: It can be speculated that bloating and, even more, abdominal distension depend on an increase in intra-abdominal contents. Since years considering the rapidly changing and fluctuating trend of such symptoms, gas has been always considered the element most heavily implicated in explaining them.
The homeostasis of intestinal gas depends on the balance between its formation and elimination. Briefly, gas input results at different level in the human being. For example, it results from swallowed air, chemical reactions (specifically, neutralization of acids and alkali in the upper gut), and diffusion from bloodstream. In the lower part of the GI tract it occurs primarily due to bacterial fermentation. Gas output is achieved by belching, absorption into the blood, bacterial consumption, and anal evacuation (Figure 2)[31].
Figure 2.
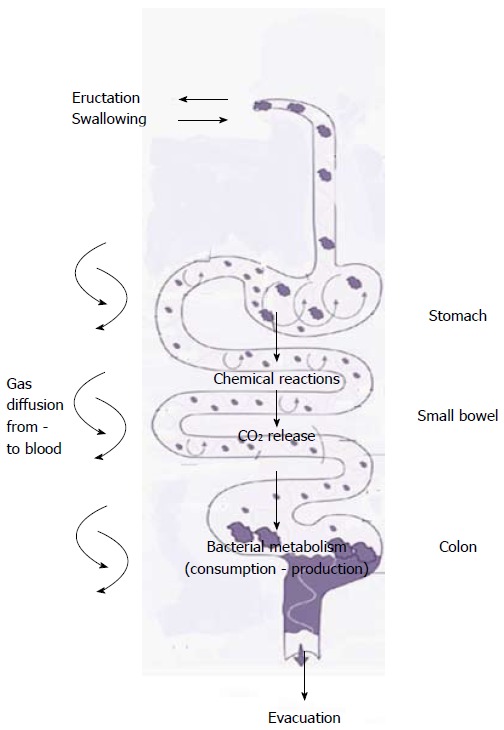
Gas physiology in the gastrointestinal tract. Gas input is determined by swallowing, chemical reactions (bacterial fermentation, food digestion) and diffusion from blood stream. Output is achieved by belching, absorption into the blood, bacterial consumption and anal evacuation. Adapted from[31].
An excessive introduction of gas, for example, occurs in aerophagia, a functional disorder in which patients swallow air so frequently and in such large quantities to result in symptoms of abdominal distention and bloating[32]. Increased air swallowing, however, is either not or rarely observed in IBS patients[33]. Although not covered in this review, eating disorders may also be associated with symptoms of gas and bloating[34].
Moreover, it has been shown that some food components in the normal diet, such as resistant starch, oligosaccharides and plant fibres, are incompletely absorbed in the small bowel and enter the colon[35] with subsequent fermentation, and secondary bacterial overgrowth with abnormal fermentation of foodstuffs within the small bowel or hyperactive colonic microflora. Some studies suggest that the absorption capacity of certain substrates (e.g., fructose) in the small bowel is reduced in IBS patients[35-37]. However, studies evaluating the benefit of exclusion diets on bloating have given contradictory results[38,39].
A potential small bowel bacterial overgrowth etiology has been suggested by some studies reporting increased positivity of the lactulose breath test in IBS[40]. However, the relevance of these data has been questioned for both the low reliability of the test, the weak clinical response to antibiotic therapy and the low placebo effect compared to other IBS studies[41]. However, there is general agreement that at least in a proportion of patients with IBS and bloating[42-44] an increased bacterial fermentation can contribute to the symptoms[45].
The net effect of colonic microflora on gas metabolism depends on the balance between gas-producing and gas-consuming microorganisms, which varies considerably among individuals[46].
For the most part, the colon hosts bacteria that produce hydrogen and carbon dioxide mainly from the fermentation of carbohydrates[47,48]. There is then, other pools of colonic microorganisms, for example sulfate-reducing bacteria, that consume a large proportion of the gases produced by fermentation and, release small amounts of odoriferous sulfur-containing gas. About 30% of the population is also home to methane-producing bacteria that consume large quantities of hydrogen and release methane[48-52]. Relevant factors contributing to the intraluminal gas homeostasis are: (1) the consume of gases (in particular hydrogen) by the bacteria strains; (2) the diffusibility of single gases (greater for oxygen and the carbon dioxide, medium for hydrogen, low for nitrogen); and (3) the gas partial pressure and the time of exposure to the diffusion surface which is influenced by intestinal transit speed[53,54]. Everything accounts for the gaseous composition of flatus and is the basis for the hydrogen and methane breath tests.
However, evaluation of intestinal gas production by breath tests has yielded inconclusive results. In one study breath hydrogen concentration was found to be similar in both IBS patients and healthy controls[55]. In contrast, another study measuring total excretion (breath plus anal) by indirect calorimetry showed that IBS patients excreted more hydrogen than healthy subjects when on a standard diet, suggesting an hyperactive gas-producing colonic microflora as potential etiology[45].
However, the stated increase in the production of intestinal gas should lead to an increase in flatulence but not necessarily of bloating, in fact several studies show that most of the normal subjects are able to excrete large amounts of gas without complain of symptoms[24,56]. Therefore, additional etiology factors are to be speculated in the genesis of bloating.
In their early studies with the washout technique Lasser et al[57] showed that the amount (and composition) of intestinal gas in IBS patients, in fasting condition, was similar to that of healthy subjects, about 100-300 mL. In these studies, gas was measured by infusing argon into the intestine at a relatively high flow rate (40 mL/min) and recovering rectal gas. The Barcelona group more than twenty years later, using a gas challenge test Figure 3), came to the same conclusion[58]. In contrast studies conducted using plain abdominal radiographs concluded that intra-abdominal gas content was larger in IBS patients than in healthy subjects[59,60]. However, the differences in the amount of gas between the two groups, reported by these studies, appears to be relatively low in absolute volume and in addition these increases did not correlate with the symptoms reported. In fact, a computed tomography study of intestinal gas could not detect significant differences between controls and patients with significant daytime abdominal girth increments and bloating[21].
Figure 3.
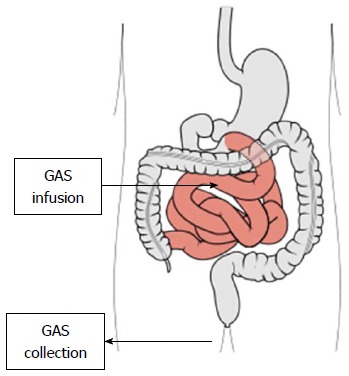
Gas challenge test. The method consists in the infusion of a mixture of gases in the jejunum while anal gas output is quantified. Adapted from[65].
These data suggest that clinical bloating may not be simply the result of too much intestinal gas, but abnormal gas handling and distribution within the gut might play a relevant role as well.
Stools: Bloating is a frequent complains in constipated patients[22]. Retained feces in the rectum causes delayed small intestinal as well as colonic transit, probably explaining the aggravated bloating in constipated patients[61]. It has been previously suggested that anorectal dysfunction underlying functional constipation may be an important contributory factor to the development of bloating and abdominal distension[62].
We found that in healthy men who underwent experimentally-controlled 35-d of bed rest, at the end of the study, 60% had new onset of functional constipation fulfilling Rome III criteria, moreover, there was a significant increase in flatulence and a significant correlation between the change in flatulence and the change in number of evacuations that significantly decreased[63]. It is possible that during bed rest due to an ineffective evacuation of stools, a larger amount of faeces were retained in the rectum, then causing rectal distension and explaining the decreased bowel frequency and the increase in desire of defecate along the experimental period. It is common knowledge that also normal subjects when provided with a sensation of stooling may show a dyssynergic pattern of defecation, particularly when tested in left lateral position[64]. Since in healthy subjects experimentally-induced rectal distension accelerates gas evacuation and prevents gas retention[65], we hypothesized this mechanism underlying the demonstrated increase in flatulence. In fact, intestinal gas handling is a dynamic and efficient process that allows the normal gut to accommodate and evacuate large gas loads without inducing symptoms[65].
Although the relationship between bloating/abdominal distension and constipation can be intuitive, contrasting data have been published showing that also IBS-D patients claim for bloating. In 2006, Houghton et al[22] showed that in IBS patients there is a real increase in abdominal circumference (up to 12 cm in IBS) compared to healthy subjects. However, a direct correlation among bloating, abdominal distension and girth increase was evident in IBS-constipation patient only. In this study, the relationship of distension to subjective bloating showed a good correlation in constipation-predominant patients but not in diarrhea-predominant IBS[66]. Conflicting results were evidenced by Chang et al[11] who correlated IBS patterns with bloating and objective abdominal distension showing that patients complaining bloating plus abdominal distension were more frequently constipated (IBS-C), while those complaining of simple bloating were more frequently diarrhea-predominant (IBS-D). These data has been subsequently confirmed by Jiang et al[14] who reported that IBS-C patients, compared to controls, experienced more frequently abdominal distension (14-fold more likely) than IBS-D who report more frequently bloating alone. In the attempt to explain possible underlining mechanisms, Authors again implicate an impaired colonic transit with subsequent bacterial overgrowth, fermentation and increased abdominal girth distension, even if definitive data are still lacking.
Liquids: Once eaten, food is diluted in a large amount of gastric secretion in order to suspend and disperse solid parts and facilitate digestion and absorption mechanisms. Typically, bloating patients do not claim for symptoms when fasten, but shortly after a meal. Thus one can speculate that the liquid, proximal, small bowel stimulation can be responsible for symptoms in bloating patients. Moreover, it has been shown that in bloating patients a liquid infusion of lipids is associated with slow gut propulsive motility significantly more than in healthy individuals[67]. This stimulus seems to begin in the proximal small bowel for an impaired motor jejunum response, while distal ileum and colon are not affected by any change in motor patters. Moreover jejunal gas infusion, but not distal infusions, was able to induce abdominal symptoms[68].
Fat: An excessive weight is known to be correlated with some clinical manifestations of functional GI disorders, such as dyspepsia, however, the relationship between bloating and BMI is contradictory. Two clinical studies demonstrated that a BMI ≥ 30 positively correlates with bloating symptoms[69,70], while a recent meta-analysis showed that there was no relationship between bloating and increasing BMI[71].
Two interesting studies showed the possible association between weight gain and bloating. In 1994, Sullivan et al[72] indicated that about 40% of bloating complainers gained weight concurrently to the onset of symptoms and, in 2001, Chang et al[11] found that 24% of patients believed that their bloating symptoms began when weight started to increase. In our experience massive obese patients on a waiting list prior to bariatric surgery do not have a significant increase in intensity-frequency scores of bloating and flatulence compared to controls[34]. Thus one can speculate that the recent fat accumulation in the abdomen, not massive obesity as chronic condition may favor the development or awareness of bloating as a symptom.
Impaired abdominal empting
Even if intestinal motility since long is recognized as one of the key features in functional bowel disorders pathophysiology[73], its central role can be questioned when it comes to bloating generation using conventional techniques[74]. A recent study has also suggested that altered colon transit correlates poorly or not at all with IBS symptoms such as bloating, flatulence or pain[75]. On the other hand the Barcelona group has undertaken a series of elegant studies on intestinal gas transit and tolerance, which have helped our understanding of how gas might cause symptoms in IBS.
They have suggested that while gas volumes may be normal in patients with bloating, they have an impaired intestinal handling of gas loads[57,67,76,77]. This may be of particular relevance since a sensory-motor gut abnormality might represent the pathophysiological basis of bloating.
Lasser et al[57] evidenced that in a number of patients complaining of bloating the gas infused into the small bowel provokes both the flow back into the stomach of the gas and symptoms such as excessive gas, abdominal pain, and bloating. Over twenty years later, it has been shown that patients with IBS and functional bloating had impaired transit and tolerance of intestinal gas[67,76,77]. For example, they retained gas and complaining about their usual symptoms in response to infusion of gas, which was well tolerated by the majority of healthy subjects. Interestingly abdominal distension correlated with the volume of gas retained in their gut.
In healthy volunteers two important mechanisms have been demonstrated to give a possible explanation of the mechanisms of gas retention increased resistance to gas flow and impaired intestinal propulsion[77]. In particular, subjective abdominal complaints were much higher when it were produced by increased resistance to flow than when gas retention was induced by inhibition of propulsion produced by glucagon-induced motor inhibition. Therefore, if abdominal distention depends largely on the volume of gas retained independently of the mechanism (i.e., high-resistance barrier obstructing flow or an impaired propulsion), symptom perception depends on the mechanism of retention: at the same volumes, flow obstruction produces more symptoms than impaired propulsion. Conceivably, small gas bubbles, pushed against high-resistance barriers (maybe simultaneously at different points of the intestine), may increase intestinal wall tension and produce symptoms also by means of a spatial summation phenomena[58]. Hence a disorder involving the pelvic floor (defecation disorder) or the voluntary inhibition of anal flatus, might contribute to gas retention and symptoms.
The origin of such bowel motor alterations would be an abnormal reflex control. The transit of gas seems to happen secondary to tonic motor activity and unrelated to phasic activity[65,74,78], finely regulated by gastro-intestinal and intestine-intestinal reflexes[65,67,78-81]. Gas transit seems also being influenced by posture and physical activity[82-85], suggesting possible somato-visceral reflexes[80,81,86].
In healthy subjects it was shown that jejunal gas infusion produced, on similar gas volume retained and abdominal distension, more symptoms than rectal infusion[87]. These results indicate that the symptoms are influenced by the segment in which the gas is trapped.
Moreover, studies performed with a scintigraphic technique, using Xenon 133, have shown that in patients with bloating there is a impaired small-intestinal propulsion of gases[88], (interestingly the proximal small bowel is the site where are normally produced large amounts of carbon dioxide). More recently this alteration in gas handling was detected in the proximal colon[86,89] that is the first physiological site of colonic gas production. While alterations of the aforementioned intestine-intestinal reflexes could explain the small intestinal impaired gas transit[68,88], the proximal colon gas retention could be due to a proximal-to-distal uncoupling: low colon proximal tone, increased resistance to flow through the distal colon or both[77]. However, the clinical correlation remains unclear.
Altered intra-abdominal volume displacement
Abdomino-phrenic theory: One of the hypotheses for abdominal distension is that if the distension is not related to increased luminal contents, but to gas displacement. In other words, the overall volume is still the same, but is shifted from its original position.
The abdomen is limited by the vertebral column, which determines the configuration of the posterior abdominal wall, the diaphragm, and the anterolateral muscles. Furthermore, it is intuitive that abdominal wall mechanics and postural activity are different in supine position compared when the trunk is erect.
It has been showed firstly that in healthy individuals, the increase of intra-abdominal volume, using colonic gas infusion, causes girth enlargement and activates muscular contractions of the anterior abdominal wall and that the same phenomena is impaired in bloating patients that showed an altered pattern of abdominal contractions and an exaggerate girth increase caused by abdominal-wall dystony[90].
Subsequently, the same group tested in a series of elegant electromyographic (EMG) studies, the hypothesis of an impaired abdomino-phrenic coordination or abdomino-phrenic dyssynergia.
Using colonic gas infusions and EMG of the diaphragm and of the anterior wall, they found that in healthy individuals, studied in the in upright position, gas infusion causes a contraction of the muscles in the anterior abdominal wall (all except the internal oblique muscles already contracted as it exerts anti-gravitational activity)[90] and an adaptative relaxation of the diaphragm[91] leading to a volumetric increase of the abdominal cavity by an upper expansion and redistribution of intra-abdominal contents to accommodate the extra volume load preventing an exaggerated girth increments. These changes were observed also during more physiological conditions such as meal ingestion[92]. In supine position, these responses to volume load express a similar posture-dependent coordination: the diaphragm in this case contracts to exert anti-gravitational activity while the anterior abdominal wall remains inactive. Conversely, in functional patients, by the same experimental model, was shown a paradoxical relaxation of the anterior wall (inhibition of the internal oblique without a significant contraction of the rest of the anterior wall muscles)[90]. Moreover, by using a simultaneous abdominal CT scans observations in supine position, a marked and significant diaphragmatic drop was evidenced which correlated with the degree of anterior wall distension[93]. In particular, functional patients exhibit a paradoxical contraction of the diaphragm (change in EMG activity) that in combination with relaxation of the anterior wall muscles determined an altered redistribution of luminal content (Figure 4). It was further proven a contribution of the thorax to abdominal accommodation during gas load with a simultaneous intercostals muscles contraction coordinated with diaphragmatic relaxation and cephalad displacement, to increase thoracic perimeter[94]. These abnormal responses to gas load in patients, may be triggered by uncomfortable abdominal sensation[95,96]. So, even modest gas retention, alone or in conjunction with other factors such as meals, stress, etc., may induce symptoms and visible abdominal distension[8].
Figure 4.
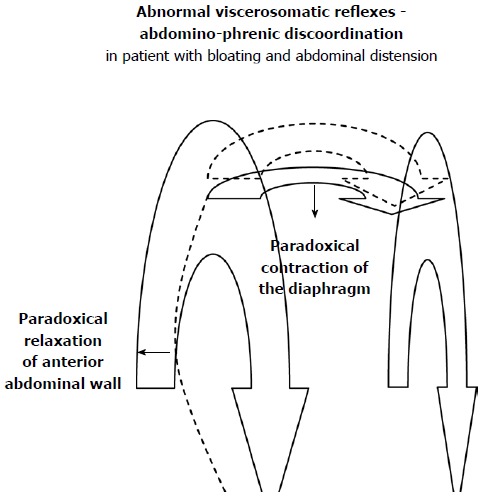
Altered intra-abdominal volume displacement as possible explanation of bloating. Bloating patients show an impaired abdomino-phrenic coordination with a paradoxical contraction of the diaphragm coupled with a relaxation of the abdominal wall leading to a modification in abdominal girth. Adapted from[129].
Increased perception of intestinal stimuli
Sensory dysfunction: As previously reported, patients with dyspepsia perceived and tolerated smaller meal volumes than healthy subjects[97,98]. Perception of intragastric content depends on stimulation of tension receptors in the gastric wall[99].
The difference between patients and healthy subjects is more pronounced in the postprandial period because the presence of nutrients increases visceral sensitivity in healthy subjects[100], but this effect is exaggerated in patients with dyspepsia[101]. Although during rectal distension not all IBS patients show visceral hypersensitivity, the presence of an altered visceral sensitivity is considered greatly important in the pathogenesis of IBS[10]. The gas challenge test proved a role of sensory disturbances in IBS patients, and recent clinical experiment has demonstrated that bloating without visible distension is associated with visceral hypersensitivity[8]. Moreover, IBS patients with bloating pay more attention to their abdominal symptoms, which is a kind of hyper-vigilance[102]. Therefore, altered sensory threshold combined with altered conscious perception may explain the mechanism of bloating. Moreover, a role for hormones has been hypothesized based on female predominance in IBS. Although healthy women may report changes in bowel function at the time of menstruation, this effect is far more pronounced in women with IBS with approximately 40%-75% claiming that bowel habit and bloating are exacerbated perimenstrually[11,72,103]. This suggests that bloating may be strongly influenced by hormonal status to modify visceral sensitivity. However, whether estrogens have pronociceptive or antinociceptive properties is still a controversial issue[104].
Psycological factors: The role of psychological factors in the etiology induction pathogenesis of bloating is still controversial. Some authors reported a strong link between symptoms of mental stress such as depression, sleeping difficulties, problems of coping and abdominal complaints, including bloating, in IBS patients[105], although this association was not confirmed by others[106].
Neri et al[107] rated abdominal bloating as one of the six useful symptoms on discriminating functional bowel disorders. More recently, Jiang et al[14] reported higher Somatic Symptom Checklist (a measure of somatization) scores in patients with bloating and distension, suggesting that the process of somatization could play a role. Further studies applying standardized psychiatric questionnaires and the Diagnostic and Statistical Manual of Mental Disorders criteria are needed to clarify the possible association between psychological factors and bloating.
TREATMENT
As a consequence of unclear pathophysiological mechanisms underlining bloating, the management remains mostly empiric. Currently, the primary therapeutic aim is limited on reducing gas production or accelerating colonic transit, but there is a lack of concrete scientific basis. Also, there are few trails assessing relief of bloating as primary end point, thus therapeutic options derive from data extrapolated from IBS studies with a consequent limited reliability.
A change in diet habits is commonly considered as first line therapy. Most of the primary care patients have already tested the efficacy of different exclusion diet before consulting a gastroenterologist. Among exclusion diets, recent evidences suggest a strong role of FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) in the genesis of IBS symptoms. Halmos et al[108] tested the effects of a low FODMAPs diet in a randomized, controlled, single-blind, cross-over trial of patients with IBS showing a reduction of overall IBS symptoms, including bloating, pain and passage of gas[39].
Evidences of a role of physical activity on bloating symptoms were studied by the Barcelona group. They showed that mild physical activity and upright position accelerate gas transit and reduce bloating and abdominal distension[87]. Theoretically, a change in hydrostatic forces distribution and increased intra-abdominal pressure during upright position or a propulsive gut motor response induced by exercise, promote gas transit. Non-pharmacological agents showed inconstant results. We speculated that mechanical compression of the abdominal wall could exert a passive effect on gas displacement and stimulate a propulsive gut motor response, making the gas evacuation easier and the symptoms to reduce. On the contrary, our data demonstrated that a mechanical massage of the abdominal wall does not modify colonic gas evacuation, perception and abdominal distension[86].
An interesting hypothesis is the possibility to correct the abnormal abdominal-phrenic coordination, which, as mentioned above, is one of the theories involved in the abdominal bloating genesis. Preliminary data show the effectiveness of a new treatments based on correction of inappropriate diaphragm contraction with bio-feedback techniques to prevent anterior protrusion of the abdominal wall and so manage bloating[109].
A second widely used approach to treat bloating is the use of antibiotics and/or probiotics. The rationale underling this drug is that an intestinal dysbiosis could increase gas production. In 2006, Sharara et al[110] demonstrated that a 10 d course of rifaximin is effective in reducing bloating symptoms without significant adverse events. Also, another interesting double blind, randomized, placebo-controlled trial, showed that patients with IBS (based on Rome I criteria) treated with rifaximin reported a significant improvement in bloating and IBS symptoms compared to patients who received placebo (P = 0.010)[111].
The same group in 2011 analyzing data from 2 large, double-blind, placebo-controlled studies for a total of 1260 non-constipated IBS patients (TARGET 1 and 2) found that, compared to placebo, patients in the rifaximin group were more likely to have adequate relief of bloating (P < 0.001)[112].
Regarding the use of probiotics, conflicting results were published in the last years. Most of the studies were limited for relevant methodological limitations, such as the use of different probiotic bacteria in each study and, the use of heterogeneous and not clearly defined study population. An interesting study in 2003 showed that in Rome II IBS-D patients, VSL#3 reduced post-treatment abdominal bloating (P = 0.046) and the same Authors in the subsequent trial in patients with IBS and significant abdominal bloating demonstrated that VSL#3 reduces specifically flatulence[113]. A recent trial of Lactobacillus acidophilus and Bifidobacterium lactis in patients with IBS found an improvement in bloating severity during an 8-wk trial period[114].
Conversely, our group found that a in IBS patients (Rome III criteria) a symbiotic mixture (Probinul, 5 g bid) for 4 wk was effective in decreasing the severity of flatulence in IBS patients, but failed to achieve an improvement in global satisfactory relief of flatulence and bloating[115] although maintenance for further 6 mo still showed a benefits on flatulence[116].
A recent metanalysis, among different items, specifically addressed the use of probiotics in bloating patients (Table 1). After including all published trials in which probiotics were tested to asses bloating or distension relief as primary or secondary endpoints in IBS or non IBS patients, conclusion was that specific probiotics can lead to bloating relief in some patients with IBS[117].
Table 1.
Summary of randomised clinical trials specifically assessing bloating reliefs
| Agent | Main outcome on bloating symptom | Rome criteria |
| Probiotics | Bifidobacterium bifidum MIMBb75: Improving bloating/distension[136] | II |
| Lactobacillus plantarum 299v: improvement[137] | III | |
| Multispecies: improvement[138] | III | |
| Symbiotics | Symbiotic mixture: no benefits[115,116] | III |
| Antibiotics | Rifaximin: reduction in bloating-specific scores[110] | II |
| Rifaximin: improvement (39.5% vs 28.7%)[112] | II | |
| Prokinetics | Tegaserod: improvement[120] | I |
| Tegaserod: improvement[139-141] | II | |
| Tegaserod: no differences vs placebo[142] | II | |
| Prucalopride: improvement[126] | II | |
| Antispasmodic | Otilonium bromide improvement[143] | II |
| Mebeverine vs Otilonium bromide: improvement significantly achieved by both treatments[144] | II | |
| Antidepressant | Paroxetine: no difference vs placebo[145] | I |
| Citalopram: improvement[146] | II | |
| Fluoxetine: improvement[147] | II |
Experimental data in healthy controls showed that glucagon, a potent muscle relaxant, reduced abdominal symptoms but gas retention and objective abdominal distension were unaffected[77]. Domperidone has been shown to be ineffective in IBS symptoms[118] whereas the administration of neostigmine, a prokinetic drug, produced gas clearance and improvement of both abdominal symptoms and objective distension[119]. Likewise the 5-HT4 receptor agonist tegaserod has demonstrated potent prokinetic effects on small intestinal and colonic transit in IBS patients with constipation, also reducing the symptoms of bloating[120]. Ischemic cardiac effects have been reported with tegaserod used in a patient population at potentially high risk of cardiovascular complications[121-123]. For these reasons, its use in clinical practice is very limited. Prucalopride is a highly selective 5-HT4 receptor agonist with strong enterokinetic activity[124,125] and requires lower dose for this therapeutic effect[119] making this drug potentially suitable for bloating patients[126]. Itopride, a dopamine receptors antagonist and acetylcholinesterase inhibitor, has shown conflicting results in patients with functional dyspepsia[127,128].
Other commonly used drugs to relieve bloating and more general IBS symptoms are antispasmodics (including peppermint oil), gas reducing agents such as simethicone and tricyclic antidepressants (TCAs).
Antispasmodics, such as mebeverine, otilonium, and trimebutine, act decreasing the tone and contractility of intestinal smooth muscle by an antimuscarinic effect. Those drugs are widely prescribed and whereas scientific evidence on relieving IBS symptom exists[129], efficacy on bloating is under discussion[130]. In a meta-analysis of therapeutic options in IBS, Ford et al[131] reviewed clinical trials on peppermint oil in IBS patients. Authors’ conclusions were that it was superior to placebo, although with a statistically significant heterogeneity among those studies (4 in total). In 2013, Khanna et al[132], reviewing published data on this drug, concluded that peppermint oil is significantly superior to placebo in controlling both IBS symptoms and abdominal pain with some minor and brief adverse events (manly heartburn).
As regard to gas adsorbing agents, the first licensed for bloating symptoms was metylpolysiloxane or syloxone then substituted by simethicone[133]. A combination of simethicone plus activated charcoal and magnesium oxide was tested against placebo in a double blind, randomized, multicenter trial in patients with FD meeting the Rome III criteria[134]. This study showed a reductions in the intensity of post-prandial fullness, epigastric pain, epigastric burning and bloating in the active treatment arm compared to placebo[134].
Low dose of antidepressants are effective treatment for IBS. The rationale behind the use of these drugs is that at low dosage they act, through an anticholinergic effect, similarly to spasmolytics agents. A recent systematic review and meta-analysis of 12 randomized controlled trials showed that TCAs are effective in the treatment of IBS while there is less high-quality evidence for psychological therapies in IBS[135].
In conclusion, advances in the understanding of functional bloating have accelerated in the past decade owing to increased attention placed on the definition and diagnostic criteria, and inroads into the understanding of the underlying pathophysiology. Other studies that may also add significant breakthroughs in therapy are welcomed.
Footnotes
P- Reviewer: Abraham P, Bortolotti M, de Medina FS, Tsujikawa T S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Panzuto F, Chiriatti A, Bevilacqua S, Giovannetti P, Russo G, Impinna S, Pistilli F, Capurso G, Annibale B, Delle Fave G. Symptom-based approach to colorectal cancer: survey of primary care physicians in Italy. Dig Liver Dis. 2003;35:869–875. doi: 10.1016/j.dld.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Croner RS, Brueckl WM, Reingruber B, Hohenberger W, Guenther K. Age and manifestation related symptoms in familial adenomatous polyposis. BMC Cancer. 2005;5:24. doi: 10.1186/1471-2407-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oustamanolakis P, Tack J. Dyspepsia: organic versus functional. J Clin Gastroenterol. 2012;46:175–190. doi: 10.1097/MCG.0b013e318241b335. [DOI] [PubMed] [Google Scholar]
- 4.Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, Patras J, Mahony BS, Andersen MR. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 5.Knill-Jones RP. A formal approach to symptoms in dyspepsia. Clin Gastroenterol. 1985;14:517–529. [PubMed] [Google Scholar]
- 6.Talley NJ, Phillips SF, Melton J, Wiltgen C, Zinsmeister AR. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–674. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 7.Lembo T, Naliboff B, Munakata J, Fullerton S, Saba L, Tung S, Schmulson M, Mayer EA. Symptoms and visceral perception in patients with pain-predominant irritable bowel syndrome. Am J Gastroenterol. 1999;94:1320–1326. doi: 10.1111/j.1572-0241.1999.01009.x. [DOI] [PubMed] [Google Scholar]
- 8.Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders--epidemiology and exploration of possible mechanisms. Aliment Pharmacol Ther. 2008;27:2–10. doi: 10.1111/j.1365-2036.2007.03549.x. [DOI] [PubMed] [Google Scholar]
- 9.Marcus SN, Heaton KW. Irritable bowel-type symptoms in spontaneous and induced constipation. Gut. 1987;28:156–159. doi: 10.1136/gut.28.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertz H, Naliboff B, Mayer EA. Symptoms and physiology in severe chronic constipation. Am J Gastroenterol. 1999;94:131–138. doi: 10.1111/j.1572-0241.1999.00783.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- 12.Caldarella MP, Azpiroz F, Malagelada JR. Antro-fundic dysfunctions in functional dyspepsia. Gastroenterology. 2003;124:1220–1229. doi: 10.1016/s0016-5085(03)00287-7. [DOI] [PubMed] [Google Scholar]
- 13.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Locke GR, Choung RS, Zinsmeister AR, Schleck CD, Talley NJ. Prevalence and risk factors for abdominal bloating and visible distention: a population-based study. Gut. 2008;57:756–763. doi: 10.1136/gut.2007.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandler RS, Stewart WF, Liberman JN, Ricci JA, Zorich NL. Abdominal pain, bloating, and diarrhea in the United States: prevalence and impact. Dig Dis Sci. 2000;45:1166–1171. doi: 10.1023/a:1005554103531. [DOI] [PubMed] [Google Scholar]
- 16.Ho KY, Kang JY, Seow A. Prevalence of gastrointestinal symptoms in a multiracial Asian population, with particular reference to reflux-type symptoms. Am J Gastroenterol. 1998;93:1816–1822. doi: 10.1111/j.1572-0241.1998.00526.x. [DOI] [PubMed] [Google Scholar]
- 17.Sach J, Bolus R, Fitzgerald L, Naliboff BD, Chang L, Mayer EA. Is there a difference between abdominal pain and discomfort in moderate to severe IBS patients? Am J Gastroenterol. 2002;97:3131–3138. doi: 10.1111/j.1572-0241.2002.07110.x. [DOI] [PubMed] [Google Scholar]
- 18.Wiklund IK, Fullerton S, Hawkey CJ, Jones RH, Longstreth GF, Mayer EA, Peacock RA, Wilson IK, Naesdal J. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38:947–954. doi: 10.1080/00365520310004209. [DOI] [PubMed] [Google Scholar]
- 19.Koloski NA, Jones M, Kalantar J, Weltman M, Zaguirre J, Talley NJ. Psychological impact and risk factors associated with new onset fecal incontinence. J Psychosom Res. 2012;73:464–468. doi: 10.1016/j.jpsychores.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Cain KC, Headstrom P, Jarrett ME, Motzer SA, Park H, Burr RL, Surawicz CM, Heitkemper MM. Abdominal pain impacts quality of life in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101:124–132. doi: 10.1111/j.1572-0241.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 21.Maxton DG, Martin DF, Whorwell PJ, Godfrey M. Abdominal distension in female patients with irritable bowel syndrome: exploration of possible mechanisms. Gut. 1991;32:662–664. doi: 10.1136/gut.32.6.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houghton LA, Lea R, Agrawal A, Reilly B, Whorwell PJ. Relationship of abdominal bloating to distention in irritable bowel syndrome and effect of bowel habit. Gastroenterology. 2006;131:1003–1010. doi: 10.1053/j.gastro.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Lewis MJ, Reilly B, Houghton LA, Whorwell PJ. Ambulatory abdominal inductance plethysmography: towards objective assessment of abdominal distension in irritable bowel syndrome. Gut. 2001;48:216–220. doi: 10.1136/gut.48.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suarez FL, Savaiano DA, Levitt MD. A comparison of symptoms after the consumption of milk or lactose-hydrolyzed milk by people with self-reported severe lactose intolerance. N Engl J Med. 1995;333:1–4. doi: 10.1056/NEJM199507063330101. [DOI] [PubMed] [Google Scholar]
- 25.Hebden JM, Blackshaw E, D’Amato M, Perkins AC, Spiller RC. Abnormalities of GI transit in bloated irritable bowel syndrome: effect of bran on transit and symptoms. Am J Gastroenterol. 2002;97:2315–2320. doi: 10.1111/j.1572-0241.2002.05985.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith RC, Greenbaum DS, Vancouver JB, Henry RC, Reinhart MA, Greenbaum RB, Dean HA, Mayle JE. Gender differences in Manning criteria in the irritable bowel syndrome. Gastroenterology. 1991;100:591–595. doi: 10.1016/0016-5085(91)80002-q. [DOI] [PubMed] [Google Scholar]
- 27.Thompson WG. Gender differences in irritable bowel symptoms. Eur J Gastroenterol Hepatol. 1997;9:299–302. doi: 10.1097/00042737-199703000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Toner BB, Akman D. Gender role and irritable bowel syndrome: literature review and hypothesis. Am J Gastroenterol. 2000;95:11–16. doi: 10.1111/j.1572-0241.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 29.Longstreth GF, Wilson A, Knight K, Wong J, Chiou CF, Barghout V, Frech F, Ofman JJ. Irritable bowel syndrome, health care use, and costs: a U.S. managed care perspective. Am J Gastroenterol. 2003;98:600–607. doi: 10.1111/j.1572-0241.2003.07296.x. [DOI] [PubMed] [Google Scholar]
- 30.Azpiroz F, Serra J. Treatment of Excessive Intestinal Gas. Curr Treat Options Gastroenterol. 2004;7:299–305. doi: 10.1007/s11938-004-0016-2. [DOI] [PubMed] [Google Scholar]
- 31.Azpiroz F, Malagelada JR. Abdominal bloating. Gastroenterology. 2005;129:1060–1078. doi: 10.1053/j.gastro.2005.06.062. [DOI] [PubMed] [Google Scholar]
- 32.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 33.Hemmink GJ, Weusten BL, Bredenoord AJ, Timmer R, Smout AJ. Aerophagia: excessive air swallowing demonstrated by esophageal impedance monitoring. Clin Gastroenterol Hepatol. 2009;7:1127–1129. doi: 10.1016/j.cgh.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 34.Santonicola A, Siniscalchi M, Capone P, Gallotta S, Ciacci C, Iovino P. Prevalence of functional dyspepsia and its subgroups in patients with eating disorders. World J Gastroenterol. 2012;18:4379–4385. doi: 10.3748/wjg.v18.i32.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernández-Bañares F, Esteve-Pardo M, de Leon R, Humbert P, Cabré E, Llovet JM, Gassull MA. Sugar malabsorption in functional bowel disease: clinical implications. Am J Gastroenterol. 1993;88:2044–2050. [PubMed] [Google Scholar]
- 36.Rumessen JJ, Gudmand-Høyer E. Functional bowel disease: malabsorption and abdominal distress after ingestion of fructose, sorbitol, and fructose-sorbitol mixtures. Gastroenterology. 1988;95:694–700. doi: 10.1016/s0016-5085(88)80016-7. [DOI] [PubMed] [Google Scholar]
- 37.Symons P, Jones MP, Kellow JE. Symptom provocation in irritable bowel syndrome. Effects of differing doses of fructose-sorbitol. Scand J Gastroenterol. 1992;27:940–944. doi: 10.3109/00365529209000167. [DOI] [PubMed] [Google Scholar]
- 38.McKee AM, Prior A, Whorwell PJ. Exclusion diets in irritable bowel syndrome: are they worthwhile? J Clin Gastroenterol. 1987;9:526–528. doi: 10.1097/00004836-198710000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Serra J. Intestinal gas: has diet anything to do in the absence of a demonstrable malabsorption state? Curr Opin Clin Nutr Metab Care. 2012;15:489–493. doi: 10.1097/MCO.0b013e328356662d. [DOI] [PubMed] [Google Scholar]
- 40.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–419. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 41.Hasler WL. Lactulose breath testing, bacterial overgrowth, and IBS: just a lot of hot air? Gastroenterology. 2003;125:1898–1900; discussion 1900. doi: 10.1053/j.gastro.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 42.Spiller RC. Postinfectious irritable bowel syndrome. Gastroenterology. 2003;124:1662–1671. doi: 10.1016/s0016-5085(03)00324-x. [DOI] [PubMed] [Google Scholar]
- 43.Madden JA, Hunter JO. A review of the role of the gut microflora in irritable bowel syndrome and the effects of probiotics. Br J Nutr. 2002;88 Suppl 1:S67–S72. doi: 10.1079/BJN2002631. [DOI] [PubMed] [Google Scholar]
- 44.Kassinen A, Krogius-Kurikka L, Mäkivuokko H, Rinttilä T, Paulin L, Corander J, Malinen E, Apajalahti J, Palva A. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 45.King TS, Elia M, Hunter JO. Abnormal colonic fermentation in irritable bowel syndrome. Lancet. 1998;352:1187–1189. doi: 10.1016/s0140-6736(98)02146-1. [DOI] [PubMed] [Google Scholar]
- 46.Suarez FL. Intestinal gas. In: Feldman M, Friedman LS, Sleisenger MH, editors. Gastrointestinal and liver diseases: pathophysiology/diagnosis/management. Philadelphia: Elsevier Medicine; 2002. pp. 155–163. [Google Scholar]
- 47.Levitt MD, Bond JH. Volume, composition, and source of intestinal gas. Gastroenterology. 1970;59:921–929. [PubMed] [Google Scholar]
- 48.Suarez F, Furne J, Springfield J, Levitt M. Insights into human colonic physiology obtained from the study of flatus composition. Am J Physiol. 1997;272:G1028–G1033. doi: 10.1152/ajpgi.1997.272.5.G1028. [DOI] [PubMed] [Google Scholar]
- 49.Flourié B, Pellier P, Florent C, Marteau P, Pochart P, Rambaud JC. Site and substrates for methane production in human colon. Am J Physiol. 1991;260:G752–G757. doi: 10.1152/ajpgi.1991.260.5.G752. [DOI] [PubMed] [Google Scholar]
- 50.Kajs TM, Fitzgerald JA, Buckner RY, Coyle GA, Stinson BS, Morel JG, Levitt MD. Influence of a methanogenic flora on the breath H2 and symptom response to ingestion of sorbitol or oat fiber. Am J Gastroenterol. 1997;92:89–94. [PubMed] [Google Scholar]
- 51.Strocchi A, Levitt MD. Factors affecting hydrogen production and consumption by human fecal flora. The critical roles of hydrogen tension and methanogenesis. J Clin Invest. 1992;89:1304–1311. doi: 10.1172/JCI115716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strocchi A, Furne J, Ellis C, Levitt MD. Methanogens outcompete sulphate reducing bacteria for H2 in the human colon. Gut. 1994;35:1098–1101. doi: 10.1136/gut.35.8.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Forster RE. Physiological basis of gas exchange in the gut. Ann N Y Acad Sci. 1968;150:4–12. doi: 10.1111/j.1749-6632.1968.tb19024.x. [DOI] [PubMed] [Google Scholar]
- 54.Pogrund RS, Steggerda FR. Influence of gaseous transfer between the colon and blood stream on percentage gas compositions of intestinal flatus in man. Am J Physiol. 1948;153:475–482. doi: 10.1152/ajplegacy.1948.153.3.475. [DOI] [PubMed] [Google Scholar]
- 55.Haderstorfer B, Psycholgin D, Whitehead WE, Schuster MM. Intestinal gas production from bacterial fermentation of undigested carbohydrate in irritable bowel syndrome. Am J Gastroenterol. 1989;84:375–378. [PubMed] [Google Scholar]
- 56.Serra J, Azpiroz F, Malagelada JR. Intestinal gas dynamics and tolerance in humans. Gastroenterology. 1998;115:542–550. doi: 10.1016/s0016-5085(98)70133-7. [DOI] [PubMed] [Google Scholar]
- 57.Lasser RB, Bond JH, Levitt MD. The role of intestinal gas in functional abdominal pain. N Engl J Med. 1975;293:524–526. doi: 10.1056/NEJM197509112931103. [DOI] [PubMed] [Google Scholar]
- 58.Serra J, Azpiroz F, Malagelada JR. Modulation of gut perception in humans by spatial summation phenomena. J Physiol. 1998;506(Pt 2):579–587. doi: 10.1111/j.1469-7793.1998.579bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chami TN, Schuster MM, Bohlman ME, Pulliam TJ, Kamal N, Whitehead WE. A simple radiologic method to estimate the quantity of bowel gas. Am J Gastroenterol. 1991;86:599–602. [PubMed] [Google Scholar]
- 60.Koide A, Yamaguchi T, Odaka T, Koyama H, Tsuyuguchi T, Kitahara H, Ohto M, Saisho H. Quantitative analysis of bowel gas using plain abdominal radiograph in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95:1735–1741. doi: 10.1111/j.1572-0241.2000.02189.x. [DOI] [PubMed] [Google Scholar]
- 61.Agrawal A, Houghton LA, Reilly B, Morris J, Whorwell PJ. Bloating and distension in irritable bowel syndrome: the role of gastrointestinal transit. Am J Gastroenterol. 2009;104:1998–2004. doi: 10.1038/ajg.2009.251. [DOI] [PubMed] [Google Scholar]
- 62.Shim L, Prott G, Hansen RD, Simmons LE, Kellow JE, Malcolm A. Prolonged balloon expulsion is predictive of abdominal distension in bloating. Am J Gastroenterol. 2010;105:883–887. doi: 10.1038/ajg.2010.54. [DOI] [PubMed] [Google Scholar]
- 63.Iovino P, Chiarioni G, Bilancio G, Cirillo M, Mekjavic IB, Pisot R, Ciacci C. New onset of constipation during long-term physical inactivity: a proof-of-concept study on the immobility-induced bowel changes. PLoS One. 2013;8:e72608. doi: 10.1371/journal.pone.0072608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feyen BJ, Rao SS. Functional disorders of defecation: evaluation and treatment. Curr Treat Options Gastroenterol. 2007;10:221–230. doi: 10.1007/s11938-007-0015-1. [DOI] [PubMed] [Google Scholar]
- 65.Harder H, Serra J, Azpiroz F, Malagelada JR. Reflex control of intestinal gas dynamics and tolerance in humans. Am J Physiol Gastrointest Liver Physiol. 2004;286:G89–G94. doi: 10.1152/ajpgi.00174.2003. [DOI] [PubMed] [Google Scholar]
- 66.Kii Y, Ito T. Effects of 5-HT4-receptor agonists, cisapride, mosapride citrate, and zacopride, on cardiac action potentials in guinea pig isolated papillary muscles. J Cardiovasc Pharmacol. 1997;29:670–675. doi: 10.1097/00005344-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 67.Serra J, Azpiroz F, Malagelada JR. Gastric distension and duodenal lipid infusion modulate intestinal gas transit and tolerance in humans. Am J Gastroenterol. 2002;97:2225–2230. doi: 10.1111/j.1572-0241.2002.05976.x. [DOI] [PubMed] [Google Scholar]
- 68.Salvioli B, Serra J, Azpiroz F, Malagelada JR. Impaired small bowel gas propulsion in patients with bloating during intestinal lipid infusion. Am J Gastroenterol. 2006;101:1853–1857. doi: 10.1111/j.1572-0241.2006.00702.x. [DOI] [PubMed] [Google Scholar]
- 69.Delgado-Aros S, Locke GR, Camilleri M, Talley NJ, Fett S, Zinsmeister AR, Melton LJ. Obesity is associated with increased risk of gastrointestinal symptoms: a population-based study. Am J Gastroenterol. 2004;99:1801–1806. doi: 10.1111/j.1572-0241.2004.30887.x. [DOI] [PubMed] [Google Scholar]
- 70.Talley NJ, Quan C, Jones MP, Horowitz M. Association of upper and lower gastrointestinal tract symptoms with body mass index in an Australian cohort. Neurogastroenterol Motil. 2004;16:413–419. doi: 10.1111/j.1365-2982.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 71.Eslick GD. Gastrointestinal symptoms and obesity: a meta-analysis. Obes Rev. 2012;13:469–479. doi: 10.1111/j.1467-789X.2011.00969.x. [DOI] [PubMed] [Google Scholar]
- 72.Sullivan SN. A prospective study of unexplained visible abdominal bloating. N Z Med J. 1994;107:428–430. [PubMed] [Google Scholar]
- 73.Cann PA, Read NW, Brown C, Hobson N, Holdsworth CD. Irritable bowel syndrome: relationship of disorders in the transit of a single solid meal to symptom patterns. Gut. 1983;24:405–411. doi: 10.1136/gut.24.5.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galati JS, McKee DP, Quigley EM. Response to intraluminal gas in irritable bowel syndrome. Motility versus perception. Dig Dis Sci. 1995;40:1381–1387. doi: 10.1007/BF02065555. [DOI] [PubMed] [Google Scholar]
- 75.Törnblom H, Van Oudenhove L, Sadik R, Abrahamsson H, Tack J, Simrén M. Colonic transit time and IBS symptoms: what’s the link? Am J Gastroenterol. 2012;107:754–760. doi: 10.1038/ajg.2012.5. [DOI] [PubMed] [Google Scholar]
- 76.Caldarella MP, Serra J, Azpiroz F, Malagelada JR. Prokinetic effects in patients with intestinal gas retention. Gastroenterology. 2002;122:1748–1755. doi: 10.1053/gast.2002.33658. [DOI] [PubMed] [Google Scholar]
- 77.Serra J, Azpiroz F, Malagelada JR. Mechanisms of intestinal gas retention in humans: impaired propulsion versus obstructed evacuation. Am J Physiol Gastrointest Liver Physiol. 2001;281:G138–G143. doi: 10.1152/ajpgi.2001.281.1.G138. [DOI] [PubMed] [Google Scholar]
- 78.Tremolaterra F, Villoria A, Serra J, Azpiroz F, Malagelada JR. Intestinal tone and gas motion. Neurogastroenterol Motil. 2006;18:905–910. doi: 10.1111/j.1365-2982.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 79.Hernando-Harder AC, Serra J, Azpiroz F, Malagelada JR. Sites of symptomatic gas retention during intestinal lipid perfusion in healthy subjects. Gut. 2004;53:661–665. doi: 10.1136/gut.2003.026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serra J, Salvioli B, Azpiroz F, Malagelada JR. Lipid-induced intestinal gas retention in irritable bowel syndrome. Gastroenterology. 2002;123:700–706. doi: 10.1053/gast.2002.35394. [DOI] [PubMed] [Google Scholar]
- 81.Passos MC, Serra J, Azpiroz F, Tremolaterra F, Malagelada JR. Impaired reflex control of intestinal gas transit in patients with abdominal bloating. Gut. 2005;54:344–348. doi: 10.1136/gut.2003.038158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 83.Dainese R, Serra J, Azpiroz F, Malagelada JR. Influence of body posture on intestinal transit of gas. Gut. 2003;52:971–974. doi: 10.1136/gut.52.7.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dainese R, Serra J, Azpiroz F, Malagelada JR. Effects of physical activity on intestinal gas transit and evacuation in healthy subjects. Am J Med. 2004;116:536–539. doi: 10.1016/j.amjmed.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 85.Villoria A, Serra J, Azpiroz F, Malagelada JR. Physical activity and intestinal gas clearance in patients with bloating. Am J Gastroenterol. 2006;101:2552–2557. doi: 10.1111/j.1572-0241.2006.00873.x. [DOI] [PubMed] [Google Scholar]
- 86.Tremolaterra F, Pascariello A, Gallotta S, Ciacci C, Iovino P. Colonic gas transit in patients with bloating: the effect of an electromechanical stimulator of the abdominal wall. Tech Coloproctol. 2013;17:405–410. doi: 10.1007/s10151-012-0951-1. [DOI] [PubMed] [Google Scholar]
- 87.Harder H, Serra J, Azpiroz F, Passos MC, Aguadé S, Malagelada JR. Intestinal gas distribution determines abdominal symptoms. Gut. 2003;52:1708–1713. doi: 10.1136/gut.52.12.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salvioli B, Serra J, Azpiroz F, Lorenzo C, Aguade S, Castell J, Malagelada JR. Origin of gas retention and symptoms in patients with bloating. Gastroenterology. 2005;128:574–579. doi: 10.1053/j.gastro.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 89.Hernando-Harder AC, Serra J, Azpiroz F, Milà M, Aguadé S, Malagelada C, Tremolaterra F, Villoria A, Malagelada JR. Colonic responses to gas loads in subgroups of patients with abdominal bloating. Am J Gastroenterol. 2010;105:876–882. doi: 10.1038/ajg.2010.75. [DOI] [PubMed] [Google Scholar]
- 90.Tremolaterra F, Villoria A, Azpiroz F, Serra J, Aguadé S, Malagelada JR. Impaired viscerosomatic reflexes and abdominal-wall dystony associated with bloating. Gastroenterology. 2006;130:1062–1068. doi: 10.1053/j.gastro.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 91.Villoria A, Azpiroz F, Soldevilla A, Perez F, Malagelada JR. Abdominal accommodation: a coordinated adaptation of the abdominal wall to its content. Am J Gastroenterol. 2008;103:2807–2815. doi: 10.1111/j.1572-0241.2008.02141.x. [DOI] [PubMed] [Google Scholar]
- 92.Burri E, Cisternas D, Villoria A, Accarino A, Soldevilla A, Malagelada JR, Azpiroz F. Abdominal accommodation induced by meal ingestion: differential responses to gastric and colonic volume loads. Neurogastroenterol Motil. 2013;25:339–e253. doi: 10.1111/nmo.12068. [DOI] [PubMed] [Google Scholar]
- 93.Accarino A, Perez F, Azpiroz F, Quiroga S, Malagelada JR. Abdominal distention results from caudo-ventral redistribution of contents. Gastroenterology. 2009;136:1544–1551. doi: 10.1053/j.gastro.2009.01.067. [DOI] [PubMed] [Google Scholar]
- 94.Burri E, Cisternas D, Villoria A, Accarino A, Soldevilla A, Malagelada JR, Azpiroz F. Accommodation of the abdomen to its content: integrated abdomino-thoracic response. Neurogastroenterol Motil. 2012;24:312–e162. doi: 10.1111/j.1365-2982.2011.01846.x. [DOI] [PubMed] [Google Scholar]
- 95.Azpiroz F, Bouin M, Camilleri M, Mayer EA, Poitras P, Serra J, Spiller RC. Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil. 2007;19:62–88. doi: 10.1111/j.1365-2982.2006.00875.x. [DOI] [PubMed] [Google Scholar]
- 96.Kellow JE, Azpiroz F, Delvaux M, Gebhart GF, Mertz HR, Quigley EM, Smout AJ. Applied principles of neurogastroenterology: physiology/motility sensation. Gastroenterology. 2006;130:1412–1420. doi: 10.1053/j.gastro.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 97.Kindt S, Tack J. Impaired gastric accommodation and its role in dyspepsia. Gut. 2006;55:1685–1691. doi: 10.1136/gut.2005.085365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 99.Tack J, Caenepeel P, Corsetti M, Janssens J. Role of tension receptors in dyspeptic patients with hypersensitivity to gastric distention. Gastroenterology. 2004;127:1058–1066. doi: 10.1053/j.gastro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 100.Accarino AM, Azpiroz F, Malagelada JR. Modification of small bowel mechanosensitivity by intestinal fat. Gut. 2001;48:690–695. doi: 10.1136/gut.48.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Feinle C, Rades T, Otto B, Fried M. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology. 2001;120:1100–1107. doi: 10.1053/gast.2001.23232. [DOI] [PubMed] [Google Scholar]
- 102.Accarino AM, Azpiroz F, Malagelada JR. Attention and distraction: effects on gut perception. Gastroenterology. 1997;113:415–422. doi: 10.1053/gast.1997.v113.pm9247458. [DOI] [PubMed] [Google Scholar]
- 103.Heitkemper MM, Jarrett M, Cain KC, Shaver J, Walker E, Lewis L. Daily gastrointestinal symptoms in women with and without a diagnosis of IBS. Dig Dis Sci. 1995;40:1511–1519. doi: 10.1007/BF02285200. [DOI] [PubMed] [Google Scholar]
- 104.Cervero F. Visceral versus somatic pain: similarities and differences. Dig Dis. 2009;27 Suppl 1:3–10. doi: 10.1159/000268115. [DOI] [PubMed] [Google Scholar]
- 105.Johnsen R, Jacobsen BK, Førde OH. Associations between symptoms of irritable colon and psychological and social conditions and lifestyle. Br Med J (Clin Res Ed) 1986;292:1633–1635. doi: 10.1136/bmj.292.6536.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song JY, Merskey H, Sullivan S, Noh S. Anxiety and depression in patients with abdominal bloating. Can J Psychiatry. 1993;38:475–479. doi: 10.1177/070674379303800703. [DOI] [PubMed] [Google Scholar]
- 107.Neri M, Laterza F, Howell S, Di Gioacchino M, Festi D, Ballone E, Cuccurullo F, Talley NJ. Symptoms discriminate irritable bowel syndrome from organic gastrointestinal diseases and food allergy. Eur J Gastroenterol Hepatol. 2000;12:981–988. doi: 10.1097/00042737-200012090-00003. [DOI] [PubMed] [Google Scholar]
- 108.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 109.Burri E, Azpiroz A, Hernandez C, Accarino A, Malagelada JR. Biofeedback treatment of abdominal distention: a proof-of-concept. Gut. 2010;59:A137. [Google Scholar]
- 110.Sharara AI, Aoun E, Abdul-Baki H, Mounzer R, Sidani S, Elhajj I. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–333. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 111.Pimentel M, Park S, Mirocha J, Kane SV, Kong Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: a randomized trial. Ann Intern Med. 2006;145:557–563. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 112.Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, Mareya SM, Shaw AL, Bortey E, Forbes WP. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. 2011;364:22–32. doi: 10.1056/NEJMoa1004409. [DOI] [PubMed] [Google Scholar]
- 113.Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, Thomforde G, Zinsmeister AR. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil. 2005;17:687–696. doi: 10.1111/j.1365-2982.2005.00695.x. [DOI] [PubMed] [Google Scholar]
- 114.Ringel-Kulka T, Palsson OS, Maier D, Carroll I, Galanko JA, Leyer G, Ringel Y. Probiotic bacteria Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 versus placebo for the symptoms of bloating in patients with functional bowel disorders: a double-blind study. J Clin Gastroenterol. 2011;45:518–525. doi: 10.1097/MCG.0b013e31820ca4d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cappello C, Tremolaterra F, Pascariello A, Ciacci C, Iovino P. A randomised clinical trial (RCT) of a symbiotic mixture in patients with irritable bowel syndrome (IBS): effects on symptoms, colonic transit and quality of life. Int J Colorectal Dis. 2013;28:349–358. doi: 10.1007/s00384-012-1552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bucci C, Tremolaterra F, Gallotta S, Fortunato A, Cappello C, Ciacci C, Iovino P. A pilot study on the effect of a symbiotic mixture in irritable bowel syndrome: an open-label, partially controlled, 6-month extension of a previously published trial. Tech Coloproctol. 2014;18:345–353. doi: 10.1007/s10151-013-1055-2. [DOI] [PubMed] [Google Scholar]
- 117.Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cann PA, Read NW, Holdsworth CD. Oral domperidone: double blind comparison with placebo in irritable bowel syndrome. Gut. 1983;24:1135–1140. doi: 10.1136/gut.24.12.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Maeyer JH, Lefebvre RA, Schuurkes JA. 5-HT4 receptor agonists: similar but not the same. Neurogastroenterol Motil. 2008;20:99–112. doi: 10.1111/j.1365-2982.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 120.Müller-Lissner SA, Fumagalli I, Bardhan KD, Pace F, Pecher E, Nault B, Rüegg P. Tegaserod, a 5-HT(4) receptor partial agonist, relieves symptoms in irritable bowel syndrome patients with abdominal pain, bloating and constipation. Aliment Pharmacol Ther. 2001;15:1655–1666. doi: 10.1046/j.1365-2036.2001.01094.x. [DOI] [PubMed] [Google Scholar]
- 121.Busti AJ, Murillo JR, Cryer B. Tegaserod-induced myocardial infarction: case report and hypothesis. Pharmacotherapy. 2004;24:526–531. doi: 10.1592/phco.24.5.526.33351. [DOI] [PubMed] [Google Scholar]
- 122.Pfannkuche HJ, Dennis E. Tegaserod-induced myocardial infarction: case report and hypothesis--an alternative viewpoint. Pharmacotherapy. 2004;24:1649–1650; discussion 1650-1651. doi: 10.1592/phco.24.16.1649.50956. [DOI] [PubMed] [Google Scholar]
- 123.Thompson CA. Novartis suspends tegaserod sales at FDA’s request. Am J Health Syst Pharm. 2007;64:1020. doi: 10.2146/news070044. [DOI] [PubMed] [Google Scholar]
- 124.Bouras EP, Camilleri M, Burton DD, McKinzie S. Selective stimulation of colonic transit by the benzofuran 5HT4 agonist, prucalopride, in healthy humans. Gut. 1999;44:682–686. doi: 10.1136/gut.44.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Schryver AM, Andriesse GI, Samsom M, Smout AJ, Gooszen HG, Akkermans LM. The effects of the specific 5HT(4) receptor agonist, prucalopride, on colonic motility in healthy volunteers. Aliment Pharmacol Ther. 2002;16:603–612. doi: 10.1046/j.1365-2036.2002.01195.x. [DOI] [PubMed] [Google Scholar]
- 126.Tack J, Stanghellini V, Dubois D, Joseph A, Vandeplassche L, Kerstens R. Effect of prucalopride on symptoms of chronic constipation. Neurogastroenterol Motil. 2014;26:21–27. doi: 10.1111/nmo.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sun J, Yuan YZ, Holtmann G. Itopride in the treatment of functional dyspepsia in Chinese patients: a prospective, multicentre, post-marketing observational study. Clin Drug Investig. 2011;31:865–875. doi: 10.1007/BF03256924. [DOI] [PubMed] [Google Scholar]
- 128.Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 129.Poynard T, Regimbeau C, Benhamou Y. Meta-analysis of smooth muscle relaxants in the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:355–361. doi: 10.1046/j.1365-2036.2001.00937.x. [DOI] [PubMed] [Google Scholar]
- 130.Boeckxstaens G, Corazziari ES, Mearin F, Tack J. IBS and the role of otilonium bromide. Int J Colorectal Dis. 2013;28:295–304. doi: 10.1007/s00384-012-1598-0. [DOI] [PubMed] [Google Scholar]
- 131.Ford AC, Talley NJ, Spiegel BM, Foxx-Orenstein AE, Schiller L, Quigley EM, Moayyedi P. Effect of fibre, antispasmodics, and peppermint oil in the treatment of irritable bowel syndrome: systematic review and meta-analysis. BMJ. 2008;337:a2313. doi: 10.1136/bmj.a2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505–512. doi: 10.1097/MCG.0b013e3182a88357. [DOI] [PubMed] [Google Scholar]
- 133.Kimura KK, Treon JF, Benson FR. Therapeutic use of methylpolysiloxane. Curr Ther Res Clin Exp. 1964;6:202–216. [PubMed] [Google Scholar]
- 134.Coffin B, Bortolloti C, Bourgeois O, Denicourt L. Efficacy of a simethicone, activated charcoal and magnesium oxide combination (Carbosymag®) in functional dyspepsia: results of a general practice-based randomized trial. Clin Res Hepatol Gastroenterol. 2011;35:494–499. doi: 10.1016/j.clinre.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 135.Ford AC, Talley NJ, Schoenfeld PS, Quigley EM, Moayyedi P. Efficacy of antidepressants and psychological therapies in irritable bowel syndrome: systematic review and meta-analysis. Gut. 2009;58:367–378. doi: 10.1136/gut.2008.163162. [DOI] [PubMed] [Google Scholar]
- 136.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33:1123–1132. doi: 10.1111/j.1365-2036.2011.04633.x. [DOI] [PubMed] [Google Scholar]
- 137.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol. 2012;18:4012–4018. doi: 10.3748/wjg.v18.i30.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, Lee HL, Yoon BC, Choi HS, Chung WS, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29:52–59. doi: 10.1111/jgh.12322. [DOI] [PubMed] [Google Scholar]
- 139.Tack J, Müller-Lissner S, Bytzer P, Corinaldesi R, Chang L, Viegas A, Schnekenbuehl S, Dunger-Baldauf C, Rueegg P. A randomised controlled trial assessing the efficacy and safety of repeated tegaserod therapy in women with irritable bowel syndrome with constipation. Gut. 2005;54:1707–1713. doi: 10.1136/gut.2005.070789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamm MA, Müller-Lissner S, Talley NJ, Tack J, Boeckxstaens G, Minushkin ON, Kalinin A, Dzieniszewski J, Haeck P, Fordham F, Hugot-Cournez S, Nault B. Tegaserod for the treatment of chronic constipation: a randomized, double-blind, placebo-controlled multinational study. Am J Gastroenterol. 2005;100:362–372. doi: 10.1111/j.1572-0241.2005.40749.x. [DOI] [PubMed] [Google Scholar]
- 141.Vakil N, Laine L, Talley NJ, Zakko SF, Tack J, Chey WD, Kralstein J, Earnest DL, Ligozio G, Cohard-Radice M. Tegaserod treatment for dysmotility-like functional dyspepsia: results of two randomized, controlled trials. Am J Gastroenterol. 2008;103:1906–1919. doi: 10.1111/j.1572-0241.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 142.Chey WD, Paré P, Viegas A, Ligozio G, Shetzline MA. Tegaserod for female patients suffering from IBS with mixed bowel habits or constipation: a randomized controlled trial. Am J Gastroenterol. 2008;103:1217–1225. doi: 10.1111/j.1572-0241.2008.01808.x. [DOI] [PubMed] [Google Scholar]
- 143.Clavé P, Acalovschi M, Triantafillidis JK, Uspensky YP, Kalayci C, Shee V, Tack J. Randomised clinical trial: otilonium bromide improves frequency of abdominal pain, severity of distention and time to relapse in patients with irritable bowel syndrome. Aliment Pharmacol Ther. 2011;34:432–442. doi: 10.1111/j.1365-2036.2011.04730.x. [DOI] [PubMed] [Google Scholar]
- 144.Chang FY, Lu CL, Luo JC, Chen TS, Chen MJ, Chang HJ. The evaluation of otilonium bromide treatment in asian patients with irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:402–410. doi: 10.5056/jnm.2011.17.4.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tabas G, Beaves M, Wang J, Friday P, Mardini H, Arnold G. Paroxetine to treat irritable bowel syndrome not responding to high-fiber diet: a double-blind, placebo-controlled trial. Am J Gastroenterol. 2004;99:914–920. doi: 10.1111/j.1572-0241.2004.04127.x. [DOI] [PubMed] [Google Scholar]
- 146.Tack J, Broekaert D, Fischler B, Van Oudenhove L, Gevers AM, Janssens J. A controlled crossover study of the selective serotonin reuptake inhibitor citalopram in irritable bowel syndrome. Gut. 2006;55:1095–1103. doi: 10.1136/gut.2005.077503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vahedi H, Merat S, Rashidioon A, Ghoddoosi A, Malekzadeh R. The effect of fluoxetine in patients with pain and constipation-predominant irritable bowel syndrome: a double-blind randomized-controlled study. Aliment Pharmacol Ther. 2005;22:381–385. doi: 10.1111/j.1365-2036.2005.02566.x. [DOI] [PubMed] [Google Scholar]


