Abstract
Background
Anopheles (Nyssorhynchus) albitarsis (Diptera: Culicidae) is one of the very few South American mosquito vectors of malaria successfully colonized in the laboratory. These vectors are very hard to breed because they rarely mate in artificial conditions. A few years ago a free-mating laboratory colony of An. albitarsis sensu stricto was established after about 30 generations of artificial-mating. To begin to understand the process of adaptation of these malaria vectors to the laboratory we have compared the insemination rates of colony mosquitoes to those from the original population in both artificial and free-mating crosses. We also carried out crossing experiments between the two types of mosquitoes for a preliminary analysis of the genetic basis of such adaptation.
Results
We show that, compared to the original population, colony males but not females have increased their insemination rates in the laboratory in both types of mating, suggesting that faster-male evolution of mating ability might have occurred during the colonization process.
Conclusions
The results are consistent with the faster-male theory, which predicts that sexual selection will cause faster rates of evolution of genes expressed in males. The data also suggests that attempts to colonize other South American malaria mosquitoes will be more successful if special attention is given to the male ability to mate in a confined space.
Background
South American mosquito vectors of malaria are notoriously difficult to breed in the laboratory. The main difficulty in the colonization of these mosquitoes (a medical entomology term meaning the establishment of a laboratory strain) lies in their near complete inability to mate in artificial conditions. One of the very few South American species to have been successfully adapted to laboratory conditions to date is Anopheles (Nyssorhynchus) albitarsis. This distant cousin of the African mosquito An. gambiae [1] is believed to be a complex of sibling species [2-6] that were implicated as important malaria vectors in some areas of South America [7-15]. A viable free-mating laboratory colony of An. albitarsis sensu stricto has existed for about 10 years now [16] and it was successfully obtained after a number of generations of artificial-mating [17], a procedure in which copulation is induced after the male's head is cut off. After about 30 generations of artificial-mating, the mosquitoes were allowed to mate freely and the An. albitarsis colony has remained viable since then (around 120 generations).
Understanding the process of adaptation of An. albitarsis to laboratory conditions might be useful to future attempts to colonize other important South American vectors such as Anopheles darlingi. Because the main difficulty in colonization is related to mating in the laboratory, we compared the insemination rates of colony mosquitoes with the F1 progeny of wild-caught females from the original population used to establish the colony [16] to verify if the former have increased their mating ability in artificial and free-mating crosses and whether the changes occurred in both sexes. We also carried out crossing experiments between the two types of mosquitoes for a preliminary analysis of the genetic basis of such changes.
Results
Table 1 shows the insemination rates in artificial-mating crosses between An. albitarsis from the laboratory colony, F1 mosquitoes of the original population (feral) and their reciprocal hybrids. Initially, we carried out a log-linear analysis comparing crosses between colony and feral mosquitoes. The results show a highly significant effect of male genotype on insemination rates (X2 = 14.437; d.f. = 2; p = 0.0007) but no effect of female origin (X2 = 1.572; d.f. = 2; p = 0.4556) or interaction between the sexes (X2 = 0.747; d.f. = 1; p = 0.3873). In other words the genotype of the females did not matter. Inspection of table 1 show that the rates observed for colony males were all around 70%, ranging from 65.5% to 77.6%. On the other hand, crosses using males from the original population (feral) showed lower insemination rates either in crosses to colony or to feral females (45.1% and 44.0%, respectively). These results indicate that only males seem to have evolved a higher artificial-mating ability during the process of adaptation to the laboratory.
Table 1.
Insemination rates in artificial-mating crosses between An. albitarsis from the laboratory colony, F1 mosquitoes of the original population (Massaranduba, Santa Catarina State, Brazil) and their reciprocal hybrids.
| cross | Number of copulas | Number of inseminated females |
| ♀ colony X ♂ colony | 85 | 57 (67.1%) |
| ♀ feral X ♂ colony | 49 | 38 (77.6%) |
| ♀ colony X ♂ feral | 51 | 23 (45.1%) |
| ♀ feral X ♂ feral | 25 | 11 (44.0%) |
| ♀ colony X ♂ hybrid A# | 44 | 37 (84.1%) |
| ♀ colony X ♂ hybrid B$ | 50 | 33 (66.0%) |
| ♀ hybrid A X ♂ colony | 29 | 19 (65.5%) |
| ♀ hybrid B X ♂ colony | 20 | 14 (70.0%) |
# hybrid A = F1 of ♀ feral X ♂ colony $ hybrid B = F1 of ♀ colony X ♂ feral
Analysis comparing crosses between colony and hybrids indicates that when crossed to colony males the two types of hybrid females have very similar insemination rates (X2 = 0.108; d.f. = 1; p = 0.7422), that are not significantly different from colony females (X2 = 0.001; d.f. = 1; p = 0.9727; after pooling hybrid females). In addition, comparison between hybrid males shows that they have rather similar insemination rates (X2 = 4.029; d.f. = 1; p = 0.0447; non-significant at 1% level, used here as multiple tests were carried out). In fact, they performed as well as colony males when crossed to colony females, with no significant difference in insemination rates (X2 = 1.189; d.f. = 1; p = 0.2756; after pooling hybrid males).
Table 2 shows the results of the free-mating experiments. The insemination rates were much lower than the ones observed in the artificial-mating experiments highlighting the difficulty these mosquitoes have in mating in a confined space. As before, we carried out a log-linear analysis comparing crosses between colony and feral mosquitoes. No significant effects on insemination rates were observed for the interaction between the sexes (X2 = 0.462; d.f. = 1; p = 0.4968) or female genotypes (X2 = 0.481; d.f. = 2; p = 0.7863). However, as observed for the artificial-mating experiments, there was a significant male effect (X2 = 11.133; d.f. = 2; p = 0.0038). In other words, feral males show much lower insemination rates than colony males, irrespective of the females used. Note that we have looked at the mortality rate of colony and feral males during this experiment as a trivial explanation for the differences in insemination rates. Despite the large number of colony (507 from most crosses) and feral males examined (393 from all crosses), no significant differences were observed in the cumulative numbers of dead males (152 colony and 135 feral) after six days (X2 = 1.947; d.f. = 1; p = 0.1629). Therefore, the results suggest again that during the process of adaptation to the laboratory only males seem to have improved their insemination rates. The two types of hybrid males show similar free-mating rates (X2 = 0.139; d.f. = 1; p = 0.7097) that are comparable to the rates observed for feral males (X2 = 0.033; d.f. = 1; p = 0.8551; after pooling hybrid males) and significantly different from the insemination rates of colony males (X2 = 7.922; d.f. = 1; p = 0.0049; after pooling hybrid males).
Table 2.
Insemination rates in free-mating crosses between An. albitarsis from the laboratory colony, F1 mosquitoes of the original population (Massaranduba, Santa Catarina State, Brazil) and their reciprocal hybrids.
| cross | Number of crosses | Number of inseminated females |
| ♀ colony X ♂ colony | 115 | 23 (20.0%) |
| ♀ feral X ♂ colony | 85 | 16 (18.8%) |
| ♀ colony X ♂ feral | 65 | 3 (4.6%) |
| ♀ feral X ♂ feral | 66 | 5 (7.6%) |
| ♀ colony X ♂ hybrid A# | 43 | 2 (4.7%) |
| ♀ colony X ♂ hybrid B$ | 30 | 2 (6.7%) |
# hybrid A = F1 of ♀ feral X ♂ colony $ hybrid B = F1 of ♀ colony X ♂ feral
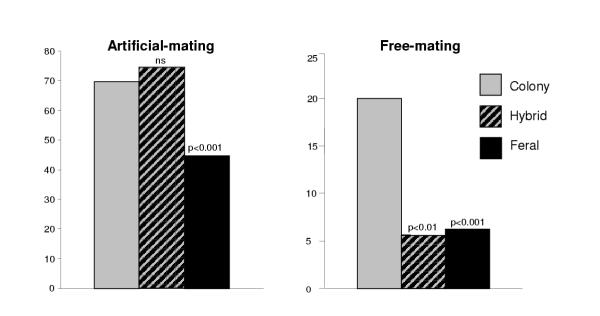
Figure 1 summarizes the results obtained with colony, hybrid and feral males in the artificial and free-mating experiments, pooling the data for the different female genotypes and the two types of hybrids. As mentioned before, the insemination rates observed for hybrid males in artificial-mating show that they perform as well as colony males suggesting that mainly dominant alleles were selected for in one or more autosomal genes since the establishment of the laboratory colony. However, in the free-mating experiments hybrid males show insemination rates comparable to feral males, suggesting that the adaptation to free-mating in the laboratory involved mainly one or more autosomal recessive factors. Therefore, it is very likely that different genes are involved in the higher artificial- and free-mating rates observed for colony males.
Figure 1.
Insemination rates in artificial- and free-mating crosses. The figure shows the insemination rates, in percentages, observed in artificial- (graph on the left) and free-mating crosses (graph on the right) using males from the colony (with all types of females) compared to the rates observed for males from the original population (feral) and its hybrids (pooled data for both types of hybrids).
Discussion
Swarming is very common in Anopheles mosquitoes [18] and it is probably an important component of mating behavior in some species. That is perhaps one of the reasons why it is so difficult to establish free-mating laboratory colonies of some important South American malaria vectors. They might not swarm properly in the confined space of laboratory cages and this might explain the low insemination rates in artificial conditions. In order to study the adaptation of our An. albitarsis colony to mating in the laboratory we intentionally used rather extreme conditions by placing three males and one female in small plastic tubes. This tends to decrease the free-mating insemination rates compared to the rates observed in larger cages using the same colony (J. B. P. Lima, unpublished observations).
The free-mating An. albitarsis colony was obtained after about 30 generations of artificial-mating [16]. Although an earlier attempt to establish a free-mating colony of this species had failed (J. B. P. Lima, unpublished observations) it is not clear whether the success in the second attempt is related to the generations of artificial mating or simply reflects improved methodology. As the genetic analysis has indicated, the genes involved in the high insemination rates shown by colony males in the two types of mating are probably different. Therefore the selection for successful insemination that most probably occurred during the artificial mating generations has not apparently selected, as a byproduct, genes that improved the insemination rates in free-mating. It is possible, however, that the selection coupled with drift caused by the small colony population size in the early generations have converted non-additive into additive genetic variation that was then available to be selected for once free-mating was tried again [19].
The fact that colony males, but not females, have improved their insemination rates in forced- and free-mating experiments, compared to the original population have at least two possible explanations. A trivial one is that males are the ones to have difficulties mating in a confined space. Perhaps females will mate more easily in the laboratory by either method used (forced-copulation or free-mating). If that is the case then only males would be under selection for improving their insemination rates.
Another possible explanation is that our results indicate that faster-male evolution of mating success occurred during the laboratory adaptation of An. albitarsis. The faster-male theory [20] predicts that sexual selection will cause faster rates of evolution of genes expressed in males. Interestingly, a recently published study shows that most of the interspecific differences in messenger RNA abundance between Drosophila melanogaster and its sibling D. simulans affect genes with male-biased expression [21].
If our results are indicative of faster-male evolution then this suggests that stronger sexual selection is acting on An. albitarsis males in our colony. Although we do not have direct evidence for that, we observed that each male can copulate with a number of females while multi-inseminated Anopheles females are rare, at least in the wild (e.g. [22]), even though rates might be higher in the laboratory [18]. Therefore, variance for reproductive success is likely to be higher in males than in females resulting in a more effective selection on male behavioral traits [23].
Whatever the explanation for the results we obtained it is clear that males might hold the key for successful colonization of other South American malaria mosquitoes such as An. darlingi and schemes which particularly select for males that are capable to mate in a restricted space might prove to be the way forward.
Conclusions
Our data show that during the colonization of An. albitarsis, males but not females have evolved higher rates of insemination in laboratory conditions. Although other explanations might be plausible, these results are consistent with the faster-male theory and suggest that the ability of males to mate in a confined space might be one of the most important aspects of mosquito biology involved in the successful laboratory colonization of South American malaria vectors.
Methods
The crossing experiments were carried using mosquitoes from the An. albitarsis s. s. colony maintained at the Instituto de Biologia do Exército, Rio de Janeiro, Brazil, and the F1 progeny of wild-caught females from the original population (Massaranduba, Santa Catarina State, Brazil, referred here as "feral") used to establish the colony [16]. An. albitarsis s. s. is the only member of the Albitarsis complex found in this locality [13].
The mosquitoes were reared as described in Horosko et al [16] with slight modifications. Wild-caught blood-fed Anopheles females were placed in screen-topped pint cartons and transferred to the laboratory in Rio de Janeiro. On day 3 after the blood meal, gravid females were anesthetized with ethyl acetate and the species identified using a taxonomic key. To obtain eggs, the An. albitarsis females were transferred from the screen-topped pint cartons, and placed into 500 ml cylindrical screened containers (9 cm wide), which were subsequently placed into a white plastic basin (15 × 8 × 4 cm) containing 100 ml of dechlorinated water. Two days after oviposition the cylindrical screened containers were removed. When the first eggs began to hatch powdered fish food (Tetramin™) was added to the basin. On day 4 after oviposition, larvae were separated into groups of 100 and placed in a 18 cm-diam × 8 cm deep white plastic basin, with 150 ml dechlorinated water, where they were reared until pupation. The pupae were transferred daily to 50 ml plastic cups and placed inside screened-top carton cages (18 cm-diam × 17 cm high). To ensure collection of virgin females [24], twice a day, the newly emerged adults were sexed and separated in smaller screened-pint cartons (10 to 20 adults per carton) and provided a 10% sucrose solution wetted cotton.
The forced-mating experiments were carried out using the artificial-mating technique described in Ow-Yang et al [17]. Mated females were placed into a screened pint carton and provided a cotton plug wetted with a 10% sucrose solution. To induce egglaying, on the third day after artificial-mating, one wing was removed from each female [25] and they were placed individually in 50 ml plastic cups filled with dechlorinated water. After oviposition, the eggs were counted, each female was dissected and the spermathecae examined for sperm by light microscopy.
The free-mating experiments were performed in 50 ml Falcon tubes enclosed by nylon mesh and containing cotton wetted on water in the bottom covered by filter paper. A 10% sucrose solution was also provided by wetted cotton on the top of the tube. Crosses were set up using three males and one female. Preliminary analysis of colony mosquitoes indicates that little change in the insemination rates occurred after five days. Therefore, they were kept together for up to 7 days or until all males were dead, when each female was dissected and the spermathecae examined for sperm by light microscopy.
Authors' contributions
JL carried out the experimental work. DV and AP participated in the analysis of the data and drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported by FIOCRUZ, FAPERJ and CNPq. AAP is supported by the Howard Hughes Medical Institute. We would like to thank Sabrina C. Gonçalvez de Carvalho, Henrique M. da Costa Ramon and Eliane de la Plata Ruiz for their technical assistance and Dr. Maria Braulia (Secretaria Estadual de Saúde de Santa Catarina), FUNASA/Joinvile and Secretaria Municipal de Saúde de Massaranduba for their help with the field work. We also would like to thank Bambos Kyriacou and Mike Ritchie for comments on an earlier version of the manuscript.
Contributor Information
José BP Lima, Email: josebento@globo.com.
Denise Valle, Email: dvalle@ioc.fiocruz.br.
Alexandre A Peixoto, Email: apeixoto@fiocruz.br.
References
- Krzywinski J, Besansky NJ. Molecular systematics of Anopheles: from subgenera to subpopulations. Annu Rev Entomol. 2003;48:111–139. doi: 10.1146/annurev.ento.48.091801.112647. [DOI] [PubMed] [Google Scholar]
- Kreutzer RD, Kitzmiller JB, Rabbani MG. Cytogenetically distinguishable sympatric and allopatric populations of mosquito Anopheles albitarsis. Acta Amazônica. 1976;6:473–481. [Google Scholar]
- Rosa-Freitas MG. Anopheles (Nyssorhynchus) deaneorum: a new species in the albitarsis complex (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 1989;84:535–543. [Google Scholar]
- Rosa-Freitas MG, Deane LM, Momen H. A morphological, isoenzymatic and behavioural study of ten populations of Anopheles (Nyssorhynchus) albitarsis Lynch-Arribalzaga, 1878 (Diptera: Culicidae) including from the Type-Locality–Baradero, Argentina. Mem Inst Oswaldo Cruz. 1990;85:275–289. [Google Scholar]
- Klein TA, Lima JBP, Toda-Tang T. Hybridization evidence supporting separate species status for Anopheles albitarsis and Anopheles deaneorum (Diptera: Culicidae) in Brazil. J Am Mosq Control Assoc. 1991;7:301–303. [PubMed] [Google Scholar]
- Narang SK, Klein TA, Pereira OP, Lima JBP, Toda-Tang A. Genetic evidence for the existence of cryptic species in the Anopheles albitarsis complex in Brazil: allozymes and mitochondrial DNA restriction fragment length polymorphism. Biochem Genet. 1993;31:97–112. doi: 10.1007/BF02399823. [DOI] [PubMed] [Google Scholar]
- Arruda M, Carvalho MB, Nussenweig RS, Maracic M, Ferreira AW, Cochrane AH. Potential vector of malaria and their different susceptibility to Plasmodium falciparum and Plasmodium vivax in Northern Brazil identified by immunoassay. Am J Trop Med Hyg. 1986;60:1010–1018. doi: 10.4269/ajtmh.1986.35.873. [DOI] [PubMed] [Google Scholar]
- Deane LM. Malaria studies and control in Brazil. Am J Trop Med Hyg. 1988;38:223–230. doi: 10.4269/ajtmh.1988.38.223. [DOI] [PubMed] [Google Scholar]
- de Oliveira–Ferreira J, Lourenço-de-Oliveira R, Teva A, Deane LM, Daniel-Ribeiro CT. Natural malaria infections in Anophelines in Rondônia State, Brazilian Amazon. Am J Trop Med Hyg. 1990;43:6–10. [PubMed] [Google Scholar]
- Klein TA, Lima JBP, Toda-Tang A. Biting behavior of Anopheles mosquitoes in Costa Marques, Rondônia, Brazil. Rev Soc Bras Med Trop. 1991;24:13–20. doi: 10.1590/s0037-86821991000100003. [DOI] [PubMed] [Google Scholar]
- Klein TA, Lima JBP, Tada MS, Miller R. Comparative susceptibility of Anopheles mosquitoes in Rondônia, Brazil, to infection by Plasmodium vivax. Am J Trop Med Hyg. 1991;45:463–470. doi: 10.4269/ajtmh.1991.45.463. [DOI] [PubMed] [Google Scholar]
- Klein TA, Lima JBP, Tada MS, Miller R. Comparative susceptibility of anopheline mosquitoes to Plasmodium falciparum in Rondônia, Brazil. Am J Trop Med Hyg. 1991;44:598–603. doi: 10.4269/ajtmh.1991.44.598. [DOI] [PubMed] [Google Scholar]
- Wilkerson RC, Gaffigan TV, Lima JBP. Identification of species related to Anopheles (Nyssorhynchus) albitarsis by random amplified polymorphic DNA-polymerase chain reaction (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 1995;90:721–732. doi: 10.1590/s0074-02761995000600013. [DOI] [PubMed] [Google Scholar]
- Póvoa MM, Wirtz RA, Lacerda RNL, Miles MA, Warhurst D. Malaria vectors in the municipality of Serra do Navio, state of Amapá, Amazon Region, Brazil. Mem Inst Oswaldo Cruz. 2001;96:179–184. doi: 10.1590/s0074-02762001000200008. [DOI] [PubMed] [Google Scholar]
- Conn JE, Wilkerson RC, Segura MN, de Souza RT, Schlichting CD, Wirtz RA, Povoa MM. Emergence of a new Neotropical malaria vector facilitated by human migration and changes in land use. Am J Trop Med Hyg. 2002;66:18–22. doi: 10.4269/ajtmh.2002.66.18. [DOI] [PubMed] [Google Scholar]
- Horosko S, Lima JBP, Brandolini MB. Establishment of a free-mating colony of Anopheles albitarsis from Brazil. J Am Mosq Control Assoc. 1997;13:95–96. [PubMed] [Google Scholar]
- Ow-Yang CF, Sta-Maria FL, Wharton RH. Maintenance of a laboratory colony of Anopheles maculates Theobald by artificial mating. Mosq News. 1963;23:34–35. [Google Scholar]
- Clements AN. Sensory and Behaviour. Vol. 2. Wallingford: CABI Publishing; 1999. The biology of mosquitoes. [Google Scholar]
- Whitlock MC, Phillips PC, Wade MJ. Gene interaction affects the additive genetic variance in subdivided populations with migration and extinction. Evolution. 1993;47:1758–1769. doi: 10.1111/j.1558-5646.1993.tb01267.x. [DOI] [PubMed] [Google Scholar]
- Wu CI, Davies AW. Evolution of post-mating reproductive isolation: the composite nature of Haldane's rule and its genetic bases. Am Nat. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Ranz JM, Castillo-Davis CI, Meiklejohn CD, Hartl DL. Sex-dependent gene expression and evolution of the Drosophila transcriptome. Science. 2003;300:1742–1745. doi: 10.1126/science.1085881. [DOI] [PubMed] [Google Scholar]
- Tripet F, Toure YT, Dolo G, Lanzaro GC. Frequency of multiple inseminations in field-collected Anopheles gambiae females revealed by DNA analysis of transferred sperm. Am J Trop Med Hyg. 2003;68:1–5. [PubMed] [Google Scholar]
- Andersson M. Sexual selection. New Jersey: Princeton University Press; 1994. [Google Scholar]
- Clements AN. Development, nutrition and reproduction. Vol. 1. London: Chapmann & Hall; 1992. The biology of mosquitoes. [Google Scholar]
- Lanzaro GC, Narang SK, Mitchell SE, Kaiser PE, Seawright JA. Hybrid male sterility in crosses between field and laboratory strains of Anopheles quadrimaculatus Say (Diptera: Culicidae) J Med Entomol. 1988;25:248–255. doi: 10.1093/jmedent/25.4.248. [DOI] [PubMed] [Google Scholar]